Abstract
Background:
Intubation with either an endotracheal tube or a rigid bronchoscope is generally preferred to provide airway protection as well as to manage unpredictable complications during transbronchial lung cryobiopsy (TBLC). The laryngeal mask airway has been described as a safe and convenient tool for airway control during bronchoscopy.
Aims and Objectives:
In this study, we evaluated the safety and outcome of using a laryngeal mask airway (LMA) as a conduit for performing TBLC by flexible video bronchoscopy (FB).
Methods:
We retrospectively analyzed the database of the patients who underwent TBLC between November 2015 and September 2019. The procedure was performed using FB through LMA under general anesthesia. Prophylactic occlusion balloon was routinely used starting January 2017 onwards. Radial endobronchial ultrasound (R-EBUS) guidance was used for TBLC in the localized lung lesions when deemed necessary. Multidisciplinary consensus diagnostic yield was determined and periprocedural complications were recorded.
Results:
A total of 326 patients were analysed. The overall diagnostic yield was 81.60% (266/326) which included a positive yield of 82.98% (161/194) in patients with diffuse lung disease and 79.54% (105/132) in patients with localized disease. Serious bleeding complication occurred in 3 (0.92%) cases. Pneumothorax was encountered in 8 (2.45%) cases. A total of 9 (2.76%) cases had at least 1 major complication.
Conclusion:
This study demonstrates that the use of LMA during TBLC by flexible bronchoscopy allows for a convenient port of entry, adequate airway support and effective endoscopic management of intrabronchial haemorrhage especially with the use of occlusion balloon.
KEY WORDS: Cryobiopsy, interstitial lung disease, radial endobronchial ultrasound, transbronchial biopsy
INTRODUCTION
Transbronchial lung cryobiopsy (TBLC) is a technique for establishing the diagnosis of a variety of pulmonary lesions, with the advantage of obtaining significantly larger biopsy specimens, as compared to the conventional transbronchial lung biopsy (TBLB).[1] In a meta-analysis,[2] TBLC when compared with TBLB increased the diagnostic yield from 20%–30% to 74.6%, with a concomitant increase in the risks of complications, especially significant bleeding (1%–4% to 14.2%) and pneumothorax (0.7%–2% to 9.4%). Another systemic analysis[3] reported moderate-to-severe bleeding during TBLC, with a pooled frequency of 39% (range 0%–78%). Accordingly, the TBLC technique has been evolving in pursuit of enhancing the safety of the procedure. It is believed that intubation with either an endotracheal tube (ETT) or a rigid bronchoscope (RB) along with the use of a prophylactic occlusion bronchial balloon blocker (OB) during the procedure is preferable to provide airway protection as well as to manage unpredictable bleeding.[4,5,6] Since first introduced in 1989, laryngeal mask airway (LMA) has been described as a safe and convenient tool for airway control during bronchoscopy.[7] Due to its larger inner diameter, LMA allows for a better view and flexibility compared to ETT[8] and has been successfully used in bronchoscopic interventions, such as balloon dilation, brachytherapy, stenting, and foreign body extraction.[9,10,11] In this study, we evaluated the safety and outcome of using an LMA as a conduit for performing TBLC by flexible video bronchoscopy in patients with parenchymal lung diseases.
SUBJECTS AND METHODS
Study subjects
This is a retrospective, single-center study which included 326 consecutive patients who underwent TBLC in the bronchoscopy suite between November 2015 and September 2019 at a tertiary care referral hospital. The study was approved by our institutional review board (Ethics Committee Project No. 2019-001-IP-27). A consent waiver was granted due to the retrospective nature of the study and the use of anonymized patient data. The cases involving a variety of diffuse and localized parenchymal lung diseases were included in the cohort after a thoughtful and careful clinical and imaging evaluation, which included high-resolution computed tomography (HRCT) of the chest with or without contrast administration in a multidisciplinary team (MDT) meeting, involving the pulmonologists, radiologists, pathologists, and if needed the oncologists. All patients were classified by the American Society of Anesthesiologists (ASA) scoring system before undergoing the procedure.[12] The patients with high risk for general anesthesia (GA, ASA category 4–6), hemodynamic instability (hypotension or uncontrolled hypertension), pulmonary hypertension (pulmonary artery systolic pressure of >50 mmHg on echocardiography), uncorrected bleeding diathesis (platelet count <50,000 cells/mm3, or INR >1.5), severe hypoxemia (partial pressure of oxygen in arterial blood <50 mmHg on room air), reduced lung function (FVC <50% of predicted or 1.5 L or FEV1 <0.8 L, DLCO <30% of predicted), and pregnancy were excluded from the study.
Study data
We analyzed the data with the primary aim to elucidate the safety of LMA for TBLC by flexible bronchoscopy with respect to various procedural complications, particularly the occurrence and management of bleeding. We also analyzed demographic data of the patients as well as their imaging findings. The morphological details of the biopsy specimens were also recorded. The diagnostic yield was evaluated in relation to diffuse or localized parenchymal lung disease.
Procedure
TBLC was performed by experienced bronchoscopists in a dedicated bronchoscopy suite with full anesthesia support and emergency equipment. Before the procedure, 0.5–1 g of tranexamic acid (TXA) adjusted to bodyweight was administered intravenously. The patients underwent GA using incremental doses of intravenous midazolam, fentanyl, propofol, and dexmedetomidine. An LMA (LarySeal, Flexicare, Glamorgan, UK) of 3, 4, or 5 size was placed to secure airway access in all cases. Spontaneous breathing was allowed in all the patients with as and when required positive pressure ventilation using a Bain Coaxial Circuit (Flexicare, CF45 4ER, UK). A flexible video bronchoscope (Olympus BF Type 1T180) and a 1.9-mm diameter nitrous oxide cooled flexible cryoprobe measuring 90 cm in length (ERBE, Germany) were used for the biopsy procedure. A 1.9-mm radial endobronchial ultrasonic (R-EBUS) probe (UM-S20-17S; Olympus, Tokyo, Japan) was used at the discretion of the bronchoscopists in the localized lesions. A bronchoscopic assessment of the airways was done initially. If an endobronchial lesion was visible, the patient was excluded from this study. TBLC was not attempted in the localized lesions, measuring less than 3.0 cm in size on imaging findings and which could not be identified on R-EBUS screening. In the initial 82 cases, prophylactic OB was not used but was readily available to manage complications.[13] From January 2017 onward, we began employing prophylactic bronchial blockade using an occlusion balloon catheter (5 or 6 Fr Fogarty balloon; Edwards Lifesciences, Irvine, CA, or 7 Fr Arndt Blocker; Cook Medical, Bloomington, IN) in all the cases. The Fogarty balloon catheter was grasped with the loop of a snare (SD-210U-10, Olympus, India) already introduced through the working channel of the bronchoscope. Both were then passed as a unit parallel to each other through the LMA into the airway. The catheter tip was directed into the desired segmental orifice under direct bronchoscopic vision. The snare was disengaged from the Fogarty catheter and was pulled out of the bronchoscope. The catheter was secured firmly throughout the procedure by the assistant at the entry port of the LMA to prevent its dislodgment following biopsy extraction [Figure 1a]. An Arndt blocker when chosen was advanced through the blocker port of the Arndt Multiport Adapter, and a fiber-optic bronchoscope was introduced through its fiber-optic port. The guide loop of a lubricated and deflated Arndt blocker was secured around the distal tip of the bronchoscope. The Arndt Multiport Adapter is then fixed over the LMA. The bronchoscope with the endobronchial blocker riding along it is then advanced through the LMA into the desired bronchus. The guide loop is loosened, releasing the OB from the bronchoscope, where it remains in place. The OB is secured by tightening the blocker port of the Arndt Multiport Adapter[14] [Figure 1b].
Figure 1.
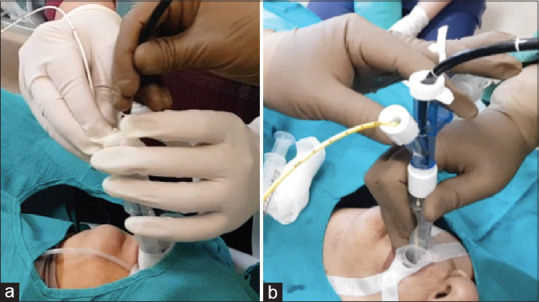
Occlusion balloons: (a) The assistant is firmly gripping the Fogarty Balloon catheter with his right hand at its proximal exit from the laryngeal mask airway which he also stabilizes with his left hand. The operator maneuvers the bronchoscope through the laryngeal mask airway. (b) An Arndt endobronchial blocker is advanced through the blocker port of the Arndt Multiport Adapter, and a fiber-optic bronchoscope is introduced through its fiber-optic port. The occlusion balloon is secured by tightening the blocker port of the Arndt Multiport Adapter
Once the OB was positioned, the cryoprobe was advanced through the working channel of bronchoscope to the distal lung parenchyma. The probe was withdrawn approximately 1.5–2 cm from the point of resistance in cases with diffuse lung disease. The cooling was activated by pressing the foot pedal for 4–8 s, causing surrounding parenchyma to rapidly freeze and adhere to the tip of the cryoprobe. The decision of longer freezing times (>4 s) was made based on the size of the initial sample at 4 s. Both the cryoprobe with the frozen tissue specimen at its tip and the bronchoscope were then pulled out of the airway together en bloc. The OB was inflated immediately to occlude the biopsied segment to limit any bleeding. The cryoprobe tip with the frozen biopsy was then submerged in saline to rapidly thaw and release the specimen. Subsequently, the cryoprobe was removed from the bronchoscope. Thereafter, the scope was re-introduced into the airway lumen and the OB was gently deflated after 1 min under direct bronchoscopic vision. In case of ongoing bleeding, the balloon was reinflated for another 1–3 min. At least two biopsies from different segments of ipsilateral lobes were performed. The consequent emergent bleeding was usually controlled by instilling saline and by wedging the bronchosocope into the bronchial lumen. Instillation of epinephrine (5 mL of 1:10,000) and/or TXA (500 mg) was done topically at the discretion of the bronchoscopists. A chest X-ray was taken in all the cases after the procedure. Patients were monitored in the endoscopy area for an additional 6 h before discharge.
Complications
We recorded various adverse events associated with TBLC, especially bleeding, pneumothorax, acute respiratory failure, acute coronary syndromes, new cardiac arrhythmias, and death occurring within 30 days of the procedure, regardless of cause. The severity of bleeding has been categorized differently in various studies including those in which OB was also used.[13,15,16,17,18,19,20] We classified the severity of bleeding as follows: grade 1: no bleeding or minimal bleeding without the need of bronchoscopic suction; Grade 2: bleeding that stopped after bronchoscopic suction; Grade 3: bleeding that required instillation of intrabronchial epinephrine; or Grade 4: bleeding that required other interventions such as intubation, insertion of OB (when prophylactic OB was not deployed), repeated re-inflation of prophylactic blocker, blood transfusion, or intensive care unit (ICU) admission or that took unusually long time to control or that prevented us from taking further biopsies or leading to termination of the procedure. As there is substantial variability among bleeding definitions,[13,15,16,17,18,19,20,21] currently used, the Nashville Working Group has recently published a consensus airway bleeding scale in response to the need to develop, test, disseminate, and adopt standardized airway bleeding definitions for patients undergoing TBLBs that could be used for general communication, clinical trials, and registries in the future.[22]
Respiratory failure was defined as hypoxemia requiring initiation or escalation of supplemental oxygen or increased level of monitoring for respiratory status. Classification of major complications also included any incident requiring escalation of care, such as ICU admission, unplanned ward admission, mechanical ventilation, the need of blood transfusion, cardiac event, or death.
Diagnostic yield
The histopathological findings were correlated with the patient's clinical history, physical examination, and results of relevant blood investigations, radiographic imaging, and bronchoalveolar lavage. The diagnosis was established by MDT in a clinical–radiologic–pathologic consensus team meeting.
Statistics
Continuous variables were expressed as mean with standard deviation or median with the interquartile range as appropriate.
RESULTS
Table 1 shows the demographic and baseline data. Overall, 326 patients with a mean age of 53.58 ± 15.346 years were analyzed. Of them, 222/326 (68.09%) were male patients.
Table 1.
Demographic and baseline data (n=326)
| Variables | Results,n(%) |
|---|---|
| Age (mean±SD) (years) | 53.58±15.346 |
| Male: female | 222:104 |
| Functional class (NYHA) | |
| Class 1 | 53 (16.26) |
| Class 2 | 107 (32.82) |
| Class 3 | 137 (42.02) |
| Class 4 | 29 (8.89) |
| ASA classification | |
| Class 2 | 152 (46.62) |
| Class 3 | 174 (53.38) |
NYHA: New York Heart Association, ASA: American Society of Anesthesiologists, SD: Standard deviation
The findings of HRCT of the chest [Table 2] revealed that 194 cases (59.51%) had diffuse lung disease and 132 (40.49%) demonstrated a localized lesion.
Table 2.
Computed tomography chest findings (n=326)
| Imaging findings | Results |
|---|---|
| Diffuse parenchymal lung disease | 194 (59.51%) |
| Predominant diffuse ground glass opacity | 9 |
| Probable UIP pattern | 28 |
| Indeterminate for UIP | 18 |
| Profuse micronodules | 11 |
| Marked mosaic attenuation | 13 |
| Ground glass attenuation, consolidation with reticular lines | 21 |
| Reticular lines, centrilobular nodules, patchy ground-glass opacity | 19 |
| Centrilobular nodules, mosaic attenuation reticular line, ground-glassing, traction bronchiectasis, honeycombing, centrilobular emphysema | 44 |
| Bilateral patchy consolidation | 31 |
| Localized lesion* | 132 (40.49%) |
*Localized lesions included cases with consolidation, collapse-consolidation, mass, solitary nodules, cavity, or localized ground-glass opacities. UIP: Usual interstitial pneumonia
Table 3 summarizes the procedure details and biopsy features. Cryobiopsy alone was performed in 211 cases and under R-EBUS guidance in 115 cases. The diagnostic yield with cryobiopsy alone was 82.93% (175/211) and with R-EBUS guidance was 79.13% (91/115). The mean duration of the procedure was 29.5 and 37.09 min, respectively. We obtained an average of four biopsies per patient, with an average largest diameter of 6.85 mm [Figure 2]. The average freezing time was 6 s. The samples were obtained with a representation of alveoli in 318 cases (97.54%).
Table 3.
Procedure details and biopsy features
| Procedure | Number of cases (n) | Duration (min), mean±SD | Diagnostic yield, n(%) |
|---|---|---|---|
| Cryobiopsy | 211 | 29.5±5.68 | 175 (82.93) |
| Radial EBUS | 115 | 37.09±5.89 | 91 (79.13) |
| Guided cryobiopsy | |||
| Biopsy specimen characteristics | Results | ||
| Number of samples per procedure, median (range, SD) | 4 (1–7, 1.26) | ||
| Average largest diameter, mean (range, SD) (mm) | 6.85 (1–15, 2.234) | ||
| Freezing time, median (range, SD) (s) | 6 (4–8, 1.674) | ||
| Presence of alveoli (n=326), n (%) | 318 (97.54) | ||
| Presence of small airways (n=326), n (%) | 313 (96.01) | ||
| Presence of bronchial wall (n=326), n (%) | 10 (3.06) | ||
| Presence of pleura (n=326), n (%) | 3 (0.92) | ||
| Artifact free sampled specimens (n=326), n (%) | 311 (95.39) |
SD: Standard deviation, EBUS: Endobronchial ultrasound
Figure 2.
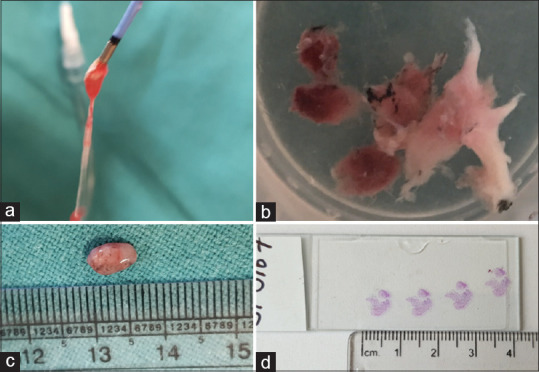
Tissue specimens obtained with transbronchial lung cryobiopsy: (a) Extracted biopsy sample attached to the tip of the cryoprobe, (b) transbronchial lung cryobiopsy samples from one of the patients, (c) a biopsy specimen measuring 8 mm in diameter, (d) fixed, stained, and cut sections of cryobiopsy specimen for a histopathology examination
Table 4 summarizes the multidisciplinary consensus diagnosis. The overall diagnostic yield was 81.60% (266/326) which included a positive yield of 82.98% (161/194) in patients with diffuse lung disease and 79.54% (105/132) in patients with localized disease. The most common diagnosis among patients with diffuse parenchymal lung disease (DPLD) was chronic hypersensitivity pneumonitis in 40 cases. However, among patients with localized lesions, malignancy was the most common diagnosis with 64 cases. Some notable diagnoses included alveolar hemorrhage in five cases, pulmonary alveolar microlithiasis, Wegener's granulomatosis, and polyarteritis nodosa in one case each; and one case of coexisting invasive aspergillosis and mucormycosis. In a total of 60 cases (18.40%), the results of cryobiopsy specimens were nondiagnostic.
Table 4.
Diagnosis made by transbronchial lung cryobiopsy
| Multidisciplinary diagnosis | Diffuse disease (n=194) | Localized lesion (n=132) | Total (n=326) |
|---|---|---|---|
| Idiopathic pulmonary fibrosis | 24 | 24 | |
| Idiopathic pulmonary fibrosis (likely) | 9 | 9 | |
| Idiopathic pulmonary fibrosis (indeterminate) | 5 | 5 | |
| Nonspecific interstitial pneumonia | 18 | 18 | |
| Lymphoid interstitial pneumonia | 2 | 2 | |
| Respiratory bronchiolitis-associated interstitial | 2 | 2 | |
| lung disease | |||
| Chronic hypersensitivity pneumonitis | 40 | 40 | |
| Acute hypersensitivity pneumonitis | 2 | 2 | |
| Cryptogenic organizing pneumonia | 13 | 6 | 19 |
| Acute interstitial pneumonia | 4 | 4 | |
| Desquamative interstitial pneumonia | 4 | 4 | |
| Alveolar microlithiasis | 1 | 1 | |
| Alveolar hemorrhage | 4 | 1 | 5 |
| Pulmonary hemosiderosis | 1 | 1 | |
| Eosinophilic pneumonia | 2 | 3 | 5 |
| Wegener’s granulomatosis | 1 | 1 | |
| Polyarteritis nodosa | 1 | 1 | |
| Sarcoidosis | 8 | 4 | 12 |
| Malignancy | 1 | 64 | 65 |
| Tuberculosis | 13 | 16 | 29 |
| Invasive fungal infection | 5 | 9 | 14 |
| Bronchiolitis | 3 | 3 | |
| Total, n (%) | 161 (82.98) | 105 (79.54) | 266 (81.60) |
| Results of nondiagnostic cryobiopsy specimens | |||
| Histopathological findings (n=326) | n (%) | ||
| Unclassifiable fibrosis | 14 (4.29) | ||
| Mild inflammation | 26 (7.97) | ||
| Inadequate specimen | 10 (3.06) | ||
| Normal lung parenchyma | (2.45) | ||
| Atypical cells | (0.61) | ||
| Total | 60 (18.40) | ||
Table 5 lists the complications during the procedure. Most patients had Grade 2 bleeding, whereas Grade 3 bleeding was observed in nine cases, and Grade 4 bleeding occurred in three cases. Pneumothorax was encountered in eight cases with three requiring chest tube drainage. Respiratory failure was noted in 11 patients, out of whom three with underlying obstructive sleep apnea needed continuous positive airway pressure support and five patients needed oxygen supplementation briefly in the postprocedure period. Escalation of care to the ICU admission was required in three patients of respiratory failure as two cases needed noninvasive ventilation due to hypoventilation and one case of pneumothorax required clinical stabilization along with chest tube drainage. Acute exacerbation of interstitial lung disease occurred in one case after 5 days of the procedure requiring unplanned admission to the ward. Overall, one patient died within 30 days of the procedure due to progression of his primary underlying disease of organizing pneumonia. A total of nine cases (2.76%) had at least one major complication. Damage to cryoprobe was another major complication that occurred during its retrieval when excessive resistance was encountered in one case.
Table 5.
Complications from transbronchial lung cryobiopy procedures
| Complications | Without OB (n=82) | With OB (n=244) | Total (n=326), n (%) |
|---|---|---|---|
| Bleeding Grade 1 | 2 | 1 | 3 (0.92) |
| Bleeding Grade 2 | 74 | 237 | 311 (95.39) |
| Bleeding Grade 3 | 5 | 4 | 9 (2.76) |
| Bleeding Grade 4 | 1 | 2 | 3 (0.92) |
| Pneumothorax | 2 | 6 | 8 (2.45) |
| Resolved with oxygen | 5 (1.53) | ||
| Supplementation | |||
| Resolved with | 3 (0.92) | ||
| Insertion of ICD | |||
| Post procedure respiratory failure | 11 (3.37) | ||
| Escalation of care | 4 (1.22) | ||
| Admission to ICU | 3 (0.92) | ||
| Unplanned admission to ward | 1 (0.31) | ||
| Acute exacerbation of ILD | 1 (0.31) | ||
| Post procedure invasive MV | 0 | ||
| Death within 30 days of procedure | 1 (0.31) | ||
| Bronchoscopy related death | 0 | ||
| Excessive resistance during cryoprobe retrieval | 2 (0.61) | ||
| Damage to cryoprobe | 1 | ||
| Conversion to ETT | 0 | ||
| Major complications (n=326) | n (%) | ||
| Bleeding Grade 4 | 3 (0.92) | ||
| Pneumothorax requiring ICD | 3 (0.92) | ||
| Postprocedure respiratory failure | 3 (0.92) | ||
| Acute exacerbation of ILD | 1 (0.31) | ||
| Escalation of care | 4 (1.22) | ||
| Damage to cryoprobe | 1 | ||
OB: Occlusion balloon, ICD: Intercostal chest tube drainage, ILD: Interstitial lung disease, MV: Mechanical ventilation, ETT: Endotracheal tube. More than 1 complication occurred in some cases; 9 cases in total (2.76%) had at least 1 major complication
DISCUSSION
TBLC is a technique currently in evolution with a promise of achieving diagnostic yield close to surgical lung biopsy.[21] Two expert statements[21,23] suggested that the procedure should be best performed after placement of an artificial airway under deep sedation or GA using OB and fluoroscopic guidance. Although intubation with ETT or RB[21,23] is preferred as an airway conduit to enhance the safety of the procedure, especially from the risk of massive bleeding, there is no real-world prospective, randomized clinical trial that is of sufficient size to compare various procedural modalities and definitively suggest the superiority of a particular technique over the others.[24] Several novel techniques of TBLC have been described such as the use of two-bronchoscope to allow rapid re-entry into the airway after each biopsy instead of using an endobronchial blocker;[25,26] and a recent report[27] of two patients who underwent TBLC by flexible bronchoscopy using only the Arndt blocker without intubation. We opted for LMA as the airway conduit because initially we lacked the facility of RB but later continued to prefer using LMA as a simpler and less invasive solution for airway support, which has been used even in high-risk patients and complicated bronchoscopic procedures.[7] The use of supraglottic airway (SGA) devices during TBLC has also been published in four studies[14,24,25,28] previously. Our study is probably the largest series to date reporting the use of LMA as an airway conduit for performing TBLC. We conjecture that the use of LMA might provide airway protection and could be used as a conduit, instead of no airway protection, especially when the expertise of RB is not available, which could be a hindrance to performing TBLC at many centers.
A recent comparative study[29] highlighted several ideal features of SGAs over ETT for interventional pulmonology (IP) procedures. With a good oropharyngeal seal and stable fit, an SGA enables ventilation even at a higher airway pressure and may be less prone to dislodgment even during prolonged procedures. Further, an SGA with a relatively short, straight, and large internal diameter reduces resistance to bronchoscopic device movements, facilitating the IP procedure. As compared to ETT, there is lesser requirement of anesthetic or neuromuscular blocking drugs. A thin bronchoscope with a 2.0-mm channel has the advantage of occupying lesser space through the airway conduit, but it cannot freely accommodate a cryoprobe of a 1.9-mm diameter. For that reason, we used a therapeutic bronchoscope with its wider working channel that could also manage intraoperative bleeding more efficiently than a thin bronchoscope. We did not find any difficulty in maneuvering a 5.9-mm OD bronchoscope alongside the occlusion balloon through the LMA, due to its wide internal diameter [Figure 1].
Sastre et al.[14] indicated that i-gel SGA is an optimum conduit for performing cryobiopsy. Dibardino et al.[24] in their series reported the use of LMA in 21 out of 25 patients undergoing TBLC. Six patients experienced major complications, including one having life-threatening bleeding. However, the direct causality of complications due to the procedure is difficult to conclude in this study which lacked a regimented protocol.[30] Schmutz et al.[28] examined the safety and feasibility of performing TBLC under GA using an SGA or an ETT. In this series, four cases required intubation, of which two due to the failure of placing SGA properly and another two due to procedural complications. One of these cases had massive endobronchial bleeding which was controlled by balloon tamponade. However, TBLC could be performed successfully in the majority of 123 patients using i-gel SGA. The bronchoscopist's satisfaction was reported as “excellent” with SGA group compared to the “good” in the intubated patients in their series.
Sriprasart et al.[25] employed LMA or ETT to perform TBLC with a two-scope technique under GA without reporting any airway-related complication. Our results are difficult to compare with these studies due to heterogeneity in the cryoprobe types and differences in protocols.
The overall diagnostic yield of TBLC using LMA in our series was 81.60% (266/326) which falls within the previously reported range of 50%–98%,[13,16,17,18,31,32] and our major complication rate of 2.76% compares favorably with the previously reported range of 0%–25%.[13,16,17,20,31,32,33,34] Our results in terms of the diagnostic yield as well as complication rates with LMA are similar to other studies,[13,16,17,18,20,31,32,34] in which either ETT or RB was used for the requisite airway conduit during TBLC.
In our cohort, Grade 3 bleeding occurred in 9 (2.76%) and Grade 4 in 3 cases (0.92%). In all these patients, hemorrhage could be effectively controlled with a combination of endoscopic measures, such as bronchoscopic wedging, suction, cold saline, topical epinephrine, topical TXA, and balloon tamponade. Failure of LMA or procedure-related complications, leading to intubation, was not noted in any of the patients. The access to airway was never lost as LMA allowed easy entry and re-entry even during severe bleeding episodes.
In the initial 82 cases, we did not deploy a prophylactic OB. An episode of serious bleeding in one of the cases in this subgroup was effectively controlled by the therapeutic insertion of the balloon catheter similar to other reports,[28,32,35] which also did not use the prophylactic OB routinely. A systemic analysis[36] revealed that in 84% of cases performed through a LMA, prophylactic OB was not employed. However, given the potential risk of life-threatening bleeding as reported in other studies,[24,37] since January 2017, we incorporated the use of prophylactic OB routinely during the TBLC procedure. The most important concern with the use of LMA is the possibility of airway loss due to a massive bleed in case there is an accidental blocker malfunction or displacement, and for specifically this reason, ETT and RB will still be preferable because of the much superior ability of rapid lung isolation. However, the OB displacement[18] or malfunction[38] is anecdotally reported and has not been observed in the studies[13,14] including ours in which LMA was used, but one has to be always mindful of occurrence of this complication. We observed that the Arndt blocker can be soundly secured by tightening it at the balloon port and the Fogarty catheter by an assistant operator who grips it firmly at its exit from the LMA [Figure 1], which is likely to minimise the risk of displacement of OB following biopsy extraction. However, we believe that RB must be a definitive backup in case of a massive bleed during the procedure with blocker malfunction.
In our study, TXA intravenously was administered before the TBLC procedure. Uncontrolled studies[39,40] have demonstrated that intravenous TXA may reduce both duration and volume of endobronchial bleeding and topical endobronchial instillation of TXA has also shown promising results.[41,42,43] We found that in none of the cases in our series, bleeding complication led to hemodynamic or respiratory instability, surgical intervention, blood transfusion, or admission to the ICU. A systemic review[44] found that the adverse reaction of TXA such as thrombosis is relatively less and reported mainly as anecdotal case reports, despite its wide use. However, the role of TXA in the prevention of bleeding following transbronchial biopsy is yet to be established.
The occurrence of pneumothorax was recorded in 2.45% of cases in our study. A meta-analysis[3] that included 11 studies reported pneumothorax related to TBLC with a mean value of 8.8% (range, 0%–25.9%). Another meta-analysis[35] described pneumothorax in 6.8% of cases and a pooled estimate[22] reported in 9.5% (5.9%–14.9%) of cases. Although we lacked the fluoroscopic guidance similar to other studies,[15,24,26] we ensured that the biopsies were taken at least 1.5–2 cm away from the point of resistance, especially in cases with diffuse lung disease. In peripheral localized lesion, the TBLC was performed only if there was no anticipated risk of pneumothorax based on the imaging findings after discussion in the MDT meeting.
It is noteworthy that in two patients, excessive resistance was encountered during retrieval of the probe after freezing for 6 s. This led to unrepairable damage of the cryoprobe during extraction in one of the cases. This possibly happened due to the lodging of the cryoprobe in a relatively small, proximal, cartilaginous bronchus during the freezing process.[20] In such an event, the probe should be left in place and allowed to defreeze before pulling it out.
There are some important limitations to the current study. The single-center retrospective nature of this series may limit the generalizability of these results, and the possibility of bias cannot be ruled out. Many patients of sarcoidosis could have been diagnosed with TBLB only and of tuberculosis (TB) and alveolar hemorrhage by standard bronchoscopic washing alone. However, these cases lacked typical imaging and laboratory criteria with a low pretest probability of such diagnosis. The study lacks direct comparison of safety and yield of LMA with other artificial airways such as ETT or RB; as a consequence, we had to rely on the previously published data for such analogy. The study cohort is also not a uniform population and the overall complication rate cannot be generalized for the two groups of diffuse and localized lesions. However, given the overall low complication rate in the study, the difference was not likely to be significant. This is one of the largest series of TBLC to date with the primary objective of the use and safety of LMA during TBLC in heterogeneous groups of patients with both diffuse and localized lung diseases. The study also incorporated need-based R-EBUS guidance in cases with localized lesions.
CONCLUSIONS
This study demonstrates that the use of LMA during TBLC by flexible bronchoscopy allows for a convenient port of entry and re-entry of the bronchoscope and various accessories, adequate airway support, stable oxygenation, secured ventilation, and effective endoscopic management of intrabronchial hemorrhage, especially with the use of occlusion balloon. Our results from the current study could be considered as hypothesis-generating. The inclusion of LMA in a standardized protocol needs further confirmation by large prospective trials comparing different procedural strategies of TBLC in both diffuse and localized parenchymal lung diseases. As this technique is still evolving, we strongly feel that TBLC should be performed by experienced operators who are adept at managing serious complications.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Griff S, Ammenwerth W, Schönfeld N, Bauer TT, Mairinger T, Blum TG, et al. Morphometrical analysis of transbronchial cryobiopsies. Diagn Pathol. 2011;6:53. doi: 10.1186/1746-1596-6-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sethi J, Ali MS, Mohananey D, Nanchal R, Maldonado F, Musani A. Are transbronchial cryobiopsies ready for prime time?: A systematic review and meta-analysis. J Bronchology Interv Pulmonol. 2019;26:22–32. doi: 10.1097/LBR.0000000000000519. [DOI] [PubMed] [Google Scholar]
- 3.Johannson KA, Marcoux VS, Ronksley PE, Ryerson CJ. Diagnostic yield and complications of transbronchial lung cryobiopsy for interstitial lung disease. A systematic review and metaanalysis. Ann Am Thorac Soc. 2016;13:1828–38. doi: 10.1513/AnnalsATS.201606-461SR. [DOI] [PubMed] [Google Scholar]
- 4.Madan K, Mehta S, Gupta N, Hadda V, Mohan A, Guleria R. Pneumomediastinum and extensive subcutaneous emphysema after cryoprobe transbronchial lung biopsy. Ann Am Thorac Soc. 2016;13:2101–3. doi: 10.1513/AnnalsATS.201605-395LE. [DOI] [PubMed] [Google Scholar]
- 5.Poletti V, Hetzel J. Transbronchial cryobiopsy in diffuse parenchymal lung disease: Need for procedural standardization. Respiration. 2015;90:275–8. doi: 10.1159/000439313. [DOI] [PubMed] [Google Scholar]
- 6.Linhas R, Marçôa R, Oliveira A, Almeida J, Neves S, Campainha S. Transbronchial lung cryobiopsy: Associated complications. Rev Port Pneumol (2006) 2017;23:331–7. doi: 10.1016/j.rppnen.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Alon D, Pertzov B, Gershman E, Frishman M, Rahman NA, Rosengarten D, et al. The safety of laryngeal mask airway-assisted bronchoscopy versus standard nasal bronchoscopy. Respiration. 2017;93:279–84. doi: 10.1159/000456551. [DOI] [PubMed] [Google Scholar]
- 8.Goudra BG, Singh PM, Borle A, Farid N, Harris K. Anesthesia for advanced bronchoscopic procedures: State-of-the-art review. Lung. 2015;193:453–65. doi: 10.1007/s00408-015-9733-7. [DOI] [PubMed] [Google Scholar]
- 9.Nussbaum E, Zagnoev M. Pediatric fiberoptic bronchoscopy with a laryngeal mask airway. Chest. 2001;120:614–6. doi: 10.1378/chest.120.2.614. [DOI] [PubMed] [Google Scholar]
- 10.Lohser J, Brodsky JB. Bronchial stenting through a ProSeal laryngeal mask airway. J Cardiothorac Vasc Anesth. 2006;20:227–8. doi: 10.1053/j.jvca.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 11.Birmingham B, Mentzer SJ, Body SC. Laryngeal mask airway for therapeutic fiberoptic bronchoscopic procedures. J Cardiothorac Vasc Anesth. 1996;10:519–20. doi: 10.1016/s1053-0770(05)80017-5. [DOI] [PubMed] [Google Scholar]
- 12.Babiak A, Hetzel J, Krishna G, Fritz P, Moeller P, Balli T, et al. Transbronchial cryobiopsy: A new tool for lung biopsies. Respiration. 2009;78:203–8. doi: 10.1159/000203987. [DOI] [PubMed] [Google Scholar]
- 13.Yarmus L, Akulian J, Gilbert C, Illei P, Shah P, Merlo C, et al. Cryoprobe transbronchial lung biopsy in patients after lung transplantation: A pilot safety study. Chest. 2013;143:621–6. doi: 10.1378/chest.12-2290. [DOI] [PubMed] [Google Scholar]
- 14.Sastre JA, Cordovilla R, Jiménez MF, López T. Management of a transbronchial cryobiopsy using the i-gel® airway and the Arndt endobronchial blocker. Can J Anaesth. 2014;61:886–8. doi: 10.1007/s12630-014-0191-0. [DOI] [PubMed] [Google Scholar]
- 15.Ussavarungsi K, Kern RM, Roden AC, Ryu JH, Edell ES. Transbronchial cryobiopsy in diffuse parenchymal lung disease-Retrospective analysis of 74 cases. Chest. 2017;151:400–8. doi: 10.1016/j.chest.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Pajares V, Puzo C, Castillo D, Lerma E, Montero MA, Ramos-Barbón D, et al. Diagnostic yield of transbronchial cryobiopsy in interstitial lung disease: A randomized trial. Respirology. 2014;19:900–6. doi: 10.1111/resp.12322. [DOI] [PubMed] [Google Scholar]
- 17.Hernández-González F, Lucena CM, Ramírez J, Sánchez M, Jimenez MJ, Xaubet A, et al. Cryobiopsy in the diagnosis of diffuse interstitial lung disease: Yield and cost-effectiveness analysis. Arch Bronconeumol. 2015;51:261–7. doi: 10.1016/j.arbres.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 18.She S, Steinfort DP, Ing AJ, Williamson JP, Leong P, Irving LB, et al. Transbronchial cryobiopsy in interstitial lung disease: Safety of a standardized procedure. J Bronchology Interv Pulmonol. 2020;27:36–41. doi: 10.1097/LBR.0000000000000633. [DOI] [PubMed] [Google Scholar]
- 19.Ravaglia C, Bonifazi M, Wells AU, Tomassetti S, Gurioli C, Piciucchi S, et al. Safety and diagnostic yield of transbronchial lung cryobiopsy in diffuse parenchymal lung diseases: A comparative study versus video-assisted thoracoscopic lung biopsy and a systematic review of the literature. Respiration. 2016;91:215–27. doi: 10.1159/000444089. [DOI] [PubMed] [Google Scholar]
- 20.Ravaglia C, Wells AU, Tomassetti S, Dubini A, Cavazza A, Piciucchi S, et al. Transbronchial lung cryobiopsy in diffuse parenchymal lung disease: Comparison between biopsy from 1 segment and biopsy from 2 segments-Diagnostic yield and complications. Respiration. 2017;93:285–92. doi: 10.1159/000456671. [DOI] [PubMed] [Google Scholar]
- 21.Hetzel J, Maldonado F, Ravaglia C, Wells AU, Colby TV, Tomassetti S, et al. Transbronchial cryobiopsies for the diagnosis of diffuse parenchymal lung diseases: Expert statement from the cryobiopsy working group on safety and utility and a call for standardization of the procedure. Respiration. 2018;95:188–200. doi: 10.1159/000484055. [DOI] [PubMed] [Google Scholar]
- 22.Folch EE, Mahajan AK, Oberg CL, Maldonado F, Toloza E, Krimsky WS, et al. Standardized definitions of bleeding after transbronchial lung biopsy: A Delphi Consensus Statement From the Nashville Working Group. Chest. 2020;158:393–400. doi: 10.1016/j.chest.2020.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dhooria S, Agarwal R, Sehgal IS, Aggarwal AN, Goyal R, Guleria R, et al. Bronchoscopic lung cryobiopsy: An Indian Association for Bronchology position statement. Lung India. 2019;36:48–59. doi: 10.4103/lungindia.lungindia_75_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DiBardino DM, Haas AR, Lanfranco AR, Litzky LA, Sterman D, Bessich JL. High complication rate after introduction of transbronchial cryobiopsy into clinical practice at an academic medical center. Ann Am Thorac Soc. 2017;14:851–7. doi: 10.1513/AnnalsATS.201610-829OC. [DOI] [PubMed] [Google Scholar]
- 25.Sriprasart T, Aragaki A, Baughman R, Wikenheiser-Brokamp K, Khanna G, Tanase D, et al. A single US center experience of transbronchial lung cryobiopsy for diagnosing interstitial lung disease with a 2-scope technique. J Bronchology Interv Pulmonol. 2017;24:131–5. doi: 10.1097/LBR.0000000000000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bango-Álvarez A, Ariza-Prota M, Torres-Rivas H, Fernández-Fernández L, Prieto A, Sánchez I, et al. Transbronchial cryobiopsy in interstitial lung disease: Experience in 106 cases-How to do it. ERJ Open Res. 2017;3:00148–2016. doi: 10.1183/23120541.00148-2016. doi.org/10.1183/23120541.00148-2016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madan K, Mittal S, Hadda V, Mohan A. Cryoprobe transbronchial lung biopsy with flexible bronchoscope using Arndt endobronchial blocker. Lung India. 2019;36:241–3. doi: 10.4103/lungindia.lungindia_35_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmutz A, Dürk T, Idzko M, Koehler T, Kalbhenn J, Loop T. Feasibility of a supraglottic airway device for transbronchial lung cryobiopsy-A retrospective analysis. J Cardiothorac Vasc Anesth. 2017;31:1343–7. doi: 10.1053/j.jvca.2017.02.055. [DOI] [PubMed] [Google Scholar]
- 29.Behrens KM, Galgon RE. Supraglottic airway versus endotracheal tube during interventional pulmonary procedures-A retrospective study. BMC Anesthesiol. 2019;19:196. doi: 10.1186/s12871-019-0872-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen AC, Feller-Kopman D. Cryobiopsy: A work in progress. Ann Am Thorac Soc. 2017;14:827–8. doi: 10.1513/AnnalsATS.201703-215ED. [DOI] [PubMed] [Google Scholar]
- 31.Hagmeyer L, Theegarten D, Wohlschläger J, Treml M, Matthes S, Priegnitz C, et al. The role of transbronchial cryobiopsy and surgical lung biopsy in the diagnostic algorithm of interstitial lung disease. Clin Respir J. 2016;10:589–95. doi: 10.1111/crj.12261. [DOI] [PubMed] [Google Scholar]
- 32.Lentz RJ, Taylor TM, Kropski JA, Sandler KL, Johnson JE, Blackwell TS, et al. Utility of flexible bronchoscopic cryobiopsy for diagnosis of diffuse parenchymal lung diseases. J Bronchology Interv Pulmonol. 2018;25:88–96. doi: 10.1097/LBR.0000000000000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pajares V, Torrego A, Puzo C, Lerma E, Gil De Bernabé MA, Franquet T. Transbronchial lung biopsy using cryoprobes. Arch Bronconeumol. 2010;46:111–5. doi: 10.1016/j.arbres.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 34.Griff S, Schönfeld N, Ammenwerth W, Blum TG, Grah C, Bauer TT, et al. Diagnostic yield of transbronchial cryobiopsy in non-neoplastic lung disease: A retrospective case series. BMC Pulm Med. 2014;14:171. doi: 10.1186/1471-2466-14-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Echevarria-Uraga JJ, Pérez-Izquierdo J, García-Garai N, Gómez-Jiménez E, Aramburu-Ojembarrena A, Tena-Tudanca L, et al. Usefulness of an angioplasty balloon as selective bronchial blockade device after transbronchial cryobiopsy. Respirology. 2016;21:1094–9. doi: 10.1111/resp.12827. [DOI] [PubMed] [Google Scholar]
- 36.Dhooria S, Sehgal IS, Aggarwal AN, Behera D, Agarwal R. Diagnostic yield and safety of cryoprobe transbronchial lung biopsy in diffuse parenchymal lung diseases: Systematic review and meta-analysis. Respir Care. 2016;61:700–12. doi: 10.4187/respcare.04488. [DOI] [PubMed] [Google Scholar]
- 37.Casoni GL, Tomassetti S, Cavazza A, Colby TV, Dubini A, Ryu JH, et al. Transbronchial lung cryobiopsy in the diagnosis of fibrotic interstitial lung diseases. PLoS One. 2014;9:e86716. doi: 10.1371/journal.pone.0086716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mittal S, Hadda V, Mohan A, Madan K. Rupture of occlusion balloon during transbronchial lung cryobiopsy. Lung India. 2019;36:263–5. doi: 10.4103/lungindia.lungindia_152_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moen CA, Burrell A, Dunning J. Does tranexamic acid stop haemoptysis? Interact Cardiovasc Thorac Surg. 2013;17:991–4. doi: 10.1093/icvts/ivt383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tscheikuna J, Chvaychoo B, Naruman C, Maranetra N. Tranexamic acid in patients with hemoptysis. J Med Assoc Thai. 2002;85:399–404. [PubMed] [Google Scholar]
- 41.Solomonov A, Fruchter O, Zuckerman T, Brenner B, Yigla M. Pulmonary hemorrhage: A novel mode of therapy. Respir Med. 2009;103:1196–200. doi: 10.1016/j.rmed.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Segrelles Calvo G, De Granda-Orive I, López Padilla D. Inhaled tranexamic acid as an alternative for hemoptysis treatment. Chest. 2016;149:604. doi: 10.1016/j.chest.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 43.Márquez-Martín E, Vergara DG, Martín-Juan J, Flacón AR, Lopez-Campos JL, Rodríguez-Panadero F. Endobronchial administration of tranexamic acid for controlling pulmonary bleeding: A pilot study. J Bronchology Interv Pulmonol. 2010;17:122–5. doi: 10.1097/LBR.0b013e3181dc8c17. [DOI] [PubMed] [Google Scholar]
- 44.Calapai G, Gangemi S, Mannucci C, Miniullo PL, Casciaro M, Calpai F, et al. Systematic review of tranexamic acid adverse reactions. J Pharmacovigilance. 2015;3:171. doi:10.4172/2329-6887.1000171. [Google Scholar]


