Abstract
The increasing prevalence of diabetes, combined with a growing global shortage of health care professionals (HCP), necessitates the need to develop new approaches to diabetes care delivery to expand access to care, lessen the burden on people with diabetes, improve efficiencies, and reduce the unsustainable financial liability on health systems and payers. Use of digital diabetes technologies and telehealth protocols within a digital/virtual diabetes clinic has the potential to address these challenges. However, several issues must be resolved to move forward. In February 2020, organizers of the Advanced Technologies & Treatments for Diabetes Annual Conference convened an international panel of HCP, researchers, patient advocates, and industry representatives to review the status of digital diabetes technologies, characterize deficits in current technologies, and identify issues for consideration. Since that meeting, the importance of using telehealth and digital diabetes technologies has been demonstrated amid the global coronavirus disease (COVID-19) pandemic. This article summarizes the panel's discussion of the opportunities, obstacles, and requisites for advancing the use of these technologies as a standard of care for the management of diabetes.
Keywords: Telemedicine, Digital tools, AGP, Type 1 diabetes, Type 2 diabetes, Connected devices
Introduction
Despite advances in antidiabetic medications and diabetes technologies, a substantial percentage of people with diabetes (PwD) are not achieving their treatment goals, resulting in inadequate clinical outcomes.1,2 A key contributor to suboptimal glycemic control is therapeutic inertia, a combination of failure to initiate or intensify therapy according to evidence-based clinical guidelines and nonadherence to prescribed treatment.2–5 Although several barriers to therapy intensification have been cited,6–10 the underlying obstacle is often the inability to readily access accurate and sufficient clinically relevant data that are presented in standardized formats that can be readily interpreted and acted upon.11–13
Technology advances create the potential to address this obstacle through use of digital diabetes technologies that automatically collect, transfer, and interpret relevant diabetes data in ways that facilitate more informed therapy decisions.
In February 2020, organizers of the Advanced Technologies & Treatments for Diabetes (ATTD) Annual Conference convened an international panel of health care professionals (HCP), researchers, patient advocates, and industry observers to review the status of digital diabetes and telehealth technologies, characterize deficits, and identify issues for consideration.
Following the meeting, principals of the panel discussed the importance of these technologies in the face of the global coronavirus disease (COVID-19) pandemic, which has prompted a dramatic restructuring of health care delivery.14–18 This article summarizes the panel's discussion of the opportunities, obstacles, and requisites for advancing the use of these technologies as a standard of care for the management of diabetes.
Efficacy of Digital Tools and Telemedicine Technologies
Digital technologies
Continuous glucose monitoring
Numerous studies have demonstrated the clinical efficacy of continuous glucose monitoring (CGM) use in individuals with type 1 diabetes (T1D) and type 2 diabetes (T2D).19–28 The recent DIAMOND trials showed that use of real-time CGM (rtCGM) resulted in lower glycated hemoglobin (HbA1c) levels, less time spent in the hypoglycemic and hyperglycemic ranges, and reductions in moderate to severe hypoglycemia in T1D and T2D individuals treated with multiple daily insulin injections (MDI) compared with traditional self-monitoring of blood glucose (SMBG).21,22
The more recent HypoDE trial showed that the use of rtCGM in T1D adults with problematic hypoglycemia resulted in fewer low-glucose events and episodes of severe hypoglycemia.29 Significant and sustained reductions in HbA1c over 3 years, with increases in the percent time in range (%TIR) and reductions in percent time below range (%TBR) in T1D adults treated with MDI or sensor-augmented pump (SAP) therapy using rtCGM compared with SMBG have also been demonstrated.23
In the recent CONCEPTT trial, investigators assessed the clinical impacts of rtCGM use versus SMBG within a cohort of 325 T1D women who were pregnant or planning to become pregnant.30 Investigators reported significant increases in %TIR with rtCGM compared with SMBG use (68% vs. 61%; P = 0 · 0034, respectively). The recommended %TIR for pregnancy in T1D is >70% at 63–140 mg/dL (3.5–7.8 mmol/L).31 rtCGM use was also associated with lower incidence of large for gestational age (P = 0.0210), fewer neonatal intensive care admissions lasting >24 h (P = 0.0157), fewer incidences of neonatal hypoglycemia (P = 0 · 0250), and 1-day shorter length of hospital stay (P = 0 · 0091).
In a large prospective cohort study, investigators reported significantly improved overall glycemic control, lower glycemic variability, and reductions in mean infant birth weight among women with gestational diabetes who used rtCGM compared with SMBG.32
Use of intermittently scanned CGM (isCGM) has also been shown to confer glycemic benefits in T1D25,26 and T2D27,28 adults treated with intensive insulin therapy. In adults with well-controlled T1D, isCGM use was associated with a 38% reduction in time spent in hypoglycemia (<70 mg/dL).25 Similar findings were reported in the REPLACE trial, which showed a 43% reduction in time spent in hypoglycemia.28 There is also emerging evidence associating iCGM use in individuals treated with less-intensive insulin or noninsulin therapy with improved HbA1c33 and reductions in acute diabetes-related events and all-cause hospitalizations.34
Insulin delivery
The benefits of SAP with predictive low-glucose suspend functionality have also been demonstrated.35–41 Weiss et al. reported that in-home use of a hybrid closed-loop was associated with reductions in nocturnal hypoglycemia events compared with use when the low-glucose suspend function was disengaged.41 Use of hybrid closed-loop in adults and adolescents with T1D has also been shown to increase time in glycemic range and reductions in HbA1c, hyperglycemia, and hypoglycemia.37 Similar results were reported by Bergenstal et al.36
We have also seen promising evidence of improved glycemic control with use of closed-loop control insulin delivery systems.42–44 In a recent randomized-controlled trial, 168 T1D patients, the use of closed-loop control was associated with a higher %TIR compared with SAP.45 A subset of the study was then randomly assigned to hybrid closed-loop with low-glucose suspend and followed for an additional 3 months.46 Similar to the previous findings, participants who switched to hybrid closed-loop experienced significant reductions in time in glycemic range and increased HbA1c, but with similar reductions in hypoglycemia in both groups.
Telemedicine/telemonitoring technologies
There is a growing body of evidence that supports various applications of telemedicine and telemonitoring technologies as effective alternative methods of health care delivery. Several recent meta-analyses and systematic reviews of randomized-controlled trials have demonstrated the addition of telemedicine and telemonitoring interventions in adult and pediatric patients with T1D and T2D.47–55 Telemedicine interventions appear to be most effective when HCP used Web portals or text messaging to communicate with patients and when telemedicine facilitated medication adjustment.47
A recent randomized-controlled trial involving 74 T1D adults followed for 1 year showed similar reductions in HbA1c between those supported by teleconsultations or standard care (−1.9 mmol/mol [−0.17%] vs. −0.8 mmol/mol [−0.07%], respectively, P = 0.60).54 However, participants randomized to teleconsultation interventions reported no severe hypoglycemia events compared with six hypoglycemia-related emergency room admissions in the standard care group. Most reported increased satisfaction with these interventions, improved self-management, time savings, and cost reductions.
An earlier study by Charpentier et al. looked at the impact of using an app-based software program (Diabeo) for remote insulin-dosing advice with and without follow-up teleconsultations among 180 adult T1D patients treated with basal-bolus therapy.55 Use of the program with teleconsults resulted in a 0.91% HbA1c reduction, and a 0.67% reduction without teleconsult follow-up.
Dixon et al. recently reported a study that investigated use of a novel telehealth technology/care model (Virtual Diabetes Clinic) that combines connected devices (e.g., blood glucose meter, rtCGM, remote lifestyle coaching, and clinical support with a mobile app.56
Preliminary data among T2D adults who used rtCGM (n = 740) suggested that participation in the Virtual Diabetes Clinic program was associated with a significant improvement in HbA1c with up to 6 months follow-up in those not meeting treatment targets. HbA1c decreased by 2.3% ± 1.9%, 0.7% ± 1.0%, and 0.2% ± 0.8% across baseline categories of >9.0%, 8.0%–9.0%, and 7.0% to <8.0%, respectively (all P < 0.001). Participation in the Virtual Diabetes Clinic program has also been associated with reductions in diabetes-related distress as measured by the Diabetes Distress Scale, specifically in the subscale score for regimen-related distress.57
In a recent meta-analysis of 32 randomized-controlled trials that included 5108 women with gestational diabetes, use of telemedicine-based interventions was associated with significant improvements in HbA1c, fasting blood glucose, and 2-h postprandial blood glucose compared with standard care.58 The telemedicine group also experienced better neonatal outcomes, including lower incidences of cesarean section, neonatal hypoglycemia, premature rupture of membranes, macrosomia, pregnancy-induced hypertension or preeclampsia, preterm birth, neonatal asphyxia, and polyhydramnios.
A 12-month prospective pediatric study reported a significant increase in the average number of clinician consultations in 54 pediatric patients using telemedicine compared with the previous year (2.9 ± 1.3 vs. 2.0 ± 1.3 times per year, respectively [P < 0.0001]), with no significant changes in HbA1c.59 More recently, Crossen et al. studied the effects of home-based video visits in a cohort of pediatric T1D patients with poor glycemic control.60 During the 12 months before the study, clinic visits averaged 3.2 ± 1.1. During the 6-month study period, total clinician interactions increased to 5.8 ± 1.5 (P < 0.001), which included 4.0 ± 1.1 video visits and 1.8 ± 0.6 clinic visits.
Opportunities
Leveraging the diabetes ecosystem
Continuous development of innovative digital health technologies has created a diabetes ecosystem that is populated with a diverse offering of digital tools and capabilities, including connected medical devices, social networking, decision support software, remote coaching programs, and rapidly advancing data analytics. The ultimate goal is create a digital virtual diabetes clinic (D/VDC) that integrates core components from the ecosystem into an overarching architecture of feedback mechanisms that facilitate seamless transfer of real-time diabetes data to monitor health status, aid in diagnosis of pertinent concerns, guide therapy decisions, and advise/adjust treatment directly between PwD and HCP.
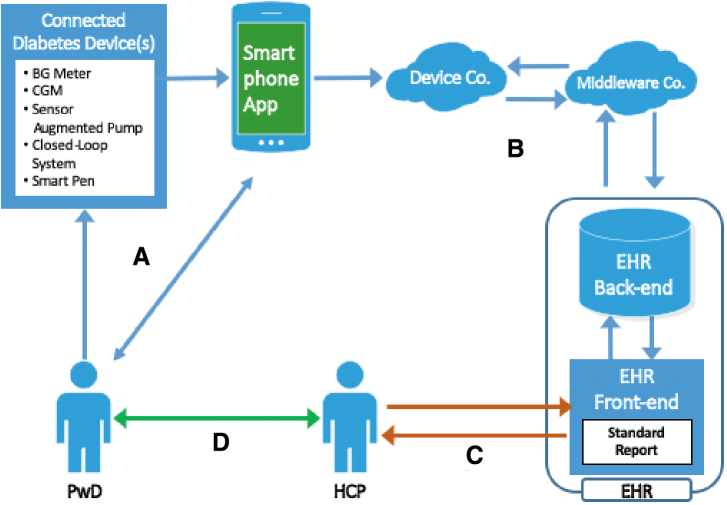
The first step in advancing the D/VDC is establishing universal protocols and mechanisms for transferring glucose and insulin data from PwD to HCP via cloud-based software in which the data are presented in standardized, easy-to-interpret formats (such as the ambulatory glucose profile)61 that are compatible with electronic health record (EHR) systems. HCP can then choose from various telehealth technologies (e.g., phone, text, videoconference) to provide feedback to PwD as needed. Figure 1 presents a conceptual representation of the D/VDC approach to diabetes care.
FIG. 1.
Conceptual representation of D/VDC information flow and feedback. (A) Data from the device(s) are automatically transmitted to a smartphone app, which provides immediate feedback to the user. (B) The app transfers the data to the EHR via cloud-based, device-specific software and middleware. (C) HCP then access the data, which are presented in standardized formats. HCP enter any therapy changes into the EHR and (D) contact the patient to set up an in-clinic visit or schedule a remote consultation via one of the telemedicine tools. D/VDC, digital/virtual diabetes clinic; EHR, electronic health record; HCP, health care professional.
Potential outcomes of the D/VDC approach
Implementation of the D/VDC has the potential to address many of the current obstacles to effective and efficient diabetes management. Importantly, the D/VDC approach directly addresses therapy inertia by facilitating more timely and informed therapy adjustments to avoid delays in treatment escalation. This approach limits the costs associated with chronic disease management due to frequent travel for face-to-face visits. The expected result is improved overall diabetes control and greater treatment satisfaction, both of which are associated with enhanced treatment adherence and quality of life.62
The ability of PwD to interact remotely via smartphones and other communication devices can significantly increase access to HCP and support programs (e.g., diabetes coaching, online support groups). However, protocols for HCP frequency of data review and how/when HCP respond to their PwD must established.
Technologies for remote monitoring, counseling, and some physical examinations (e.g., retinal scanning, foot examinations, blood pressure measurement) are especially valuable in providing care in rural areas or many low-income countries where even basic health care services are scarce or nonexistent. These technologies greatly expand the capacity of health systems to meet the needs of the growing diabetes populations despite the current and projected shortage of HCP.
Another expectation is enhanced population health management, which can manifest in several ways. The D/VDC creates a large database of interventions and outcomes that can be mined for risk stratification within various diabetes populations and to standardize evidence-based best practices that can be disseminated to HCP. This not only improves efficiency in health resource allocation by providing evidence-based guidance but also overcomes limitations in HCP expertise. In particular, the use of remote monitoring and subsequent interventions may minimize the number of unnecessary F2F clinic visits, thereby freeing up HCP time to address the needs of PwD who have more complicated needs.
All of these outcomes have the potential to lower the direct and indirect costs of diabetes care. Improvements in diabetes control will significantly reduce the number and severity of preventable hospitalizations, emergency department visits, and other health care services/medications associated with treating the acute and long-term complications of diabetes. Although time efficiencies realized from the D/VDC approach may also reduce the costs associated with delivery of health care services, the costs associated with setting up and maintaining equipment and infrastructure (e.g., broadband networks/facilities, computers, and licenses) cannot be ignored. However, D/VDC should become cost effective in the long run.
Obstacles
Current tools and technologies provide the basic functionality for the development of the envisioned D/VDC approach to care. However, a number of technical, policy, regulatory, and logistical obstacles must be addressed to bring this approach to fruition.
Lack of interoperability and data compatibility
Lack of interoperability between manufacturers and third-party devices and software remains a critical obstacle to optimizing use of the full array of tools within the diabetes ecosystem and forces end users to navigate with multiple disparate manufacturers' platforms, none of which will, alone, serve their needs.63 Integrating data downloads from incompatible devices also poses significant challenges to efficient clinic workflow and reduces the time HCP can spend with PwD.
Although the European Union imposed standards for interoperability in 2009, these standards are yet to be implemented. No standards are in place in the United States. Moreover, incompatibility between diabetes digital devices and EHRs often requires HCP to “cut and paste” glucose and insulin data into patient records, which limits retrospective analysis of patient data, negatively impacts efficiency, and contributes to clinician burnout.64 A dedicated, interoperable diabetes record is often lacking.65
Lack of clarity on data ownership and accessibility
There is an ongoing debate about the ownership of medical data among PwD, HCP, insurance companies, device manufacturers, and software developers. Moreover, health data captured in EHRs and manufacturer cloud portals are not always accessible by PwD and their HCP. It was the agreement of the ATTD panel that PwD should retain ownership and unfettered access to their data.
Inadequate or inconsistent HCP reimbursement
Inadequate and/or inconsistent reimbursement for digital-based interventions (e.g., downloading and interpreting glucose data) and use of telemedicine have inhibited the widespread use of such approaches. Many HCP are reluctant to invest in information technology out of concern that their time and investment may not be reimbursed. In some countries, such as the United States, uniformity in reimbursement rates from region to region is lacking. Of key concern in the United States are restrictions on practicing medicine across state lines. This significantly limits opportunities to utilize telemedicine technologies to their fullest potential.
Restrictive PwD insurance coverage
Although insurance coverage for diabetes medical devices has improved over the years, eligibility criteria continue to restrict the use of these devices among many PwD who would benefit. For example, Medicare and many other private insurers only cover CGM systems for PwD who are treated with intensive insulin therapy (≥3 injections/day or insulin pump) and currently performing SMBG ≥4 times daily.
Sparse evidence supporting D/VDC approach
There is a lack of definitive evidence from appropriately designed and well-conducted clinical trials. Because digital-based interventions encompass a wide range of technologies and applications, it is difficult to generalize findings from any specific study. Although numerous studies have evaluated the efficacy and usability of various tools and approaches, systematic reviews and meta-analyses have failed to provide definitive guidance due to differences in study designs, durations of observation, populations, and the tools/technologies studied. The cost and complexity of generating a robust body of evidence that supports the viability of the D/VDC approach remain a key challenge.
Requisites for Advancing D/VDC Approaches
Stakeholders include device manufacturers, online diabetes service providers, software developers (apps and network systems), EHR providers and developers, payers (public and private), regulatory agencies, health system administrators, and HCP.
Initiate stakeholder collaborations to achieve full interoperability and data compatibility
Seamless transmission and interpretation of data can only occur through universal interoperability among medical devices, device-specific software, EHR systems, and other HCP interface portals. The ATTD panel agreed that there is a clear need to re-engineer EHRs to facilitate integration of patient-generated data across the care continuum through protocols that support traditional face-to-face and alternative care delivery models while advancing data analytics for personalized, precision medicine and ensuring the delivery of many elements of diabetes management advice remotely (e.g., telehealth).
The ideal would be to have the EHR communicate data directly to the HCP. Current protocols for entering diabetes data add an extra step that makes data interpretation more burdensome for the HCP. Importantly, there is an ongoing need to ensure privacy, confidentiality, and security with seamless flow of data. This will require ongoing collaborations between all stakeholders to develop device/app software and EHR systems to achieve standardized data interfaces and reports.
Medical organizations should consider formalizing advocacy initiatives to pass legislation and/or regulatory policies that enforce standards that require interoperability between all devices and medically approved apps and all device download software and EHR systems. Requiring data downloading capability in all new glucose monitoring devices should also be considered.
Ensure that PwD retain ownership and unfettered access to their data
Studies have shown that access to medical records is beneficial for both patients and clinicians.66 Since 1997, the European legislation has protected patients' rights to access their clinical data when requested and have control over who can see and change that information.67 However, this does not often occur. It was the agreement of the ATTD panel that PwD should retain ownership and unfettered access to their data.
Provide adequate HCP reimbursement for use of diabetes digital and telehealth technologies
The ATTD panel recommended initiation of collaborations/coordination between stakeholders to develop and implement reimbursement models that adequately support HCP in the efficient utilization of D/VDC approaches. Moreover, reimbursement should be extended to all relevant HCP, including diabetes nurses, diabetes educators, dietitians, and psychologists who expand the workforce by providing important education and counseling services. Given the rapid adoption and demonstrated critical importance of telemedicine in response to the COVID-19 pandemic, it is likely that the relaxed regulations and reimbursement policies become permanent. It recommended that medical organizations advocate for regulatory changes that further simplify and expand the medical licensure across state and national boundaries.
Ease restrictions for PwD insurance coverage
The coverage requirements for frequent SMBG and intensive insulin therapy are unnecessarily restrictive and medically unfounded. In a subanalysis of PwD who participated in the DIAMOND T1D21 and T2D22 trials, Ruedy et al. reported a significant improvement in glycemic control among older CGM users (age 67 ± 5 years), but no association between A1C reductions and SMBG frequency was observed; 52% of the CGM users reported performing SMBG <4 times daily at baseline.68
Moreover, results from recent database analyses of individuals who switched from SMBG to CGM showed no correlation between prior daily frequency of blood glucose monitoring and adverse diabetes-related events.69,70 The ATTD panel agreed that the eligibility criteria for coverage of digital diabetes technologies should be based on current scientific evidence and frequently updated as new evidence becomes available.
Generate evidence that addresses the information needs of all stakeholders
D/VDC approaches must be adaptable, scalable, and clearly demonstrate safety, efficacy, and cost effectiveness using study designs and methodologies that address the needs of all stakeholders. Importantly, as reported by Mayberry et al., new approaches to the design, implementation, and evaluation of mobile and Internet interventions for disadvantaged and vulnerable PWD are needed.71 Research programs should utilize both RCT designs in combination with real-world observational studies.
Summary
Clinicians, health systems, payers, and policy makers are challenged to develop effective strategies to address the increasing global prevalence of diabetes and the growing shortage of HCP. Digital diabetes technologies have the potential to increase access to care, reduce costs, and improve clinical outcomes and quality of life. Although often considered to be more futuristic than realistic, telemedicine technologies have now proven to be the best option and, in many cases, the only option for providing critical health care for PwD as the COVID-19 pandemic runs its course.
Indeed, one benefit of COVID-19 has been the rapid adoption of telemedicine in many countries. In India, for example, any form of telemedicine (including telephone consultations) was considered illegal until the COVID-19 emergency hit the country. Within a week, telemedicine was legalized in India and the government established guidelines for all forms of telemedicine. It was widely adopted, and, in fact, it may change the way medicine is practiced in the future. These data facilitate ongoing quality improvement within health systems and provide robust guidance for payers, policy makers, and regulatory agencies.
However, several obstacles must be addressed. Achieving interoperability is a critical factor. Adequate reimbursement schedules for devices and HCP services must be established. Most critical is protecting data/device integrity and safeguarding privacy. In addition, insurers and regulatory agencies must play a role in supporting D/VDC approaches through less restrictive reimbursement policies and regulations. Finally, all digital strategies must be scalable and properly validated in carefully designed research programs to demonstrate efficacy, safety, and cost effectiveness.
Overcoming these obstacles will require close collaboration between all stakeholders. Also, it will require compromise, particularly in bringing manufacturers and software developers together to establish standards for interoperability and data sharing, while keeping clinicians and PwD in the loop. The recommendations presented here are intended to provide a starting point for advancing D/VDC approaches to diabetes management.
Acknowledgments
The authors wish to thank the Advanced Technologies and Treatments for Diabetes (ATTD) organizers for planning and coordinating the meeting. They also wish to thank Rachel Naveh for assistance in organizing the meeting. Panel Attendees—Participants: Katharine Barnard-Kelly, UK; Ananda Basu, USA; Tadej Battelino, Slovenia; Roy Beck, USA; Richard Bergenstal, USA; Torben Biester, Germany; Bruce Bode, USA; Pratik Choudhary, UK; Mark Clements, USA; Kelly Close, USA; Claudio Cobelli, Italy; Deniz Dalton, USA; Thomas Danne, Germany; Eyal Dassau, USA; J. Hans DeVries, the Netherlands; Kim Donaghue, Australia; Klemen Dovc, Slovenia; Frank Doyle III, USA; Leon Farhy, USA; Gregory Forlenza, USA; Satish Garg, USA; Lutz Heinemann, Germany; Irl Hirsch, USA; Neal Kaufman, USA; David Klonoff, USA; Boris Kovatchev, USA; Aaron Kowalski, USA; Lori Laffel, USA; Courtney Lias, USA; David Maahs, USA; Sanjeev Mehta, USA; Laurel Messer, USA
V. Mohan, India; Pablo Mora, USA; Revital Nimri, Israel; Tal Oron, Israel; Christopher G. Parkin, USA; Moshe Phillip, Israel; Manel Puig Domingo, Spain; Eric Renard, France; Michael Riddell, Canada; Helena Rodbard, USA
David Rodbard, USA; Julio Rosenstock, USA; Banshi Saboo, India; Jay Skyler, USA; Tatsuhiko Urakami, Japan
Henk J Veeze, the Netherlands; Paul Wadwa, USA; Stuart Weinzimer, USA; Lorena Wright, USA—Industry Observers: Howard Look, Tidepool; Eran Atlas, DreaMed; Alejando Galindo, Medtronic; Ali Dianaty, Medtronic; Marlene Gyldmark, Roche; Rolf Hinzmann, Roche; Ron Dixon, Onduo; Jennifer Layne, Onduo; Bryan Mazlish, Bigfoot; Howard Zisser, Verily; David Price, Dexcom; Keri Leone, Dexcom; Galit Kopatz, Novo Nordisk; Anders Dhyr Toft, Novo Nordisk; Howard Wolpert, Eli Lilly; Marie Schiller, Eli Lilly; Maurizio Guidi, Eli Lilly; Xavier De Anda, Tandem; Manuel Jaime, Tandem; Maeve Germe, Sanofi; Zslot Bosnyak, Sanofi; Reman McDonagh, Insulet; Eric Benjamin, Insulet; Jeff Halpern, Abbott; Alexander Seibold, Abbott; Brian Levine, Onduo; Albert Cai, Close Concern; Martin Kurian, Close Concern; Cherise Shokley, Close Concern; Natalie Bellini, Close Concern; Emily Fitts, DiaTribe.
Authors' Contributions
All authors participated in writing/revising the initial drafts and approved the final article for submission. M.P. is the guarantor of this work and takes full responsibility for the integrity of the information included in the report.
Author Disclosure Statement
M.P. is a member of the Advisory Board of AstraZeneca, Sanofi, Medtronic, Eli Lilly, Novo Nordisk, Insulet, and Pfizer and is a consultant to Qulab Medical. The Institute headed by M.P. received research support from Medtronic, Novo Nordisk, Eli Lilly, Dexcom, Sanofi, Insulet, OPKO, and DreaMed Diabetes. M.P. is a stock/shareholder of DreaMed Diabetes, NG Solutions, and Nutriteen Professionals and reports two patent applications. R.M.B. has received research funding, served as a consultant or served on advisory boards for Abbott Diabetes Care, Ascensia, Dexcom, Eli Lilly and Company, Glooko, Helmsley Charitable Trust, Hygieia, Johnson & Johnson, Medtronic, Merck, Novo Nordisk, Onduo, Roche, Sanofi, Senseonics and United Healthcare; his employer, nonprofit HealthPartners Institute, contracts for his services and no personal income goes to R.M.B. K.L.C. is an employee of Close Concerns and diaTribe, which receive funding for CGM manufacturers, including Medtronic, Dexcom and Abbott Diabetes Care. T.D. has received speaker honoraria, research support, and consulting fees from Abbott Diabetes Care, Bayer, BMS, AstraZeneca, Boehringer Ingelheim, Dexcom, Eli Lilly & Company, Medtronic, Novo Nordisk, Sanofi, and Roche Diabetes Care; he is a shareholder of DreaMed Diabetes. S.K.G. has received consulting fees and honoraria for participation on advisory boards for Medtronic, Roche Diabetes Care, Merck, Lexicon, Novo-Nordisk, Sanofi, MannKind, Senseonics, Zealand, and Eli Lilly & Company, and research grants from Eli Lilly and Company, Novo-Nordisk, Merck, Lexicon, Medtronic, Dario, NCI, T1D Exchange, NIDDK, JDRF, Animas, Dexcom, and Sanofi. L.H. is a consultant for companies developing novel diagnostic and therapeutic options for diabetes. He is a shareholder of Profil Institut für Stoffwechselforschung GmbH, Neuss, Germany, and ProSciento, San Diego, USA. I.B.H. receives research funding from Medtronic Diabetes and Insulet, and has received consulting fees from Abbott Diabetes Care, Bigfoot, Roche, and Becton Dickinson. B.P.K. declared research support handled by the University of Virginia from Dexcom, Roche, Sanofi, Tandem; patent royalties handled by the University of Virginia from Johnson & Johnson, Sanofi, Dexcom; consultant: Sanofi, Tandem; advisory board/speaker's bureau: Dexcom. L.M.L. receives consulting fees from Eli Lilly, Sanofi, Novo Nordisk, Roche, Dexcom, Insulet, Boehringer Ingelheim, Janssen, Unomedical, Medtronic, Laxmi and Insulogic. V.M. has received honoraria, research grants, consulting and speaking fees from Medtronic, Abbott, J & J, LifeScan, Novo Nordisk, Sanofi, MSD (Merck), Novartis, Boehringer Ingelheim, and several Indian pharmaceutical companies, including USV Ltd., Dr. Reddy's Labs, Intas Pharma, Sun Pharma, Lupin, and IPCA. C.G.P. has received consulting fees from Abbott Diabetes Care, American Diabetes Association, American Association of Clinical Endocrinologists, Dexcom, Diasome, Onduo, Novo Nordisk, and Roche Diabetes Care. T.B. has received honoraria for participation on advisory boards for Novo Nordisk, Sanofi, Eli Lilly & Company, Boehringer, Medtronic, and Bayer Health Care, and as a speaker for Astra Zeneca, Eli Lilly & Company, Bayer, Novo Nordisk, Medtronic, Sanofi, and Roche. T.B. owns stocks of DreaMed Diabetes; his institution has received research grant support and travel expenses from Abbott Diabetes Care, Medtronic, Novo Nordisk, GluSense, Sanofi, Sandoz, and Diamyd.
Funding Information
Support for the D/VDC meeting and development of this article was provided by the Advanced Technologies and Treatments for Diabetes (ATTD). Some panel participants were reimbursed for travel to the ATTD conference and one/two nights of lodging; no honoraria were provided. No industry participants received reimbursement for travel/lodging. ATTD provided funding to Christopher G. Parkin, MS, CGParkin Communications, Inc., for his editorial support.
References
- 1. Carls G. Huynh J. Tuttle E, et al.: Achievement of glycated hemoglobin goals in the US remains unchanged through 2014. Diabetes Ther 2017;8:863–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Khunti K, Gomes MB, Pocock S, et al. : Therapeutic inertia in the treatment of hyperglycaemia in patients with type 2 diabetes: a systematic review. Diabetes Obes Metab 2018;20:427–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khunti K, Millar-Jones D. Clinical inertia to insulin initiation and intensification in the UK: a focused literature review. Prim Care Diabetes 2017;11:3–12 [DOI] [PubMed] [Google Scholar]
- 4. Khunti K, Nikolajsen A, Thorsted BL, et al. Clinical inertia with regard to intensifying therapy in people with type 2 diabetes treated with basal insulin. Diabetes Obes Metab 2016;18:401–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khunti K, Seidu S, Kunutsor S, Davies M. Association between adherence to pharmacotherapy and outcomes in type 2 diabetes: A meta-analysis. Diabetes Care 2017;40:1588–1596 [DOI] [PubMed] [Google Scholar]
- 6. Peyrot M, Rubin RR, Lauritzen T, et al. on behalf of the International DAWN Advisory Panel. Psychosocial problems and barriers to improved diabetes management: results of the Cross-National Diabetes Attitudes, Wishes and Needs (DAWN) Study, Diabet Med 2005;22:1379–1385 [DOI] [PubMed] [Google Scholar]
- 7. Costagliola D, Chwalow AJ, Varenne P, et al. Initiation of insulin treatment after 70 years of age: patient status 2 years later. Diabet Med 1991;8:773–777 [DOI] [PubMed] [Google Scholar]
- 8. Wallace TM, Matthews DR. Poor glycaemic control in type 2 diabetes: a conspiracy of disease, suboptimal therapy and attitude. QJM 2000;93:369–374 [DOI] [PubMed] [Google Scholar]
- 9. Kunt T, Snoek FJ. Barriers to insulin initiation and intensification and how to overcome them. Int J Clin Pract 2009;63(Suppl. 164):6–10 [DOI] [PubMed] [Google Scholar]
- 10. Zafar A, Stone MA, Davies MJ, Khunti K. Acknowledging and allocating responsibility for clinical inertia in the management of type 2 diabetes in primary care: a qualitative study. Diabet Med 2015;32:407–413 [DOI] [PubMed] [Google Scholar]
- 11. Hirsch IB. Blood glucose monitoring technology: translating data into practice. Endocr Pract 2004;10:67–76 [DOI] [PubMed] [Google Scholar]
- 12. Given JE, O'Kane MJ, Bunting BP, Coates VE. Comparing patient-generated blood glucose diary records with meter memory in diabetes: a systematic review. Diabet Med 2013;30:901–913 [DOI] [PubMed] [Google Scholar]
- 13. Kazlauskaite R, Soni S, Evans AT, et al. Accuracy of self-monitored blood glucose in type 2 diabetes. Diabetes Technol Ther 2009;11:385–392 [DOI] [PubMed] [Google Scholar]
- 14. Keesara S, Jonas A, Schulman K.. Covid-19 and Health Care's Digital Revolution. N Engl J Med 2020. [Epub ahead of print]; DOI: 10.1056/NEJMp2005835 [DOI] [PubMed] [Google Scholar]
- 15. Galindo RG, Aleppo G, Klonoff DC, et al. Implementation of continuous glucose monitoring in the hospital: emergent considerations for remote glucose monitoring during the COVID-19 pandemic. J Diabetes Sci Technol 2020;1932296820932903. [Epub ahead of print]; DOI: 10.1177/1932296820932903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jones MS, Goley AL, Alexander BE, et al. Inpatient transition to virtual care during COVID-19 pandemic. Diabetes Technol Ther 2020;22:444–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19). Using Telehealth to Expand Access to Essential Health Services during the COVID-19 Pandemic. 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/telehealth.html (accessed June26, 2020)
- 18. Peters AL, Garg SK. The Silver Lining to COVID-19: avoiding diabetic ketoacidosis admissions with telehealth. Diabetes Technol Ther 2020;22:449–453 [DOI] [PubMed] [Google Scholar]
- 19. Lind M, Polonsky W, Hirsch IB: Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the GOLD randomized clinical trial. JAMA 2017;317:379–387 [DOI] [PubMed] [Google Scholar]
- 20. Aleppo G, Ruedy KJ, Riddlesworth TD, et al. : REPLACE-BG: a randomized trial comparing continuous glucose monitoring with and without routine blood glucose monitoring in adults with well-controlled type 1 diabetes. Diabetes Care 2017;40:538–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Beck RW, Riddlesworth T, Ruedy K, et al. : Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA 2017;317:371–378 [DOI] [PubMed] [Google Scholar]
- 22. Beck RW, Riddlesworth TD, Ruedy K, et al. : Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med 2017;167:365–374 [DOI] [PubMed] [Google Scholar]
- 23. Šoupal J, Petruželková L, Grunberger G, et al. : Glycemic outcomes in adults with T1D are impacted more by continuous glucose monitoring than by insulin delivery method: 3 years of follow-up from the COMISAIR study. Diabetes Care 2020;43:37–43 [DOI] [PubMed] [Google Scholar]
- 24. Beck RW, Riddlesworth TD, Ruedy K, et al. : Effect of initiating use of an insulin pump in adults with type 1 diabetes using multiple daily insulin injections and continuous glucose monitoring (DIAMOND): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol 2017;5:700–708 [DOI] [PubMed] [Google Scholar]
- 25. Bolinder J, Antuna R, Geelhoed-Duijvestijn P, et al. : Novel glucose-sensing technology and hypoglycemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet 2016;388:2254–2263 [DOI] [PubMed] [Google Scholar]
- 26. Oskarsson P, Antuna R, Geelhoed-Duijvestijn P, et al. : Impact of flash glucose monitoring on hypoglycaemia in adults with type 1 diabetes managed with multiple daily injection therapy: a pre-specified subgroup analysis of the IMPACT randomised controlled trial. Diabetologia 2018;61:539–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haak T, Hanaire H, Ajjan R, et al. : Use of flash glucose sensing technology for 12 months as a replacement for blood glucose monitoring in insulin-treated type 2 diabetes. Diabetes Ther 2017;8:573–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Haak T, Hanaire H, Ajjan R, et al. : Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther 2017;8:55–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Heinemann L, Freckmann G, Faber-Heinemann G, et al. : Benefits of continuous glucose monitoring use in adults with type 1 diabetes and impaired hypoglycaemia awareness and/or severe hypoglycaemia treated with multiple daily insulin injections: Results of the multicentre, randomised controlled HypoDE study. The Lancet 2018;391:1367–1377 [DOI] [PubMed] [Google Scholar]
- 30. Feig DS, Donovan LE, Corcoy R, et al. : Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. Lancet 2017;390:2347–2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Battelino T, Danne T, Bergenstal RM, et al. : Clinical targets for continuous glucose monitoring data interpretation: Recommendations from the International Consensus on Time in Range. Diabetes Care 2019;42:1593–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yu F, Lv L, Liang Z, et al. : Continuous glucose monitoring effects on maternal glycemic control and pregnancy outcomes in patients with gestational diabetes mellitus: a prospective cohort study. J Clin Endocrinol Metab 2014;99:4674–4682 [DOI] [PubMed] [Google Scholar]
- 33. Wright E, Kerr MSD, Reyes I, et al. : HbA1c reduction associated with a FreeStyle Libre® system in people with type 2 diabetes not on bolus insulin therapy. Diabetes 2020;(Supplement 1):78-LB-P [Google Scholar]
- 34. Miller E, Kerr MSD, Roberts GJ, et al. : FreeStyle Libre® system use associated with reduction in acute diabetes events and all-cause hospitalizations in patients with type 2 diabetes without bolus insulin. Diabetes 2020;(Supplement 1):85-LB [Google Scholar]
- 35. Choudhary P, Olsen BS, Conget I, et al. : Hypoglycemia prevention and user acceptance of an insulin pump system with predictive low glucose management. Diabetes Technol Ther 2016;18:288–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bergenstal RM, Garg S, Weinzimer SA, et al. : Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA 2016;316:1407–1408 [DOI] [PubMed] [Google Scholar]
- 37. Garg SK, Weinzimer SA, Tamborlane WV, et al. : Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther 2017;19:155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Danne T, Kordonouri O, Holder M, et al. : Prevention of hypoglycemia by using low glucose suspend function in sensor-augmented pump therapy. Diabetes Technol Ther 2011;13:1129–1134 [DOI] [PubMed] [Google Scholar]
- 39. Davis T, Salahi A, Welsh JB, Bailey TS: Automated insulin pump suspension for hypoglycaemia mitigation: development, implementation and implications. Diabetes Obes Metab 2015;17:1126–1132 [DOI] [PubMed] [Google Scholar]
- 40. Ly TT, Nicholas JA, Retterath A, et al. : Effect of sensor-augmented insulin pump therapy and automated insulin suspension vs standard insulin pump therapy on hypoglycemia in patients with type 1 diabetes: a randomized clinical trial. JAMA 2013;310:1240–1247 [DOI] [PubMed] [Google Scholar]
- 41. Weiss R, Garg SK, Bode BW, et al. : Hypoglycemia reduction and changes in hemoglobin A1c in the ASPIRE In-Home Study. Diabetes Technol Ther 2015;17:542–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Karageorgiou V, Papaioannou TG, Bellos I, et al. : Effectiveness of artificial pancreas in the non-adult population: a systematic review and network meta-analysis. Metabolism 2019;90:20–30 [DOI] [PubMed] [Google Scholar]
- 43. Bekiari E, Kitsios K, Thabit H, et al. : Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis. BMJ 2018;361. [Epub ahead of print]; DOI: 10.1136/bmj.k1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weisman A, Bai J-W, Marina Cardinez M, et al. : Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol 2017;5:501–512 [DOI] [PubMed] [Google Scholar]
- 45. Brown SA, Kovatchev BP, Raghinaru D, et al. : Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med 2019;381:1707–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brown SA, Beck RW, Raghinaru D, et al. : Glycemic outcomes of use of CLC versus PLGS in type 1 diabetes: a randomized controlled trial. Diabetes Care 2020;dc200124. [Epub ahead of print]; DOI: 10.2337/dc20-0124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Faruque LI, Wiebe N, Ehteshami-Afshar, et al.: Effect of telemedicine on glycated hemoglobin in diabetes: a systematic review and meta-analysis of randomized trials. CMAJ 2017;189:E341-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tchero H, Kangambega P, Briatte C, et al. Clinical effectiveness of telemedicine in diabetes mellitus: a meta-analysis of 42 randomized controlled trials. Telemed J E Health 2019;25:569–583 [DOI] [PubMed] [Google Scholar]
- 49. Lee WC, Balu S, Cobden D, et al. : Medication adherence and the associated health-economic impact among patients with type 2 diabetes mellitus converting to insulin pen therapy: an analysis of third-party managed care claims data. Clin Ther 2006;28:1712–1725 [DOI] [PubMed] [Google Scholar]
- 50. Salehi, S., Olyaeemanesh, A., Mobinizadeh, M.. et al.: Assessment of remote patient monitoring (RPM) systems for patients with type 2 diabetes: a systematic review and meta-analysis. J Diabetes Metab Disord 2020;19:115–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Xu T, Pujara S, Sutton S, Rhee M: Telemedicine in the management of type 1 diabetes. Prev Chronic Dis 2018;15:E13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang X, Shu W, Du J, et al. : Mobile health in the management of type 1 diabetes: a systematic review and meta-analysis. BMC Endocr Disord 2019;19:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. De Guzman KR, Snoswell CL, Taylor ML, et al. : A Systematic Review of Pediatric Telediabetes Service Models 2020. Diabetes Technol Ther 2020;10.1089/dia.2019.0489. [Epub ahead of print]; DOI: 10.1089/dia.2019.0489 [DOI] [PubMed] [Google Scholar]
- 54. Bertuzzi F, Stefani I, Rivolta B, et al. : Teleconsultation in type 1 diabetes mellitus (TELEDIABE). Acta Diabetol 2018;55:185–192 [DOI] [PubMed] [Google Scholar]
- 55. Charpentier G, Benhamou PY, Dardari D, et al. : The Diabeo software enabling individualized insulin dose adjustments combined with telemedicine support improves HbA1c in poorly controlled type 1 diabetic patients: a 6-month, randomized, open-label, parallel-group, multicenter trial (TeleDiab 1 Study). Diabetes Care 2011;34:533–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dixon RF, Zisser H, Layne JE, et al. : A smartphone-based type 2 diabetes clinic using video endocrinology consultations and CGM. J Diabetes Sci Technol. 2019. [Epub ahead of print]; DOI: 10.1177/1932296819888662 [DOI] [Google Scholar]
- 57. Polonsky WH, Layne JE, Parkin CG, et al. : Impact of participation in a virtual diabetes clinic on diabetes-related distress in individuals with type 2 diabetes. Clin Diabetes 2020;cd190105. [Epub ahead of print]; DOI: 10.2337/cd19-0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Xie W, Dai P, Qin Y, et al. : Effectiveness of telemedicine for pregnant women with gestational diabetes mellitus: an updated meta-analysis of 32 randomized controlled trials with trial sequential analysis. BMC Pregnancy Childbirth 2020;20:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wood CL, Clements SA, McFann K, et al. : Use of telemedicine to improve adherence to American Diabetes Association standards in pediatric type 1 diabetes. Diabetes Technol Ther 2016;18:7–14 [DOI] [PubMed] [Google Scholar]
- 60. Crossen S, Glaser N, Sauers-Ford H, et al. : Home-based video visits for pediatric patients with poorly controlled type 1 diabetes 2019. J Telemed Telecare. 2019;1357633X19828173. [Epub ahead of print]; DOI: 10.1177/1357633X19828173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. American Diabetes Association: 6. Glycemic targets: standards of care in diabetes 2020.Diabetes Care 2020;43(Suppl. 1):S66–S76 [DOI] [PubMed] [Google Scholar]
- 62. Bradley C, Eschwege E, dePablos-Velasco P, et al. : Predictors of quality of life and other patient-reported outcomes in the PANORAMA multinational study of people with type 2 diabetes. Diabetes Care 2018;41:267–276 [DOI] [PubMed] [Google Scholar]
- 63. Carfazzo JA: A digital-first model of diabetes care. Diabetes Technol Ther 2019;21(S2):S252–S258 [DOI] [PubMed] [Google Scholar]
- 64. Health Information Technology Policy Committee: Report to Congress: challenges and barriers to interoperability. 2015. www.healthit.gov/facas/sites/faca/files/HITPC_Final_ITF_Report_2015-12-16%20v3.pdf (accessed June26, 2020)
- 65. American Diabetes Association: Summary of Proceedings of the American Diabetes Association Overcoming Therapeutic Inertia: Accelerating Diabetes Care for Life. 2018, Arlington, VA. https://professional.diabetes.org/sites/professional.diabetes.org/files/media/ada_therapeutic_inertia_interior_final.pdf (accessed April12, 2020)
- 66. Ferreira A, Correia A, Silva A, et al.: Why facilitate patient access to medical records Stud Health Technol Inform 2007;127:77–90 [PubMed] [Google Scholar]
- 67. Recommendation No. R (97) 5 of the Committee of Ministers to Member States on the Protection of Medical Data. Council of Europe—Committee of Ministers 1997 [PubMed] [Google Scholar]
- 68. Ruedy KJ, Parkin CG, Riddlesworth TD, et al. : Continuous glucose monitoring in older adults with type 1 and type 2 diabetes using multiple daily injections of insulin: results from the DIAMOND trial. J Diabetes Sci Technol 2017;11:1138–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hirsch IB, Kerr MSD, Roberts GJ, et al. : Utilization of continuous glucose monitors is associated with reduction in inpatient and outpatient emergency acute diabetes events regardless of prior blood test strip usage. Diabetes 2020;(Supplement 1):875-P [Google Scholar]
- 70. Roussel R, Bruno Guerci B, Vicaut E, et al. : Dramatic drop in ketoacidosis rate after FreeStyle Libre™ system initiation in type 1 and type 2 diabetes in France, especially in people with low self-monitoring of blood glucose (SMBG): a nationwide study. Diabetes 2020;(Supplement 1):68-OR [Google Scholar]
- 71. Mayberry LS, Lyles CR, Oldenburg B, et al. : mHealth interventions for disadvantaged and vulnerable people with type 2 diabetes. Curr Diab Rep 2019;19:148. [DOI] [PMC free article] [PubMed] [Google Scholar]