Abstract
Background and Objectives:
Concomitant biliary and duodenal malignant obstruction are a severe condition mainly managed by duodenal and biliary stenting, which can be performed simultaneously (SAMETIME) or in two distinct procedures (TWO-TIMES). We conducted a single-center retrospective study to evaluate the feasibility of a SAMETIME procedure and the impact of endoscopic ultrasound (EUS)-hepaticogastrostomy in double malignant obstructions.
Patients and Methods:
From January 1, 2011, to January 1, 2018, patients with concomitant malignant bilioduodenal obstruction treated endoscopically were included. The primary endpoint was hospitalization duration. The secondary endpoints were bilioduodenal reintervention rates, adverse event rates, and overall survival. Patients were divided into groups for statistical analysis: (i) divided according to the timing of biliary drainage: SAMETIME vs. TWO-TIMES group, (ii) divided based on the biliary drainage method: EUS-HG group underwent hepaticogastrostomy, while DUODENAL ACCESS group underwent endoscopic retrograde cholangiopancreatography (ERCP), percutaneous transhepatic drainage (PCTD) or EUS-guided choledocoduodenostomy (EUS-CD).
Results:
Thirty-one patients were included (19 women, median age = 71 years). Stenosis was mainly related to pancreatic cancer (17 patients, 54.8%). Sixteen patients were in the SAMETIME group, and 15 were in the TWO-TIMES group. Biliary drainage was performed by EUS-HG in 11 (35.%) patients, PCTD in 11 (35.%), ERCP in 8 (25.8%) and choledoduodenostomy in 1. Thirty patients died during follow-up. The median survival was 77 days (9% confidence interval [37–140]). The mean hospitalization duration was lower in the SAMETIME group: 7.5 vs. 12.6 days, P = 0.04. SAMETIME group patients tended to have a lower complication than TWO-TIMES (26.7% vs. 56.3%, P = 0.10). The EUS-HG group tended to have a lower complication rate (5% vs. 18.2%, P = 0.07) and less biliary endoscopic revision (30% vs. 9.1%, P = 0.37) than DUODENAL ACCESS.
Conclusions:
SAMETIME drainage is associated with a lower hospital stay without increased morbidity. EUS-HG could provide better access because it did not exhibit a higher complication rate and showed a tendency toward better patency and fewer complications.
Keywords: biliary drainage, biliary stricture, ERCP, EUS, EUS biliary drainage, gastric outlet obstruction, hepaticogastrostomy, pancreatic cancer
INTRODUCTION
Concomitant biliary and duodenal malignant stenosis is a rare event, reflecting a locally advanced neoplastic process.
Japanese recommendations favor endoscopic biliary drainage for the management of biliary stenosis, but surgical management either with gastrojejunal anastomosis or the endoscopic placement of a duodenal stent can be conducted for patients with sufficient estimated survival time.[1,2]
However, for concomitant bilioduodenal stricture, many case series have shown advantages of endoscopic treatment (ERCP associated with duodenal stenting) of patients with advanced disease. Biliary access can be challenging in such cases, and the classic management is dilation of the duodenal stenosis to reach the papilla. Some studies have suggested that in cases of failed ERCP due to tight duodenal stenosis, biliary stenting should be performed in a second procedure soon after the placement of a duodenal self-expandable metallic stent (SEMS).[3,4] However, the performance of ERCP is rare in patients with indwelling duodenal stents,[5] and percutaneous transhepatic biliary drainage has a high morbidity rate. The need for these two modalities of drainage was reassessed following the development of EUS-guided drainage in the 2000s.[6] EUS-guided hepaticogastrostomy (EUS-HG) has since become an interesting alternative for patients with inaccessible papillae,[7] but little is known about the feasibility of this procedure when duodenal stenting is conducted simultaneously. In addition, this procedure can be controversial for several reasons. First, duodenal stenting with insufflation and potential gastric stasis may complicate the EUS procedure, and second, efficient duodenal drainage is considered a prerequisite for transgastric biliary drainage.
Because of the availability of the three modalities of biliary drainage (EUS-HG, ERCP, and percutaneous transhepatic biliary drainage [PCTBD]) and the current use of CO2 insufflation in our unit since 2010, the management of concomitant bilioduodenal stricture differs and is at the discretion of the operator in terms of timing of drainage (one procedure [SAMETIME] vs. two procedures [TWO-TIMES]) and biliary drainage modalities (ERCP, PCTBD, or EUS-HG). We studied the impact of the timing of biliary drainage and duodenal stenting (as simultaneous procedures or two distinct procedures) and the outcomes regarding the type of biliary drainage.
PATIENTS AND METHODS
Study design
This is a retrospective single-center study based on a registry obtained with the software ConSore, allowing the extraction of patients managed in our hospital. Patients with duodenal stenting were extracted from the database, and then patients with biliary drainage were selected. To obtain patients with concomitant strictures, we included all the patients who underwent biliary and duodenal stenting within a period of 7 days; these patients were included in either the SAMETIME group (simultaneous procedures) or in the TWO-TIMES group (two procedures performed within 7 days of each other), regardless of which procedure was performed first (duodenal stenting or biliary stenting).
Global endoscopic management strategy
The timing of the treatment of the biliary and gastric outlet obstructions (GOOs) was at the endoscopist's discretion. In all cases, GOO was managed with the placement of an uncovered SEMS. In the case of visible papillae after duodenal dilation, if necessary, in order to cross the duodenal stenosis, retrograde biliary drainage was first attempted with the placement of an uncovered biliary SEMS by ERCP. In the case of SAMETIME drainage with accessible papillae, ERCP was performed first followed by duodenal stenting. In the other cases of TWO-TIMES drainage or in cases of SAMETIME drainage with inaccessible papillae or ERCP failure, duodenal stenting was performed first followed by biliary drainage. The timing (SAMETIME vs. TWO-TIMES) and the modalities of biliary drainage in case of ERCP failure were at the discretion of the operators. In cases of ERCP failure, biliary drainage was performed by EUS-HG, percutaneous biliary drainage or EUS-guided choledocoduodenostomy (EUS-CD) at the physician's discretion. An uncovered SEMS was used in cases of ERCP or percutaneous drainage, and a partially covered metallic stent was used in cases of EUS-guided biliary drainage (EUS-BD) (Giobor Taewong).
Drainage techniques
All procedures were performed on an intubated patient in the supine position. All patients were given intravenous antibiotics immediately before the biliary procedure.
Biliary drainage procedures
ERCP, EUS-HG, and percutaneous transhepatic drainage (PCTD) were performed according to the European Society of Gastrointestinal Endoscopy (ESGE) Guidelines,[8] as described in our a previous work.[9]
Duodenal stenting
The procedures were performed under fluoroscopic control. The proximate flange was placed straddling the pylorus, and the stents used ranged from 9 to 12 cm, as described in our a previous work.[10]
Inclusion criteria and data
The inclusion criteria were as follows: (i) concomitant biliary and duodenal strictures and (ii) advanced malignant disease without the possibility of curative surgical treatment. The exclusion criteria were (i) previous treatment for biliary or GOO, (ii) an interval between the two interventions (biliary and gastric) longer than 7 days, (iii) nonmalignant obstruction, and (iv) hilar biliary obstruction.
The data collected were as follows: (i) patient demographics, including sex, age at diagnosis, type of cancer, the American Society of Anesthesiologists (ASA) score, OMS score, bilirubin level and GOO score (GOOS)[11] at the first endoscopy; (ii) the type of duodenal stenosis, and (iii) the modality of biliary and GOO drainage and complications associated with both.
Definitions
Biliary obstruction was defined as biliary duct stenosis located at least 2 cm below the hepatic hilum associated with cholestasis.
Duodenal obstruction was defined as obstructive symptoms that resulted in decreased oral intake (anorexia, nausea, vomiting, and/or abdominal pain) and a duodenal stricture observed on endoscopy.
Localization of the duodenal stenosis was classified according to the involvement of the ampulla of Vater: Type I was proximal to and without involvement of the ampulla of Vater; type II affected the second part of the duodenum and the ampulla of Vater; and type III affected the third part of the duodenum without involvement of the ampulla of Vater.[12]
In the SAMETIME group, the biliary and duodenal drains were performed in the same procedure.
In the TWO-TIMES group, the two drains were placed in different procedures (within 7 days).
The duration of hospitalization was calculated from the day of the first endoscopic procedure for the TWO-TIMES group and the day of the only endoscopic session for the SAMETIME group.
Morbidity was defined by the occurrence of any type of complication within 30 days after the procedure. Postoperative mortality was defined as death occurring within 1 month.
Biliary complications were defined as cholangitis, cholestasis, biliary leakage, or any other adverse event related to biliary drainage.
Duodenal complications were defined as the malposition of the duodenal stent requiring the placement of a new stent or bleeding, duodenal perforation, or abdominal pain occurring within 30 days as a result of the stent placement. Postoperative complications within 30 days were categorized according to the Clavien–Dindo classification system.[13] We classified the complications into two groups: Minor to moderate complications if the Clavien–Dindo score was <III and significant complications if the Clavien-Dindo score was >III, indicating the need for reoperation.
The baseline date for analysis was defined as the date of the first therapeutic endoscopic procedure. Follow-up started from the date of the first endoscopic procedure and continued to the date of death or the last follow-up, if the patient was alive at the end of the data collection. The occurrence of complications was collected from the patients' medical records. The time of the occurrence of biliary or duodenal complications was collected from the date of biliary or duodenal endoscopy, respectively.
Study objectives
The primary endpoint was the duration of the hospital stay.
The secondary endpoints were the rates of mortality and morbidity (as defined by the Clavien –Dindo classification system), the duration of biliary and duodenal patency and the rate of subsequent chemotherapy after endoscopic procedures. Comparisons were made between patients who had the same drainage durations and between patients who benefited from hepaticogastrostomy vs. other biliary drainage modalities.
Institutional review board approval
The study was approved by the institutional review board from our center (authorization number: IPC 2018-026).
Statistical analysis
The database was obtained by ConSore, a new generation of big data health software developed by Unicancer that is used in our center. All statistical analyses were performed at the significance level α = 0.05 and with SAS® 9.4 software (SAS, SAS Institute Inc, Cary, North Carolina, USA). Qualitative data are described by counts (frequencies), and quantitative data are described by means (standard deviations) and medians (min-max). Comparisons between the SAMETIME and TWO-TIMES groups and between patients who benefited from hepaticogastrostomy vs. other biliary drainage modalities were performed by Chi-square or Fisher's exact test(qualitative data) or Wilcoxon's tests (quantitative data).
Overall survival (OS) was defined as the duration from the date of endoscopic drainage to the date of death. Biliary (duodenal) stent patency was defined as the duration from the date of the endoscopic drainage to the date of biliary (duodenal) revision. Patients without events were right-censored at the date of their last follow-up for all time-to-event endpoints. Estimations were performed by using the Kaplan–Meier method. Log-rank tests were used to compare time-to-event endpoints between the SAMETIME and TWO-TIMES groups and between patients who benefited from hepaticogastrostomy vs. other biliary drainage modalities.
RESULTS
Population
Between January 2011 and January 2018, 111 patients came to our unit with biliary and duodenal obstruction and received double stenting. Of these, 78 patients were excluded because the time required to place both stents was more than 1 week. Of the 78 excluded patients, 40 (51%) had a duodenal stent placed first, and 38 (49%) had a biliary stent placed first. Thirty-three patients with concomitant stenosis as defined above (interval <7 days between the two stenting sessions) were finally selected for analysis. Of these 33 patients, 2 were excluded due to missing data. Therefore, 31 patients with concomitant double obstruction were considered for analysis: 19 women and 12 men, with a median age at diagnosis of 71 years (30–88). Thirteen patients (41.9%) had a Type I duodenal invasion, 16 (51.6%) had a Type II duodenal invasion, and 2 (6.4%) had a Type II invasion, as described above in the methods.
One patient (3.2%) had an ASA score of 1, 8 (25.8%) had an ASA score of 2, 18 (58.1%) had an ASA score of 3, 4 (12.9%) had an ASA score of 4 (13%), and the mean Karnofsky index was 71.9% (19.6%).
The obstruction was mainly due to locally advanced pancreatic cancer (n = 17, 54.8%), followed by colorectal metastasis (n = 4, 12.9%), breast cancer metastasis (n = 3, 9.7%), locally advanced gallbladder cancer (n = 3, 9.7%), uterine cancer (n = 1, 3.2%), gastric carcinoma (n = 1, 3.2%), duodenal cancer (n = 1, 3.2%), and ovarian cancer (n = 1, 3.2%).
Before endoscopy, the average bilirubin level was 143.4 μmol/L, and 22 (71%) patients had a GOOS (6) of 0 before duodenal stenting.
Fifteen patients underwent biliary drainage and gastroduodenal stenting in the SAMETIME group, and sixteen underwent both interventions in the TWO-TIMES group. The characteristics of both groups were comparable [Table 1].
Table 1.
Baseline characteristics of the SAME TIME and TWO TIMES groups
| Characteristics | All patients (n=31), n (%) | SAME TIME (n=15), n (%) | TWO TIMES (n=16), n (%) | P* |
|---|---|---|---|---|
| Sex | ||||
| Female | 19 (61.3) | 9 (60) | 10 (62.5) | 0.89 |
| Male | 12 (38.7) | 6 (40) | 6 (37.5) | |
| Age at first endoscopy (years) | ||||
| Median (minimum-maximum) | 71 (30-88) | 72 (38-88) | 67 (30-81) | 0.25 |
| Mean (SD) | 66.2 (15.5) | 69.5 (14.8) | 63.1 (16.0) | |
| ASA score | ||||
| Median (minimum-maximum) | 3 (1-4) | 3 (1-4) | 3 (2-4) | 0.53 |
| 1 | 1 (3.2) | 1 (6.7) | 0.48 | |
| 2 | 8 (25.8) | 3 (20) | 5 (31.25) | |
| 3 | 18 (58.1) | 8 (53.3) | 10 (62.5) | |
| 4 | 4 (12.9) | 3 (20) | 1 (6.25) | |
| Karnofsky index | ||||
| Median (minimum-maximum) | 70 (20-100) | 70 (40-100) | 80 (20-100) | 0.73 |
| Mean (SD) | 71.9 (19.6) | 71.3 (19.2) | 72.5 (20.5) | |
| Bilirubin (µmol/L) | ||||
| Median (minimum-maximum) | 116 (4-430) | 86 (4-430) | 134.5 (16-335.2) | 0.17 |
| Mean (SD) | 143.4 (109.6) | 123.5 (117.3) | 164.7 (100.6) | |
| Diagnosis | ||||
| Colorectal cancer | 4 (12.9) | 3 (20) | 1 (6.25) | 0.61 |
| Duodenal carcinoma | 1 (3.23) | 1 (6.25) | ||
| Gastric carcinoma | 1 (3.23) | 1 (6.25) | ||
| Ovarian carcinoma | 1 (3.23) | 1 (6.7) | ||
| Pancreatic cancer | 17 (54.84) | 7 (46.7) | 10 (62.5) | |
| Breast cancer | 3 (9.68) | 2 (13.3) | 1 (6.25) | |
| Gallbladder carcinoma | 3 (9.68) | 2 (13.3) | 1 (6.25) | |
| Uterine sarcoma | 1 (3.23) | 1 (6.25) | ||
| Preoperative gastric outlet obstruction score | ||||
| 0: No oral intake | 22 (71) | 11 (73.3) | 11 (68.8) | 1 |
| 1: Liquids only | 7 (22.6) | 3 (20) | 4 (25) | |
| 2: Soft solids | 2 (6.4) | 1 (6.7) | 1 (6.2) |
*P <0.05. SD: Standard deviation
The types of biliary drainage used were not significantly different between the SAMETIME and TWO-TIMES groups [Table 2].
Table 2.
Comparison of the modalities of treatment between the SAME TIME and TWO TIMES groups
| Characteristics | All patients (n=31), n (%) | SAME TIME (n=15), n (%) | TWO TIMES (n=16), n (%) | P* |
|---|---|---|---|---|
| Postdrainage chemotherapy | ||||
| No | 16 (51.6) | 9 (60) | 7 (43.75) | 0.37 |
| Yes | 15 (48.4) | 6 (40) | 9 (56.25) | |
| Hospital stay (days) | ||||
| Median (minimum-maximum) | 8 (1-26) | 6 (1-22) | 9.5 (3-26) | 0.04 |
| Mean (SD) | 10.1 (7.4) | 7.5 (6.8) | 12.6 (7.3) | |
| Drainage | ||||
| EUS-HG | 11 (35.5) | 7 (46.7) | 4 (25) | 0.21 |
| Other | 20 (64.5) | 8 (53.3) | 12 (75) | |
| Biliary drainage modality | ||||
| ERCP | 8 (25.8) | 4 (26.6) | 4 (25) | 0.45 |
| EUS-CD | 1 (3.2) | 1 (6.25) | ||
| EUS-HG | 11 (35.5) | 7 (46.7) | 4 (25) | |
| PCTBD | 11 (35.5) | 4 (26.7) | 7 (43.75) |
PCTBD: Percutaneous transhepatic biliary drainage; SD: Standard deviation; CD: Choledocoduodenostomy; ERCP: Endoscopic retrograde cholangio-pancreatography; HG: Hepaticogastrostomy
Outcomes
Technical success of biliary drainage was obtained in all cases by either ERCP, EUS-HG, or PCTBD during the dedicated biliary procedure.
After bilioduodenal stenting, the median OS was 77 days (9% confidence interval, [37–140]). The morbidity rate was 4% (15 patients). Two had general complications (one ischemic stroke, and one had acute renal failure), and 13 had postintervention complications within 30 days after the procedures: (i) the biliary complications were cholangitis in 8 patients (25.8%, n = 8/31), biliary leakage in 2 patients (6.%, n = 2/31, all in the PCTBD group), mild pancreatitis in one patient (3.2%, n = 1/31, ERCP group), and (ii) duodenal complications in 3 patients, with early obstruction due to malposition of the stent in 2 patients (6.%, n = 2/31), and fatal duodenal bleeding in one patient.
The postprocedure mortality rate was 19.4% (n = 6/31). One patient died because of a postoperative ischemic stroke, one because of biliary peritonitis and one because of tumoral duodenal bleeding. Three other patients died because of the evolution of the disease.
Three patients had delayed complications: One had cholangitis and 2 had delayed obstruction of the stent (6.%, n = 2/31).
The analysis of SAMETIME versus TWO-TIMES groups [Table 3].
Table 3.
Comparison of the outcomes between the SAME TIME and TWO TIMES groups
| Characteristics | All patients (n=31), n (%) | SAME TIME (n=15), n (%) | TWO TIMES (n=16), n (%) | P* |
|---|---|---|---|---|
| Hospital stay (days) | ||||
| Median (minimum-maximum) | 8 (1-26) | 6 (1-22) | 9.5 (3-26) | 0.04 |
| Mean (SD) | 10.1 (7.4) | 7.5 (6.8) | 12.6 (7.3) | |
| Duodenal revision | ||||
| No | 21 (67.7) | 9 (60) | 12 (75) | 0.46 |
| Yes | 10 (32.3) | 4 (40) | 4 (25) | |
| Biliary revision | ||||
| No | 24 (77.4) | 13 (86.7) | 11 (68.75) | 0.39 |
| Yes | 7 (22.6) | 2 (13.3) | 5 (31.25) | |
| Duodenal complication | ||||
| No | 26 (83.9) | 14 (93.3) | 12 (75) | 0.33 |
| Yes | 5 (16.1) | 1 (6.7) | 4 (25) | |
| Biliary complication | ||||
| No | 13 (41.9) | 8 (53.3) | 5 (31.25) | 0.21 |
| Yes | 18 (58.1) | 7 (46.7) | 11 (68.75) | |
| Clavien-Dindo classification | ||||
| I | 5 (27.7) | 3 (42.9) | 2 (18.2) | 0.32 |
| IIIb | 10 (55.6) | 3 (42.9) | 7 (63.6) | |
| IVa | 1 (5.6) | 1 (14.2) | ||
| V | 2 (11.1) | 2 (18.2) | ||
| Significant complication (Clavien-Dindo classification) | ||||
| No | 18 (58.1) | 11 (73.3) | 7 (43.75) | 0.10 |
| Yes | 13 (41.9) | 4 (26.7) | 9 (56.25) | |
| Death before 30 days | ||||
| No | 25 (80.6) | 13 (86.7) | 12 (75) | 0.65 |
| Yes | 6 (19.4) | 2 (13.3) | 4 (25) |
*Chi-square or Fisher’s exact or Wilcoxon’s test P values. SD: Standard deviation
The median hospitalization duration was significantly lower in the SAMETIME group than in the TWO-TIMES group (6 days vs. 9.5 days, P = 0.04).
OS was not different (P = 0.52, log-rank test) between the SAMETIME group (median 71 days [34–172]) and TWO-TIMES group (median 91.5 days [19–207]) [Figure 1]. The postoperative mortality rate was 2% in the TWO-TIMES group and 13% in the SAMETIME group (P = 0.65).
Figure 1.
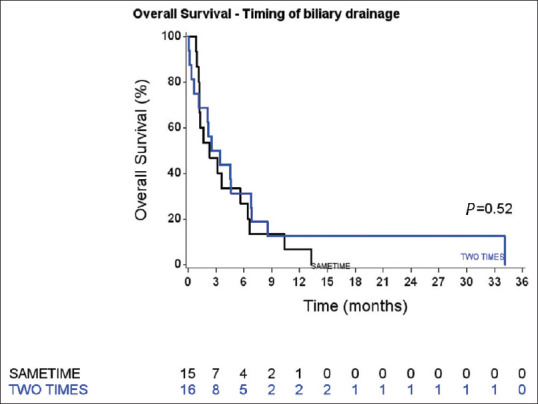
Overall survival curves of the SAMETIME and TWO-TIMES groups
There was no difference in biliary stent patency [Figure 2] between the SAMETIME and TWO-TIMES groups (log-rank test, P = 0.10). There was also no significant difference between the groups regarding duodenal stent patency (P = 0.47) [Figure 3].
Figure 2.
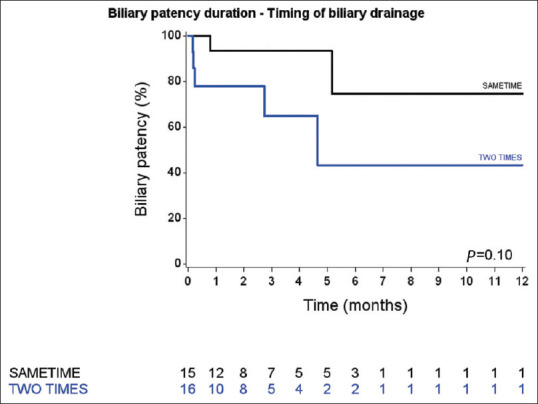
Biliary stent patency duration curves of the SAMETIME and TWO-TIMES groups
Figure 3.
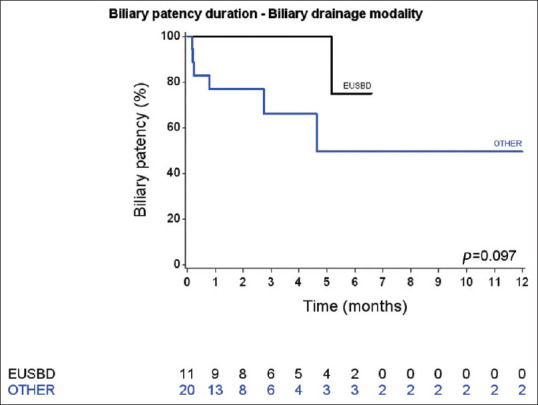
Biliary stent patency duration curves of EUS-BD and other biliary drainage modality patients
The overall complication rate was not different between the SAMETIME group and the TWO-TIMES group: 7 (47%) patients in the SAMETIME group vs. 11 (68.8%) in the TWO-TIMES group (P = 0.38). However, patients in the SAMETIME group tended to have less severe complications, with fewer significant post-endoscopy complications according to the Clavien–Dindo classification system: 26.7% (4/15) vs. 56.2% (n = 9/16) in the TWO-TIMES group. In addition, patients in the SAMETIME group trended to undergo EUS-HG for biliary drainage more often than those in the TWO-TIMES group (47% vs. 2%); however, this difference was not significant (P = 0.10).
Analysis of EUS-HG vs. biliary drainage through the duodenal stenosis is shown in Table 4.
Table 4.
Comparison of the outcomes between the EUS-biliary drainage and other biliary drainage modalities groups
| Characteristics | All patients (n=31), n (%) | Other (n=20), n (%) | EUS-HG (n=11), n (%) | P* |
|---|---|---|---|---|
| Duodenal revision | ||||
| No | 21 (67.7) | 14 (70) | 7 (63.6) | 1 |
| Yes | 10 (32.3) | 6 (30) | 4 (36.4) | |
| Biliary revision | ||||
| No | 24 (77.4) | 14 (70) | 10 (90.9) | 0.37 |
| Yes | 7 (22.6) | 6 (30) | 1 (9.1) | |
| Duodenal complication | ||||
| No | 26 (83.9) | 16 (80) | 10 (90.9) | 0.63 |
| Yes | 5 (16.1) | 4 (20) | 1 (9.1) | |
| Biliary complication | ||||
| No | 13 (41.9) | 6 (30) | 7 (63.6) | 0.13 |
| Yes | 18 (58.1) | 14 (70) | 4 (36.4) | |
| Clavien-Dindo classification | ||||
| I | 5 (27.7) | 3 (21.4) | 2 (50) | 0.18 |
| IIIb | 10 (55.6) | 9 (64.3) | 1 (25) | |
| IVa | 1 (5.6) | 1 (25) | ||
| V | 2 (11.1) | 2 (14.3) | ||
| Significant complication (Clavien-Dindo classification) | ||||
| No | 18 (58.1) | 9 (45) | 9 (81.8) | 0.07 |
| Yes | 13 (41.9) | 11 (55) | 2 (18.2) | |
| Death before 30 days | ||||
| No | 25 (80.6) | 16 (80) | 9 (81.8) | 1 |
| Yes | 6 (19.4) | 4 (20) | 2 (18.2) |
*Chi-square or Fisher’s exact or Wilcoxon’s test P values. HG: Hepaticogastrostomy
When comparing the type of biliary drainage (EUS-HG vs. biliary drainage through the duodenal stenosis), EUS-HG patients tended to have markedly fewer significant postprocedure complications (18.2% (2/11) vs. 5% (11/20); P = 0.07)) and fewer biliary complications (36.4% (4/11) vs. 70% (14/20); P = 0.13), although the differences were not significant. The biliary patency tended to be better in the EUS-HG group [Figure 3], but the difference did not reach statistical significance (log-rank test, P = 0.10).
DISCUSSION
Our study shows a shorter hospital stay in patients with concomitant malignant bilioduodenal obstruction who underwent dual stenting in the same session rather than in two different sessions. These patients are not eligible for treatment with curative intent and had a very poor median survival time in our series (76 days); in these patients, best supportive care and comfort are the goals of the physician. Here, the simultaneous stenting strategy allowed us to decrease the time spent in the hospital by 5 days, which may seem trivial in the general population but represents almost one-tenth of the remaining life expectancy of these patients.
Moreover, the simultaneous stenting strategy did not increase the rate of complications associated with the procedures, and no differences were seen in terms of survival and the subsequent rates of chemotherapy.
Performing biliary and duodenal drainage in two distinct procedures may seem counter-intuitive, but the strategy was not consensual within our team. In cases of ERCP failure, some physicians preferred to perform biliary drainage in a second procedure because EUS-HG is an examination conducted without insufflation, and the preliminary installation under insufflation of the duodenal stent in a full stomach could introduce an additional difficulty factor. In addition, some physicians want to ensure good function of the duodenal stent to decrease the risk of complications related to the performance of EUS-HG in case of gastric stasis (migration or dysfunction). We arbitrarily included patients who underwent both procedures within <1 week in the TWO-TIMES group because, usually in our unit, when the drainage strategy is performed in two steps, patients are scheduled to undergo the procedure in <7 days during the same hospitalization. We did not include rare patients who underwent double stenosis with the two procedures performed more than 7 days apart so that the primary endpoint (length of hospital stay) would not be significantly biased.
In our center, EUS-BD and PCTD are performed by the gastroenterology team, and each procedure can be selected after ERCP failure. However, some data suggest that EUS-BD should be preferred over PCTD when possible in such cases because EUS-BD is associated with better clinical success, fewer postprocedure adverse events and a lower rate of reintervention.[14]
EUS-HG is known to have a high morbidity rate, with approximately 20% of patients experiencing postprocedure adverse events.[15] Interestingly, in our study, EUS-HG biliary drainage did not result in a higher morbidity rate compared to other biliary drainage modalities (18% of patients with significant complications in the EUS-HG group vs. 5% of patients undergoing other modalities of drainage). Moreover, biliary patency tended to be better in the EUS-HG group than in the other groups, although the difference did not reach statistical significance. This must be explained by the fact that patients with concomitant bilioduodenal obstruction are in very poor condition, suffering from very aggressive local tumors. This specific local context could make transduodenal drainage less efficient because trans-duodenal biliary drainage is particularly difficult in such a setting, which could potentially lead to biliary stent malposition and related complications. Moreover, stent tumoral ingrowth could occur faster in this very aggressive condition, which could potentially cause a higher rate of biliary stent dysfunction in the medium term after the procedure.
Our findings are in agreement with those of studies in which duodenal invasion and duodenal stenting were found to be independently associated with twice as much biliary stent dysfunction in patients with pancreatic cancer. In the first study by Hamada et al., the rate of early dysfunction was 42% in patients with duodenal invasion and 24% in patients without duodenal invasion,[16] and in the second study, the hazard ratio (HR) for biliary stent dysfunction was doubled (HR: 2.00; confidence interval-95: 1.16–3.45).[17] Furthermore, Ogura et al. showed that in patients with concomitant bilioduodenal invasion, when EUS-BD is performed, EUS-HG should be selected rather than EUS-CD to improve biliary patency. In their study, EUS-HG resulted in significantly longer biliary stent patency than EUS-CD, with a median of 133 vs. 37 days (P =0.045). Moreover, in this study, only EUS-CD was associated with adverse events, particularly reflux cholangitis (odds ratio 10.285, P=0.012).[18] This might suggest that in patients with very aggressive local bilioduodenal tumors, placement of the stent away from the tumor ingrowth with EUS-HG could ensure better biliary patency.
In addition, ERCP in the setting of duodenal stricture is associated with a relatively higher failure rate, and placement of a temporary duodenal stent to allow papillae access is associated with success in only 76% of the cases,[3] whereas EUS-BD has a more than 9% success rate in cases of ERCP failure.[19] In the setting of an indwelling gastroduodenal stent, EUS-HG was better than ERCP in terms of the technical success rate 95.2% vs. 56% and clinical success rate (90.% vs. 52.0%).[20]
Our results are consistent with these findings because we observed a tendency toward greater efficacy without an increase in morbidity in favor of EUS-HG. This tendency was also observed in the SAMETIME group, where EUS-HG was performed more frequently than in the TWO-TIMES group (46.7 vs. 2%).
CONCLUSIONS
Concomitant bilioduodenal procedures for patients with dual malignant bilioduodenal stricture allowed for a shorter hospital stay and a similar morbidity rate in patients with very short life expectancies. EUS-HG could be preferred over other modalities of biliary drainage through the duodenal stenosis. Moreover, EUS-HG showed strong but nonsignificant trends toward better safety and biliary patency, but these findings need to be confirmed in studies with larger sample sizes.
Financial support and sponsorship
Nil.
Conflicts of interest
Drs Debourdeau, Caillol, Zemmour, Winkler, Decoster, Pesenti, Ratone and Boher have no conflicts of interest or financial ties to disclose. Dr Giovannini is consultant for Cook and Taewong.
REFERENCES
- 1.Yamaguchi K, Okusaka T, Shimizu K, et al. EBM-based clinical guidelines for pancreatic cancer (2013) issued by the Japan pancreas society: A synopsis. Jpn J Clin Oncol. 2014;44:883–8. doi: 10.1093/jjco/hyu127. [DOI] [PubMed] [Google Scholar]
- 2.Yamaguchi K, Okusaka T, Shimizu K, et al. Clinical practice guidelines for pancreatic cancer 2016 from the Japan pancreas society. Pancreas. 2017;46:595–604. doi: 10.1097/MPA.0000000000000816. [DOI] [PubMed] [Google Scholar]
- 3.Goutorbe F, Rouquette O, Mulliez A, et al. Temporary placement of a covered duodenal stent can avoid riskier anterograde biliary drainage when ERCP for obstructive jaundice fails due to duodenal invasion. Surg Endosc. 2017;31:625–31. doi: 10.1007/s00464-016-5008-5. [DOI] [PubMed] [Google Scholar]
- 4.Manta R, Conigliaro R, Mangiafico S, et al. Multimodal, one-session endoscopic approach for management of patients with advanced pancreatic cancer. Surg Endosc. 2016;30:1863–8. doi: 10.1007/s00464-015-4403-7. [DOI] [PubMed] [Google Scholar]
- 5.Khashab MA, Valeshabad AK, Leung W, et al. Multicenter experience with performance of ERCP in patients with an indwelling duodenal stent. Endoscopy. 2014;46:252–5. doi: 10.1055/s-0033-1359214. [DOI] [PubMed] [Google Scholar]
- 6.Bories E, Pesenti C, Caillol F, et al. Transgastric endoscopic ultrasonography-guided biliary drainage: Results of a pilot study. Endoscopy. 2007;39:287–91. doi: 10.1055/s-2007-966212. [DOI] [PubMed] [Google Scholar]
- 7.Poincloux L, Rouquette O, Buc E, et al. Endoscopic ultrasound-guided biliary drainage after failed ERCP: Cumulative experience of 101 procedures at a single center. Endoscopy. 2015;47:794–801. doi: 10.1055/s-0034-1391988. [DOI] [PubMed] [Google Scholar]
- 8.Dumonceau JM, Tringali A, Papanikolaou I, et al. Endoscopic biliary stenting: Indications, choice of stents, and results: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline – Updated October 2017. Endoscopy. 2018;50:910–30. doi: 10.1055/a-0659-9864. [DOI] [PubMed] [Google Scholar]
- 9.Caillol F, Bories E, Zemmour C, et al. Palliative endoscopic drainage of malignant stenosis of biliary confluence: Efficiency of multiple drainage approach to drain a maximum of liver segments. United European Gastroenterol J. 2019;7:52–9. doi: 10.1177/2050640618803812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ratone JP, Caillol F, Zemmour C, et al. Outcomes of duodenal stenting: Experience in a French tertiary center with 220 cases. Dig Liver Dis. 2020;52:51–6. doi: 10.1016/j.dld.2019.06.025. [DOI] [PubMed] [Google Scholar]
- 11.Adler DG, Baron TH. Endoscopic palliation of malignant gastric outlet obstruction using self-expanding metal stents: Experience in 36 patients. Am J Gastroenterol. 2002;97:72–8. doi: 10.1111/j.1572-0241.2002.05423.x. [DOI] [PubMed] [Google Scholar]
- 12.Mutignani M, Tringali A, Shah SG, et al. Combined endoscopic stent insertion in malignant biliary and duodenal obstruction. Endoscopy. 2007;39:440–7. doi: 10.1055/s-2007-966327. [DOI] [PubMed] [Google Scholar]
- 13.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharaiha RZ, Khan MA, Kamal F, et al. Efficacy and safety of EUS-guided biliary drainage in comparison with percutaneous biliary drainage when ERCP fails: A systematic review and meta-analysis. Gastrointest Endosc. 2017;85:904–14. doi: 10.1016/j.gie.2016.12.023. [DOI] [PubMed] [Google Scholar]
- 15.Wang K, Zhu J, Xing L, et al. Assessment of efficacy and safety of EUS-guided biliary drainage: A systematic review. Gastrointest Endosc. 2016;83:1218–27. doi: 10.1016/j.gie.2015.10.033. [DOI] [PubMed] [Google Scholar]
- 16.Hamada T, Isayama H, Nakai Y, et al. Duodenal invasion is a risk factor for the early dysfunction of biliary metal stents in unresectable pancreatic cancer. Gastrointest Endosc. 2011;74:548–55. doi: 10.1016/j.gie.2011.04.046. [DOI] [PubMed] [Google Scholar]
- 17.Hamada T, Nakai Y, Isayama H, et al. Duodenal metal stent placement is a risk factor for biliary metal stent dysfunction: An analysis using a time-dependent covariate. Surg Endosc. 2013;27:1243–8. doi: 10.1007/s00464-012-2585-9. [DOI] [PubMed] [Google Scholar]
- 18.Ogura T, Chiba Y, Masuda D, et al. Comparison of the clinical impact of endoscopic ultrasound-guided choledochoduodenostomy and hepaticogastrostomy for bile duct obstruction with duodenal obstruction. Endoscopy. 2015;48:156–63. doi: 10.1055/s-0034-1392859. [DOI] [PubMed] [Google Scholar]
- 19.Park DH, Jang JW, Lee SS, et al. EUS-guided biliary drainage with transluminal stenting after failed ERCP: Predictors of adverse events and long-term results. Gastrointest Endosc. 2011;74:1276–84. doi: 10.1016/j.gie.2011.07.054. [DOI] [PubMed] [Google Scholar]
- 20.Yamao K, Kitano M, Takenaka M, et al. Outcomes of endoscopic biliary drainage in pancreatic cancer patients with an indwelling gastroduodenal stent: A multicenter cohort study in West Japan. Gastrointest Endosc. 2018;88:66–75.e2. doi: 10.1016/j.gie.2018.01.021. [DOI] [PubMed] [Google Scholar]


