Abstract
Introduction:
Pulmonary neuroendocrine tumors (NETs) comprise a spectrum of tumors ranging from indolent to highly aggressive neoplasm. This study aims to study the clinicopathological and immunohistochemical features of NETs and assess the sensitivity of various IHC markers.
Materials and Methods:
All consecutive cases of pulmonary NETs diagnosed from January 2016 to June 2019 were analyzed retrospectively. The routine hematoxylin- and eosin-stained sections along with immunohistochemistry (IHC) slides were reviewed. IHC was done using a panel of markers which included synaptophysin, chromogranin, CD56, thyroid transcription factor-1 (TTF-1), p-40, napsin-A, and ki67.
Results:
Of total number of 53 patients, diagnosis was made on biopsy in 40 patients and resection specimen in 13 patients. Small cell lung carcinoma was the most common (31 cases), followed by 16 cases of typical carcinoid, 5 cases of atypical carcinoid, and 1 case of combined SCLC. Both synaptophysin and chromogranin were positive in all the cases of typical carcinoid. Synaptophysin had better sensitivity than chromogranin in atypical carcinoid and small cell carcinoma. CD56 was positive in 8 out of 9 cases done. TTF-1 was negative in all the cases of typical carcinoid. The sensitivity of TTF-1 in small cell carcinoma was 85.19%. The mean Ki67 labeling index was 1.4%, 6.6%, and 65.6% in typical, atypical carcinoid, and small cell carcinomas, respectively.
Conclusion:
Synaptophysin was more sensitive than chromogranin, especially in atypical carcinoid and small cell carcinoma. TTF-1 along with high Ki67 differentiates small cell carcinoma from carcinoid.
KEY WORDS: Immunohistochemistry, neuroendocrine tumors, small cell lung carcinoma
INTRODUCTION
The neuroendocrine tumors (NETs) of the lung encompass a spectrum of lesions ranging from indolent typical carcinoid to intermediate atypical carcinoid and aggressive large cell neuroendocrine carcinoma and small cell lung carcinoma (SCLC).[1] Diagnosis is primarily based on constellation of various morphological features such as architecture, cytological features, necrosis, and mitotic activity. Definite diagnosis of atypical carcinoid and large cell neuroendocrine carcinoma is difficult on small biopsies. In some instances, even if the neuroendocrine nature of the tumor is evident on morphology and immunohistochemistry (IHC), crushing artifact may lead to overdiagnosis of atypical carcinoid as SCLC. Hence, in this article, we have emphasized the clinicopathological and immunohistochemical features of NETs and evaluate the sensitivity of various neuroendocrine immunohistochemical markers.
MATERIALS AND METHODS
All consecutive cases of pulmonary NETs diagnosed from January 2016 to June 2019 were analyzed retrospectively. The demographic data, clinical features, and imaging findings were retrieved from the medical records. Of the total 53 cases, the diagnosis was made on biopsy in 40 patients and resection specimen in 13 patients. The biopsy specimens comprised 19 computed tomography (CT)-guided biopsies, 20 fiberoptic bronchial biopsies, and 1 ultrasound-guided biopsy. The resected specimens included lobectomy (11 patients) and pneumonectomy (2 patients).
The hematoxylin- and eosin-stained sections were reviewed in all the cases. The tumors were histologically classified based on the World Health Organization (WHO) classification of NETs of the lung.[1] The other non-small cell carcinoma and preneoplastic lesions such as tumorlet and diffuse idiopathic pulmonary neuroendocrine cell hyperplasia were excluded from the study. IHC was done using polymer horseradish peroxidase (HRP) technique on fully automated immunostainer (Xmatrx Elite; Biogenex). The panel of immunohistochemical markers comprised chromogranin, synaptophysin, CD56, thyroid transcription factor-1 (TTF-1), napsin-A, p-40, and Ki67. The list of the IHC markers is tabulated in Table 1. The pattern of immunohistochemical staining included nuclear staining for TTF-1 and p40, granular cytoplasmic staining for napsin-A, cytoplasmic staining for neuroendocrine markers synaptophysin and chromogranin, and membranous pattern of staining for CD56. A cutoff limit of 5% and 20% Ki67 labeling index was considered for typical and atypical carcinoids, respectively. The sensitivity of various IHC markers was also determined.
Table 1.
Panel of immunohistochemical markers
| IHC marker | Clone | Supplier |
|---|---|---|
| TTF1 | 8G7G3 | Pathn Situ |
| Chromogranin | LK2H10 | Biogenex |
| Synaptophysin | Snp88 | Biogenex |
| CD56 | 123C3 | DAKO |
| p-40 | BC 28 | Biocare |
| Ki67 | GM001 | Pathn Situ |
IHC: Immunohistochemistry
RESULTS
The age of the patients of SCLC ranged from 41 to 80 years with a mean of 58.4 years and a marked male preponderance of M:F ratio of 15:1. The carcinoids prevailed in the age group of 30–60 years with a mean age of 45.3 years and almost equal gender distribution with M:F ratio of 1.1:1. The duration of the symptoms ranged from 1 month–2.5 years. The patients presented with cough (26 patients), shortness of breath (22 patients), hemoptysis (12 patients), chest pain (2 patients), and superior vena cava (SVC) syndrome (2 patients). None of the patients had carcinoid syndrome. On contrast-enhancing CT, mediastinal and hilar lymphadenopathy were detected in 4 patients, and there was evidence of metastatic deposits in the liver, brain, and bone in one patient. Another patient had a lytic lesion in the vertebral body suggestive of metastasis. One patient was diagnosed as pulmonary Koch's elsewhere and was on treatment with antitubercular drugs. Later on imaging, a right hilar mass was detected on imaging with SVC obstruction and internal jugular vein thrombosis. Another patient had received chemotherapy for non-small cell carcinoma diagnosed previously. On follow-up, progressive disease on positron emission tomography scan prompted the clinician for a repeat biopsy. The right lung was more commonly involved than the left accounting for 69% and 31%, respectively. The size of the tumors ranged from 1.5 cm to 5.5 cm. The endobronchial tumors were polypoidal in nature with smooth reddish to slightly irregular contours.
On histopathology, SCLC was the most common subtype accounting for 31 cases, followed by 16 cases of typical carcinoid, 5 cases of atypical carcinoid, and 1 case of combined SCLC. Diagnosis was made solely based on morphology in 9 cases which included 5 cases of SCLC and 4 typical carcinoids. Morphologically SCLC displayed nests and sheets of small round-to-oval cells with scant cytoplasm, high N: C ratio, nuclear molding, finely granular chromatin, frequent mitosis, necrosis, and apoptotic debris [Figure 1]. The typical carcinoid predominantly had organoid, trabecular, and pseudoglandular pattern. Spindling of the cells was noted in one case. The cells were uniform with finely granular nuclear chromatin and inconspicuous nucleoli. Mitosis was <2 per 10 HPF and necrosis was not seen [Figure 2]. Of these 4 typical carcinoids, 2 were diagnosed on lobectomy specimen. The typical and atypical carcinoids had similar morphological growth pattern. The latter was differentiated from the former by the presence of necrosis and/mitosis (2–10 per 10 HPF). Necrosis was seen in 3 out of the 5 cases of atypical carcinoid [Figure 3].
Figure 1.
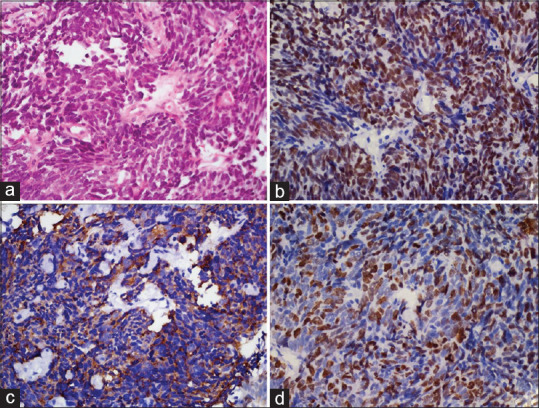
Small cell lung carcinoma: (a) Nests and sheets of small round cells with scant cytoplasm, nuclear hyperchromasia, molding, and inconspicuous nucleoli (H and E, ×400). (b) Cells show nuclear positivity for TTF-1 (poly-HRP, ×400). (c) Tumor cells were positive for synaptophysin (poly-HRP, ×400). (d) Cells show a high ki67 labeling index (poly-HRP, ×400)
Figure 2.
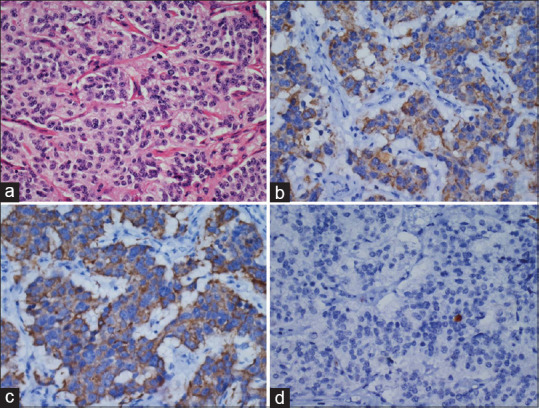
Typical carcinoid: (a) Nests of cells having uniform nucleus with granular nuclear chromatin (H and E, ×400). Tumor cells were positive for (b) synaptophysin and (c) chromogranin (poly-HRP, ×400). (d) Ki67 labeling index 1% (poly-HRP, ×400)
Figure 3.
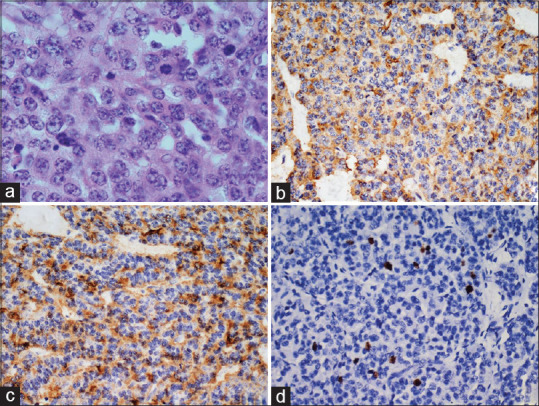
Atypical carcinoid (a) Sheets of cells with fairly uniform chromatin and frequent mitosis (H and E, ×400). Cells are positive for (b) chromogranin and (c) synaptophysin (poly-HRP, ×400). (d) Ki67 labeling index 4% (poly-HRP, ×400)
Histopathological examination of the resected specimens comprising 11 lobectomy and 2 pneumonectomy specimens revealed 11 cases of typical carcinoid and 2 cases of atypical carcinoid.
Immunohistochemistry
The IHC results are summarized in Table 2. Among the neuroendocrine markers, both synaptophysin and chromogranin were positive in all the cases of typical carcinoid. Synaptophysin was more sensitive than chromogranin in atypical carcinoid and SCLC (100% vs. 80% and 95.83% vs. 76%). The sensitivity of the IHC markers is tabulated in Table 3. CD56 done in 9 cases was positive in all except one case of SCLC. The negative predictive value of TTF-1 in typical carcinoid was 100%. Two out of three cases showed nuclear positivity for TTF-1 in atypical carcinoid. The SCLC showed TTF-1 expression in 85.19%. Negative staining for p-40 in all the 18 cases of SCLC done ruled out basaloid squamous cell carcinoma. Negative staining for napsin-A ruled out adenocarcinoma in all the cases done. A case of combined SCLC was diagnosed based on positivity for neuroendocrine markers and p-40 in two different components. The Ki67 ranged from 1% to 4% in typical carcinoid, 5%–10% in atypical carcinoid, and 35%–95% in SCLC with a mean labeling index of 1.4, 6.6, and 65.6, respectively.
Table 2.
Immunohistochemistry of neuroendocrine tumors
| Histopathology | TTF1 | Napsin-A | Chromogranin | Synaptophysin | CD56 | p-40 | Ki67 (mean) (%) |
|---|---|---|---|---|---|---|---|
| Typical carcinoid (n=16) | 0/7 | 0/1 | 12/12 | 7/7 | 0/2 | 1.4 | |
| Atypical carcinoid (n=5) | 2/3 | 0/1 | 4/5 | 3/3 | 1/1 | 6.6 | |
| Small cell lung carcinoma (n=31) | 23/27 | 0/16 | 19/25 | 23/24 | 7/8 | 0/18 | 53.9 |
| Combined small cell carcinoma | 1/1 | 0/1 | 1/1 | 1/1 | 1/1 | - |
Table 3.
Sensitivity of the immunohistochemistry markers
| Histopathology | IHC markers | Sensitivity (%) | 95% CI | Positive likelihood ratio |
|---|---|---|---|---|
| Small cell lung carcinoma (n=31) | TTF1 | 85.19 | 66.27-95.81 | 0.85 |
| Chromogranin | 76 | 54.87-90.64 | 0.76 | |
| Synaptophysin | 95.83 | 78.88-99.89 | 0.96 | |
| Typical carcinoid tumor (n=16) | Chromogranin | 100 | 73.54-100 | 1.00 |
| Synaptophysin | 100 | 59.04-100 | 1.00 | |
| Atypical carcinoid tumor (n=5) | Chromogranin | 80 | 28.36-99.49 | 0.80 |
| Synaptophysin | 100 | 29.24-100 | 1.00 |
IHC: Immunohistochemistry, CI: Confidence interval
DISCUSSION
The NETs of the lung account for 25% of all lung tumors, of which 20% are SCLC, followed by 3% of large cell neuroendocrine carcinoma, 2% of typical carcinoid, and 0.2% of atypical carcinoid.[1,2,3,4] The former two are considered poorly differentiated, and the later ones are classified as well-differentiated and intermediate-grade tumors, respectively.[1,3] These four histological variants have been clubbed together as a separate category under NETs in the latest WHO 2015 classification of lung tumors.[1] In addition, typical and atypical carcinoids are still referred to as carcinoid tumors of the lung, whereas in other locations like gastrointestinal tract, these are replaced by the term well-differentiated NETs.[1,5,6] Studies have shown a rising trend of carcinoid in the recent years as opposed to a declining tendency of SCLC.[7] The tumors arise from Kulchitzky cells present in the bronchial mucosa.[3,4] There are a stronger predilection in older individuals and an association with smoking history in SCLC and large cell neuroendocrine carcinoma as opposed to carcinoid tumors.[3] The mean age of the patients of SCLC was seen a decade older with marked male preponderance compared to carcinoid in the present study.
Carcinoid tumors display a wide variety of morphological patterns. The nested pattern and glandular pattern should be differentiated from adenocarcinoma. Cells with spindle cell morphology should be distinguished from other mesenchymal neoplasms involving the lung and pleura.[3]
Nicholson et al. in their study on 100 cases of SCLC in surgically resected specimens found a prevalence of combined SCLC in 28 cases. The non-small cell carcinoma component included adenocarcinoma, squamous cell carcinoma, large cell carcinoma, spindle cell carcinoma, or giant cell carcinoma.[8] In this study, there was one case of combined SCLC where the non-small cell component was squamous cell carcinoma highlighted on staining with p-40.
In one case where biopsy was repeated following chemotherapy, the second biopsy revealed histological transformation to SCLC after treatment with tyrosine kinase inhibitors. Although the initial biopsy was diagnosed as adenocarcinoma, there was no adenocarcinoma component in the repeat biopsy. The absence of resistant clones T790M on EGFR analysis pointed histological transformation to SCLC as the underlying mechanism of EGFR resistance in this case. This case has been reported by the authors earlier.[9]
Biopsies frequently show crush artifact in small cell carcinoma. The differential diagnosis in such cases includes lymphoma, non-small cell carcinoma, carcinoid, and chronic inflammation. Another problem while dealing with the surgical lung biopsies in comparison to the bronchial biopsies is the deceptive appearance of larger cell size and vesicular nucleus with prominent nucleoli in some cases. IHC helps in resolving diagnostic dilemma.[2] Although cytomorphology and architectural features are indistinguishable in typical and atypical carcinoids, cell size, nuclear morphology, and architectural features help in differentiating small cell and large cell neuroendocrine carcinoma.[10]
The optimal panel of markers used in this study for diagnosis included TTF-1, napsin-A, chromogranin, synaptophysin, CD56, p63, and Ki67. There is a diffuse and intense expression of neuroendocrine markers in carcinoid except for a minority of atypical carcinoid.[11] Curioni-Fontecedro et al. in their study analyzed the efficacy of neuroendocrine markers in 192 patients and found synaptophysin and chromogranin to be more sensitive in carcinoid in comparison to SCLC.[12] A similar sensitivity pattern was noted in the present study as well. Around one-fourth of SCLCs are negative for both synaptophysin and chromogranin. In this study, there was one case which was negative for both synaptophysin and chromogranin, but neuroendocrine differentiation was evident based on positivity for CD56. Although CD56 is considered to be less specific at other sites, it is considered to be the most sensitive marker for SCLC. CD56 was not performed in all the cases of SCLC in the present study, but it was positive in 8 out of 9 cases done. If all the above markers are negative, then additional IHC should be done by LCA, S100, and CD99 to rule out lymphoma, melanoma, and primitive NETs.[2]
Synaptophysin, chromogranin, and CD56 are used to confirm the neuroendocrine nature. However, TTF-1 is required to specifically subtype different types of NET as TTF-1 is intensely positive in 70%–90% of SCLC cases.[3,12] The present study showed a slightly higher sensitivity rate of 85.19% compared to 76%, as documented by Curioni-Fontecedro et al.[12] Unlike pulmonary adenocarcinoma, it cannot be used as a site-specific marker as TTF-1 can be expressed in 20%–80% of small cell carcinomas of extrapulmonary sites such as prostate, bladder, cervix, and gastrointestinal tract.[3]
The reported incidence of TTF-1 expression in pulmonary carcinoid is variable in the literature ranging from 0% to 94% and 0%–100% in typical and atypical carcinoids, respectively. Unlike lung adenocarcinoma and SCLC where the staining pattern is strong and diffuse, carcinoids show focal and weak staining. On the one hand, Rugge et al. and Sturm et al. did not detect TTF-1 expression in either typical or atypical carcinoid, whereas Du et al. reported TTF-1 positivity in 27.8% of typical carcinoids and 29.4% of atypical carcinoids.[13,14,15] Interestingly, most of the positive cases were predominantly peripherally located and had spindle cell morphology Curioni-Fontecedro et al. also noted TTF-1 positivity in 46% and 45% of atypical and typical carcinoids, respectively.[12] None of the typical carcinoid tumors were positive for TTF-1, and 2 out of 3 atypical carcinoids were positive for TTF-1 in the present study.
A compilation of various studies on Ki67 proliferation in NETs has revealed a mean (range) Ki67 labeling index of 1.5 (0%–2.3%) for typical carcinoid, 7.7 (0%–17%) for atypical carcinoid, and 64 (25%–96%) for SCLC.[3] This is in concordance with the results of our study. Ki67 is used to distinguish SCLC or large cell neuroendocrine carcinoma from carcinoid as the former shows a high proliferative index. It is particularly of great help in small biopsies with crushed morphology. However, it is not reliable in distinguishing typical carcinoid from atypical carcinoid in small biopsies. Unlike pulmonary NETs, gastroenteropancreatic NETs (GEP-NETs) incorporates Ki67 along with mitosis to grade tumors with greater impact of Ki67 in determining the survival outcome. The revised criteria in GEP-NET includes well-differentiated grade III NET in addition to poorly differentiated grade III NET. This distinction is still not applicable to lung NET despite a recent grading proposal based on the assessment of Ki67, mitosis, and necrosis.[16] A recent study combining all the above three parameters and stratification into three-tier grading system revealed that all histologically classified typical carcinoids were grade I whereas 29 out of 75 atypical carcinoids were downgraded to grade I and one upgraded to grade III. Of the 86 SCLC, most of them were grade III except 6 tumors which were downgraded to grade II. Hence, behavioral heterogeneity was observed among the atypical carcinoid group which was split into all three tumor grades.[1,16] Studies on phenotypic-genotypic correlation has shown poor prognosis among atypical carcinoid with Ki-67 LI over 10% outperforming necrosis and mitotic count.
The updated 2015 WHO classification for lung NETs proposed guidelines on Ki67 labeling index which includes 50%–100% for SCLC, up to 20% for atypical carcinoid, and 5% for typical carcinoid.[1,17] There were 2 cases in the present study which had a Ki67 of 35%. It will be interesting to find out if these tumors with a ki67 index ranging from 21% to 40% will behave like grade 3 NETs applying a similar classification system which is recommended in the gastrointestinal tract and other organs. A study on 244 resected neuroendocrine neoplasm showed similar clinicopathological and immunohistochemical features in all well-differentiated neuroendocrine neoplasm except for worse prognosis in patients with ki67 >20%.[18] Future research with special emphasis on NETs with high Ki67 is required to determine if the present classification of pulmonary NET needs to focus on the inclusion of ki67 in stratifying tumors. Due to lack of substantial evidence as an independent prognostic factor and overlap of cutoff threshold, at present, Ki67 is still not included as a diagnostic criterion in grading of pulmonary NET.[10,19,20] However, its inclusion in surgical practice helps the oncologist to individualize treatment.[6,21]
CONCLUSION
SCLC was the most common pulmonary NET. Among the neuroendocrine markers, synaptophysin was more sensitive than chromogranin. TTF-1 along with high Ki67 differentiates SCLC from carcinoid, especially in small biopsies with crushed morphology.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. WHO Classification of Tumors of the Lung, Pleura, Thymus and Heart. 4th ed. Lyon: IARC; 2015. [DOI] [PubMed] [Google Scholar]
- 2.Travis WD. Advances in neuroendocrine lung tumors. Ann Oncol. 2010;21:765–71. doi: 10.1093/annonc/mdq380. [DOI] [PubMed] [Google Scholar]
- 3.Rekhtmann N. Neuroendocrine tumors of the lung. Arch Pathol Lab Med. 2010;134:1628–38. doi: 10.5858/2009-0583-RAR.1. [DOI] [PubMed] [Google Scholar]
- 4.Bertino EM. Pulmonary neuroendocrine/carcinoid tumors. Cancer. 2009;115:4434–41. doi: 10.1002/cncr.24498. [DOI] [PubMed] [Google Scholar]
- 5.Öberg KE. Gastrointestinal neuroendocrine tumors. Ann Oncol. 2010;21(Suppl 7):vii72–80. doi: 10.1093/annonc/mdq290. [DOI] [PubMed] [Google Scholar]
- 6.Hendifar AE, Marchevsky AM, Tuli R. Neuroendocrine tumors of the lung: Current challenges and advances in the diagnosis and management of well-differentiated disease. J Thorac Oncol. 2017;12:425–36. doi: 10.1016/j.jtho.2016.11.2222. [DOI] [PubMed] [Google Scholar]
- 7.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–59. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 8.Nicholson SA, Beasley MB, Brambilla E, Hasleton PS, Colby TV, Sheppard MN, et al. Small cell lung carcinoma (SCLC): A clinicopathologic study of 100 cases with surgical specimens. Am J Surg Pathol. 2002;26:1184–97. doi: 10.1097/00000478-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Hui M, Uppin SG, Stalin BJ, Sadashivudu G. Histological transformation of adenocarcinoma to small cell carcinoma lung as a rare mechanism of resistance to epidermal growth factor receptor-tyrosine kinase inhibitors: Report of a case with review of literature. Lung India. 2018;35:160–3. doi: 10.4103/lungindia.lungindia_347_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Righi L, Gatti G, Volante M, Papotti M. Lung neuroendocrine tumors: Pathological characteristics. J Thorac Dis. 2017;9:S1442–S1447. doi: 10.21037/jtd.2017.01.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beasley MB, Thunnissen FB, Brambilla E, Hasleton P, Steele R, Hammar SP, et al. Pulmonary atypical carcinoid: Predictors of survival in 106 cases. Hum Pathol. 2000;31:1255–65. doi: 10.1053/hupa.2000.19294. [DOI] [PubMed] [Google Scholar]
- 12.Curioni-Fontecedro A, Soldini D, Seifert B, Eichmueller T, Korol D, Moch H, et al. A comprehensive analysis of markers for neuroendocrine tumors of the lungs demonstrates estrogen receptor beta to be a prognostic markers in SCLC male patients. J Cytol Histol. 2014;5:268. [Google Scholar]
- 13.Rugge M, Fassan M, Clemente R, Rizzardi G, Giacomelli L, Pennelli G, et al. Bronchopulmonary carcinoid: Phenotype and long-term outcome in a single-institution series of Italian patients. Clin Cancer Res. 2008;14:149–54. doi: 10.1158/1078-0432.CCR-07-1631. [DOI] [PubMed] [Google Scholar]
- 14.Sturm N, Rossi G, Lantuejoul S, Papotti M, Frachon S, Claraz C, et al. Expression of thyroid transcription factor-1 in the spectrum of neuroendocrine cell lung proliferations with special interest in carcinoids. Hum Pathol. 2002;33:175–82. doi: 10.1053/hupa.2002.31299. [DOI] [PubMed] [Google Scholar]
- 15.Du EZ, Goldstraw P, Zacharias J, Tiffet O, Craig PJ, Nicholson AG, et al. TTF-1 expression is specific for lung primary in typical and atypical carcinoids: TTF-1-positive carcinoids are predominantly in peripheral location. Hum Pathol. 2004;35:825–31. doi: 10.1016/j.humpath.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 16.Rindi G, Klersy C, Inzani F, Fellegara G, Ampollini L, Ardizzoni A, et al. Grading the neuroendocrine tumors of the lung: An evidence-based proposal. Endocr Relat Cancer. 2014;21:1–6. doi: 10.1530/ERC-13-0246. [DOI] [PubMed] [Google Scholar]
- 17.Pelosi G, Papotti M, Rindi G, Scarpa A. Unraveling tumor grading and genomic landscape in lung neuroendocrine tumors. Endocr Pathol. 2014;25:151–64. doi: 10.1007/s12022-014-9320-0. [DOI] [PubMed] [Google Scholar]
- 18.Kasajima A, Konukiewitz B, Oka N, Suzuki H, Sakurada A, Okada Y, et al. Clinicopathological profiling of lung carcinoids with a Ki67 index>20% Neuroendocrinology. 2019;108:109–20. doi: 10.1159/000495806. [DOI] [PubMed] [Google Scholar]
- 19.Pelosi G, Pattini L, Morana G, Fabbri A, Faccinetto A, Fazio N, et al. Grading lung neuroendocrine tumors: Controversies in search of a solution. Histol Histopathol. 2017;32:223–41. doi: 10.14670/HH-11-822. [DOI] [PubMed] [Google Scholar]
- 20.Pelosi G, Sonzogni A, Harari S, Albini A, Bresaola E, Marchiò C, et al. Classification of pulmonary neuroendocrine tumors: New insights. Transl Lung Cancer Res. 2017;6:513–29. doi: 10.21037/tlcr.2017.09.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naheed S, Holden C, Tanno L, Jaynes E, Cave J, Ottensmeier CH, et al. The utility of Ki-67 as a prognostic biomarker in pulmonary neuroendocrine tumours: Protocol for a systematic review and meta-analysis. BMJ Open. 2019;9:e031531. doi: 10.1136/bmjopen-2019-031531. [DOI] [PMC free article] [PubMed] [Google Scholar]


