Abstract
Traumatic spinal cord injury (SCI) often causes micturition dysfunction. We recently discovered a low level of spinally-derived dopamine (DA) that regulates recovered bladder and sphincter reflexes in SCI female rats. Considering substantial sexual dimorphic features in the lower urinary tract, it is unknown if the DA-ergic mechanisms act in the male. Histological analysis showed a similar distribution of tyrosine hydroxylase (TH)+ neurons in the lower cord of male rats and the number increased following thoracic SCI. Subsequently, focal electrical stimulation in slices obtained from L6/S1 spinal segments of SCI rats elicited detectable DA release with fast scan cyclic voltammetry. Using bladder cystometrogram and external urethral sphincter (EUS) electromyography in SCI male rats, intravenous (i.v.) administration of SCH 23390, a D1-like receptor (DR1) antagonist, induced significantly increased tonic EUS activity and a trend of increased residual volume, whereas activation of these receptors with SKF 38393 did not influence the reflex. Meanwhile, blocking spinal D2-like receptors (DR2) with remoxipride had no effect but stimulating these receptors with quinpirole elicited EUS bursting to increase voiding volume. Further, intrathecal delivery of SCH 23390 and quinpirole resulted in similar responses to those with i.v. delivery, respectively, which indicates the central action regardless of delivery route. In addition, metabolic cage assays showed that quinpirole increased the voiding frequency and total voiding volume in spontaneous micturition. Collectively, spinal DA-ergic machinery regulates recovered micturition reflex following SCI in male rats; spinal DR1 tonically suppress tonic EUS activity to enable voiding and activation of DR2 facilitates voiding.
Keywords: bladder, external urethral sphincter, sex difference, spontaneous recovery
Introduction
Traumatic spinal cord injury (SCI) rostral to the lumbosacral spinal cord often interrupts the spinobulbospinal micturition reflex pathway. As a result, bladder voiding is abolished immediately after the injury. However, an involuntary micturition reflex is gradually developed over time if the bladder is frequently emptied by caregivers and antibiotic treatment is applied. This is due to the establishment of spinal neuronal circuits.1,2 Although urinary function can partially recover, the occurrence of bladder hyperactivity and detrusor-sphincter dyssynergia causes inefficient emptying and incontinence that may lead to lower urinary tract (LUT) infections and ultimately renal failure.3,4 Currently, there is no effective therapeutic approach to cure the disease.
Dopamine (DA) is a crucial neurotransmitter in the central nervous system (CNS) and modulates a variety of biological functions. DA-producing neurons express two essential enzymes for DA synthesis: tyrosine hydroxylase (TH) and dopamine decarboxylase (DDC), which convert L-tyrosine to L-DOPA and then to DA, respectively.5-7 Though DA neurons were believed to be solely restricted to the brain in mammals, we recently demonstrated that a population of TH+ neurons is located within the lower spinal cord in female rats.8 After SCI interrupts supraspinal DA transportation, this group of neurons undergoes plasticity and contributes to a low level of DA sustention in the lumbosacral spinal cord. Further, we found that spinal TH+ neurons are involved in the establishment of spinal bladder reflex circuitry following SCI, and pharmacological manipulation of the spinal DA-ergic pathway profoundly influences bladder and sphincter reflexes in the female. This indicates that spinal endogenous DA-ergic mechanisms regulate involuntary micturition function, and that stimulating the spinal reflex pathway may be an effective treatment of LUT dysfunction after SCI.
Previous studies demonstrated sexually dimorphic micturition on anatomical and functional bases in rats.9,10 In contrast to the female, the urethra in male rats is much longer and thicker and is surrounded by the prostate, which means greater urine flow resistance during voiding.10 Accordingly, the muscle of external urethral sphincter (EUS) is poorly developed in female rats but extensive and thick in the male that enables stronger contraction to expulsion. At the histological level, the striated urethral muscle in female rats is composed of both type I (slow-twitch) and II fibers (fast-twitch), while it entirely consists of type II fibers in males.11 In the bladder reflex, it was observed that the amplitude of the intravesical pressure in voiding is greater in males than in females.12 Moreover, organization of neural activity controlling the muscular activity and synergia is probably different between males and females because electrical stimulation of the hypogastric nerve raises urethral resistance in the male but reduces it in the female.13
In line with differences in the peripheral organization, it has been reported that many neurotransmitter receptors are distributed with sex disparity in humans and animals.14,15 For instance, previous studies showed that there is a greater proportion of purinergic neurotransmission into the bladder in male than female rats.16 Additionally, it has been reported that the density of alpha-2 adrenergic receptors (AR) in the urethra is higher in female than male rabbits, while the expression of beta-AR in the bladder is opposite.17,18 Given marked differences in the male and female urogenital system, it remains unclear if the spinal DA-ergic mechanism present in male rats influences micturition following SCI.
In the present study, we examined DA-ergic profiles in the lumbosacral spinal cord of male rats and their responses to thoracic SCI. Using systemic or central drug administration to inhibit or activate spinal DA receptors, we evaluated the effect on the recovered micturition reflex in SCI male rats.
Methods
Animals
A total of 62 adult male and four female Wistar rats (weight 200-250 g, Charles River) were used in this study, including two naïve male and two naïve and two SCI female rats for basal micturition reflex recording, nine naïve male rats for histology, six SCI male rats for fast scan cyclic voltammetry, 38 SCI male for micturition reflex assays (containing five males for metabolic cages and 11 males for histological analysis), and seven SCI male rats for voiding efficiency assessments. Institutional Animal Care and Use Committee and National Institutes of Health guidelines on animal care were strictly followed to minimize the number of animals used and any potential suffering.
SCI
A complete spinal cord transection was performed at the 10th thoracic (T10) level to interrupt the spinobulbospinal micturition reflex pathway while lower motor neurons for urinary function kept intact.8 After animals were anesthetized with 2% isoflurane, the skin on the back was shaved and disinfected with xenodine. A drop of eye lube was put on the eyes for protection. Under a surgical microscope (M651, Leica), animals were placed on the surgical station with a heating pad on the bottom to maintain body temperature. A skin incision in the midline of the back was made, and then muscles on the spine were detached to expose the dorsal part of T8 vertebrae. Subsequently, dorsal laminectomy was performed with a rongeur to expose T10 spinal cord segment. The cord was completely transected with a No. 11 blade. To ensure the lesion completeness, transection was performed at least twice until two spinal stumps were retracted. Then, a small piece of gelfoam was placed between the two stumps to stop bleeding. Afterwards, the muscles were closed with 4-0 silk suture and the skin was clipped with staples.
Each animal received subcutaneous (s.c.) injections of 3 mL Lactated Ringer's solution, 0.2 mL cefazolin (10 mg/kg), and 0.2 mL buprenex (0.1 mg/kg, Reckitt Benckiser) after surgery. Animals were then put back in new cages and given purified soft diet (Dietgel 76A) for supplement. The bladder was manually expressed at least 3-4 times per day on the first 2 weeks until spontaneous bladder reflex was established, and then three times daily until sacrifice to expel residual urine.
Fast scan cyclic voltammetry (FSCV)
Three or 7 weeks after T10 spinal cord transection, rats (n = 3 per time-point) were sacrificed by decapitation after being fully anesthetized with isoflurane.19 The lumbosacral spinal cord was dissected immediately. A vibrating microtome was used to produce 300-μm thick horizontal longitudinal slices of the spinal cord samples (L2-S3), which were incubated with oxygenated, ice-cold artificial cerebrospinal fluid (aCSF) containing NaCl (126 mM), KCl (2.5 mM), NaH2PO4 (1.2 mM), CaCl2 (2.4 mM), MgCl2 (1.2 mM), NaHCO3 (25 mM), glucose (11 mM), L-ascorbic acid (0.4 mM), pH adjusted to 7.4, for approximately 30-60 min. Then, one section including the lateral parasympathetic region was placed on a perfusion chamber at 32°C.
A carbon fiber microelectrode and a bipolar stimulating electrode were placed in the lateral autonomic region of the section, where abundant TH+ neurons are located.8 DA release was elicited every 5 min using five electrical stimulation pulses at 10 Hz (400 μA, 4 msec, monophasic).20 The electrode potential was linearly scanned from -0.4 to 1.3 V and back to -0.4 V versus Ag /AgCl. Cyclic voltammograms were recorded every 100 msec. Cyclic voltammograms that were biased by electrical stimulation artifact were removed from the current versus time plots prior to analysis. DA release magnitude was calculated relative to post hoc electrode calibrations using 3 μM DA. The magnitude of electrically-evoked DA release was recorded under calcium and calcium-free conditions to ensure that release was calcium dependent.
Bladder cystometrogram and EUS electromyography
Following complete SCI, spontaneous micturition reflex was usually reestablished within 2 weeks in rats due to reestablished spinal segmental circuitry.1,2 To measure the involuntary micturition reflex, SCI male rats (n = 38) underwent bladder cystometrogram (CMG) and EUS electromyography (EMG) recordings 3-4 weeks after injury.
SCI rats were anesthetized with 2% isoflurane and the skin of abdomen and right leg was shaved. After the bladder was manually emptied, rats were placed on the surgical station with supine position. An incision was made in the midline of low abdominal wall to expose the bladder. Next, a purse string suture of 6-0 silk thread was placed around the bladder dome, and a small hole was produced by a 20-G needle in the center of the purse string sutured bladder dome. A polyethylene catheter (PE-60, Clay Adams) with a collar on the tip was then inserted into the bladder dome through the hole and the suture was tightened around the catheter.21 Next, the rostral part of the pubic bone was removed and the EUS was exposed. Two fine-wire electrodes (Astro-Med) were guided by a needle and transcutaneously inserted into both sides of the EUS (external).22 Briefly, one end of the electrode was bent and hooked on the tip of a 30 G needle to wind the wire around the needle. Subsequently, the needle tip was inserted through the perineal zone, 2-3 mm lateral to the vagina, into the EUS, and the needle was withdrawn but the electrode was left in the muscle. As an alternative approach, two additional electrodes were inserted into both sides of the EUS from opened abdomen (internal) and their ends extruded outside. The internal electrodes were used in case of failure from external ones.23 Finally, the incision was closed with 4-0 suture while a small piece of abdominal muscle was left out of the incision for connecting to the ground wire.
A separate PE-10 catheter was used for i.v. drug administration. An incision perpendicular to the femoral vein was made on the skin of the right thigh to expose the femoral blood vessel bundle. The vein was then separated from the artery and saphenous nerve with fine forceps. Two 7-0 silk thread segments were put underneath the femoral vein at the proximal and distal ends, respectively, and the distal one was ligated to stop blood flow. The catheter was implanted in the femoral vein through a small hole between the two threads poked guided by a 23-G needle with the bent tip, and was anchored by the ligation of two threads. The skin was closed with 4-0 suture and the other end of the catheter was connected to a syringe for drug delivery.
For i.t. administration, a midline incision on the back was made to expose the T12 spine. Muscles on the spine were separated and laminectomy was performed at the dorsal vertebrae to expose the lower lumbosacral spinal cord. A small hole was made on the dura mater with a 25 G needle and a small diameter catheter (ITC-32 G, ReCath) was inserted into the intrathecal space caudally about 0.5 cm long reaching to approximate L6/S1 spinal level. The catheter was fixed with tissue adhesive glue (3M) and the incision was closed with 4-0 suture leaving the other end of the catheter outside the skin for drug delivery.
After the surgery, animals were placed in a restraining cage (KN-326, Natsume). The electrodes were connected to an alternating current amplifier (1700, A-M System) at a sample frequency of 1.0 kHz and the ground wire was connected to the abdominal muscles. The bladder catheter was connected to a pressure transducer (Transbridge, WPI) and a microinjection pump (SP2001, WPI) via a three-way stopcock. The amplifier and transducer connected to a recording system of Windaq Data Acquisition (DI-1100, DATAQ Instruments). A container was put right under the animal for collecting the urine. Room temperature saline was infused into the bladder constantly at a rate of 0.3 mL/min. After 30-40 min for equilibration, rhythmic micturition reflex became stable and bladder/EUS activity was recorded.8,23 Bladder voiding events were marked in the trace and the volume of urine was manually measured in four continuous voids under the basal condition and after vehicle or drug administration.
Protocol of pharmacological interventions
Drugs including SCH 23390, SKF 38393, remoxipride, and quinpirole were dissolved in saline and administered after baseline and vehicle delivery during bladder CMG and sphincter EMG (Table 1). The doses of the drugs used were chosen on the basis of previously published data.24-26 The information of half-life in each drug except SKF 3839327 was available from literatures, including 4.8 h of remoxipride,28,29 9.5 h of quinpirole,30,31 and 30 min of SCH 23390.32,33
Table 1.
Normalized Parameters in Bladder CMG and EUS EMG (I.V. Drug Delivery)
| Drug | Animal number | Dose (mg/kg) | Bladder CMG |
EUS Tonic |
VV | ||
|---|---|---|---|---|---|---|---|
| VA | VI | MA | RMS | ||||
| Saline | 1.02 ± 0.03 | 1.09 ± 0.06 | 0.99 ± 0.03 | 1.02 ± 0.02 | 1.00 ± 0.07 | ||
| SCH 23390 (DR1 antagonist) | n = 6 | Low 0.01 | 1.15 ± 0.03* | 0.99 ± 0.10 | 1.82 ± 0.15* | 1.23 ± 0.08 | 0.87 ± 0.15 |
| Mid 0.1 | 1.19 ± 0.03* | 1.20 ± 0.14 | 2.20 ± 0.25* | 1.34 ± 0.04* | 0.81 ± 0.10 | ||
| High 1.0 | 1.30 ± 0.05* | 1.57 ± 0.30 | 2.22 ± 0.22* | 1.37 ± 0.08* | 1.27 ± 0.20 | ||
| Saline | 1.00 ± 0.07 | 0.97 ± 0.03 | 0.93 ± 0.04 | 1.01 ± 0.01 | 0.99 ± 0.05 | ||
| SKF 38393 (DR1 agonist) | n = 4 | Low 0.3 | 1.12 ± 0.08 | 1.05 ± 0.12 | 1.06 ± 0.05 | 1.01 ± 0.00 | 1.16 ± 0.04 |
| Mid 1.0 | 1.24 ± 0.15 | 1.07 ± 0.17 | 1.25 ± 0.10 | 1.03 ± 0.01 | 1.13 ± 0.04 | ||
| High 3.0 | 1.46 ± 0.27 | 0.92 ± 0.16 | 1.43 ± 0.17 | 1.06 ± 0.03 | 1.19 ± 0.10 | ||
| Saline | 1.00 ± 0.01 | 0.84 ± 0.20 | 0.98 ± 0.05 | 1.01 ± 0.02 | 1.12 ± 0.03 | ||
| Remoxipride (DR2 antagonist) | n = 3 | Low 0.1 | 1.08 ± 0.05 | 0.97 ± 0.14 | 1.17 ± 0.27 | 1.14 ± 0.11 | 1.76 ± 1.05 |
| High 1.0 | 1.03 ± 0.03 | 1.67 ± 0.84 | 1.07 ± 0.31 | 1.10 ± 0.11 | 2.30 ± 1.69 | ||
| Saline | 1.05 ± 0.03 | 1.13 ± 0.06 | 1.87 ± 0.95 | 1.03 ± 0.04 | 1.10 ± 0.05 | ||
| Quinpirole (DR2 agonist) | Cat #1 n = 3 |
Low 0.03 | 1.17 ± 0.08 | 1.20 ± 0.17 | 1.73 ± 0.77 | 1.11 ± 0.20 | 1.13 ± 0.06 |
| Mid 0.1 | 1.39 ± 0.13 | 1.15 ± 0.01 | 1.45 ± 0.54 | 1.05 ± 0.14 | 0.95 ± 0.08 | ||
| High 0.3 | 1.37 ± 0.19 | 1.41 ± 0.39 | 0.99 ± 0.25 | 0.94 ± 0.08 | 1.32 ± 0.32 | ||
| Saline | 1.06 ± 0.04 | 1.36 ± 0.47 | 0.92 ± 0.09 | 0.99 ± 0.02 | 1.07 ± 0.04 | ||
| Cat #2 n = 6 |
Low 0.03 | 1.28 ± 0.13 | 1.63 ± 0.20 | 1.43 ± 0.29 | 1.02 ± 0.04 | 1.46 ± 0.16 | |
| Mid 0.1 | 1.40 ± 0.17 | 1.91 ± 0.80 | 1.15 ± 0.24 | 1.02 ± 0.06 | 1.96 ± 0.29 | ||
| High 0.3 | 1.34 ± 0.16 | 2.13 ± 0.32* | 1.00 ± 0.20 | 0.98 ± 0.04 | 2.43 ± 0.18** | ||
| Saline | 1.02 ± 0.08 | 1.11 ± 0.14 | 0.74 ± 0.19 | 0.97 ± 0.04 | 1.00 ± 0.05 | ||
| Cat #3 n = 4 |
Low 0.03 | 1.09 ± 0.05 | 1.72 ± 0.78 | 1.08 ± 0.07 | 1.04 ± 0.01 | 1.02 ± 0.11 | |
| Mid 0.1 | 1.16 ± 0.07 | 1.32 ± 0.58 | 0.96 ± 0.07 | 1.03 ± 0.01 | 0.95 ± 0.13 | ||
| High 0.3 | 1.19 ± 0.05 | 1.67 ± 0.90 | 0.81 ± 0.16 | 0.98 ± 0.04 | 1.02 ± 0.16 | ||
p < 0.05, **p < 0.01
CMG, cystometrogram; EUS, external urethral sphincter; EMG, electromyography; I.V., intravenous; VA, voiding amplitude of intravesical bladder pressure, VI, voiding interval, MA, maximum amplitude of tonic activity, RMS, root mean square, VV, voiding volume.
Drug delivery (i.v.)
After four basal voids were recorded, a total of 100 μL of saline (vehicle) was delivered through the femoral vein catheter as a control. The voiding volume (VV) was measured. Then, the same volume of drug (Table. 1) was delivered. In consideration of possible effect from injection, the first void was always skipped, and the VV was measured in the following four continuous voids in each dose of drug. To ensure that the drug was washed off, there was an interval with at least 40 min between administration of different doses or drugs until signals of bladder pressure and sphincter activity returned to the baseline.25,34
Drug delivery (i.t.)
After four basal voids were recorded, a volume of 10 μL of saline was delivered by a Hamilton syringe through intrathecal catheter and the VV was measured. For drug delivery, 10 μL of the solution (Table 2) was injected. The VV was measured as described above. There was an interval with at least 20 min between administration of different doses or drugs until signals of bladder pressure and sphincter activity returned to the baseline.
Table 2.
Normalized Parameters of Bladder CMG and EUS EMG (I.T. Drug Delivery)
| Drug (Animal) | Dose (μg/kg) | Bladder CMG |
EUS EMG |
VV | ||||
|---|---|---|---|---|---|---|---|---|
| VA | VI | #NVC | Tonic MA | Tonic RMS | BP | |||
| Saline | 1.02 ± 0.04 | 0.93 ± 0.13 | 1.25 ± 0.51 | 0.93 ± 0.04 | 1.00 ± 0.01 | 1.01 ± 0.07 | 0.93 ± 0.10 | |
| SCH 23390 (DR1 antagonist) n = 6 | Low 1.0 | 1.21 ± 0.09 | 0.99 ± 0.09 | 2.85 ± 0.41 | 1.31 ± 0.15 | 1.09 ± 0.06 | 1.00 ± 0.07 | 0.70 ± 0.16 |
| Mid 10 | 1.34 ± 0.11* | 1.02 ± 0.18 | 4.54 ± 0.54 | 1.86 ± 0.24* | 1.34 ± 0.16 | 0.88 ± 0.08 | 0.77 ± 0.13 | |
| High 100 | 1.35 ± 0.08** | 0.98 ± 0.20 | 7.92 ± 1.99* | 2.66 ± 0.38* | 1.65 ± 0.28 | 0.90 ± 0.13 | 0.80 ± 0.08 | |
| Quinpirole (DR2 agonist) n = 6 | Saline | 1.06 ± 0.07 | 1.02 ± 0.14 | 9.28 ± 2.86 | 0.95 ± 0.09 | 0.99 ± 0.01 | - | 0.95 ± 0.08 |
| Low 3.0 | 0.90 ± 0.10 | 0.46 ± 0.09 | 2.00 ± 0.51 | 1.04 ± 0.12 | 1.05 ± 0.04 | 0.86 ± 0.08 | 1.62 ± 0.73 | |
| Mid 10 | 0.79 ± 0.17 | 0.33 ± 0.05* | 1.00 ± 0.38* | 0.92 ± 0.11 | 1.03 ± 0.03 | 0.84 ± 0.14 | 3.32 ± 1.96 | |
| High 30 | 0.87 ± 0.21 | 0.41 ± 0.08* | 1.14 ± 0.41* | 1.02 ± 0.12 | 1.05 ± 0.03 | 0.79 ± 0.12 | 4.53 ± 1.63* | |
p < 0.05, **p < 0.01
CMG, cystometrogram; EUS, external urethral sphincter; EMG, electromyography; I.T., intrathecal; VA, voiding amplitude of intravesical bladder pressure; VI, voiding interval; NVCs, non-voiding contractions (#unnormalized data); MA, maximum amplitude of tonic activity; RMS root mean square; BP, bursting period; VV, voiding volume.
Urodynamic parameters and data analysis
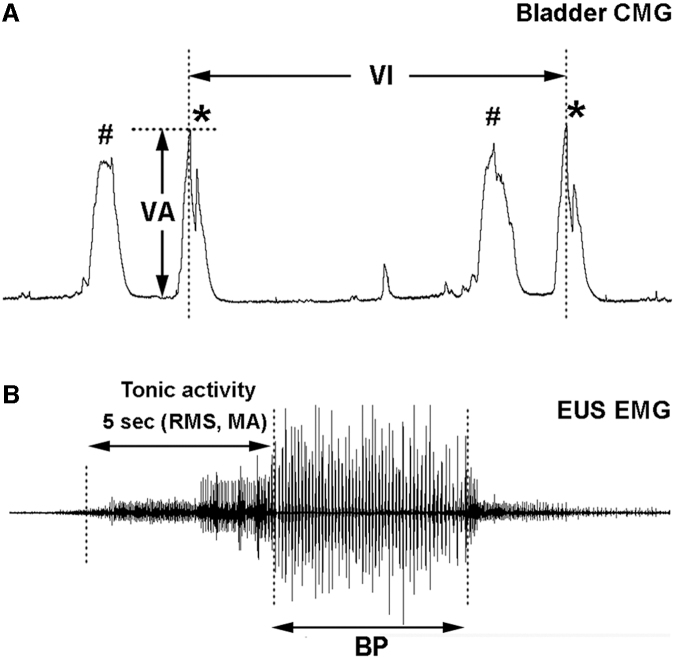
The Windaq Browser software (DATAQ Instruments) was employed to measure CMG and EMG parameters. Bladder CMG parameters included the voiding amplitude (VA) of intravesical bladder pressure, the voiding intervals between two sequential voids (VI), and the number of non-voiding contractions (NVCs) before each voiding contraction (Fig. 1A). The urine volume was measured manually in each voiding contraction. To evaluate EUS tonic activity, the root mean square (RMS) of recorded voltage value of tonic activity was calculated during 5 sec right before the onset of voiding, which represents the filling phase and reflects the capability of continence. The maximum amplitude of tonic activity (MA) in the period of this duration was also measured. Depending on the extent of recovery, EUS bursting during voiding that often indicates the ability of urinary elimination appeared in some SCI rats but not in others. However, those who initially had no bursting might display the activity after drug administration. In both situations, the bursting period during the voiding (Fig. 1B) was measured in the recorded EUS activity. All parameters were measured in 4 continuous voiding cycles and the values were averaged for statistical analysis.
FIG. 1.
Illustrations indicate parameters measured during bladder and sphincter reflex recordings. These include (A) the voiding amplitude (VA) of intravesical bladder pressure (*indicates a voiding contraction), the voiding interval (VI), non-voiding contractions (NVCs, represents as #) in bladder cystometrogram (CMG), and (B) tonic or bursting activity in external urethral sphincter (EUS) electromyography (EMG). Tonic EUS activity in the filling phase is evaluated by root mean square (RMS) and the maximum amplitude (MA) of recorded voltage value during 5 sec right before the onset of voiding, while bursting EUS activity, which exhibits as clusters of high-amplitude spikes separated by silent periods, is measured as the duration of bursting period (BP).
To elucidate the voiding efficiency, rats (n = 7) underwent pharmacological interventions during single bladder CMG assessment. After each void, the infusion pump was stopped and the residual volume (RV) in the bladder was measured by withdrawing through the intravesical catheter with a syringe. The sum of VV and RV was the bladder capacity (BC), and then the voiding efficiency (VE) was determined by this equation: VE (%) = [(VV/BC) × 100].35
Metabolic cage assays
To measure micturition pattern of spontaneous voiding, metabolic cage assays were employed.36,37 Three weeks after SCI, rats (n = 5) were placed in metabolic cages (Braintree Scientific) after the bladder was manually emptied, with free access to ample food and water. A plastic cup was put under the cage on the transducer for collecting the urine, which was filled with 3 mL of mineral oil to avoid evaporation. After 3 h equilibration, animals received s.c. administration of a total of 300 μL of saline as a control or the D2-like receptor (DR2) agonist quinpirole (0.03 mg/kg). The spontaneous voiding was recorded continuously for 6 h each day with Windaq Data Acquisition software (DI-158). Experiments were performed at the same time frame every day. Saline vehicle or drug administration were alternately repeated three times. Micturition parameters, including the urine volume per void, total volumes, voiding intervals or frequencies, as well as volumes of drinking water, were measured. Data that were collected from 3 days per rat were averaged for statistical analysis.
Tissue processing for histological analysis
Naïve (n = 9) or SCI rats (n = 11) that survived for an additional 3 weeks following injury were euthanized with intraperitoneal injection of 0.3 mL euthasol. Animals underwent transcardial perfusion with saline and then 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS). The spinal cord was dissected out and post-fixed overnight and later transferred into 30% sucrose in PBS. For coronal sections (n = 5 per group), approximately 3 cm lumbosacral cord spanning from T12-S3 was dissected to remove the dura and nerve roots, then six spinal samples were placed in a plastic mold with the caudal spinal end evenly aligned and embedded in tragacanth dissolved in sucrose solution. For longitudinal sections (n = 4 in naïve, n = 6 in SCI), each lumbosacral spinal segment was embedded separately in one mold. The tissue block was snap frozen in dry ice and stored at -20°C until sectioning.
Cryosectioning was performed with a cryostat (Microtome). Coronal sections were obtained in 20-μm thickness and directly mounted on five series of subsided slides. Each slide contained 10 sections and each series contained 10 slides. In this manner, adjacent sections on the same slide were separated by 100 μm.38 One series was picked for each immunostaining assay. In contrast, the spinal segment that was sectioned horizontally and longitudinally with 35-μm thickness was kept free floating. Every six sections were consecutively placed into six wells of a 24-well plate filled with tissue cryosection solution (TCS). Thus, adjacent sections in one well were separated by 210 μm.39 Likewise, serial sections were randomly selected for each immunostaining.
Immunostaining and quantification
Mounted spinal coronal sections were rinsed in Tris-buffered saline (TBS) three times, 10 min each time. The sections were blocked in blocking buffer (TBS containing 5% goat serum and 0.5% triton X-100) for 1 h and were then incubated with primary antibody against TH (rabbit, 1:1000, Millipore) overnight at room temperature. After being rinsed in TBS three times again, sections were incubated in Alexa 594-conjugated goat-anti-rabbit secondary antibodies (1:500, Invitrogen) for 6 h, blocked from light. Following rinse, slides were cover-slipped after adding Fluorosave mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI; Southern). For longitudinal sections, free-floating spinal cord sections were incubated with primary antibodies against TH (rabbit, 1:1000, Millipore), mouse, 1:500, Millipore), and mature neuronal marker NeuN (mouse, 1:200, Millipore). The sections were incubated in secondary antibodies including Alexa 594 or 488-conjugated goat anti-rabbit IgG (both 1:500, Invitrogen) for 3 h at room temperature. After cover-slipped, the edge of slides was sealed with nail polish and then kept in -20°C. Images were obtained using a Leica DM5500B microscope.
Quantification of lumbosacral TH+ cells was performed in 10 coronal sections of each animal (n = 5 per group). All TH+ cells displaying typical neuronal morphology with cellular processes and a DAPI+ nucleus in the lateral parasympathetic nuclei were counted at 200 × magnification. The number of cells in each section was added together for statistical analysis.
Statistical analysis
As micturition function was recovered at different degrees in SCI rats, variable CMG and EMG parameters were recorded between individuals. Accordingly, these data were normalized to the basal values for statistics and were analyzed using one-way repeated measures analysis of variance (ANOVA) followed by Dunnett's adjustment. Nonparametric data, such as NVCs, which was not normalized, was analyzed with a Friedman test followed by Dunn's multiple comparisons. FSCV and metabolic cage assay data were compared via a paired t-test whereas histological data were assessed by an unpaired t-test. For all statistical analyses, p < 0.05 was considered a value of significance. All data were expressed as mean ± standard error of the mean.
Results
TH+ neurons reside in the lower spinal cord of male rats
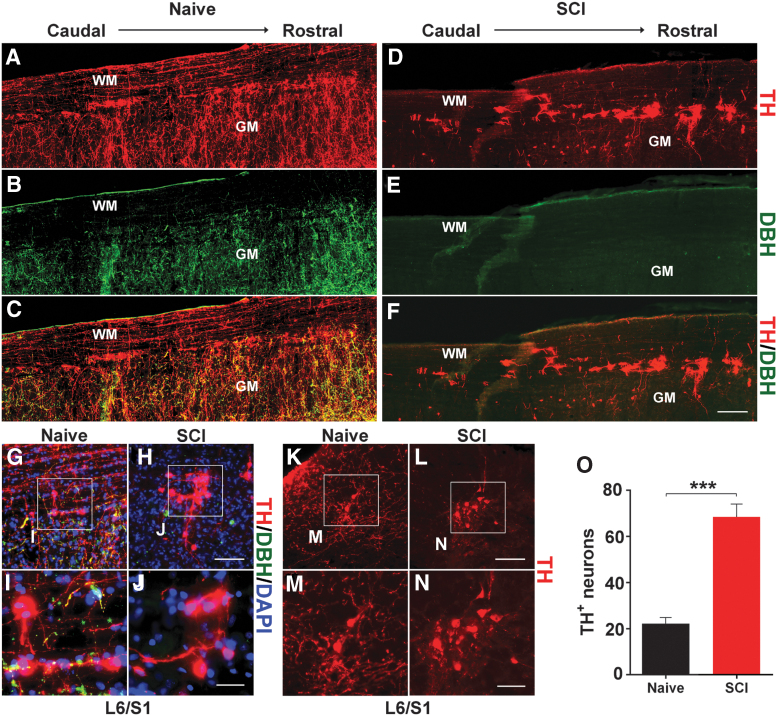
In the longitudinal sections of naïve male rats, immunostaining showed that TH+ cells are mainly located in the marginal area of the dorsal and lateral gray matter spanning L6-S3 segments (Fig. 2A-F). These TH+ cells were labeled by neuronal marker NeuN but did not co-express DBH, indicating that they were neurons but not adrenergic or noradrenergic (Fig. 2G-J). In coronal sections, it was observed that these TH+ neurons were distributed in the lateral parasympathetic region, lamina X, and superficial dorsal horn (Fig. 2K-N), which is a similar pattern to that was previously observed in the female.
FIG. 2.
The distribution of tyrosine hydroxylase (TH)+ cells in the male rat lumbosacral spinal cord. (A-J) Immunostaining demonstrates that TH+ neurons reside in the lateral parasympathetic region from L6-S3 segments in a longitudinal cord section of a naïve (A-C) or spinal cord injury (SCI) rat (D-F). Three weeks after T10 spinal cord transection (D-F), TH+/dopamine-β-hydroxylase (DBH)+/- supraspinal catecholaminergic pathways have degenerated below the injury while TH+/DBH- neurons are present in the lower cord. (G-N) TH+ cells in the lateral parasympathetic region are shown in the longitudinal (G-J) or coronal (K-N) spinal cord sections at L6/S1 level under high magnification. (O) Quantification indicates that the number of TH+ cells in one series of section samples is significantly (unpaired t-test; ***p < 0.001) greater in SCI rats compared with the naïve. WM, white matter; GM, gray matter. Scale bars: F, 200 μm; H, L, 100 μm; J, 30 μm; N, 50 μm. Color image is available online.
Three weeks after T10 spinal cord transection, immunostaining demonstrated that TH/DBH double labeled supraspinal descending fibers disappeared below the injury, while numerous TH+ neurons were observed in the lumbosacral cord (Fig. 2D-F, K-N). Quantification showed, compared with naïve rats, the number of TH+ cells in one series of section samples significantly (naïve 22.20 ± 2.56, SCI 68.4 ± 5.56; p < 0.001, unpaired t-test) increased after SCI (Fig. 2O). This suggests that TH+ cells exist in the lower spinal cord of male rats and the amount elevates in response to SCI.
Electrical stimulation elicits DA release within the lumbosacral cord
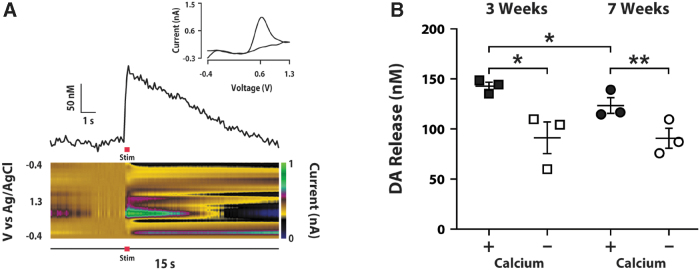
To determine if TH+ neurons in the male rat spinal cord can release DA, we used FSCV to test electrically evoked DA release in spinal cord slices at 3 and 7 weeks post-injury. Following electrical stimulation of the parasympathetic region, where TH+ cells aggregated, DA release was detected in the lumbosacral cord tissue. Cyclic voltammograms with peak oxidation at 0.6 mV and peak reduction at -0.4 mV were used to determine that signals were DA-ergic and not derived from other chemical sources (Fig. 3A). Evoked DA signals were observed at 3 and 7 weeks (Fig. 3B), with a modest decrease between Weeks 3 and 7 (3 weeks 0.143 ± 0.004, 7 weeks 0.123 ± 0.008 μM; p = 0.046, unpaired t-test). This suggests that DA was constantly produced in the spinal cord following injury. When calcium was removed from the preparation, DA release significantly decreased at both 3 (0.143 ± 0.004 vs. 0.091 ± 0.016; p < 0.05, paired t-test) and 7 weeks (0.123 ± 0.008 vs. 0.091 ± 0.010; p < 0.01, paired t-test). This indicates that DA release in the injured spinal cord is only partially dependent on extracellular calcium, which is consistent with the proposed mechanisms for somatodendritic release of DA.40 Together, these data demonstrate that DA can be synthesized and released from neurons within the male spinal cord following SCI.
FIG. 3.
Dopamine (DA) signal is detected by fast scan cyclic voltammetry (FSCV) in the lumbosacral cord slices after T10 spinal cord transection. (A) Example current vs. time plot (above) and color plot (below) from an individual rat 3 weeks post–spinal cord injury (SCI). Current has been converted to micromolar concentration in the current vs. time plot. Green pseudo color in the color plot depicts DA release. Red horizontal bar indicates the time of electrical stimulation (Stim). Inset upper shows a cyclic voltammogram that illustrates the peak oxidation and reduction potentials for DA. (B) DA concentrations at 3 and 7 weeks post-SCI under baseline conditions in which calcium is included in the artificial cerebrospinal fluid (aCSF; +; closed symbols) and in calcium free conditions (-; open symbols). Stimulated DA release is achieved even 7 weeks after SCI, although there is a modest decrease (*p < 0.05, unpaired t-test) compared with 3 weeks. When calcium is removed from the preparation, DA release significantly (*p < 0.05, **p < 0.01, paired t-test) decreases, suggesting calcium-dependence. Color image is available online.
Blocking D1-like receptors increases EUS tonic activity and bladder pressure
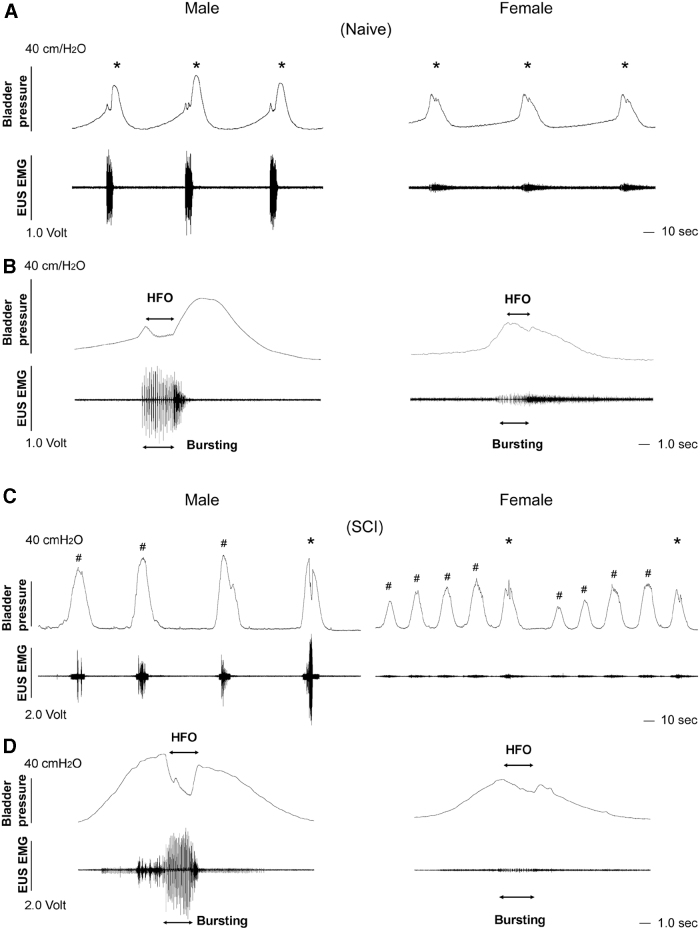
During bladder CMG and EUS EMG recordings, both naïve male and female rats had typical bladder voiding contraction accompanied by EUS bursting activity. Particularly, the amplitude of bladder pressure and EUS activity in voiding appeared high in male rats (Fig. 4A, 4B), which could be due to a strong sphincter muscle. Three weeks after SCI, bladder NVCs emerged in some male and female rats (Fig. 4C, 4D). Bursting EUS activity might or might not occur during voiding depending on the extent of recovery.
FIG. 4.
Representative traces show sexual dimorphic micturition patterns of bladder cystometrogram (CMG) and external urethral sphincter (EUS) electromyography (EMG) recordings in naïve or spinal cord injury (SCI) male and female rats. (A, B) In naïve rats, the voiding amplitude of intravesical pressure and the amplitude of bursting EUS activity during voiding (*) are low in the female but high in the male. (C, D) Three weeks after T10 spinal cord transection, spontaneous spinal micturition reflex is established while bladder non-voiding contractions (#) may occur in both sexes. Time-stretched recordings (B, D) show the high frequency oscillation (HFO) of bladder contractions and bursting EUS activity during voiding.
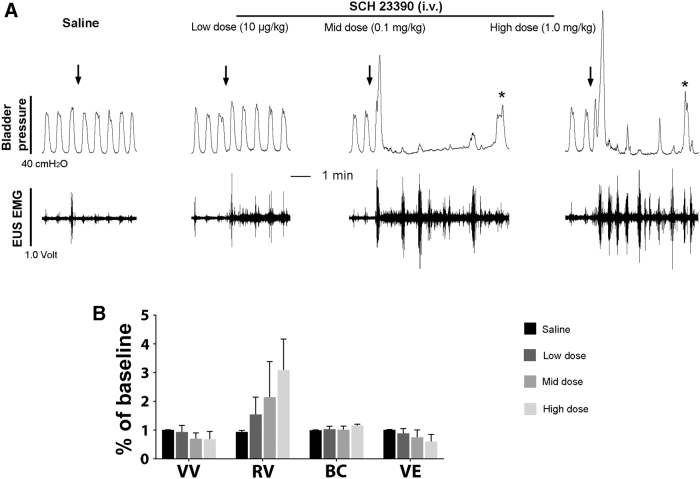
Three to 4 weeks after spinal cord transection, DA receptor agonists or antagonists were administered intravenously (Table 1) during CMG and EMG recordings. Blocking D1-like receptors (DR1) with different doses of antagonist SCH 23390 (0.01, 0.1, 1.0 mg/kg) significantly increased the VA (all p < 0.05, repeated measures ANOVA followed by Dunnett's post hoc) compared with vehicle, and non-significantly prolonged the VI in the middle and high doses. Importantly, the drug elicited dramatic effects on the EUS activity. The maximum of EUS tonic activity in the filling phase increased in all three doses (all p < 0.05), and RMS values of the tonic activity before the onset of voiding significantly increased when the middle and high doses of the drug were administered (both p < 0.05; Fig. 5A). In contrast, stimulating spinal DR1 with SKF 38393 (0.3, 1.0, 3.0 mg/kg) had no significant effect on these parameters. Neither blockage nor stimulation of these receptors significantly changed the VV. Since the majority of rats did not show bladder NVCs and EUS bursting activity, these two indices could not be statistically analyzed.
FIG. 5.
Blocking spinal D1-like receptors (DR1) with SCH 23390 increases voiding amplitude (VA) of intravesical pressure in bladder contractions and tonic external urethral sphincter (EUS) activity in spinal cord injury (SCI) male rats. (A) Representative traces show increased VA of bladder contractions and EUS activity after intravenous (i.v.) administration of DR1 antagonist SCH 23390 during bladder cystometrogram (CMG) and EUS electromyography (EMG; asterisks represent voiding contractions). (B) In voiding efficiency tests, SCH 23390 shows a trend of decreased voiding volume (VV) and voiding efficiency (VE) but increased residual volume (RV). BC, bladder capacity.
In voiding efficiency assays, blocking DR1 with SCH 23390 (n = 3) resulted in non-significantly (all p > 0.05) decreased VV (saline 1.01 ± 0.01, low 0.94 ± 0.23, mid 0.71 ± 0.20, and high 0.69 ± 0.27), increased RV (saline 0.94 ± 0.05, low 1.55 ± 0.60, mid 2.15 ± 1.24, and high 3.09 ± 1.08), and decreased VE (saline 1.01 ± 0.01, low 0.89 ± 0.17, mid 0.76 ± 0.25, and high 0.61 ± 0.24). However, there was an obvious trend in either the increased RV or decreased VE despite of non-statistical difference (Fig. 5B). Collectively, the results indicate that spinal DR1 appear to exert tonic suppression of tonic EUS activity to enable voiding in SCI male rats.
Stimulating DR2 improves micturition reflex
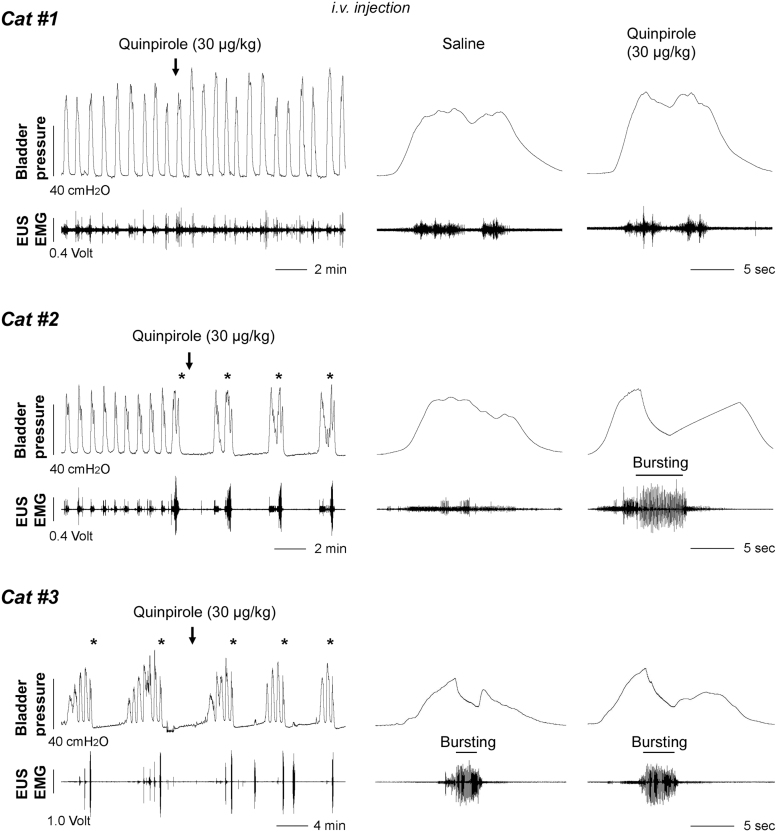
Blocking spinal DR2 with remoxipride did not induce obvious changes during CMG and EMG recordings (Table 1). This suggests the receptors do not tonically modulate the spinal micturition reflex. The number of bladder NVCs and EUS bursting periods could not be analyzed due to their absence in the majority of animals. When quinpirole, a DR2 agonist, was administered (0.03, 0.1, 0.3 mg/kg), the response of bladder and EUS activity was variable. There were three different patterns of bladder and EUS activity when urine was voided (Fig. 6). Rats in category #1 had no bursting EUS activity during voiding at baseline or after drug delivery. Accordingly, a small volume of urine was expelled in voiding without significant difference prior and after drug usage (all p > 0.05).
FIG. 6.
Stimulation of spinal D2-like receptors (DR2) with quinpirole elicits three different patterns of bladder cystometrogram (CMG) and external urethral sphincter (EUS) electromyography (EMG) activity in spinal cord injury (SCI) male rats. In category #1, bladder activity displays only rhythmic contractions with short-intervals accompanied by tonic EUS activity during voiding at baseline or following saline delivery. Quinpirole (30 μg/kg) triggers only high-amplitude EUS tonic activity but no bursting during voiding. In category #2, reflex patterns initially exhibit shortly frequent bladder contractions and tonic EUS activity. Subsequently, quinpirole evokes a typical pattern of bladder voiding contractions (asterisks) and elicits bursting EUS activity during voiding. In category #3, there is a typical voiding pattern at baseline and bursting EUS activity occurs during voiding. Administration of quinpirole prolongs the duration of bursting with a dose-dependent fashion.
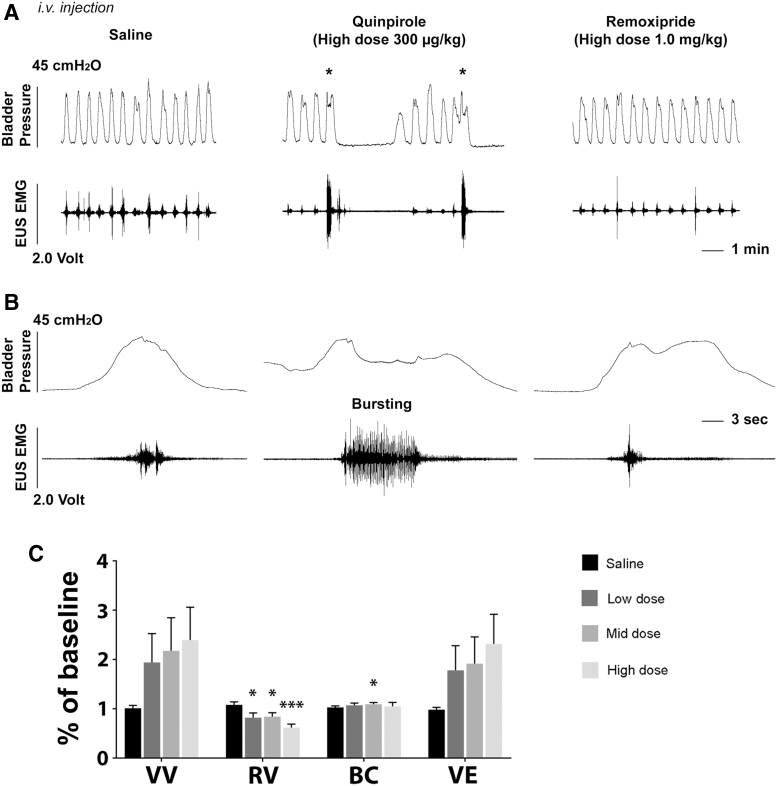
Rats in category #2 initially did not have EUS bursting in the basal/saline condition, but then displayed phasic bursting activity after quinpirole administration. Notably, the VV dramatically increased following the high dose compared with vehicle (p < 0.05) in this group. Those in category #3 had EUS bursting activity initially and the duration significantly prolonged after quinpirole delivery with the high dose (saline 1.12 ± 0.08, low 1.46 ± 0.23, mid 1.44 ± 0.17, high 1.50 ± 0.16, p < 0.05 between saline and the high dose; Fig. 7A, 7B). However, the VV was not significantly increased. Additionally, the number of bladder NVCs was non-significantly decreased after the drug delivery (basal 2.67 ± 1.26, saline 2.4 ± 1.18, low 1.00 ± 0.58, mid 0.92 ± 0.43, high 0.83 ± 0.44; Friedman test followed by Dunn's, all p > 0.05). After administration of remoxipride to block activated DR2, established typical bladder voiding pattern disappeared. Bladder activity changed back to identical rhythmic contractions (Fig. 7A), and EUS bursting was masked again (Fig. 7B). In those with prolonged bursting duration after quinpirole delivery, remoxipride eliminated the effect by shortening the duration. This suggests that effects of quinpirole were provoked via DR2.
FIG. 7.
A representative trace shows that remoxipride, a D2-like receptor (DR2) antagonist, blocks the effect induced by quinpirole during bladder cystometrogram (CMG) and external urethral sphincter (EUS) electromyography (EMG) recordings in a spinal cord injury (SCI) male rat. (A) In the basal condition or following saline delivery, bladder activity exhibits rhythmic contractions with short-intervals without. After intravenous administration of quinpirole (300 μg/kg), a typical voiding pattern (asterisks) occurs, which is accompanied by strong EUS activity during voiding and several non-voiding contractions (NVCs) preceding the void. However, remoxipride (1.0 mg/kg) eliminates the effects and the pattern of bladder and EUS activity changes to the condition before quinpirole delivery. (B) In time-stretched traces, there is no bursting but only high-amplitude tonic EUS activity during voiding after saline delivery. Administration of quinpirole elicits bursting EUS activity during voiding. Following remoxipride injection, bursting EUS activity disappears and high-amplitude tonic EUS activity reemerges. (C) In voiding efficiency tests, quinpirole significantly reduces residual volume (RV) with three doses and increases bladder capacity (BC) with the middle dose. In addition, there is a trend of increased voiding volume (VV) and voiding efficiency (VE) (repeated measures of one-way analysis of variance followed by Dunnett's adjustment, *p < 0.05, ***p < 0.001).
In voiding efficiency assays, stimulating DR2 with quinpirole (n = 4) increased the VV (saline 1.01 ± 0.06, low 1.94 ± 0.59, mid 2.18 ± 0.67, and high 2.39 ± 0.67, all p > 0.05) with a trend and significantly decreased the RV (saline 1.08 ± 0.06, low 0.82 ± 0.10, mid 0.84 ± 0.08, and high 0.62 ± 0.07; p < 0.05 in the low and middle doses, p < 0.001 in the high dose). As a result, the VE was markedly improved (saline 0.98 ± 0.05, low 1.78 ± 0.50, mid 1.92 ± 0.54, and high 2.32 ± 0.60) with a trend to significance (Fig. 7C). Therefore, the results indicate that spinal DR2 are not actively regulating the micturition reflex but stimulating the receptors elicits EUS bursting to facilitate voiding in SCI male rats.
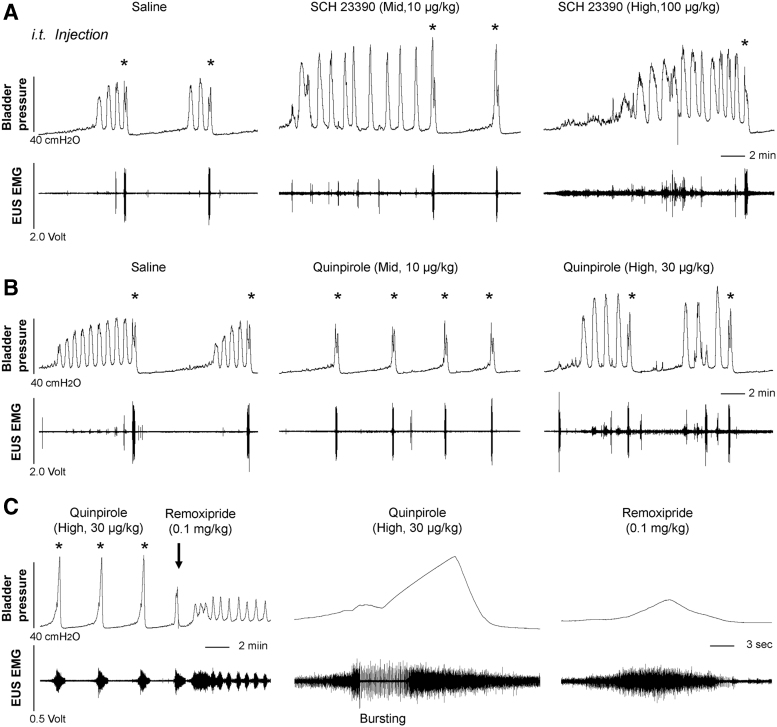
Intrathecal drug administration elicits similar effects
Systemic administration of drugs could affect both central and peripheral nervous system. Although it was never recognized that DA exerts a peripheral role in the LUT, we selected SCH 23390 and quinpirole, in consideration of their dramatic effects via i.v. delivery, to verify central effects by i.t. injection during bladder CMG and EUS EMG. Administration of the middle or high dose of DR1 antagonist SCH 23390 significantly elevated the VA of intravesical pressure (repeated measures of one-way ANOVA followed by Dunnett's adjustment, p < 0.05 in middle dose, p < 0.01 in high dose) and the maximum amplitude of tonic EUS activity (both p < 0.05), similar to the results from i.v. delivery (Fig. 8A). Particularly, the number of bladder NVCs markedly increased after blocking DR1 with the high dose (Friedman test followed by Dunn's, both p < 0.05 between basal/saline and high dose). Hence, SCH 23390 elicits similar response via either systemic or central delivery.
FIG. 8.
Intrathecal (i.t.) administration of spinal DR antagonists/agonists elicits effects similar to that is induced with intravenous (i.v.) delivery during bladder cystometrogram (CMG) and external urethral sphincter (EUS) electromyography (EMG) in spinal cord injury (SCI) male rats. (A) Inhibiting D1-like receptors (DR1) with SCH 23390 with the middle or high dose significantly leads to increased voiding amplitude (VA) of intravesical pressure, elevated tonic EUS activity in the filling phase, and increased bladder non-voiding contractions (NVCs). (B) A trace shows that stimulating D2-like receptors (DR2) with quinpirole reduces the number of bladder NVCs and decreased VI with the middle or high dose. (C) Subsequent blockage of spinal DR2 with remoxipride causes dramatically decreased VA of bladder contractions and bursting EUS activity during voiding is masked.
Injection of DR2 agonist quinpirole in the middle and high doses significantly reduced the number of bladder NVCs (both p < 0.05 compared with basal/saline) and decreased VI (both p < 0.05). The high dose significantly (p < 0.05) increased VV (Fig. 8B). In addition, quinpirole did not significantly influence the tonic EUS activity in the filling whereas elicited bursting phase during voiding (n = 3). Subsequently, administration of remoxipride dramatically changed the voiding pattern. The VA of bladder pressure was reduced by ∼40% and EUS bursting activity was masked, resulting in decreased VV by ∼54%. The results confirm the central action of quinpirole by i.t. delivery, which is similar to that with i.v. administration, on the micturition reflex in SCI male rats.
Specific stimulation of spinal DR2 improves spontaneous voiding
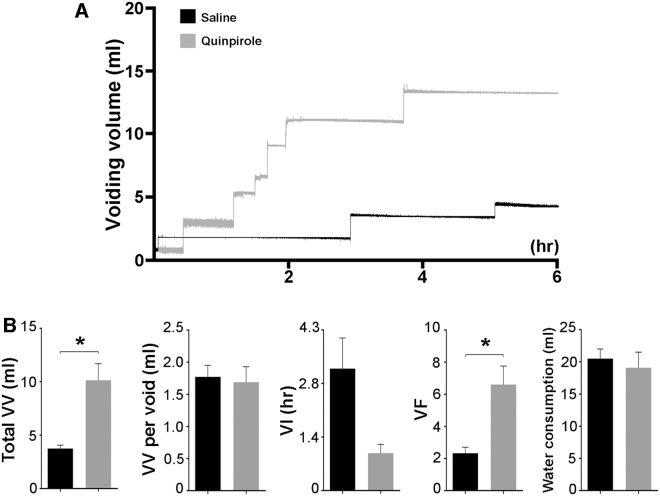
Regarding that stimulating spinal DR2 improved the VV when it provoked bursting EUS activity during bladder CMG, we further tested if it is effective on spontaneous micturition using metabolic cage assays in SCI male rats. Compared with the control treated with vehicle, s.c. administration of the DR2 agonist quinpirole did not induce a difference in the VV per void (saline 1.77 ± 0.18 vs. quinpirole 1.69 ± 0.24 mL; p > 0.05, paired t-test). However, it significantly improved the total VV (saline 3.75 ± 0.31 vs. quinpirole 10.13 ± 1.55 mL; p < 0.05), increased the number of voids (saline 2.33 ± 0.37 vs. quinpirole 6.60 ± 1.15; p < 0.05) and shortened the VI (saline 3.16 ± 0.80 vs. quinpirole 0.97 ± 0.23 h; p = 0.079). Note that there was no significant difference in water consumption (saline 20.50 ± 1.48 vs. quinpirole 19.07 ± 2.43 mL; p > 0.05) between saline and drug delivery (Fig. 9). Therefore, the results illustrate that pharmacological activation of spinal DR2 may improve spontaneous voiding in SCI male rats.
FIG. 9.
Stimulation of spinal D2-like receptors (DR2) improves spontaneous voiding in spinal cord injury (SCI) male rats. Three weeks after T10 spinal cord transection, rats were placed in metabolic cages to assess spontaneous micturition function. (A) Representative traces show markedly increased total voiding volume (VV) and voiding frequency (VF) in SCI rats during 6 h after subcutaneous (s.c.) administration of quinpirole (0.03 mg/kg). (B) Statistical analysis reveals that, compared with vehicle injection, quinpirole induces significantly increased total VV and VF.
Discussion
Although it was believed that DA-ergic neurons in the CNS are restricted to the brain, we previously demonstrated that a subpopulation of TH+ interneurons exists within the lower spinal cord in female rats which may regulate the recovered micturition reflex after SCI.8 In the present study, a similar distribution of TH+ neurons was observed within the lumbosacral spinal cord in male rats, and SCI might possibly induce their plasticity that enables DA synthesis. Subsequently, focal electrical stimulation in the injured spinal cord slices elicited detectable DA release. Pharmacological interventions of spinal DA receptors influenced the bladder and sphincter reflexes. This indicates that similar spinal DA-ergic mechanisms modulate the involuntary micturition reflex in SCI male rats, regardless of sexual dimorphic LUT in anatomy and physiology.
In neonatal rats when bulbospinal pathways are immature, a spinal reflex dominates bladder function and mediates involuntary voiding.41,42 During postnatal development, the spinal bladder reflex is gradually suppressed and eventually eliminated via activity-based competition-related synapse reorganization43,44 after bulbospinal projections reach to the lower cord and establish the spinobulbospinal micturition reflex.45,46 Traumatic SCI often interrupts this reflex pathway. Over a few weeks, partial urinary function spontaneously recovers due to the reestablishment of a spinal bladder reflex.2,4 Albeit spinal interneurons appear to be substantially involved in this process, mechanistic basics are still unclear. According to our recent findings,8 we postulated that spinal TH+ neurons are a remnant of primitive spinal circuits involved in pelvic visceral activity during development and become silent in the adult. Following SCI to interrupt descending DA-ergic pathways, their TH+ phenotype reemerges allowing them to play a compensatory role in regulating the spinal micturition reflex. Immunostaining confirmed that the majority of TH+/DBH- neurons are distributed in the lateral parasympathetic region, lamina X, and superficial dorsal horn in the lower cord.
After SCI, an increase in the amount of these cells suggests their plasticity after interruption of supraspinal modulation. DA release was detected by FSCV in spinal cord slices at two time-points of measurement, 3 and 7 weeks post-injury, which indicates stable DA production over this time course. Previous studies reported that SCI enables aromatic L-amino acid decarboxylase (AADC or DDC)-containing cells, such as pericytes of capillaries and neurons, to upregulate the expression for synthesis of monoamines.47,48 AADC cells distal to the lesion acquire the ability to produce 5-HT from its immediate precursor, 5-hydroxytryptophan,49 or produce DA if supplied L-dopa.50 This can increase the excitability of spinal motoneurons, and the phenotypic change in these cells appears to result from a loss of inhibition by descending catecholaminergic pathways. Together with sustained TH expression, upregulation of AADC affords necessary machinery to produce DA in the injured spinal cord.
Following SCI, the recovered bladder function varies due to different degrees of recovery of the peripheral organs. Rats with good recovery may show a typical voiding cycle and bursting activity in the EUS whereas those with poor recovery may not display bursting but only high-amplitude tonic activity during voiding. Previous studies reported that, in neuroaxis intact rats, DR1 at the brain level have a tonic inhibitory effect on micturition reflex.24,25,51 In SCI male rats, blocking spinal DR1 with SCH 23390 during bladder CMG and EUS EMG increased the tonic EUS activity in the filling phase, similar to that in the female. Since the EUS tonic phase before bursting reflects the closure of the urethral outlet for urine storage, it is therefore considered that physiological activation of spinal DR1 reduces outlet resistance or has an inhibitory effect on continence. Increased VA of intravesical bladder pressure after blocking these receptors could be the subsequent reaction induced by increased outlet resistance because stimulation of these receptors with SKF 38393 did not influence the reflex. Notably, higher frequency of bladder NVCs after delivery of the high dose of SCH 23390 could be explained by increased tonic EUS activity during filling which might cause elevated bladder threshold for voiding. Hence, spinal DR1 mainly suppress the EUS resistance to facilitate voiding. This effect is opposite to that evoked by DR1 in the brain.
In neuroaxis intact male rats, activation of DR2 in the CNS induces hyperactive bladder response.52,53 In the present study, stimulating spinal DR2 with quinpirole increased the VA of intravesical pressure in SCI males. It elicited the EUS bursting during voiding to increase the VV or prolonged the duration of existing bursting. Increased EUS bursting duration often means a decrease in urethral outlet resistance while enhanced bladder contraction reflects an excitatory effect on the parasympathetic outflow to the bladder.54 Meanwhile, decreased VI and frequency of NVCs with administration of quinpirole may be due to reduced residual volumes or decreased threshold of bladder pressure for voiding. Therefore, it appears that spinal DR2 participate in both the bladder and sphincter activity, and their activation improves voiding capability in SCI male rats.
The effects are very similar to what was observed in female rats (unpublished data). In addition, administration of SCH 23390 or quinpirole i.t. induced similar effects to those with i.v. drug delivery. This confirms the role of these drugs were indeed from central actions even via systemic delivery. In metabolic cage assays, injection of quinpirole to stimulate spinal DR2 dramatically increased the voiding frequency and total VV in spontaneous micturition function. One may conclude that activation of these receptors improves urinary function following SCI. However, previous studies reported that activation of DR2 in the kidney increases sodium excretion and urine flow.55-57 Accordingly, an increase in total urine volume in SCI rats could be due to combined effects from an increase in voiding efficiency as well as in urine production by the kidney, which can also account for the decrease in the VI and increase in frequency of voids. Although it is difficult to dissect the effect of spinal DR2 from these results of spontaneous micturition assays, their function of increasing voiding capability has been interpreted in bladder CMG and EUS EMG reflex recordings. In these experiments, saline was fast infused into the bladder and made the production from the kidney unmeaningful to impact the reflex.
Sexual dimorphism of the LUT exists in all mammalian species. The different micturition pattern between the male and female is mainly due to anatomical differences in peripheral organs. The urethra and EUS are two important parts in the LUT and they are substantially different in each sex. Firstly, the urethra is longer and thicker in males than those in females. Because of the existence of a penis, there are two physiological curves on the urethra in males but none in females. In males, both the roughness of the urethral surface and elasticity of the urethral wall add to the resistance of urine flow. The longer male urethra also costs more energy10 so that it requires stronger contraction activity of the bladder and EUS to expel the urine out.
Correspondingly, the EUS in male rats is longer and thicker than that in the female, which complies with the longer urethra and the requirement for stronger contraction. It was demonstrated that the striated muscle of EUS in male rats consists of only type II fibers, which accounts for fast twitch and provides stronger contraction while the proportion of type II is approximately 86-94% in female rats.11 In regard to these differences, male rats exhibited higher bladder pressure and amplitudes of EUS activity during voiding in the procedure of bladder CMG and EUS EMG recordings. It is worthwhile to note that, during our daily bladder care, it was more difficult to manually expel the urine in SCI male rats than in the female due to more resistance in urine flow. However, the bladder recovered to great extent in most male rats after 2 weeks, which was reflected as a clear EUS bursting pattern in voiding with EMG assessments. Despite the sexual difference, we demonstrated similar spinal endogenous DA-ergic mechanisms and regulation of micturition reflex in SCI male versus female rats.
In conclusion, this study demonstrated that spinal DA-ergic machinery is present in the lower spinal cord of male rats after SCI. Plasticity of TH+ neurons following SCI contributes to a low level of DA in the cord which regulates the partial recovered micturition reflex. Additionally, pharmacological stimulation of spinal DR2 improves the micturition reflex while tonic activation of DR1 suppresses tonic EUS activity to facilitate voiding. This is very similar to that observed in female rats. Further, the DA-ergic mechanisms may modulate other pelvic organ activity since disrupting DA signaling causes abnormal sexual and bowel functions and visceral pain. In fact, Ishizuka and colleagues reported that apomorphine induces penile erection in SCI rats,58 suggesting this possibility. Collectively, the results may provide guidance to design a novel therapeutic strategy for micturition and other pelvic dysfunctions following SCI.
Acknowledgments
We gratefully thank Dr. William C. de Groat and Stephanie L. Daugherty at the University of Pittsburgh for technical training. Thank Jeremy Weinberger, Jaclyn H. DeFinis and Silvia Fernandes for experimental assistance, and Emily A. Oatman for proofreading. The support for this work was provided by NIH NINDS R01NS099076, to S.H. and R01DA03190 to R.A.E, and NINDS R01NS085426, R01NS106908, and DoD/CDMRPW81XWH-14-1-0605 to V.J.T., and Shandong Provincial Key Research and Development Project 2019GSF107062 to Z.L.
Authors' Contributions
S.H., R.A.E., V.J.T., Z.D.B., and Z.L. designed the experiments. Y.Q., Z.D.B., S.Z., and C.T.T. carried out the experiments. Y.Q., Z.D.B., S.Z., and S.H. analyzed data and prepared figures. Y.Q., S.H., Z.D.B., C.T.T., S.Z., R.A.E., V.J.T., and Z. L. wrote the paper.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. de Groat, W.C. (1995). Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Paraplegia 33, 493–505 [DOI] [PubMed] [Google Scholar]
- 2. de Groat, W.C. and Yoshimura, N. (2012). Plasticity in reflex pathways to the lower urinary tract following spinal cord injury. Exp. Neurol. 235, 123–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Groat, W.C. and Yoshimura, N. (2015). Anatomy and physiology of the lower urinary tract. Handb. Clin. Neurol. 130, 61–108 [DOI] [PubMed] [Google Scholar]
- 4. Fowler, C.J., Griffiths, D., and de Groat, W.C. (2008). The neural control of micturition. Nature reviews. Neuroscience 9, 453–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gabriel, L.R., Wu, S., Kearney, P., Bellve, K.D., Standley, C., Fogarty, K.E., and Melikian, H.E. (2013). Dopamine transporter endocytic trafficking in striatal dopaminergic neurons: differential dependence on dynamin and the actin cytoskeleton. J. Neurosci. 33, 17836–17846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hoffman, B.J., Hansson, S.R., Mezey, E., and Palkovits, M. (1998). Localization and dynamic regulation of biogenic amine transporters in the mammalian central nervous system. Front. Neuroendocrinol. 19, 187–231 [DOI] [PubMed] [Google Scholar]
- 7. Bjorklund, A. and Dunnett, S.B. (2007). Dopamine neuron systems in the brain: an update. Trends Neurosci. 30, 194–202 [DOI] [PubMed] [Google Scholar]
- 8. Hou, S., Carson, D.M., Wu, D., Klaw, M.C., Houle, J.D., and Tom, V.J. (2016). Dopamine is produced in the rat spinal cord and regulates micturition reflex after spinal cord injury. Exp. Neurol. 285, 136–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cruz, Y. and Downie, J.W. (2005). Sexually dimorphic micturition in rats: relationship of perineal muscle activity to voiding pattern. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289, R1307–R1318 [DOI] [PubMed] [Google Scholar]
- 10. Streng, T., Santti, R., and Talo, A. (2002). Similarities and differences in female and male rat voiding. Neurourol. Urodyn. 21, 136–141 [DOI] [PubMed] [Google Scholar]
- 11. Chen, S., Lai, C., Fan, W., and Peng, C. (2012). Sex differences in the external urethral sphincter activity of rats. J. Exp. Clin. Med. 4, 157–164 [Google Scholar]
- 12. Maggi, C.A., Giuliani, S., Santicioli, P., and Meli, A. (1986). Analysis of factors involved in determining urinary bladder voiding cycle in urethan-anesthetized rats. Am. J. Physiol. 251, R250–R257 [DOI] [PubMed] [Google Scholar]
- 13. Kontani, H. and Shiraoya, C. (2000). Sex differences in urethral pressure response to electrical stimulation of the hypogastric nerves in rats. J. Urol. 163, 1364–1368 [PubMed] [Google Scholar]
- 14. Fan, W.J., Li, Y.T., Chen, J.J., Chen, S.C., Lin, Y.S., Kou, Y.R., and Peng, C.W. (2013). Sexually dimorphic urethral activity in response to pharmacological activation of 5-HT1A receptors in the rat. Am. J. Physiol. Renal Physiol. 305, F1332–F1342 [DOI] [PubMed] [Google Scholar]
- 15. Patra, P.B. and Patra, S. (2013). Sex differences in the physiology and pharmacology of the lower urinary tract. Curr. Urol. 6, 179–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Creed, K.E., Loxley, R.A., and Phillips, J.K. (2010). Functional expression of muscarinic and purinoceptors in the urinary bladder of male and female rats and guinea pigs. J. Smooth Muscle Res. 46, 201–215 [DOI] [PubMed] [Google Scholar]
- 17. Latifpour, J., Kondo, S., O'Hollaren, B., Morita, T., and Weiss, R.M. (1990). Autonomic receptors in urinary tract: sex and age differences. J. Pharmacol. Exp. Ther. 253, 661–667 [PubMed] [Google Scholar]
- 18. Morita, T., Masuda, H., Tosaka, A., Ishizaka, K., Tsujii, T., and Kondo, S. (1998). Sex differences in function and distribution of beta-adrenoceptors in rabbit urinary bladder. J. Urol. 159, 555–558 [DOI] [PubMed] [Google Scholar]
- 19. Brodnik, Z.D. and Espana, R.A. (2015). Dopamine uptake dynamics are preserved under isoflurane anesthesia. Neurosci. Lett. 606, 129–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brodnik, Z.D., Batra, A., Oleson, E.B., and Espana, R.A. (2019). Local GABAA receptor-mediated suppression of dopamine release within the nucleus accumbens. ACS Chem. Neurosci. 10, 1978–1985 [DOI] [PubMed] [Google Scholar]
- 21. Mitsui, T., Fischer, I., Shumsky, J.S., and Murray, M. (2005). Transplants of fibroblasts expressing BDNF and NT-3 promote recovery of bladder and hindlimb function following spinal contusion injury in rats. Exp. Neurol. 194, 410–431 [DOI] [PubMed] [Google Scholar]
- 22. Jiang, H.H., Salcedo, L.B., Song, B., and Damaser, M.S. (2010). Pelvic floor muscles and the external urethral sphincter have different responses to applied bladder pressure during continence. Urology 75, 1515 e1511–e1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee, Y.S., Lin, C.Y., Jiang, H.H., Depaul, M., Lin, V.W., and Silver, J. (2013). Nerve regeneration restores supraspinal control of bladder function after complete spinal cord injury. J. Neurosci. 33, 10591–10606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ogawa, T., Seki, S., Masuda, H., Igawa, Y., Nishizawa, O., Kuno, S., Chancellor, M.B., de Groat, W.C., and Yoshimura, N. (2006). Dopaminergic mechanisms controlling urethral function in rats. Neurourol. Urodyn. 25, 480–489 [DOI] [PubMed] [Google Scholar]
- 25. Seki, S., Igawa, Y., Kaidoh, K., Ishizuka, O., Nishizawa, O., and Andersson, K.E. (2001). Role of dopamine D1 and D2 receptors in the micturition reflex in conscious rats. Neurourol. Urodyn. 20, 105–113 [DOI] [PubMed] [Google Scholar]
- 26. Yoshimura, N., Mizuta, E., Yoshida, O., and Kuno, S. (1998). Therapeutic effects of dopamine D1/D2 receptor agonists on detrusor hyperreflexia in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned parkinsonian cynomolgus monkeys. J. Pharmacol. Exp. Ther. 286, 228–233 [PubMed] [Google Scholar]
- 27. Setler, P.E., Sarau, H.M., Zirkle, C.L., and Saunders, H.L. (1978). The central effects of a novel dopamine agonist. Eur. J. Pharmacol. 50, 419–430 [DOI] [PubMed] [Google Scholar]
- 28. Movin-Osswald, G. and Hammarlund-Udenaes, M. (1991). Remoxipride: pharmacokinetics and effect on plasma prolactin. Br. J. Clin. Pharmacol. 32, 355–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. King, D.J., Devaney, N., Cooper, S.J., Blomqvist, M., and Mitchell, M.J. (1990). Pharmacokinetics and antipsychotic effect of remoxipride in chronic schizophrenic patients. J. Psychopharmacol. 4, 83–89 [DOI] [PubMed] [Google Scholar]
- 30. Ballester Gonzalez, J., Dvorkin-Gheva, A., Silva, C., Foster, J.A., and Szechtman, H. (2015). Nucleus accumbens core and pathogenesis of compulsive checking. Behav. Pharmacol. 26, 200–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pertile, R.A.N., Corvino, M.E., Marchette, R.C.N., Pavesi, E., Cavalli, J., Ramos, A., and Izidio, G.S. (2017). The quinpirole hypolocomotive effects are strain and route of administration dependent in SHR and SLA16 isogenic rats. Behav. Genet. 47, 552–563 [DOI] [PubMed] [Google Scholar]
- 32. Hietala, J., Seppala, T., Lappalainen, J., and Syvalahti, E. (1992). Quantification of SCH 39166, a novel selective D1 dopamine receptor antagonist, in rat brain and blood. Psychopharmacology 106, 455–458 [DOI] [PubMed] [Google Scholar]
- 33. Giorgi, O., Pibiri, M.G., Loi, R., and Corda, M.G. (1993). Chronic treatment with SCH 23390 increases the production rate of dopamine D1 receptors in the nigro-striatal system of the rat. Eur. J. Pharmacol. 245, 139–145 [DOI] [PubMed] [Google Scholar]
- 34. Yoshimura, N., Kuno, S., Chancellor, M.B., De Groat, W.C., and Seki, S. (2003). Dopaminergic mechanisms underlying bladder hyperactivity in rats with a unilateral 6-hydroxydopamine (6-OHDA) lesion of the nigrostriatal pathway. Br. J. Pharmacol. 139, 1425–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cheng, C.L. and de Groat, W.C. (2016). Effect of orchiectomy and testosterone replacement on lower urinary tract function in anesthetized rats. Am. J. Physiol. Renal Physiol. 311, F864–F870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chancellor, M.B., Rivas, D.A., Huang, B., Kelly, G., and Salzman, S.K. (1994). Micturition patterns after spinal trauma as a measure of autonomic functional recovery. J. Urol. 151, 250–254 [DOI] [PubMed] [Google Scholar]
- 37. Hubscher, C.H., Montgomery, L.R., Fell, J.D., Armstrong, J.E., Poudyal, P., Herrity, A.N., and Harkema, S.J. (2016). Effects of exercise training on urinary tract function after spinal cord injury. Am. J. Physiol. Renal Physiol. 310, F1258–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hou, S., Duale, H., Cameron, A.A., Abshire, S.M., Lyttle, T.S., and Rabchevsky, A.G. (2008). Plasticity of lumbosacral propriospinal neurons is associated with the development of autonomic dysreflexia after thoracic spinal cord transection. J. Comp. neurol. 509, 382–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cameron, A.A., Smith, G.M., Randall, D.C., Brown, D.R., and Rabchevsky, A.G. (2006). Genetic manipulation of intraspinal plasticity after spinal cord injury alters the severity of autonomic dysreflexia. J. Neurosci. 26, 2923–2932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rice, M.E. and Patel, J.C. (2015). Somatodendritic dopamine release: recent mechanistic insights. Philos. Trans. R Soc. Lond. B Biol. Sci. 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. de Groat, W.C., Douglas, J.W., Glass, J., Simonds, W., Weimer, B., and Werner, P. (1975). Changes in somato-vesical reflexes during postnatal development in the kitten. Brain Res. 94, 150–154 [DOI] [PubMed] [Google Scholar]
- 42. Maggi, C.A., Santicioli, P., and Meli, A. (1986). Postnatal development of micturition reflex in rats. Am. J. Physiol. 250, R926–R931 [DOI] [PubMed] [Google Scholar]
- 43. Hua, J.Y., Smear, M.C., Baier, H., and Smith, S.J. (2005). Regulation of axon growth in vivo by activity-based competition. Nature 434, 1022–1026 [DOI] [PubMed] [Google Scholar]
- 44. Okawa, H., Hoon, M., Yoshimatsu, T., Della Santina, L., and Wong, R.O. (2014). Illuminating the multifaceted roles of neurotransmission in shaping neuronal circuitry. Neuron 83, 1303–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Araki, I. and De Groat, W.C. (1996). Unitary excitatory synaptic currents in preganglionic neurons mediated by two distinct groups of interneurons in neonatal rat sacral parasympathetic nucleus. J. Neurophysiol. 76, 215–226 [DOI] [PubMed] [Google Scholar]
- 46. Araki, I. and de Groat, W.C. (1997). Developmental synaptic depression underlying reorganization of visceral reflex pathways in the spinal cord. J. Neurosci. 17, 8402–8407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li, Y., Li, L., Stephens, M.J., Zenner, D., Murray, K.C., Winship, I.R., Vavrek, R., Baker, G.B., Fouad, K., and Bennett, D.J. (2014). Synthesis, transport, and metabolism of serotonin formed from exogenously applied 5-HTP after spinal cord injury in rats. J. Neurophysiol. 111, 145–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li, Y., Lucas-Osma, A.M., Black, S., Bandet, M.V., Stephens, M.J., Vavrek, R., Sanelli, L., Fenrich, K.K., Di Narzo, A.F., Dracheva, S., Winship, I.R., Fouad, K., and Bennett, D.J. (2017). Pericytes impair capillary blood flow and motor function after chronic spinal cord injury. Nat. Med. 23, 733–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wienecke, J., Ren, L.Q., Hultborn, H., Chen, M., Moller, M., Zhang, Y., and Zhang, M. (2014). Spinal cord injury enables aromatic L-amino acid decarboxylase cells to synthesize monoamines. J. Neurosci. 34, 11984–12000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ren, L.Q., Wienecke, J., Hultborn, H., and Zhang, M. (2016). Production of Dopamine by Aromatic l-Amino Acid Decarboxylase Cells after Spinal Cord Injury. J. Neurotrauma 33, 1150–1160 [DOI] [PubMed] [Google Scholar]
- 51. Hashimoto, K., Oyama, T., Sugiyama, T., Park, Y.C., and Kurita, T. (2003). Neuronal excitation in the ventral tegmental area modulates the micturition reflex mediated via the dopamine D1 and D2 receptors in rats. J. Pharmacol. Sci. 92, 143–148 [DOI] [PubMed] [Google Scholar]
- 52. Kontani, H., Inoue, T., and Sakai, T. (1990). Dopamine receptor subtypes that induce hyperactive urinary bladder response in anesthetized rats. Jpn. J. Pharmacol. 54, 482-486 [DOI] [PubMed] [Google Scholar]
- 53. Kontani, H., Inoue, T., and Sakai, T. (1990). Effects of apomorphine on urinary bladder motility in anesthetized rats. Jpn. J. Pharmacol. 52, 59–67 [DOI] [PubMed] [Google Scholar]
- 54. Dolber, P.C., Gu, B., Zhang, X., Fraser, M.O., Thor, K.B., and Reiter, J.P. (2007). Activation of the external urethral sphincter central pattern generator by a 5-HT(1A) receptor agonist in rats with chronic spinal cord injury. Am. J. physiol. Regul. Integr. Comp. Physiol. 292, R1699–R1706 [DOI] [PubMed] [Google Scholar]
- 55. Felder, R.A., Felder, C.C., Eisner, G.M., and Jose, P.A. (1989). The dopamine receptor in adult and maturing kidney. Am. J. Physiol. 257, F315–F327 [DOI] [PubMed] [Google Scholar]
- 56. Ozono, R., Ueda, A., Oishi, Y., Yano, A., Kambe, M., Katsuki, M., and Oshima, T. (2003). Dopamine D2 receptor modulates sodium handling via local production of dopamine in the kidney. J. Cardiovasc. Pharmacol. 42 Suppl 1, S75–S79 [DOI] [PubMed] [Google Scholar]
- 57. Siragy, H.M., Felder, R.A., Howell, N.L., Chevalier, R.L., Peach, M.J., and Carey, R.M. (1990). Evidence that dopamine-2 mechanisms control renal function. Am. J. Physiol. 259, F793–F800 [DOI] [PubMed] [Google Scholar]
- 58. Ishizuka, O., Gu, B.J., Nishizawa, O., Mizusawa, H., and Andersson, K.E. (2002). Effect of apomorphine on intracavernous pressure and blood pressure in conscious, spinalized rats. Int. J. impot. Res. 14, 128–132 [DOI] [PubMed] [Google Scholar]