Abstract
EUS has opened a new frontier in endoscopic techniques for accessing pancreatic ducts in patients with failed ERCP. The major indications of EUS-guided pancreatic duct intervention (EUS-PDI) are main pancreatic duct (MPD) strictures due to chronic pancreatitis or strictures of pancreaticojejunal or pancreaticogastric anastomosis after Whipple resection, which lead to recurrent acute pancreatitis. EUS-guided pancreaticogastro or duodenostomy offers an alternative to surgery when transpapillary drainage fails or is not possible. We provide an expert commentary and a brief overview on this relatively novel technique utilizing EUS-PDI creation in patients with impaired drainage of the MPD who have failed other conventional endoscopic techniques for MPD drainage and either are poor surgical candidates or are reluctant to undergo surgery.
Keywords: endosonography, acute pancreatitis, interventional endosonography
INTRODUCTION
Impaired drainage of the main pancreatic duct (MPD) from a stenosed pancreaticoenteral tract anastomosis after a pancreatoduodenectomy, pancreatic duct strictures in chronic pancreatitis, or in patients with disconnected pancreatic duct syndrome (DPDS) after necrotizing pancreatitis may cause recurrent acute or chronic pancreatitis in the upstream gland.[1,2] Surgical approaches for the reestablishment of the pancreatic flow in these conditions are associated with significant morbidity. In addition, surgical pancreaticojejunostomy is often not feasible with small ducts, and surgical resection of the upstream pancreas may result in diabetes.[3,4] Utilization of ERCP for endoscopic pancreatic ductal drainage has now become the mainstay of endoscopic pancreatic duct drainage.[5,6,7] However, ERCP transpapillary therapy fails in 3%–10% of the cases due to complete pancreatic ductal obstruction and/or disconnected duct after necrotizing pancreatitis.[8,9,10,11,12,13]
EUS has opened a new frontier in endoscopic techniques for accessing pancreatic ducts in patients with failed ERCP.[14,15,16,17] The major indications of EUS-guided pancreatic duct intervention (EUS-PDI) are main MPD strictures due to chronic pancreatitis or strictures of pancreaticojejunal or pancreaticogastric anastomosis after Whipple resection, which lead to recurrent acute pancreatitis. EUS-guided pancreaticogastro or duodenostomy offers an alternative to surgery when transpapillary drainage fails or is not possible. Indications for EUS-PDI are summarized in Table 1. It should be noted that EUS-guided pancreatic duct drainage is a technically challenging procedure with a good success rate of over 70% when carried out by experts. However, the rate of adverse events (AEs) can be as high as 20%, requiring careful selection of patients.
Table 1.
Indications and Contraindications for EUS-guided Intervention of the Pancreatic Duct
| Indications |
| Failed pancreatic duct cannulation by conventional ERCP |
| Pancreatic duct disruption as a result of acute pancreatitis |
| Severe pancreatic ductal stenosis and obstruction due to |
| chronic pancreatitis |
| Anastomotic stricture at the pancreatico-jejunostomy site |
| Pancreatic duct fistula |
| Contraindications |
| Inability to visualize the pancreatic duct by EUS |
| Severe coagulopathy |
| Intervening blood vessels |
| Medical conditions making patient unfit for endoscopy |
We provide an expert commentary and a brief overview on this relatively novel technique utilizing EUS-PDI creation in patients with impaired drainage of the MPD who have failed other conventional endoscopic techniques for MPD drainage and either are poor surgical candidates or are reluctant to undergo surgery.[14]
EUS-GUIDED PANCREATIC DUCT INTERVENTION
Technical techniques for EUS-pancreatic duct intervention
In order to visualize the MPD, a linear echoendoscope with a therapeutic channel is utilized. Under combined fluoroscopic and EUS guidance, the MPD is accessed trans-gastrically or trans-duodenally via a 19-gauge or 22-gauge fine aspiration needle. A pancreatogram is then performed and a guidewire is then passed into the MPD under fluoroscopic guidance using a 0.035- or 0.025-inch guidewire.
EUS-guided rendezvous approach
The rendezvous approach is performed by placing the guidewire into the MPD and then advancing it across the papilla or surgical anastomosis and into the small bowel. The echoendoscope is removed and leaving the guidewire in place. Based on the anatomy, a duodenoscope, colonoscope, or balloon-assisted enteroscope is then advanced to the papilla or the anastomosis, where the PD can be accessed with the guidance of the EUS-placed wire to perform retrograde interventions. This approach is summarized in Figure 1a-c.
Figure 1.
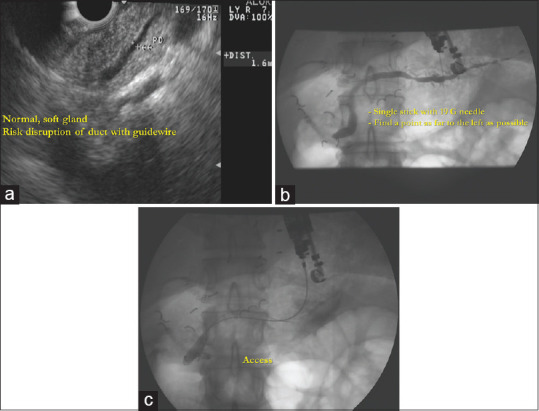
EUS-guided rendezvous approach in a patient with acute recurrent idiopathic pancreatitis with ductal stenosis in the pancreatic head; (a) linear ultrasound endoscope is used to identify the major pancreatic duct with the tip of the echoendoscope positioned in the antrum; (b) under combined fluoroscopic and EUS guidance, access into the main pancreatic duct is achieved using a 19-gauge fine aspiration needle and a pancreatogram is performed; (c) the guidewire passes the stenosis, penetrates the papilla, and travels into the duodenum. A rendezvous technique is then performed by exchanging the EUS scope for a duodenoscope and “conventional” pancreatic endotherapy is performed
EUS-guided antegrade approach
EUS-guided antegrade is utilized by puncturing the MPD and forming a tract for antegrade placement of a stent across the pancreatic-gastric anastomosis, pancreatic-duodenal anastomosis, major papilla, or pancreaticojejunal anastomosis. The tract size is then expanded using cautery-assisted devices such as a needle knife or small caliber-ringed catheter followed by dilated using a hydrostatic balloon. A stent is then placed, so it traverses the MPD obstruction, surgical anastomosis, or papilla. This approach is utilized when the papilla or anastomosis cannot be accessed or passed with a wire via a rendezvous approach [Figure 2].
Figure 2.
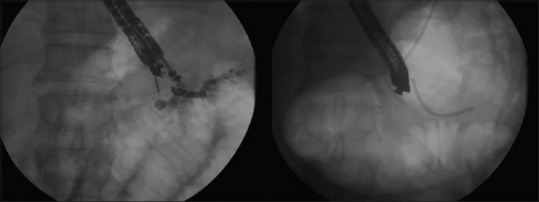
EUS-guided antegrade approach: The echoendoscope is used for the placement of a pancreatic stent into the main pancreatic duct via the stomach; once guidewire access is achieved into the main pancreatic duct, dilation of the transmural tract is performed using hydrostatic balloons prior to stent placement
Outcomes for EUS-guided pancreatic duct intervention
Francois et al. were the pioneers of the EUS-PDI via a pancreaticogastrostomy (EUS-guided PG) procedure. They published successful utilization of EUS-guided PG on four chronic pancreatitis patients presenting with pain due to ductal hypertension and insufficient pancreatic ductal drainage that had failed conventional ERCP.[14] Kahaleh et al. also described EUS-guided PG as an alternative to ERCP in two patients with failed access to the major and minor papillae due to surgical reconstruction.[15] Since then, multiple case series have described the application of EUS-guided PG in several different pathologies. Sakamoto and et al. performed a reconstruction of the surgical PG performed for pancreatic head resection for neoplasm, using EUS approach.[18] Another case also reported the utilization of EUS approach to establish PG in patients with dilated MPD secondary to occluded gastropancreatic anastomosis postpancreaticoduodenectomy.[19] EUS-guided PG can also be used to relieve obstructive symptoms caused by a displaced pancreatic stent placed during ERCP as described by Lu et al.[20] or for extrusion of a displaced pancreatic stent.[21] EUS-guided PG in combination with transgastric per-oral pancreatoscopy with electrohydraulic lithotripsy (EHL) has also been used in a patient with chronic hereditary pancreatitis and intraductal stones for the evacuation of stones and subsequent alleviation of symptoms.[22] One further case report described a 42-year-old male who had failed a trial of the conventional ERCP procedure for pancreatic duct stricture at genu secondary to chronic obstructive pancreatitis. Therefore, EUS-guided PG was performed with a fully covered, self-expandable, 10-mm diameter metallic stent with relief of symptoms.[23]
With the evidence from case reports, several studies have retrospectively evaluated the efficacy of EUS-guided PG in patients with impaired drainage of MPD. Tessier et al. performed a retrospective case review in which 36 patients with chronic pancreatitis and complete obstruction. These patients either underwent EUS-guided PG or EUS-guided pancreatobulbostomy. Relief of symptoms was demonstrated in 25 (69%) of the studied population.[12] Recently, a retrospective cohort of 5 patients demonstrated a 100% success rate of EUS-guided PG in combination with anterograde pancreaticoscopy and intraductal EHL for pancreaticolithiasis.[24] The biggest retrospective study done thus far has evaluated a total of 44 patients who underwent EUS-guided pancreatic ductal intervention. Transgastric EUS rendezvous technique was carried out in 23/44 (52.3%) patients, transgastric EUS-PG in 18/44 (40.9%), and transduodenal pancreaticobulbostomy in 3/44 (6.8%). Technical success was lower in the PG group (77.8%) compared to the transgastric rendezvous procedure (95.6%).[25]
Few prospective studies have evaluated on EUS-guided PDI. Kahaleh et al. prospectively evaluated 13 patients with abdominal pain due to pancreatic stenosis from chronic pancreatitis. There patients underwent EUS-guided PDI after a failed ERCP. Out of the patients studied, 10 had successful MPD stent placement via EUS-PDI across the pancreaticogastric fistula. The mean follow-up time period was 14 months, in which the mean pancreatic duct size in these patients decreased from 4.6 to 3.0 mm (P = 0.01) and pain score decreased from 7.3 to 3.6 (P = 0.01). Adverse events included one case of bleeding requiring hemoclip placement and 1 case of contained perforation.[26] A Korean study evaluated placement of a fully covered self-expandable metal stent (FCSEMS) for patients with obstructive pancreatitis who failed ERP. A total of 23/25 patients underwent EUS-guided PG with placement of the FCSEMS. The rest of the patients underwent pancreaticoduodenostomy (1/25) and pancreaticojejunostomy (1/25). The results showed significant improvement of pain scores (P = 0.001). In the mean follow-up period (221 days), no complications related to stent migration, stent clogging, infection, and/or stent-induced ductal stricture were seen.[27]
There are several integral steps of the EUS-guided PG procedure. One of the most important parts is the technique used for the creation of the pancreaticogastrostomy. Several different techniques have been employed by different endoscopists including needle-knife cautery,[12,14,27] bougie dilation,[26] stent extraction screw, and standard balloon dilators to allow stent passage across the muscular gastric wall and pancreatic parenchyma into the MPD. These techniques can cause significant trauma to the pancreatic tissue and can result in serious complications including bleeding, perforation, perigastric collections, or acute pancreatitis. In addition, standard balloon dilators have a larger shaft diameter of up to 6 Fr and can result in a significant leak if access to the MPD is lost after initial puncture. More recently, the authors at the University of Minnesota have described a relatively atraumatic technique using a peripheral angioplasty balloon to dilate the PG tract. The angioplasty balloon is long enough to fit the entire length of the echoendoscope and has a narrower shaft diameter of 4 Fr allowing it to pass through the PG with minimal resistance.[28] The optimal technique for the creation of the PG tract is yet to be determined and largely remains dependent on the comfort of the endoscopist with the different techniques and availability of the equipment.
The second integral part of the EUS-guided PG procedure is the type of conduit/stent placed within the created PG to maintain drainage. The stent helps form a communication between the MPD and the gastric lumen. This communicating channel (PG) can decompress the pancreatic duct and can also be used for procedures such as wire-guided basket stone retrieval and multiple types of pancreatoscopy-assisted lithotripsy.[29] Several different types of stents have been described in the published literature. The more commonly used are the plastic stents (straight, single, and double-pigtail) though there are case reports of PG creation using FCSEMSs or lumen-apposing metal stents (LAMSs).[27,30,31] Plastic stents have been preferred over the metal stents due to easier and less traumatic insertion and a theoretically lower risk of complications if the stent is displaced. When metal stents are considered, covered stents are preferred over uncovered stents due to less risk of leakage and easy removal and replacement as there is less tissue in-growth.[32] There is also a case report showing successful deployment of LAMSs for MPD drainage; Gornals et al. presented their case with EUS-guided pancreatic duct drainage using LAMS plus a double-pigtail stent in a 44-year-old male patient with chronic pancreatitis and MPD stricture.[30] A more recent study has shown a decreased rate of migration with placement of a single pigtail in a reverse fashion with the pigtail curling partway in the MPD, which anchors it in place and the straight end is left extending into the lumen of the stomach by at least 3–4 cm. A second side-by-side stent is then placed after 8 weeks.[28]
As with any intervention, EUS-guided PG has adverse events reported in the literature. In fact, it has been noted that the complication rate for EUS-guided PG is higher than a EUS-guided rendezvous procedure.[11] Patients who undergo placement of stents for the sole purpose of decompression have a high rate of stent dysfunction (occlusion, migration, or both), which can be as high as 55%. In one study, 4/26 participants were observed to have spontaneous stent dislocation after a median time of 285 days. Since the authors did not observe any damage to the ducts in this study, they used clinical and radiographic response to index drainage as an indicator for stent replacement.[33] In order to counteract this, some of the authors have modified their practice by offering endoscopy with stent exchange and caliber upsizing (which theoretically will prevent displacement) or by inserting a second side-by-side stent in the preexisting fistula.[12] The second side-by-side stent placement allows separate motion of the stents during gastric peristaltic movements which act as a wick creating space for not only drainage from within the stents but also from between the stents. Indwelling stent migration and complete obstruction of the PG have been seen which have previously been treated using the EUS-guided rendezvous procedure and a diathermic dilator.[34] Another potential complication is development of peripancreatic fluid collection, which in most cases has resolved without any intervention.[31] Transpancreatic as well as transmural stent displacement leading to distal perforation has also been described.[25,35] A retrospective study done on 18 patients in 2019 in India described several EUS-guided techniques for the resolution of external pancreatic fistulae in patients with DPDS. The study concluded that patients who underwent EUS-guided PG were at a higher risk for external migration of transmural stents.[36] Other minor complications noted previously are fever, minor bleeding, hematoma formation, contained perforation, stripping of wire, and acute pancreatitis.[25,26]
In conclusion, EUS-PDI is a relatively new procedure frequently employed in patients where undertaken standard ERCP fails or is not possible due to altered surgical anatomy, with an overall success rate of around 70%–90%.[29] However, it has several complications that have previously been observed in small studies. Due to lack of larger more controlled studies, the exact efficacy as well safety of this procedure is yet to be determined. Therefore, multicenter trials and large-scale prospective studies need to be conducted to further evaluate this promising procedure. We recommend that EUS-PDI should be performed in carefully selected patients by well-trained and experienced interventional endoscopists.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Schlitt HJ, Schmidt U, Simunec D, et al. Morbidity and mortality associated with pancreatogastrostomy and pancreatojejunostomy following partial pancreatoduodenectomy. Br J Surg. 2002;89:1245–51. doi: 10.1046/j.1365-2168.2002.02202.x. [DOI] [PubMed] [Google Scholar]
- 2.Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: Pathology, complications, and outcomes. Ann Surg. 1997;226:248–57. doi: 10.1097/00000658-199709000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byrne RL, Gompertz RH, Venables CW, et al. Surgery for chronic pancreatitis: A review of 12 years experience. Ann R Coll Surg Engl. 1997;79:405–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Kalady MF, Broome AH, Meyers WC, et al. Immediate and long-term outcomes after lateral pancreaticojejunostomy for chronic pancreatitis. Am Surg. 2001;67:478–83. [PubMed] [Google Scholar]
- 5.Dumonceau JM, Devière J, Le Moine O, et al. Endoscopic pancreatic drainage in chronic pancreatitis associated with ductal stones: Long-term results. Gastrointest Endosc. 1996;43:547–55. doi: 10.1016/s0016-5107(96)70189-x. [DOI] [PubMed] [Google Scholar]
- 6.Ponchon T, Bory RM, Hedelius F, et al. Endoscopic stenting for pain relief in chronic pancreatitis: Results of a standardized protocol. Gastrointest Endosc. 1995;42:452–6. doi: 10.1016/s0016-5107(95)70049-8. [DOI] [PubMed] [Google Scholar]
- 7.Cremer M, Devière J, Delhaye M, et al. Stenting in severe chronic pancreatitis: Results of medium-term follow-up in seventy-six patients. Bildgebung. 1992;59(Suppl 1):20–4. [PubMed] [Google Scholar]
- 8.Gabbrielli A, Pandolfi M, Mutignani M, et al. Efficacy of main pancreatic-duct endoscopic drainage in patients with chronic pancreatitis, continuous pain, and dilated duct. Gastrointest Endosc. 2005;61:576–81. doi: 10.1016/s0016-5107(05)00295-6. [DOI] [PubMed] [Google Scholar]
- 9.Rösch T, Daniel S, Scholz M, et al. Endoscopic treatment of chronic pancreatitis: A multicenter study of 1000 patients with long-term follow-up. Endoscopy. 2002;34:765–71. doi: 10.1055/s-2002-34256. [DOI] [PubMed] [Google Scholar]
- 10.Eleftherladis N, Dinu F, Delhaye M, et al. Long-term outcome after pancreatic stenting in severe chronic pancreatitis. Endoscopy. 2005;37:223–30. doi: 10.1055/s-2005-860988. [DOI] [PubMed] [Google Scholar]
- 11.Widmer J, Sharaiha RZ, Kahaleh M, et al. Endoscopic ultrasonography-guided drainage of the pancreatic duct. Gastrointest Endosc Clin N Am. 2013;23:847–61. doi: 10.1016/j.giec.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Tessier G, Bories E, Arvanitakis M, et al. EUS-guided pancreatogastrostomy and pancreatobulbostomy for the treatment of pain in patients with pancreatic ductal dilatation inaccessible for transpapillary endoscopic therapy. Gastrointest Endosc. 2007;65:233–41. doi: 10.1016/j.gie.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 13.Wright BE, Cass OW, Freeman ML, et al. ERCP in patients with long-limb Roux-en-Y gastrojejunostomy and intact papilla. Gastrointest Endosc. 2002;56:225–32. doi: 10.1016/s0016-5107(02)70182-x. [DOI] [PubMed] [Google Scholar]
- 14.François E, Kahaleh M, Giovannini M, et al. EUS-guided pancreaticogastrostomy. Gastrointest Endosc. 2002;56:128–33. doi: 10.1067/mge.2002.125547. [DOI] [PubMed] [Google Scholar]
- 15.Kahaleh M, Yoshida C, Yeaton P, et al. EUS antegrade pancreatography with gastropancreatic duct stent placement: Review of two cases. Gastrointest Endosc. 2003;58:919–23. doi: 10.1016/s0016-5107(03)02297-1. [DOI] [PubMed] [Google Scholar]
- 16.Will U, Meyer F, Manger T, et al. Endoscopic ultrasound-assisted rendezvous maneuver to achieve pancreatic duct drainage in obstructive chronic pancreatitis. Endoscopy. 2005;37:171–3. doi: 10.1055/s-2004-826151. [DOI] [PubMed] [Google Scholar]
- 17.Mallery S, Matlock J, Freeman ML, et al. EUS-guided rendezvous drainage of obstructed biliary and pancreatic ducts: Report of 6 cases. Gastrointest Endosc. 2004;59:100–7. doi: 10.1016/s0016-5107(03)02300-9. [DOI] [PubMed] [Google Scholar]
- 18.Sakamoto H, Kitano M, Komaki T, et al. Endoscopic ultrasound-guided pancreaticogastrostomy reconstruction. Endoscopy. 2007;39(Suppl 1):E70–1. doi: 10.1055/s-2007-966150. [DOI] [PubMed] [Google Scholar]
- 19.Katanuma A, Maguchi H, Fukazawa M, et al. Endoscopic ultrasonography-guided pancreaticogastrostomy for a case of occlusion of gastro-pancreatic anastomosis after pancreaticoduodenectomy. Dig Endosc. 2009;21(Suppl 1):S87–91. doi: 10.1111/j.1443-1661.2009.00854.x. [DOI] [PubMed] [Google Scholar]
- 20.Lu L, Jin HB, Yang JF, et al. Endoscopic ultrasound-guided pancreaticogastrostomy for symptomatic pancreatic duct obstruction caused by migrated pancreatic stent. World J Gastrointest Endosc. 2017;9:535–9. doi: 10.4253/wjge.v9.i10.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doi S, Mabuchi M, Tsujikawa T, et al. Retrograde pancreatic stent extrusion through the EUS-guided pancreaticogastrostomy route. VideoGIE. 2017;2:353–5. doi: 10.1016/j.vgie.2017.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maubach J, Macpherson AJ, Gloor B, et al. EUS-guided pancreaticogastrostomy and transgastric per-oral pancreatoscopy with electrohydraulic lithotripsy in a patient with chronic hereditary pancreatitis and several intraductal stones. VideoGIE. 2018;3:238–40. doi: 10.1016/j.vgie.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang A, Aswakul P, Prachayakul V, et al. Chronic pancreatic pain successfully treated by endoscopic ultrasound-guided pancreaticogastrostomy using fully covered self-expandable metallic stent. World J Clin Cases. 2016;4:112–7. doi: 10.12998/wjcc.v4.i4.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.James TW, Baron TH. Antegrade pancreatoscopy via EUS-guided pancreaticogastrostomy allows removal of obstructive pancreatic duct stones. Endosc Int Open. 2018;6:E735–8. doi: 10.1055/a-0607-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dalal A, Patil G, Maydeo A, et al. Six-year retrospective analysis of endoscopic ultrasonography-guided pancreatic ductal interventions at a tertiary referral center. Dig Endosc. 2020;32:409–16. doi: 10.1111/den.13504. [DOI] [PubMed] [Google Scholar]
- 26.Kahaleh M, Hernandez AJ, Tokar J, et al. EUS-guided pancreaticogastrostomy: Analysis of its efficacy to drain inaccessible pancreatic ducts. Gastrointest Endosc. 2007;65:224–30. doi: 10.1016/j.gie.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 27.Oh D, Park DH, Cho MK, et al. Feasibility and safety of a fully covered self-expandable metal stent with antimigration properties for EUS-guided pancreatic duct drainage: Early and midterm outcomes (with video) Gastrointest Endosc. 2016;83:366–73.e2. doi: 10.1016/j.gie.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 28.Hayat U, Freeman ML, Trikudanathan G, et al. Endoscopic ultrasound-guided pancreatic duct intervention and pancreaticogastrostomy using a novel cross-platform technique with small-caliber devices. Endosc Int Open. 2020;8:E196–202. doi: 10.1055/a-1005-6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krafft MR, Nasr JY. Anterograde endoscopic ultrasound-guided pancreatic duct drainage: A technical review. Dig Dis Sci. 2019;64:1770–81. doi: 10.1007/s10620-019-05495-9. [DOI] [PubMed] [Google Scholar]
- 30.Gornals JB, Consiglieri C, Vida F, et al. Endoscopic ultrasound-guided pancreaticogastrostomy using a lumen-apposing metal stent plus a double-pigtail plastic stent. Endoscopy. 2016;48 Suppl 1:E276–7. doi: 10.1055/s-0042-113186. [DOI] [PubMed] [Google Scholar]
- 31.Saumoy M, Xu MM, Tyberg A, et al. Endoscopic ultrasound-guided pancreaticogastrostomy in a pediatric patient. Endoscopy. 2017;49:E229–30. doi: 10.1055/s-0043-113557. [DOI] [PubMed] [Google Scholar]
- 32.Shimamura Y, Mosko J, Teshima C, et al. Endoscopic ultrasound-guided pancreatic duct intervention. Clin Endosc. 2017;50:112–6. doi: 10.5946/ce.2017.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Will U, Reichel A, Fueldner F, et al. Endoscopic ultrasonography-guided drainage for patients with symptomatic obstruction and enlargement of the pancreatic duct. World J Gastroenterol. 2015;21:13140–51. doi: 10.3748/wjg.v21.i46.13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawakami H, Kuwatani M, Kawakubo K, et al. Endoscopic ultrasonography-guided antegrade diathermic dilation for the treatment of complete obstruction of a pancreaticogastrostomy. Endoscopy. 2014;46(Suppl 1 UCTN):E517–8. doi: 10.1055/s-0034-1377600. [DOI] [PubMed] [Google Scholar]
- 35.Maubach J, Christen S, Macpherson AJ, et al. Endoscopic rescue therapy of a distally perforated, retroperitoneal stent after EUS-guided pancreaticogastrostomy. VideoGIE. 2019;4:169–71. doi: 10.1016/j.vgie.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rana SS, Sharma R, Gupta R, et al. Endoscopic treatment of refractory external pancreatic fistulae with disconnected pancreatic duct syndrome. Pancreatology. 2019;19:608–13. doi: 10.1016/j.pan.2019.05.454. [DOI] [PubMed] [Google Scholar]


