Abstract
Rationale
chronic obstructive pulmonary disease (COPD) is a leading cause of mortality and common in older adults. The BODE Index is the most recognised mortality risk score in COPD but includes a 6-minute walk test (6MWT) that is seldom available in practise; the BODE Index may be better adopted if the 6MWT was replaced.
Objectives
we investigated whether a modified BODE Index in which 6MWT was replaced by an alternative measure of physical capacity, specifically the short physical performance battery (SPPB) or components, retained its predictive ability for mortality in individuals with COPD.
Methods
we analysed 630 COPD patients from the ERICA cohort study for whom UK Office for National Statistics verified mortality data were available. Variables tested at baseline included spirometry, 6MWT, SPPB and its components (4-m gait speed test [4MGS], chair stand and balance). Predictive models were developed using stratified multivariable Cox regression, and assessed by C-indices and calibration plots with 10-fold cross-validation and replication.
Results
during median 2 years of follow-up, 60 (10%) individuals died. There was no significant difference between the discriminative ability of BODE6MWT (C-index 0.709, 95% confidence interval [CI], 0.680–0.737), BODESPPB (C-index 0.683, 95% CI, 0.647–0.712), BODE4MGS (C-index 0.676, 95% CI, 0.643–0.700) and BODEBALANCE (C-index 0.686, 95% CI, 0.651–0.713) for predicting mortality.
Conclusions
the SPPB, and its 4MGS and balance components, can potentially be used as an alternative to the 6MWT in the BODE Index without significant loss of predictive ability in all-cause mortality.
Keywords: chronic obstructive pulmonary disease, mortality, biomarkers, skeletal muscle, older people
Key points
COPD is common amongst older individuals, accurate prediction of mortality risk improves care planning.
The BODE Index, a mortality index for COPD, has failed to be widely adopted, likely due to the impractical 6MWT component.
SPPB, and its 4MGS and balance components, is a practical alternative to 6MWT without significant loss in predicting mortality.
Introduction
Chronic obstructive pulmonary disease (COPD) is predominantly a disease of older people, with a prevalence of ~5% in individuals aged <65 years but rising to 20–25% in those aged >85 [1]. Importantly, like other diseases prevalent in older people, COPD is recognised to involve multiple organ systems with recognised extrapulmonary manifestations such as frailty, cardiovascular disease, osteoporosis and cognitive changes [2,3], as well as changes in skeletal muscle, which appear directly related to COPD [4]. COPD has also been considered on the basis of telomere shortening to be a disease of premature ageing [5].
Prognostication for people living with COPD is key for future care planning. Accurately predicting prognosis also helps people better understand their condition and decide on safety or desirability of interventional procedures such as general anaesthesia or chemotherapy. Mortality prediction is equally important for clinical guidelines and government health departments to plan for healthcare system delivery and funding provision.
The best known prognostic index for COPD is the BODE Index, a composite score of body mass index (BMI), spirometry, breathlessness and exercise capacity defined by the 6-minute walk test (6MWT), which on its own is predictive of all-cause mortality [6,7]. However, both BODE and 6MWT have received limited adoption in clinical practise globally, likely because the 6MWT requires a 30-m corridor and a training walk [8]; thus, the 6MWT in practise takes >30 minutes. Unsurprisingly, the NICE (National Institute for Health and Care Excellence) UK 2018 guidelines for COPD advised against the BODE Index for prognostication in COPD. Since the other three components of the BODE Index can be measured with relative ease, it is likely that the 6MWT is an important barrier to more widespread use of the BODE Index in clinical settings, and yet assessment of functional capacity contributes significantly to mortality prediction.
Several studies have tried to improve the BODE Index by exploring additional markers; replacing the 6MWT component with alternative measures of exercise capacity such as the incremental shuttle walk test [9] and maximum oxygen uptake [10] has shown equivalence. However, both tests are at least as time consuming and resource intensive as the 6MWT. Celli et al. [11] demonstrated that the addition of interleukin 6 to the BODE Index only improved the models’ predictive performance marginally, and venepuncture is again resource intensive. Interestingly, adding exacerbation history has not been shown to substantially improve the prognostic capacity of the BODE Index [12].
Recognition of a lack of suitable predictive biomarkers has led to extensive research to evaluate functional assessments that can be easily used in the clinical setting. Of the many different assessments studied, the short physical performance battery (SPPB), a composite score of 4-m gait speed test (4MGS), balance and chair stand, is an attractive tool that fits these criteria. It has been validated in different clinical settings, is prognostic for mortality in older individuals in the general population and takes <5 minutes to complete [13–15].
We hypothesised that the SPPB may have comparable predictive power to the 6MWT, and could thus replace it within the BODE Index. We firstly aimed to evaluate the association between all-cause mortality with 6MWT, SPPB and its components in COPD patients. Next, we sought to investigate whether a BODE Index in which the 6MWT was replaced by either the SPPB or its components retained predictive ability. Finally, we aimed to assess whether addition of the SPPB or its individual components to the BODE Index improved the predictive ability for all-cause mortality.
Methods
Study design and participants
The Evaluation of the Role of Inflammation in Chronic Airways disease (ERICA) study is a multicentre observational study of 729 stable global initiative for obstructive lung disease (GOLD) stage II–IV COPD patients, registered with the UK Clinical Trials Gateway. Co-morbidities were not considered as exclusion criteria. Full details of the protocol and baseline results are provided elsewhere [16] (Supplementary Text S1). Following baseline assessments, mortality data were obtained from the UK Office for National Statistics (ONS). Analyses presented here were limited to a maximum of 3 years of follow-up that ended in August 2016.
Informed written patient consent was obtained from all study participants. The National Research Ethics Service Committee East of England—Cambridge South (reference 11/EE/0357) approved the study.
Point assignment for components of BODE Index and SPPB
The BODE Index is a multidimensional weighted model. The model is made up of BMI (kg/m2), Obstruction (FEV1% predicted), Dyspnoea (Medical Research Council [MRC] score) and Exercise capacity (6MWT distance) [6].
Short physical performance battery is a lower-extremity physical assessment consisting of three separate components scored 0–4 each, comprising the 4MGS, standing balance and five repetition chair stand test [17,18]. The only equipment required is a stopwatch, standard chair and measuring tape to mark a distance of 4 m. Total SPPB score ranges 0–12 points, with higher scores indicating better performance. To preserve a four-category system, we combined one and two points.
Points for BODE Index (0–10) were assigned in quartiles (Supplementary Table S2), with higher scores indicating higher risk of mortality [6]. The SPPB itself was divided into 10–12, 7–9, 4–6 and <4 based on the distribution of data and cut-off score of <10 to define functional limitation [19,20].
Statistical analysis
Hazard ratios (HR) with 95% confidence intervals (CI) were estimated using multivariable Cox regression. All statistical models were stratified by recruitment centre, adjusted for age and sex and developed according to the TRIPOD and guidelines for clinical prediction models. The preselected prediction models were: (i) BMI, (ii) BMI + MRC dyspnoea, (iii) BMI + MRC dyspnoea + FEV1%, (iv) BMI + MRC dyspnoea + FEV1% + 6MWT, (v) BMI + MRC dyspnoea + FEV1% + SPPB. Linearity of continuous predictors was assessed visually. We tested for violation of the proportional hazards assumption by including time interactions and visually examining Arjas plots. Discrimination (i.e. Harrell’s C-statistic [21,22]) and calibration (i.e. Gronnesby and Borgan test [23] and calibration plots) were assessed using 10-fold cross-validation with 200 replications [24]. Effect of missing data was assessed in sensitivity analyses using multivariable imputation by chained equations (MICE). Detailed methods are provided in the online supplement (Supplementary Text S2).
Analyses were performed using Stata version 13.0 (College Station, TX) and R (R Foundation). Observational data are reported according to the STROBE statement. All tests were two-sided and statistical significance was defined by 95% CI for HRs not traversing 1 or P value <0.05.
Results
Of 714 individuals with COPD followed by the ONS for survival status, 630 had complete baseline data and were included in the primary analysis (Supplementary Figure S1); of these 630 individuals, 386 (61%) were male, 192 (30%) current smokers, 358 (57%) GOLD grade II and the median age was 67 years (range 43–84 years; Table 1 and Supplementary Tables S3–S5). In total, 245 (39%) had functional limitation (SPPB score <10) with a median (interquartile range [IQR]) SPPB score of 10 (8–12) and 6MWT distance of 370 (268–440) m.
Table 1.
Baseline characteristics (analytical population, n = 630).
| Characteristics | All individuals (%) |
|---|---|
| Description | |
| Age (years), median (IQR) | 67 (62–73) |
| Male | 386 (61) |
| BMI (kg/m2), median (IQR) | 27 (23–31) |
| Lung function | |
| FEV1% predicted, median (IQR) | 53 (40–65) |
| Current smoker | 192 (30) |
| MRC dyspnoea score | |
| 1 | 54 (9) |
| 2 | 261 (41) |
| 3 | 138 (22) |
| 4 | 125 (20) |
| 5 | 52 (8) |
| GOLD | |
| Stage II | 358 (57) |
| Stage III | 216 (34) |
| Stage IV | 56 (9) |
| Musculoskeletal measures | |
| 6MWT distance (m), median (IQR) | 370 (268–440) |
| SPPB (0–12), median (IQR) | 10 (8–12) |
| No functional limitation ≥10 | 385 (61) |
| Functional limitation <10 | 245 (39) |
| 4MGS score (0–4), median (IQR) | 4 (3–4) |
| Balance points (0–4), median (IQR) | 4 (4–4) |
| Chair stand score (0–4), median (IQR) | 3 (1–4) |
| QMVC peak (kg), median (IQR) | 30 (22–39) |
Values are given as the median and IQR, or no. of cases (%). Baseline data of 630 individuals are included. QMVC, quadriceps maximum voluntary contraction.
Factors associated with all-cause mortality
Sixty patients (10%) died during the follow-up period. The three-year survival probability was 90% (88–93% CI) with an event rate of 3.3 (95% CI, 2.6–4.3) per 100 person-years. Age-adjusted multivariable analysis identified multiple markers associated with mortality including BMI (HR 0.91 per 1-point increase, 95% CI, 0.86–0.97, P = 0.002), 6MWT (HR 0.85 per 30-m increase, 95% CI, 0.78–0.92, P < 0.001), SPPB (HR 0.81 per 1-point increase, 95% CI, 0.72–0.92, P = 0.002), 4MGS (HR 0.67 per 1-point increase, 95% CI, 0.49–0.93, P = 0.015) and balance (HR 0.63 per 1-point increase, 95% CI, 0.48–0.82, P = 0.001), Supplementary Figure S2 and Supplementary Table S6). Chair stand was not associated with all-cause mortality in multivariable analysis.
Predictive models
Predictive modelling indicated slightly higher HR for SPPB and its components compared with BODE6MWT (Figure 1 and Supplementary Figure S3). The C-statistic was the highest for BODE6MWT (C = 0.709, 95% CI, 0.680–0.737), but there was no significant difference in discriminative ability compared with BODESPPB (C = 0.683, 95% CI, 0.647–0.712; Figure 1 and Table 2). Neither was there a significant difference in risk discrimination when compared with the BODE4MGS (C = 0.676, 95% CI, 0.643–0.700) or BODEBALANCE (C = 0.686, 95% CI, 0.651–0.713). When comparing BODESPPB with its components, there were no significant differences in risk discrimination between indices. Calibration tests and plots of the hazard models indicate good model fit and calibration for 3-year prediction of mortality (Table 2 and Supplementary Figure S4, and Supplementary Text S3). Risk quartile estimates for each BODE model are displayed using Kaplan–Meier plots (Figure 2).
Figure 1.

HRs and C-indices with change scores for various BODE models. All models were stratified by recruitment centre.
Table 2.
Cox proportional hazards regression analyses for all-cause mortality, using continuous data.
| Model | Model 1: BMI | Model 2: BMI, MRC | Model 3: BMI, MRC, FEV1% | Model 4: BODE6MWT | Model 5: BODESPPB | Model 6: BODE4MGS | Model 7: BODEBALANCE |
|---|---|---|---|---|---|---|---|
| Variables | HR (95% CI) | ||||||
| BMI—per 1-point increase | 0.90 (0.85–0.95) | 0.91 (0.86–0.95) | 0.91 (0.87–0.96) | 0.89 (0.85–0.94) | 0.90 (0.86–0.95) | 0.91 (0.86–0.95) | 0.91 (0.87–0.96) |
| MRC dyspnoea score—per 1-point increase | 1.38 (1.10–1.72) | 1.27 (0.99–1.63) | 0.92 (0.68–1.23) | 1.05 (0.80–1.38) | 1.15 (0.89–1.49) | 1.15 (0.90–1.49) | |
| FEV1—per 5% increase % predicted | 0.93 (0.85–1.02) | 0.98 (0.89–1.07) | 0.93 (0.84–1.02) | 0.94 (0.85–1.03) | 0.92 (0.83–1.01) | ||
| 6MWT—per 30-m increase | 0.84 (0.78–0.91) | ||||||
| SPPB (component)—per increase of 1 point | 0.80 (0.71–0.90) | 0.61 (0.45–0.84) | 0.63 (0.49–0.82) | ||||
| C-index | 0.634 (0.600–0.658) | 0.650 (0.620–0.676) | 0.646 (0.607–0.672) | 0.709 (0.680–0.737) | 0.682 (0.647–0.712) | 0.676 (0.642–0.700) | 0.685 (0.651–0.712) |
| Goodness of fit, chi2(3) | 10.63 | 2.57 | 1.95 | 2.64 | 1.57 | 0.89 | 4.28 |
| P > chi2 | 0.014 | 0.464 | 0.583 | 0.451 | 0.665 | 0.827 | 0.233 |
| Change in C-statistic | −0.075 (−0.106 to −0.048) | −0.060 (−0.082 to −0.037) | −0.064 (−0.083 to −0.041) | Reference | −0.027 (−0.052 to −0.009) | −0.033 (−0.057 to −0.013) | −0.024 (−0.047 to −0.005) |
All models were stratified by recruitment centre. Goodness of fit estimates was based on quartiles of risk. FEV1% = predicted forced expiratory volume 1 second.
Figure 2.
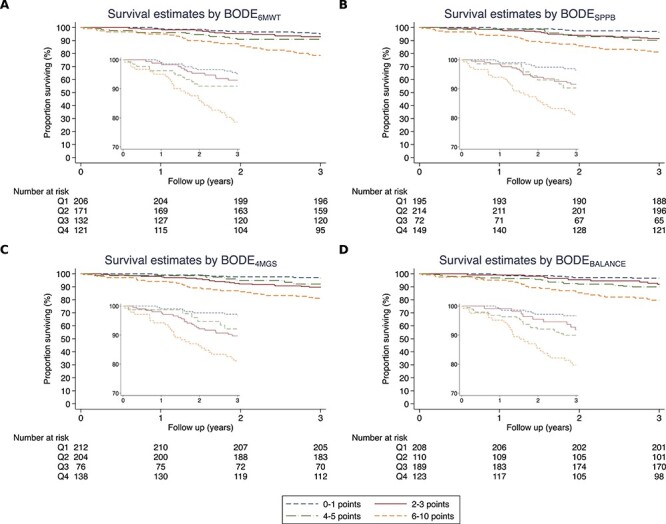
Survival risk indices, by quartiles. (A) BODE6MWT, (B) BODESPPB, (C) BODE4MGS and (D) BODEBALANCE. Mortality data obtained from the UK ONS.
Compared with the composite scoring, use of continuous data did not significantly improve discriminative ability for any of the BODE indices (Supplementary Table S7). When assessing BODEs’ individual scoring components, most of BODEs’ predictive ability was attributed to 6MWT (C = 0.648, 95% CI, 0.609–0.673; Supplementary Figure S5). When replacing 6MWT with the SPPB, or its components 4MGS or balance, the C-index changed from 0.671 (95% CI, 0.641–0.693) to 0.667 (95% CI, 627–0.694), 0.670 (95% CI, 0.634–0.694) and 0.682 (95% CI, 0.646–0.702), respectively. The C-index (95% CI) for the SPPB total score alone was 0.617 (0.580–0.645), 0.602 (0.536–0.639) for balance and 0.600 (0.545–0.631) for 4MGS.
Sensitivity analysis
All 714 individuals (n = 71 deaths after 3 years of follow-up) were included in sensitivity analyses using multiple imputation of missing baseline values (Supplementary Figures S6–S9). Cross-validated C-indices decreased but were unchanged between the different models (Supplementary Table S8).
Discussion
Short physical performance battery or its 4MGS and balance components can replace 6MWT in BODE for the prediction of all-cause mortality in stable COPD patients without loss of predictive power. The study confirms prior observations that the total SPPB or 4MGS or balance test individually is associated with prognosis in simple age- and sex-adjusted analysis.
Older patients are frequently co-morbid and have functional limitation. It is this property that is reflected in the BODE score. Often a patient’s consideration for therapies, for example surgery or chemotherapy, with significant side effects or which are resource intensive may be determined by their overall prognosis. In this context, a diagnosis of COPD may be used as a reason not to offer therapies that are otherwise beneficial. Thus, being able to determine an accurate medium-term prognosis is often helpful in determining the best advice for patients and the most appropriate use of resources. In the present study, the average age of participants was 67 with an IQR 62–73 making them very typical of older patients with COPD in the UK.
The majority of our cohort could be classified as ‘normal’ physical function based on the SPPB and very few patients had low SPPB scores. Furthermore, the majority of our patients had low BODE Index scores. This is important to consider when interpreting our results, and is likely reflective of a general outpatient population of patients with COPD rather than a more severe COPD population engaging in pulmonary rehabilitation, therefore making the results from our models more generalisable to clinicians seeing patients in a primary or secondary outpatient setting. The study population in which a model is examined is important, especially when considering that most models are not externally validated due to limitations in similarities across study populations [25].
Unlike 6MWT, the SPPB is simple to measure, requiring only a standard chair, stopwatch (or smartphone) and a 4-m flat surface, taking <5 minutes. In 2018, the European Medicines Agency approved the SPPB as a measure of frailty for diseases associated with musculoskeletal decline [26]. Furthermore, our data suggest that even substitution of a single component (e.g. balance) does not result in any significant loss in predictive ability compared with BODE6MWT.
We were unable to demonstrate any significant improvement in the predictive ability of the BODE6MWT by adding the SPPB as an additional test. Strong correlations between 6MWT and SPPB (and its components) have been described previously [27] and were to be expected as they both assess lower limb function (Supplementary Figure S10). We suspect this explains not only why the SPPB can be easily substituted for 6MWT but also why it conferred no additional value when added to 6MWT.
The superiority of the balance component is of interest and may reflect that balance is an integrative test reflecting multiple pathologies or the effects of polypharmacy [28] beyond musculoskeletal weakness. Since, only 128 (20%) of our cohort had a score below the maximum four points, this may indicate that there is a threshold effect where any reduction in the balance score confers a higher risk of mortality.
This study has limitations. Firstly, there is no independent validation cohort with a fully comparable data set. We mitigated this issue using a cross-validation technique approach and estimated C-indices through random partitioning of the dataset. Secondly, baseline data differed amongst the recruitment centres but was addressed through stratification by centre. Thirdly, there were missing data with evidence that some were not at random (Supplementary Figure S11). Analysing complete-case data may have introduced bias, and although HRs and C-statistics of the models shifted following MICE, the main conclusions were unchanged.
Additional deaths occurred beyond the 3 years of follow-up included in the primary analysis. However, 3 years of follow-up was chosen because insufficient deaths occur over a shorter time frame, whereas over a long time period, the predictive ability of BODE diminishes, both because ageing is a strong predictive variable and because measured variables at baseline are so distant from the point of death. Consistent with this, some very large COPD trials such as the TOwards a Revolution in COPD Health (TORCH) [29] and Study to Understand Mortality and Morbidity in COPD (SUMMIT) [30] have used 3-year follow-up, and furthermore, this is the time frame that regulatory agencies such as the Food and Drug Administration and European Medicines Agency also consider for outcomes data in COPD. Pragmatically when considering the wisdom of an unrelated intervention in an older person, 3 years is a reasonable time frame.
Conclusion
The SPPB, and its 4MGS and balance components, can potentially be used as an alternative to the 6MWT in the BODE Index without significant loss of predictive ability in all-cause mortality. Adoption of the SPPB might potentially enhance the uptake of risk indices such as the BODE Index, and subsequently prognostication of COPD, in clinical practise.
Supplementary Material
Acknowledgements
Authors thank Dr Victoria Tribble and Dr Nicholas Hopkinson for their comments on the work.
Declaration of Conflicts of Interest
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: GSK, a consortium partner, funded JMFs PhD. D.M. is a current and R.T.S. and H.M. are former employees of GSK, and all are current shareholders. H.M. is a current employee of AstraZeneca. J.C. is employed by Cambridge University Hospitals NHS Foundation Trust and obligated to spend 50% of his time on GSK clinical trial activity, representing a significant relationship; however, he receives no other benefits or compensation from GSK. I.B.W. received grants from GSK during the conduct of the study and outside the submitted work. M.I.P. received grants from GSK outside the submitted work. M.I.P.’s institution has received payment from GSK for M.I.P.’s time served as chief investigator for a trial of an anabolic medicine. The views expressed are those of the authors and not necessarily those of the National Institute of Health, the National Institute for Health Research or the Department of Health and Social Care.
Declaration of Sources of Funding
This work was supported by a grant (9157–61188) from Innovate UK (formerly known as Technology Strategy Board) with contributory funding in kind from GlaxoSmithKline, a consortium partner, who also funded the corresponding author's PhD. This work was supported by core funding from: the UK Medical Research Council (MR/L003120/1), the British Heart Foundation (RG/13/13/30194; RG/18/13/33946) and the National Institute for Health Research [Cambridge Biomedical Research Centre at the Cambridge University Hospitals NHS Foundation Trust] [*]. This work was also supported by Health Data Research UK, which is funded by the UK Medical Research Council, Engineering and Physical Sciences Research Council, Economic and Social Research Council, Department of Health and Social Care (England), Chief Scientist Office of the Scottish Government Health and Social Care Directorates, Health and Social Care Research and Development Division (Welsh Government), Public Health Agency (Northern Ireland), British Heart Foundation and Wellcome. *The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
References
- 1. Incalzi RA. An epidemiological overview and clinical picture of COPD in the elderly. J Gerontol Geriatr 2016; 64: 119–25. [Google Scholar]
- 2. Agustí AGN. Systemic effects of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2005; 2: 367–70. [DOI] [PubMed] [Google Scholar]
- 3. Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J 2009; 33: 1165–85. [DOI] [PubMed] [Google Scholar]
- 4. Natanek SA, Gosker HR, Slot IGMet al. . Heterogeneity of quadriceps muscle phenotype in chronic obstructive pulmonary disease (COPD); implications for stratified medicine? Muscle Nerve 2013; 48: 488–97. [DOI] [PubMed] [Google Scholar]
- 5. Lee J, Sandford AJ, Connett JEet al. . The relationship between telomere length and mortality in chronic obstructive pulmonary disease (COPD). PLoS One 2012; 7: e35567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Celli BR, Cote CG, Marin JMet al. . The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004; 350: 1005–12. [DOI] [PubMed] [Google Scholar]
- 7. Spruit MA, Polkey MI, Celli Bet al. . Predicting outcomes from 6-minute walk distance in chronic obstructive pulmonary disease. J Am Med Dir Assoc 2012; 13: 291–7. [DOI] [PubMed] [Google Scholar]
- 8. Holland AE, Spruit MA, Troosters Tet al. . An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J 2014; 44: 1428–46. [DOI] [PubMed] [Google Scholar]
- 9. Williams JEA, Green RH, Warrington V, Steiner MC, Morgan MDL, Singh SJ. Development of the i-BODE: validation of the incremental shuttle walking test within the BODE index. Respir Med 2012; 106: 390–6. [DOI] [PubMed] [Google Scholar]
- 10. Cardoso F, Tufanin AT, Colucci M, Nascimento O, Jardim JR. Replacement of the 6-min walk test with maximal oxygen consumption in the BODE index applied to patients with COPD: an equivalency study. Chest 2007; 132: 477–82. [DOI] [PubMed] [Google Scholar]
- 11. Celli BR, Locantore N, Yates Jet al. . Inflammatory biomarkers improve clinical prediction of mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 185: 1065–72. [DOI] [PubMed] [Google Scholar]
- 12. Soler-Cataluna JJ, Martinez-Garcia MA, Sanchez LS, Tordera MP, Sanchez PR. Severe exacerbations and BODE index: two independent risk factors for death in male COPD patients. Respir Med 2009; 103: 692–9. [DOI] [PubMed] [Google Scholar]
- 13. Legrand D, Vaes B, Mathei C, Adriaensen W, Van Pottelbergh G, Degryse JM. Muscle strength and physical performance as predictors of mortality, hospitalization, and disability in the oldest old. J Am Geriatr Soc 2014; 62: 1030–8. [DOI] [PubMed] [Google Scholar]
- 14. Volpato S, Cavalieri M, Sioulis Fet al. . Predictive value of the short physical performance battery following hospitalization in older patients. J Gerontol A Biol Sci Med Sci 2011; 66: 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Studenski S, Perera S, Wallace Det al. . Physical performance measures in the clinical setting. J Am Geriatr Soc 2003; 51: 314–22. [DOI] [PubMed] [Google Scholar]
- 16. Mohan D, Gale NS, McEniery CMet al. . Evaluating the role of inflammation in chronic airways disease: the ERICA study. COPD 2014; 11: 552–9. [DOI] [PubMed] [Google Scholar]
- 17. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 1995; 332: 556–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guralnik JM, Simonsick EM, Ferrucci Let al. . A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994; 49: M85–94. [DOI] [PubMed] [Google Scholar]
- 19. Pavasini R, Guralnik J, Brown JCet al. . Short physical performance battery and all-cause mortality: systematic review and meta-analysis. BMC Med 2016; 14: 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bernabeu-Mora R, Medina-Mirapeix F, Llamazares-Herran E. The short physical performance battery is a discriminative tool for identifying patients with COPD at risk of disability (vol 10, pg 2619, 2015). Int J Chron Obstruct Pulmon Dis 2016; 11: 623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Altman DG, Vergouwe Y, Royston P, Moons KGM. Prognosis and prognostic research: validating a prognostic model. BMJ 2009; 338: b605. [DOI] [PubMed] [Google Scholar]
- 22. Newson RB. Comparing the predictive powers of survival models using Harrell’s C or Somers’ D. Stata J 2010; 10: 339–58. [Google Scholar]
- 23. Altman DG. Practical Statistics for Medical Research. CRC Press: London, 1990. [Google Scholar]
- 24. Smith GC, Seaman SR, Wood AM, Royston P, White IR. Correcting for optimistic prediction in small data sets. Am J Epidemiol 2014; 180: 318–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bellou V, Belbasis L, Konstantinidis AK, Tzoulaki I, Evangelou E. Prognostic models for outcome prediction in patients with chronic obstructive pulmonary disease: systematic review and critical appraisal. BMJ 2019; 367: l5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. European Medicines Agency . Reflection paper on physical frailty: instruments for baseline characterisation of older populations in clinical trials. European Medicines Agency: London, 2018. [Google Scholar]
- 27. Kon SS, Patel MS, Canavan JLet al. . Reliability and validity of 4-metre gait speed in COPD. Eur Respir J 2013; 42: 333–40. [DOI] [PubMed] [Google Scholar]
- 28. Hanlon P, Nicholl BI, Jani BDet al. . Examining patterns of multimorbidity, polypharmacy and risk of adverse drug reactions in chronic obstructive pulmonary disease: a cross-sectional UK Biobank study. BMJ Open 2018; 8: e018404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McGarvey LP, John M, Anderson JA, Zvarich M, Wise RA. Ascertainment of cause-specific mortality in COPD: operations of the TORCH Clinical Endpoint Committee. Thorax 2007; 62: 411–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Suissa S. Will SUMMIT reach the peak in COPD? Thorax 2014; 69: 405–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


