Abstract
Objectives/Hypothesis
Bilateral stimulation of the posterior cricoarytenoid (PCA) muscles offers a physiologic approach to rehabilitate ventilation to a normal level in case of bilateral laryngeal paralysis. The objective was to evaluate the safety and efficacy of a new generation stimulator in restoring glottal opening, ventilation and exercise tolerance.
Study Design
A prospective study in three canines over 6-17 months.
Methods
A Genesis XP stimulator and electrodes were surgically implanted and the recurrent laryngeal nerves sectioned/repaired bilaterally. In bimonthly sessions, vocal fold movement was measured endoscopically in the anesthetized animal. The movement resulted from PCA stimulation or hypercapnea during spontaneous breathing. Exercise tolerance was measured on a treadmill using pulse oximetry and swallowing function examined by videofluoroscopy.
Results
During the denervation phase, there was minimal ventilatory compromise and near normal exercise tolerance with the device off (12 minutes up to 8 mph). PCA stimulation produced only nominal abduction. During the reinnervation phase, synkinetic reinnervation became significant with narrowed passive airway and paradoxical closure of the glottis during hypercapnea. Animals were stridorous and could walk for only 1-2 minutes @ 4 mph. With the device activated, bilateral PCA stimulation increased glottal area from 50 mm2 to 250 mm2, even during hypercapnea, equaling that of a normally innervated animal. Exercise tolerance was normal. There was no evidence of aspiration during deglutition.
Conclusions
This study demonstrates that severe ventilatory compromise only occurs following faulty reinnervation of laryngeal muscles. Bilateral PCA stimulation can result in complete rehabilitation of ventilation and exercise tolerance without impairment of swallowing.
Keywords: laryngeal pacing, bilateral vocal fold paralysis, electrical stimulation, posterior cricoarytenoid muscle, ventilation, aspiration
INTRODUCTION
Bilateral laryngeal paralysis presents a serious ventilatory problem, often requiring emergent tracheotomy. In case of persistent paralysis, the patient can be relieved of the tracheotomy and breathing returned through the mouth by surgical resection and enlargement of the airway (e.g. cordotomy, arytenoidectomy).1-3 However, voice is impaired and aspiration may occur with swallowing. A more physiologic approach termed laryngeal pacing involves functional neuromuscular stimulation (FNS) of the posterior cricoarytenoid (PCA) muscle to abduct the vocal folds during inspiration. During noninspiratory phases, the vocal folds passively relax to the midline allowing for normal voice and airway protection. Both animal and human trials have demonstrated the feasibility of laryngeal pacing.4-15
The first human trial was performed using an external pacing circuit, an inspiratory transducer worn around the chest to trigger the circuit, and percutaneous needle electrodes to deliver stimuli to the abductor muscle.12 More recently, clinical trials with an implantable stimulation device (Itrel II) were conducted under the auspices of Medtronic, Inc (Minneapolis, MN), in a multi-institutional study.13-15 In five of six patients, unilateral laryngeal stimulation restored airflow through the mouth equal to that through the tracheotomy without impairment of voice or swallowing. Three patients were subsequently decannulated. However, two problems were encountered in the translation of this technology for laryngeal reanimation. First, the electrode was originally designed for spinal cord stimulation and not optimally configured for the tiny PCA muscle. Second, the device interfaced with just one electrode lead, limiting abductor muscle stimulation and vocal fold opening to one side.
The focus of the present study was to evaluate the utility of a new generation device (Genesis XP, ANS, Inc) in a chronic canine model of bilateral laryngeal paralysis. The device could be interfaced with two deep brain stimulation (DBS) electrodes to deliver stimuli to the PCA muscles bilaterally to potentially restore a normal level of ventilation. In a preliminary study in acute canines, the DBS electrode was found optimal for PCA muscle reanimation.16 The purpose of the current study was to assess the efficacy and safety of laryngeal stimulation in the chronic canine model using vocal fold endoscopy, treadmill performance, and barium swallow videofluoroscopy.
MATERIALS AND METHODS
This study was performed in accordance with the PHS Policy on Humane Care and Use of Laboratory Animals, the NIH Guide for the Care and Use of Laboratory Animals, and the Animal Welfare Act (7 U.S.C. et seq.); the animal use protocol was approved by the Institutional Animal Care and Use Committee of Vanderbilt University.
Implant surgery
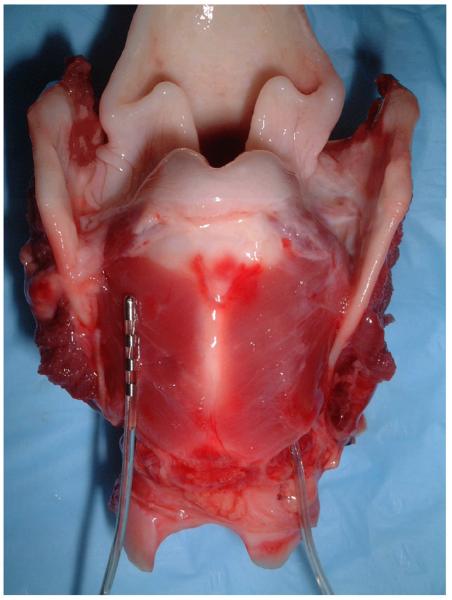
Three canines (body weight 25 kg) were entered into the study. Each animal was anesthetized with Telazol (Wyeth, Inc, Madison, Wisconsin) intravenously followed by Isoflurane in oxygen through intubation. All procedures were performed under aseptic conditions. The animal was placed in a supine position and a midline neck incision made from the thyroid notch to manubrium. The trachea was dissected free from the esophagus and the inferior border of the cricoid cartilage was exposed. On each side, a submuscular pocket was created between the PCA muscle and the underlying cricoid cartilage using a periosteal elevator. A DBS electrode was inserted into each pocket halfway between the point of RLN entry and median raphe with a trajectory parallel to the midline (fig. 1). After endoscopic confirmation that stimulation produced vocal fold abduction for each channel, the electrodes were anchored to the cricoid cartilage by 4-0 silk. The channels were numbered 1 to 4 on the left side and 5 to 8 on the right side from tip to base of each electrode. The electrode leads were interfaced with the implantable pulse generator (IPG) which, in turn, was positioned in a submuscular pocket beneath either the sternocleidomastoid or trapezius muscle (fig. 2). After implantation, IPG stimulus parameters could be changed transcutaneously with an external programmer through a radio frequency link. The recurrent laryngeal nerves (RLNs) were transected 5 cm from the cricoid bilaterally and anastomosed using 6-0 prolene. The neck incision was closed and antibiotics administered for 4 days perorally. Because of the risk of ventilatory compromise and aspiration following nerve section, the animals were periodically monitored and given soft food by hand for 7 days postoperatively.
Figure 1.
Posterior view of the larynx showing the DBS electrode inserted into the submuscular pocket of the right PCA. Detail of the left PCA muscle electrode can be appreciated prior to insertion.
Figure 2.
Photograph of DBS electrodes used for bilateral PCA stimulation interfaced with the ANS implantable pulse generator.
Chest x-ray and videofluoroscopy with barium swallow were performed preoperatively, every 6 months, and following any suspected aspiration event (GE-OEC 9900 Elite Mobile X-ray C-arm system (General Electric Healthcare, Inc).
Endoscopic session
Endoscopic sessions were conducted on each animal every two weeks during the reinnervation period (first six months) and monthly thereafter under telazol anesthesia (.4 mg/kg per hour IV). With the animal in a supine position, an endoscope with an attached Sony CCD (Tokyo, Japan) video camera was inserted through a laryngoscope to visualize and record vocal fold motion at the level of the glottis. Both stimulated PCA responses and spontaneous motion induced by hypercapnea were recorded, digitized, and saved on hard disk for off-line analysis. Because the magnification of each image varied with endoscopic position, a 3 mm ruler was placed on the vocal fold for calibration. During PCA stimulation, selected still frame images indicative of the passive and stimulated airway were analyzed with computer software (Photoshop CS3, Adobe System Inc, San Jose, California). In each session, the numbers of pixels within the circumscribed whole glottal and hemiglottal areas were counted for each image over a range of stimulus amplitude (mA). Stimulus frequency and pulse duration were fixed at 40 Hz and 1 msec during delivery of a pulse train to the muscle.
CO2 administration trial
In order to assess any return of vocal fold motion with reinnervation in the anesthetized animal, carbon dioxide mixed with room air was administered to boost respiration toward that which might occur in the awake exercising animal. A pletysmographic belt transducer was positioned around the chest wall to monitor expansion of the chest with respiration on an oscilloscope. The oscilloscope trace was videomonitored and the image superimposed on the endoscope CCD image using a digital AV mixer. The split screen television imaging allowed inspiratory and expiratory chest movements to be compared with spontaneous opening or closing of the glottis. Carbon Dioxide mixed with room air was administered orally at a rate of 2L/min for 60 seconds. The split screen video recordings were taken during the CO2 delivery and for an additional 120 seconds to allow full return to normal respiration. Still frame images were obtained every 10 seconds at peak inspiration and peak expiration for computer measurements of whole glottal area. Additional CO2 trials were conducted during unilateral and bilateral PCA stimulation to determine if stimulation would still produce glottal opening in face of any spontaneous vocal fold activity.
Treadmill test
Before implant surgery, each canine was acclimated to run on the treadmill over 8 trials and to check its ability to exercise during baseline data collection. A sensor was placed on the tail to measure blood hemoglobin oxygen saturation and heart rate with a pulse oximeter (Ultra cap N-6000, Nellcor, Inc.). The tail was carefully shaved the day before each treadmill test to enhance detection of the sensor infrared signal. A relatively strenuous exercise protocol was adopted in order to distinguish animals with normal exercise tolerance from those that were deficient. The full treadmill test comprised a 12 minute course of 3 minute runs at four levels of sequentially increasing speed (4, 5.3, 6.7, and 8 mph). If the hemoglobin oxygen saturation level dropped below 90% or the animal developed stridor at any time over the course, the treadmill was stopped and the exercise time recorded to obtain the endpoint of tolerance. Animals were tested with no stimulation, unilateral stimulation, and bilateral stimulation in separate trials.
Swallowing study with endoscope
During the endoscopic sessions, a study of an animal's swallow was performed to determine the competency of airway protection and the degree of glottal closure during swallowing. To induce a swallow the pharyngeal mucosa was touched with a cotton swab or a TECA needle for delivering a weak electrical stimulus. The glottal response was visualized and videotaped using a supraglottic intraoral endoscope. However, elevation of the larynx and obstruction of the view by supralaryngeal structures during the swallow was problematic. Therefore, a subglottic approach to viewing glottal movement was taken by inserting a 1.9 mm, 30° rigid telescope (Karl Storz Endoscopy-America, Inc, Culver City, CA) percutaneously into the trachea through a small needle.
RESULTS
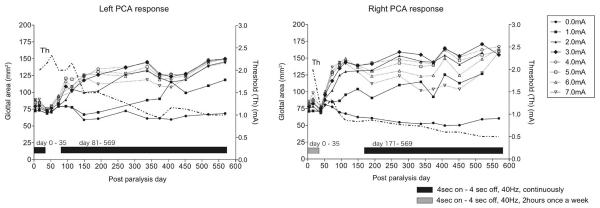
Data are shown from the first animal in the following figures, which is representative of data from the other two animals. The glottal responses to PCA muscle stimulation are shown as a function of post paralysis time in this animal in figure 3. Each data point represents the average glottal area measured from stimulation across all electrode channels on one side. The family of curves illustrate the glottal area obtained at each stimulus level. The curve at “0” mA demarcates the glottal area of the nonstimulated passive airway. It can be noted that a maximum stimulus level was required to reach peak glottal area during the initial denervation phase. The responses obtained were nominal until the reinnervation phase commenced at 70 days on the left and 40 days on the right. After this time, the responses grew rapidly with progressive reinnervation until 100-150 days and then tapered off for the remainder of the 19 month study. Following the rapid reinnervation period, the peak response was obtained at less than 3 mA. The current level required for a threshold response was also measured during endoscopic sessions (dashed lines). In general, the threshold curves varied inversely to the peak response curve at 3 mA; the threshold curves showed an initial drop followed by a tapered decrease over time in each graph. These changes reflected the lowering of stimulus requirements as activation switched from PCA muscle fibers to their reinnervating motor axons.
Figure 3.
Average glottal areas measured across 4 channels during left PCA stimulation and 4 channels during right PCA stimulation at each post operative session. Current levels approaching 3 mA tended to enhance the response (solid symbols, solid lines), while higher currents did not (open symbols, dotted lines). Current levels required for a threshold response are shown to the right of each graph (dashed lines). During the denervation period, continuous stimulation with 50% duty cycle was applied on the left from 0-35 days while intermittent stimulation was applied on the right. The delay and decrease in the response on the left was consistent with findings in the other animals that early intervention with continuous stimulation inhibited reinnervation of the muscle. Intermittent stimulation had no such effect. When synkinetic reinnervation compromised ventilation after 81 days, unilateral stimulation and eventually bilateral stimulation was applied to prevent dyspnea.
It may be noted that after the rapid reinnervation phase at 100-150 days, currents greater than 3 mA failed to generate peak abductory responses (dotted lines, open symbols). This was attributed to stray activation of anterior branch axons reinnervating adductor muscles that antagonized the stimulated PCA response. It seemed reasonable that any antagonistic responses to PCA stimulation would only appear later during the course of reinnervation in face of the longer path length for regenerating motor axons to reach adductor muscles. It might also be noted that the passive airway slightly decreased over time due to an increased tone of adductor muscles that accompanied their reinnervation.
Finally, it must be pointed out that the onset of the enhanced response with reinnervation on the left side lagged behind that on the right (70 days versus 40 days) and the final level of stimulated glottal opening achieved on the left was less than that on the right (140 mm2 versus 170 mm2). This delay in onset of response with decrease in final outcome was attributed to an inhibitory influence on left PCA muscle reinnervation stemming from early intervention with continuous laryngeal pacing. In particular, continuous stimulation was applied to the left PCA for 35 days after nerve section in this animal while only intermittent stimulation (2 hours/week) was applied to the right PCA. A similar delay and decrease in PCA responsivity was observed in the second animal exposed to the same continuous stimulation protocol. In contrast, the third animal in this study was not exposed to any programmed PCA stimulation and did not exhibit any difference in responsivity on the two sides.
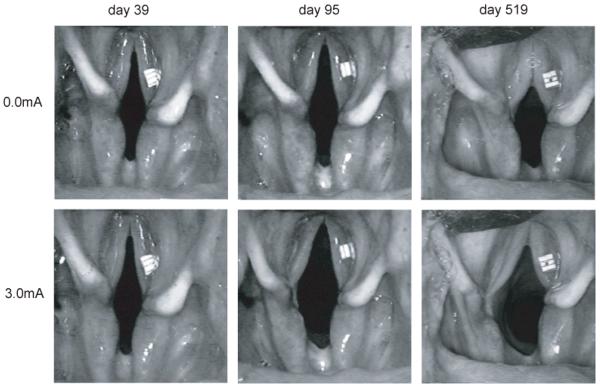
In figure 4, a series of endoscopic images of the glottis are shown to characterize the nature of stimulated responses obtained at different time points in figure 3. The still frames are representative of the passive airway (upper row) and stimulated airway (lower row) during the denervation phase (day 39), after the start of rapid reinnervation (day 95) and after reinnervation was complete (day 519). Stimulation was applied on the left side. These images reiterate two major findings. First, there was a gradual decrease in the passive airway as shown in the top row that was attributed to a gradual change in the balance of tone between abductor and adductors. The change stemmed from the delay in reinnervation of adductors, which ultimately favored a more medialized vocal fold position. Second, the abductory response was nominal during the denervation phase but increased dramatically with progressive reinnervation of the PCA muscle, as illustrated in 3rd frame in figure 4.
Figure 4.
Still frame images obtained from the passive airway (top row) and stimulated airway (bottom row) representative of the denervated PCA, initially reinnervated PCA, and finally reinnervated PCA (from left to right). Left PCA muscle was stimulated in each case.
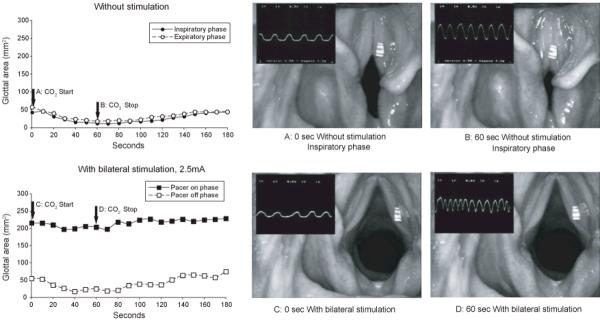
After studying stimulated glottal responses, CO2 was administered to accelerate respiration and examine spontaneous vocal fold activity at the end of each animal session. Figure 5 shows a typical finding at a time point after final reinnervation outcome (day 374). The severely compromised passive airway shown at the start of CO2 administration (A) became almost entirely occluded by the end of the 60 second administration (B). The airway measurements obtained during the trial are graphed in the left frame. This finding demonstrated the aberrant nature of adductor muscle reinnervation by inspiratory motoneurons. The observation that the embarrassed passive airway was exacerbated by hypecapnea showed that the balance of inspiratory drive also favored adductor over abductor muscles after laryngeal reinnervation. There was concern that PCA stimulation during pacing might not overcome this erroneous spontaneous drive and paradoxical closure would prevail. However, the wide glottal opening achieved with bilateral stimulation shown in figure 5C was minimally impaired by CO2 administration as evidenced in 5D. The graphed data obtained during this trial confirmed that opening of the airway (>200 mm2) was maintained during the on phase of the stimulus duty cycle but fell to the paradoxically closed glottal position during the off phase (<50 mm2).
Figure 5.
Split screen images of the glottis superimposed on oscillographic tracing of chest expansion (running trace, inspiration up). Frames “a” and “b” taken from the nonstimulated airway and “c” and “d” from the stimulated airway during laryngeal pacing. Stimulus parameters during pacing were 4 seconds on/4 seconds off, 40 Hz, 1 msec pulse duration, 2.5 mA. Data graphs show changes in airway measurements during CO2 administration and recovery. Note
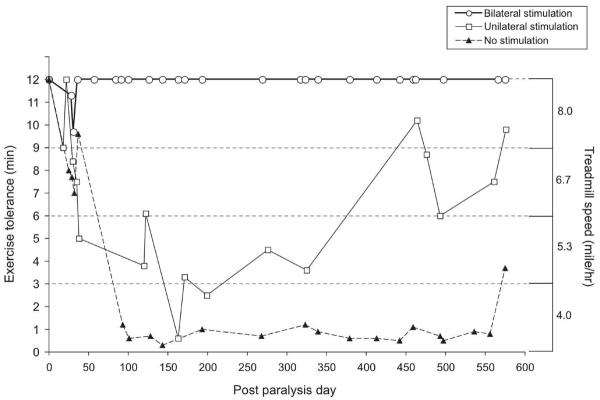
Figure 6 illustrates the exercise tolerance of this animal through postoperative sessions performed on the treadmill with the device off (closed triangles), with unilateral stimulation (open squares) and with bilateral stimulation (open circles). During the initial denervation phase (0-50 days), exercise tolerance with the device off was only minimally impaired as shown. As was expected, unilateral and bilateral stimulation only marginally improved exercise tolerance, and usually fell short of reaching normalcy in these early sessions. During the reinnervation phase, the narrowing of the passive airway and paradoxical closure in the anesthetized animal presented as dyspnea and impaired treadmill performance in the awake animal: the animal could walk less than one minute at 4 mph. Following synkinetic reinnervation, after 100 days, unilateral stimulation significantly improved the exercise tolerance so that the animal could jog at 5.3 mph, and in some cases could achieve a running speed of 6.7 mph. The most impressive finding in this study was that the animal's exercise tolerance could be restored to a normal level with bilateral stimulation. It was remarkable that an animal with severe ventilatory compromise and dyspnea with the device off could immediately recover normal ventilation and exercise tolerance when the device was switched on for bilateral stimulation. As shown in the graph, the animal maintained a normal level of exercise tolerance over the 19 months duration of the study.
Figure 6.
Exercise tolerance plotted against postoperative day with device off (solid triangles), unilateral stimulation (open squares), and bilateral stimulation (open circles).
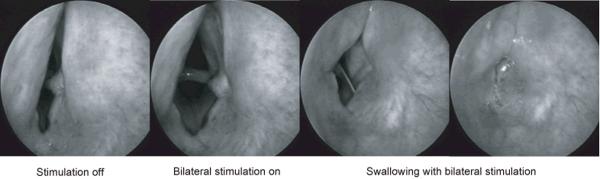
During the endoscopic sessions, it was possible to visualize the glottal movement accompanying a swallow by introducing a rigid endoscope through a small needle penetrating the trachea. In this way, a subglottal image could be obtained without any epiglottal or supralaryngeal obstruction. The glottal closure in the absence or presence of laryngeal pacing could be studied to identify any glottal incompetence and tendency for aspiration. In figure 7, a sequence of images are presented over time. In the first (left) frame, the compromised passive airway can be noted during the 4 second off phase of the pacing cycle. In the second frame there was a marked increase in glottal area when the stimulator switched to its 4 second on phase. While the stimulus train was still on, a swallow was initiated by probing the supralaryngeal region, as shown in the 3rd and 4th frames. The resultant protective reflex led to a complete occlusion of the glottis. This demonstrated that reinnervation of adductor muscles by appropriate reflex glottic closure motor units produced normal spontaneous activity sufficient to overcome the glottal opening during bilateral PCA stimulation. Similar results were obtained on all animals.
Figure 7.
Telescopic subglottic images of the vocal folds during coincidental bilateral PCA stimulation and initiation of a swallow. Compete glottal closure occurred during stimulation.
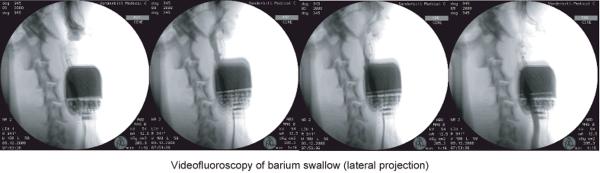
The competency of airway protection during swallowing in the paced larynx was also demonstrated by videofluoroscopic barium exam. Both barium laced liquid and food of various consistencies was utilized. As shown in the series of images in figure 8, the accumulation of a liquid bolus at the soft palate (1st frame) resulted in filling of the upper, middle, and then lower esophagus (2nd-4th frames). There was no evidence of penetration of the airway or trachea in these images. Aspiration was not observed in any animal in this study when either unilateral or bilateral pacing was employed.
Figure 8.
Videofluoroscopy of barium swallow showed no penetration of the airway during bilateral laryngeal pacing. The IPG implanted in the lateral neck and pair of 4 channel electrodes can be appreciated in this lateral projection.
DISCUSSION
It became apparent over the course of study in this animal model that narrowing of the glottis, ventilatory compromise, and the requirement for therapeutic intervention only occurred after faulty laryngeal muscle reinnervation. During the denervation phase, the paralyzed passive airway was sufficient, ventilation adequate, and exercise tolerance near normal. It was fortuitous that pacing was not required during denervation, because laryngeal stimulation produced only minimal vocal fold abduction and marginal improvement in exercise tolerance. In contrast, bilateral pacing of the synkinetically reinnervated PCA muscles increased the glottal area 3-4 times and remarkably restored exercise tolerance to normal. Unilateral stimulation was also effective in improving ventilation and exercise performance.
The significant decrease in ventilation and exercise tolerance with device off at 100 days coincided with the rapid increase of PCA muscle responsivity and the decrease in stimulus threshold. These changes in muscle stimulus requirements are indicative of the activation changing from muscle fibers to nerve fibers with reinnervation.11 Specifically, myelinated axons have a lower capacitance and faster charging time than muscle fibers, so the stimulus intensity required for activation with a 1 msec pulse is lower. Further, the muscle contraction produced by activation of a single axon is many times greater than that produced by activation of a single muscle fiber, the factor depending upon the number of muscle fibers the axon has reinnervated. In this study faulty reinnervation was the purported cause of ventilatory compromise. The gradual decrease in the passive airway over time provided evidence of reinnervation imbalance between abductor and adductor, implicating tonic inspiratory motoneurons as the source. However, paradoxical closing of the airway with each inspiratory cycle at the beginning of CO2 administration, leading to a sustained near occlusion of the airway by the end of administration, provided direct evidence of the faulty inspiratory drive on adductors as the major cause of ventilatory compromise. Previous reports using chronic electromyographic recording in this animal model have provided direct evidence for laryngeal synkinesis as the primary cause of glottal dysfunction.17 This technical approach was not used in the present study because of the risk of possible PCA muscle injury that could be falsely attributed to chronic PCA muscle stimulation.
The first observation of paradoxical closure induced by hypercapnea was both surprising and disconcerting. Although caution should be used in extrapolating animal data to human medicine, it may be that faulty inspiratory drive of adductor muscles discovered in this study represents the fundamental reason most patients with bilateral laryngeal paralysis are restricted in their level of activity. Fortunately, the inappropriate glottal closure was overcome by PCA stimulation and exercise tolerance restored to normal.
It was interesting that PCA stimulation overrode inappropriate adductor activity during inspiration, but it did not overcome appropriate adductor activity during swallowing. Even during bilateral stimulation, the adductor reinnervation by native reflex glottic closure motoneurons was strong enough to prevent the stimulated glottal opening. Perhaps the amount of native reinnervation was simply greater than that of foreign reinnervation. Or, it may be that native motor units were more powerful and generated stronger contractions than foreign ones. In general, inspiratory and reflex glottic closure motor units fall into different classes, slow and fast, respectively.17,18 Fast limb motor units are faster contracting, innervate more muscle fibers, and therefore generate more power than slow motor units.18,19
The safety and efficacy of pacing with the ANS stimulator was clearly demonstrated in this study. Each of the three animals required intervention and were successfully treated with unilateral stimulation throughout the study. Bilateral stimulation was primarily reserved for exercise to increase ventilation and improve tolerance. None of the animals demonstrated any evidence of aspiration with either unilateral or bilateral stimulation as confirmed by videofluoroscopy. Further, the 1st animal demonstrated no aspiration over a 13 month period of continuous bilateral stimulation. The only problem encountered in this study was the loss of stimulation channels. Fortunately, there is sufficient redundancy built into the electrode array that stimulation could be switched to another functional channel. Although the study is still in progress on animals 1 and 3, postmortem examination of the second animal revealed that the channel loss was due to wire breakage at the IPG interface resulting from device migration. Movement of the IPG implant site from the sternocleidomastoid to the trapezius has remedied this problem.
CONCLUSION
Laryngeal pacing with the Genesis XP offers a potential for treatment that could far exceed any conventional intervention. This study demonstrates that severe ventilatory compromise only occurs following faulty reinnervation of laryngeal muscles. Bilateral PCA stimulation can result in complete rehabilitation of ventilation and exercise tolerance without impairment of swallowing.
ACKNOWLEDGMENTS
Special thanks go to Rohan Hoare, Pat Cullen, Tracy Cameron, Jeff Huyne, and Chris Chavez at ANS, Inc for support of this research.
Research supported by NIH Grants R01-DC001149 & R01-DC008429.
Financial Disclosure: Dr. David Zealear has received NIH grant funds to support this research and has a signed license agreement with Advanced Neuromodulation Systems, Inc. including royalties to market this technology for treatment of laryngeal paralysis.
Footnotes
Research performed at Vanderbilt University.
REFERENCES
- 1.Ossoff RH, Sisson GA, Duncavage JA, Moselle HI, Andrews PE, McMillan WG. Endoscopic laser arytenoidectomy for the treatment of bilateral vocal cord paralysis. Laryngoscope. 1984;94:1293–7. doi: 10.1288/00005537-198410000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Eckel HE, Thumfart M, Vossing M, Wasserman K, Thumfart W. Cordectomy versus arytenoidectomy in the management of bilateral vocal cord paralysis. Ann Otol Rhinol Laryngol. 1994;103:852–7. 4. doi: 10.1177/000348949410301105. [DOI] [PubMed] [Google Scholar]
- 3.Remacle M, Mayne A, Lawson G, Jamart J. Subtotal carbon dioxide laser arytenoidectomy by endoscopic approach for treatment of bilateral cord immobility in adduction. Ann Otol Rhinol Laryngol. 1996;105:438–45. doi: 10.1177/000348949610500604. [DOI] [PubMed] [Google Scholar]
- 4.Zealear DL, Dedo HH. Control of paralyzed axial muscles by electrical stimulation. Acta Otolaryngol (Stockh) 1977;83:514–27. doi: 10.3109/00016487709128880. [DOI] [PubMed] [Google Scholar]
- 5.Obert PM, Young KA, Tobey DN. Use of direct posterior cricoarytenoid stimulation in laryngeal paralysis. Arch Otolaryngol. 1984;110:88–92. doi: 10.1001/archotol.1984.00800280022007. [DOI] [PubMed] [Google Scholar]
- 6.Otto RA, Temper J, Davis W, Homeyer D, Stroble M. Coordinated electrical pacing of vocal cord abductors in recurrent laryngeal nerve paralysis. Otolaryngol Head Neck Surg. 1985;93:634–8. doi: 10.1177/019459988509300512. [DOI] [PubMed] [Google Scholar]
- 7.Bergmann K, Warzel H, Eckhardt H-U, Gerhardt H-J. Respiratory rhythmically regulated electrical stimulation of paralyzed laryngeal muscles. Laryngoscope. 1984;94:1376–80. doi: 10.1288/00005537-198410000-00022. [DOI] [PubMed] [Google Scholar]
- 8.Broniatowski M, Kaneko S, Jacobs G, Nose Y, Tucker HM. Laryngeal pacemaker II. Electrical pacing of reinnervated posterior cricoarytenoid muscles in the canine. Laryngoscope. 1985;95:1194–8. doi: 10.1288/00005537-198510000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Sanders I. Electrical stimulation of laryngeal muscle. Otolaryngol Clin North Am. 24:1253–74. 991. [PubMed] [Google Scholar]
- 10.Zrunek M, Streinzer W, Mayr W, Burian K, Thoma H, Gruber H. Experiments of acute and chronic direct electrical stimulation of glottis opening muscles. Eur Arch Otorhinolaryngol. 1991;248:445–8. doi: 10.1007/BF00627631. [DOI] [PubMed] [Google Scholar]
- 11.Zealear DL, Rainey CL, Jerles ML, Tanabe T, Herzon GD. Technical approach for reanimation of the chronically denervated larynx by means of functional electrical stimulation. Ann Otol Rhinol Laryngol. 1994;103(9):705–712. doi: 10.1177/000348949410300908. [DOI] [PubMed] [Google Scholar]
- 12.Zealear DL, Rainey CL, Netterville JL, Herzon GD, Ossoff RH. Electrical pacing of the paralyzed human larynx. Ann Otol, Rhinol, Laryngol. 1996;105(9):689–93. doi: 10.1177/000348949610500904. [DOI] [PubMed] [Google Scholar]
- 13.Zealear DL, Billante CR, Courey MS, Sant'Anna GD, Netterville JL. Electrically stimulated glottal opening combined with adductor muscle botox blockade restores both ventilation and voice in a patient with bilateral laryngeal paralysis. Ann Otol Rhinol Laryngol. 2002;111(6):500–6. doi: 10.1177/000348940211100605. [DOI] [PubMed] [Google Scholar]
- 14.Billante CR, Zealear DL, Courey MS, Netterville JL. Effect of chronic electrical stimulation of laryngeal muscle on voice. Ann Otol Rhinol Laryngol. 2002;111:328–32. doi: 10.1177/000348940211100408. [DOI] [PubMed] [Google Scholar]
- 15.Zealear DL, Billante CR, Courey MS, Netterville JL, Paniello RC, Sanders I, Herzon GD, Goding GS, Mann W, Ejnell H, Habets AMMC, Testerman R, Van de Heyning P. Reanimation of the paralyzed human larynx with an implantable electrical stimulation device. Laryngoscope. 2003;113:1149–1156. doi: 10.1097/00005537-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Katada K, Van Himbergen D, Kunibe I, Nonaka S, Harabuchi Y, Huang S, Billante C, Zealear DL. Evaluation of a deep brain stimulation electrode for laryngeal pacing. Ann Otol.Rhinol.Laryngol. 2008;117(8):621–9. doi: 10.1177/000348940811700813. [DOI] [PubMed] [Google Scholar]
- 17.Zealear DL, Rodriguez RJ, Kenny T, Billante MJ, Cho Y, Billante CR, Garren KC. Electrical stimulation of a denervated muscle promotes selective reinnervation by native over foreign motoneurons. J. Neurophysiology. 2002;87(4):2195–99. doi: 10.1152/jn.00451.2001. [DOI] [PubMed] [Google Scholar]
- 18.Zealear DL. Thesis. 1979. Specialization of Laryngeal Motor Units for Their Functions. [Google Scholar]
- 19.Burke RE, Levine DN, Zajac FE, III, Tsairis P, Engel WK. Mammalian motor units: Physiological-histochemical correlation in three types in cat gastrocnemius. Science. 1971;174:709–12. doi: 10.1126/science.174.4010.709. [DOI] [PubMed] [Google Scholar]