Abstract
A 9-day-old girl presented during the 2020 SARS-CoV-2 pandemic in wide-complex tachycardia with acute, symptomatic COVID-19 infection. Because the potential cardiac complications of COVID-19 were unknown at the time of her presentation, we chose to avoid the potential risks of haemodynamic collapse associated with afterload reduction from adenosine. Instead, a transoesophageal pacing catheter was placed. Supraventricular tachycardia (SVT) with an aberrated QRS morphology was diagnosed and the catheter was used to pace-terminate tachycardia. This presentation illustrates that the haemodynamic consequences of a concurrent infection with largely unknown neonatal sequelae present a potentially high-risk situation for pharmacologic conversion. Oesophageal cannulation can be used to diagnose and terminate infantile SVT.
Keywords: arrhythmias, pacing and electrophysiology, COVID-19, paediatrics
Background
Cardiovascular consequences of COVID-19 have been described in children, including myocarditis, arrhythmia and coronary artery dilation.1 2 While current data suggest that the incidence of cardiac complications in children with COVID-19 is low, particularly in the acute phase, the incidence of cardiac complications is incompletely defined due to the rapidly evolving pandemic, including ongoing genotype shifts in the virus.3 In addition, virtually no information is available about COVID-19 infection in the first several months of life.
Wide-complex tachycardia (WCTs) are infrequent in children.4 While more benign aetiologies of WCT, such as supraventricular tachycardia (SVT), with aberration can occur, myocarditis can present with ventricular tachycardia, which can progress rapidly and be life-threatening.5 Even when there is clinical evidence for a supraventricular mechanism, pharmacologic atrioventricular nodal blocking agents (eg, adenosine) can be used to diagnose and potentially terminate SVT. However, the decrease in systemic vascular resistance (SVR) associated with these agents has potential morbidity in the setting of concurrent infection. Transoesophageal electrograms and pacing manoeuvres are alternative diagnostic and therapeutic strategies that can avoid an acute pharmacologic decrease in SVR.
Case presentation
A 9-day-old previously healthy full-term girl presented to the emergency department with reported fever at home (38.3°C), tachycardia (210 beats per minute) and tachypnea (62 breaths per minute) and with a history of nasal congestion. Her blood pressure was 87/63 mm Hg and her peripheral oxygen saturation was 97%. Her lungs were clear to auscultation. She had no murmur or hepatosplenomegaly, and her capillary refill was 2 s. Three-lead telemetry revealed a regular, WCT. Her mother, the primary caregiver, was acutely symptomatic with suspected COVID-19 illness (later verified by PCR) and other household contacts were COVID-19 positive. The infant had no family history of sudden death or rhythm disorders.
Investigations
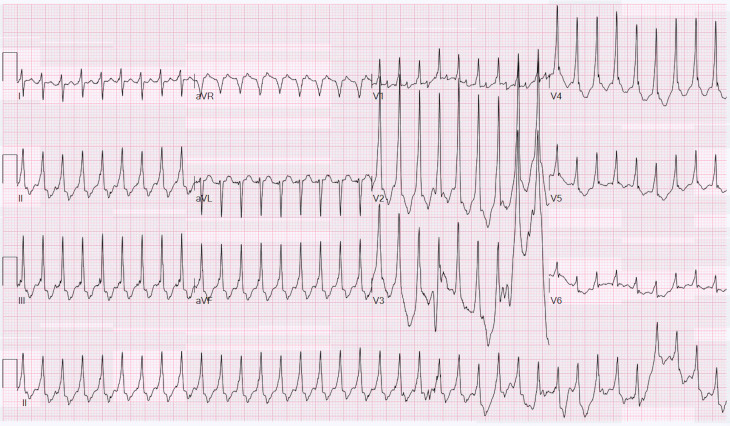
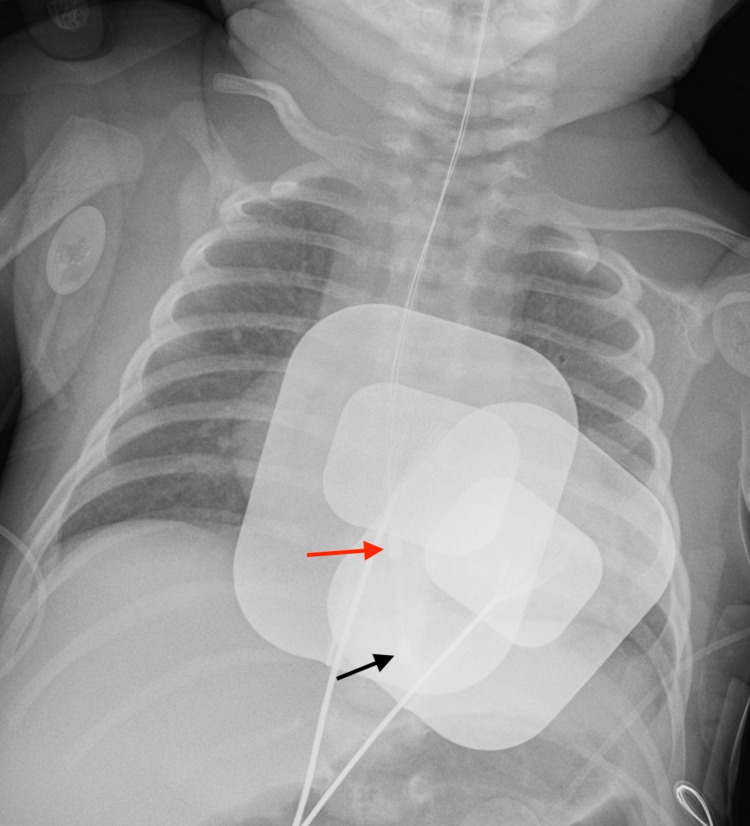
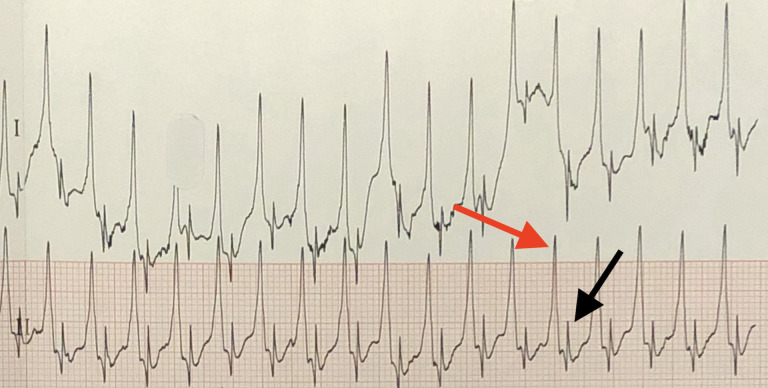
The initial 12-lead ECG recorded regular tachycardia at 215 beats per minute (figure 1). A possible retrograde p-wave inscription was present 140 ms after the QRS onset with a 1:1 ventricular–atrial (VA) relationship. The maximum QRS duration was 108 ms in lead I (normal for infants 28 ms–75 ms). A 5-French bipolar transoesophageal pacing (TOP) catheter (CardioCommand, Tampa, Florida) was placed in the nose and advanced to 17 cm based on a length-based nomogram.6 Chest radiograph confirmed position of the TOP and defibrillation pads (figure 2). A bipolar atrial electrogram was established by connecting the anode to the right arm lead and the cathode to the left arm lead. Standard ECG amplitude, speed and filtering were used (0.1 mV/mm, 25 mm/s). The atrial electrogram confirmed the 1:1 VA relationship with a tachycardia cycle length of 275 ms and a VA interval of 140 ms (figure 3).
Figure 1.
Initial presentation of tachycardia. A 12-lead ECG with leads in standard position has a maximum QRS duration of 108 ms and a minimum QRS duration of 78 ms. Retrograde p waves at 142 ms are best seen in leads II and III. Movement artefact is seen toward the end of the recording.
Figure 2.
Transoesophageal pacing catheter (TOP) at the level of the atrium. A portable anterior–posterior radiograph demonstrates good positioning of the TOP (red arrow at proximal electrode and black arrow at distal electrode) and defibrillation pads in the appropriate position.
Figure 3.
Bipolar atrial electrogram. Left and right arm leads are attached to the bipolar TOP leads. Leg limb leads are in standard position. The QRS complex (red arrow) demonstrates a regular tachycardia at cycle length of 275 ms (215 beats per minute). An atrial electrogram is present 140 ms after the QRS (black arrow) demonstrating a 1:1 ventricular:atrial ratio.
A rapid COVID-19 PCR test was positive. Initial laboratory data are given in table 1. Blood, cerebrospinal fluid and urine were obtained for microscopy, gram stain and culture (all normal), according to practice guidelines for infants with fever.7 On transthoracic echocardiogram, the heart was structurally normal with normal biventricular function. The left and right atria were mildly dilated.
Table 1.
Initial laboratory data
| Investigation | Result | Normal range for age |
| White blood cell count | 11.1 x 109 (76% lymphocytes) | 5.0–21.0 x 109 |
| Haemoglobin | 122 g/L | 135–215 g/L |
| Platelet count | 466 x 109 | 150–450 x 109 |
| Sodium | 139 mEq/L | 136–149 mEq/L |
| Potassium | 5.8 mEq/L | 4.4–6.3 mEq/L |
| Chloride | 103 mEq/L | 98–108 mEq/L |
| Bicarbonate | 21 mEq/L | 20.0–27.0 mEq/L |
| Glucose | 76 mg/dL | 40–80 mg/dL |
| Blood urea nitrogen | 10 mg/dL | 5.0–15.0 mg/dL |
| Creatinine | 0.42 mg/dL | 0.25–0.54 mg/dL |
| Calcium | 9.9 mg/dL | 7.6–11.0 mg/dL |
| Protein | 5.6 g/dL | 3.6–7.4 g/dL |
| Albumin | 3.5 g/dL | 2.3–3.9 g/dL |
| Total bilirubin | 1.5 mg/dL | 2.0–7.0 mg/dL |
| Alkaline phosphatase | 144 IU/L | 37–371 IU/L |
| Alanine aminotransferase | 11 IU/L | 5–51 IU/L |
| Aspartate aminotransferase | 31 IU/L | 18–96 IU/L |
Differential diagnosis
The most important diagnosis in the differential for WCT is ventricular arrhythmia, especially in the setting of an infant with a concurrent febrile illness and risk factors for myocarditis. However, in a haemodynamically stable child, other arrhythmia mechanisms should be excluded when feasible, including orthodromic SVT with aberrancy, antidromic SVT, ectopic atrial tachycardia with aberrancy or pre-excited atrial fibrillation with rapid ventricular response. The last is an important consideration before giving adenosine since the rapidity of the ventricular response in small children during atrial fibrillation can give the impression of a regular tachycardia. We placed cardioversion pads on the front and back of the patient and were ready to cardiovert quickly if needed. Finally, sinus tachycardia is a possibility in an infant with tachycardia and an intercurrent illness.
Treatment
While preparing for placement of a diagnostic transoesophageal catheter, empiric treatment for infection with dehydration was begun. Two fluid boluses (10 mL/kg normal saline each) were administered, rectal antipyretics were given and broad-spectrum intravenous antibiotics were initiated. None of these interventions changed the tachycardia cycle length or the clinical evaluation, making sinus tachycardia less likely.
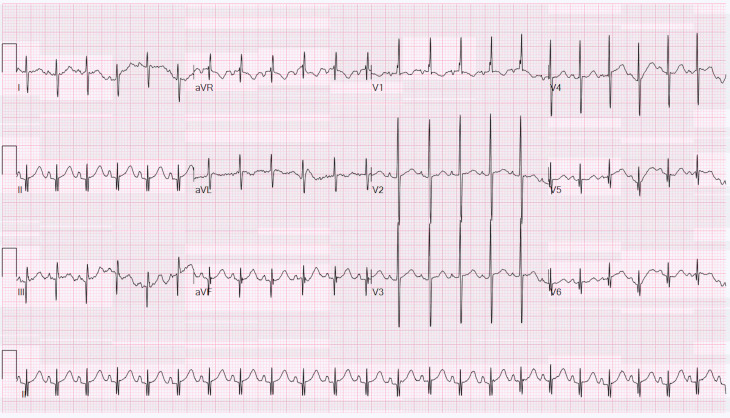
Intravenous fentanyl 1 mcg/kg was injected for analgesia. After the atrial electrogram excluded atrial fibrillation by demonstrating a regular 1:1 VA relationship, a drive train of 31 pulses at 20 mA and a pulse width of 40 ms was administered via transoesophageal catheter at the level of the left atrium using a cycle length of 200 ms. Atrial capture was achieved with beat-to-beat termination of SVT and resumption of sinus rhythm (figure 4). This therapeutic manoeuvre ruled out sinus tachycardia or ectopic atrial tachycardia as the aetiology of the previous tachycardia. A 12-lead ECG in sinus rhythm (figure 5) confirmed no ventricular pre-excitation, ruling out antidromic reciprocating tachycardia, and therefore confirmed the diagnosis of orthodromic SVT with aberrancy for the infant’s presenting arrhythmia.
Figure 4.
Transoesophageal atrial pacing with termination of supraventricular tachycardia. Pacing from transoesophageal pacing with atrial capture is demonstrated following by termination of the tachycardia and resumption of sinus rhythm.
Figure 5.
Sinus rhythm after cardioversion. A 12-lead ECG with leads in standard position demonstrated normal sinus rhythm at 140 beats per minute without ventricular pre-excitation.
Outcome and follow-up
After resumption of sinus rhythm and admission for supportive therapy for COVID-19, she had a recurrence of narrow-complex SVT, without aberration, during lumbar puncture and had spontaneous termination back to sinus rhythm. Incessant SVT in an infant can lead to heart failure, cardiovascular collapse or death.8 Therefore, oral propranolol therapy was initiated at a low dose and titrated to 2 mg/kg/day. Her symptomatic COVID-19 illness resolved without sequelae. Five months after presentation, she has had no further recurrence of clinical arrhythmia while prescribed propranolol with periodic rhythm surveillance by intermittent heart rate counting. Most neonatal SVT resolves in the first year of life.8 We plan to stop treatment at 1 year of age and monitor clinically for arrhythmia recurrence.
Discussion
This case is unique because the patient presented with a primary wide-complex arrhythmia in the setting of acute COVID-19 infection during a pandemic. The arrhythmia was orthodromic SVT, which was mediated by a concealed accessory pathway. The acute infection with fever likely lowered her threshold for developing clinical arrhythmia.
It is important to distinguish this presentation from myocarditis. While supraventricular and ventricular tachyarrhythmias have been described during the acute phase of COVID-19 in adults, the putative aetiology is secondary to cardiomyocyte injury, pericardial inflammation or microvascular ischaemia.9 In contrast, the infection in this child uncovered her underlying cardiovascular substrate (an accessory pathway), rather than suggesting a primary myocardial aetiology secondary to infection. This is consistent with the larger existing literature in COVID-19. Adults often present with respiratory distress and cardiac involvement in the acute phase,10 11 whereas children more commonly present with fever and respiratory symptoms, with rare cardiac manifestations.1 Older children with COVID-19 have shown sinus tachycardia with ventricular repolarisation abnormalities,12 but even this has not been well described in newborns. Ultimately, myocarditis was excluded in this case based on clinical features, including the elimination of the child’s tachycardia and tachypnea symptoms after cardioversion, the presence of normal cardiac function, a post conversion ECG without evidence of acute myocardial injury and a rhythm diagnosis of SVT dependent on an accessory pathway, rather than VT.
Cardiac involvement in children is more common during the hyperinflammatory state some children experience called paediatric inflammatory multisystem syndrome (PIMS) or multisystem inflammatory syndrome in children. PIMS occurs approximately 4 weeks after acute infection with SARS-CoV-2. This patient’s age, clear timeline of exposure 2 days prior to fever onset, duration of fever, unremarkable echocardiogram and haemodynamic stability once the arrhythmia was converted excluded PIMS by current criteria.13
In patients with wide complex tachycardia, the differential always includes ventricular tachycardia, especially when myocarditis or progression of a viral infection remains a possibility. Our first priority was to prepare for cardioversion and cardiopulmonary resuscitation in the event that the infant became unstable. Then, because of the uncertainty surrounding the infection, we chose to use specialised electrophysiology techniques, namely transoesophageal electrogram and pacing, to completely define the arrhythmia and to ultimately terminate it. We avoided pharmacologic diagnostic and therapeutic manoeuvres, namely adenosine, for two major reasons. First, it causes peripheral vasodilation, which could lead to cardiovascular collapse in a patient with systemic infection. Second, until the TOP verified a regular atrial electrogram and a 1:1 VA relationship, there was a small risk for rapid pre-excited atrial fibrillation for which adenosine is contraindicated. While other antiarrhythmic medications, such as amiodarone, can be used to control SVT in an infant, our first priority was to diagnose the arrhythmia correctly followed by terminating it in as safe of a manner as possible. Finally, due to the association of orthodromic SVT and congenital heart disease, a transthoracic echocardiogram was performed to rule this out.8
The main teaching point is that acute infection should trigger clinicians to think carefully about the consequences of diagnosing and terminating tachycardia. Extra precaution and thought should be taken to fully define the diagnosis and choose the most appropriate therapy.
Patient’s perspective.
My daughter and I were home for about 5 days when my young sister came home from daycare sneezing and coughing. I tried to keep my baby away from her, but it was nearly impossible in our home. My sister was tested and 2 days later, we found out she was positive for COVID-19.
I became extremely anxious as my daughter was just 1 week old and I did not know what was going to happen if she caught the virus. I called her paediatrician for advice and was told to take her temperature a few times per day. If it rose above a certain point, I was to take her straight to the hospital. At one temperature check in the middle of the night, her temperature was 101.5° so we went straight to the hospital.
Although we went to the emergency room for a fever, I was quickly told that my daughter had a heart problem. It was a blur from there. She was moved to a larger room and was surrounded by many doctors and nurses. I was told many scary things, including that her heart could stop. I was afraid that with visitor restrictions, if I left, I may not be able to come back. So many things were running through my head.
My baby was just 9 days old. When the doctors treated her arrhythmia and told me she responded as she should, I cried with relief. We found out she was positive for COVID-19 and I feared she may get sicker or have long-lasting effects. We were both infected while she was in the hospital and I am so grateful for the care the nurses were able to provide during that really difficult time. I worried continuously as there is so much we have to learn about COVID-19. Still to this day, I feel like COVID-19 is not completely solved and I worry we could get reinfected.
COVID-19 continues to scare me, but in a way, I am thankful that the fever from COVID-19 actually got us to the hospital where we found out about her heart condition. Now I am able to check her heart rate and give her medicine every day. I can sleep at night knowing her condition is not life-threatening. I have learnt so much and know what to look for to keep my daughter safe.
Being in the emergency room surrounded by doctors and nurses in masks, gloves and gowns with my infant on the examination table was the scariest day of my life. I found comfort knowing my daughter was in great hands. I never felt alone, despite the pandemic.
I am so thankful they helped me get my healthy, happy daughter back home.
Learning points.
During acute infection, the differential diagnosis for arrhythmia is broader than in a healthy child. Extra precautions should be taken to ensure the correct arrhythmia diagnosis is made and to choose the most appropriate therapy.
The differential diagnosis for wide-complex tachycardia (WCT) in infants includes ventricular tachycardia and multiple types of supraventricular tachycardia, including orthodromic supraventricular tachycardia (SVT) with aberrancy, antidromic SVT, ectopic atrial tachycardia with aberrancy or pre-excited atrial fibrillation with rapid ventricular response.
Transoesophageal atrial recording and pacing may be superior to pharmacologic cardioversion in patients at risk for decompensation with peripheral vasodilation.
The presence of an infectious illness may lower the threshold for a child to develop clinical arrhythmia or complicate acute diagnosis and therapy for SVT.
Be prepared to defibrillate or cardiovert WCT. Cardioversion is effective for atrial and ventricular re-entrant tachycardias.
Footnotes
Contributors: Both authors contributed to the conception of the work, drafting and critically revising of the report and have approved the final version of the draft submitted. Both authors are in agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: This study was funded by the National Institutes of Health (K23HL13055).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Alsaied T, Tremoulet AH, Burns JC, et al. Review of cardiac involvement in multisystem inflammatory syndrome in children. Circulation 2021;143:78–88. 10.1161/CIRCULATIONAHA.120.049836 [DOI] [PubMed] [Google Scholar]
- 2. Wei M, Yuan J, Liu Y, et al. Novel coronavirus infection in hospitalized infants under 1 year of age in China. JAMA 2020;323:1313–4. 10.1001/jama.2020.2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wise J. Covid-19: new coronavirus variant is identified in UK. BMJ 2020;371:m4857. 10.1136/bmj.m4857 [DOI] [PubMed] [Google Scholar]
- 4. Sekar RP. Epidemiology of arrhythmias in children. Indian Pacing Electrophysiol J 2008;8:S8–13. [PMC free article] [PubMed] [Google Scholar]
- 5. Canter CE, Simpson KE, Simpson KP. Diagnosis and treatment of myocarditis in children in the current era. Circulation 2014;129:115–28. 10.1161/CIRCULATIONAHA.113.001372 [DOI] [PubMed] [Google Scholar]
- 6. Saul JP, Kygler JD. Electrophysiology studies and electrophysiologic therapeutic catheterization. ThoracicKey. Available: https://thoracickey.com/electrophysiology-studies-and-electrophysiologic-therapeutic-catheterization/
- 7. American College of Emergency Physicians Clinical Policies Committee, American College of Emergency Physicians Clinical Policies Subcommittee on Pediatric Fever . Clinical policy for children younger than three years presenting to the emergency department with fever. Ann Emerg Med 2003;42:530–45. 10.1067/S0196-0644(03)00628-0 [DOI] [PubMed] [Google Scholar]
- 8. Jaeggi E, Öhman A. Fetal and neonatal arrhythmias. Clin Perinatol 2016;43:99–112. 10.1016/j.clp.2015.11.007 [DOI] [PubMed] [Google Scholar]
- 9. Siripanthong B, Nazarian S, Muser D, et al. Recognizing COVID-19-related myocarditis: the possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm 2020;17:1463–71. 10.1016/j.hrthm.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peltzer B, Manocha KK, Ying X, et al. Arrhythmic complications of patients hospitalized with COVID-19: incidence, risk factors, and outcomes. Circ Arrhythm Electrophysiol 2020;13:e009121. 10.1161/CIRCEP.120.009121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. TY H, Lee JZ, Asirvatham SJ. Cardiovascular considerations in coronavirus disease 1029 with a special focus on arrhythmia. J Innov Cardiac Rhythm Manage 2020;11:4191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ece İbrahim, Koçoğlu M, Kavurt AV, et al. Assessment of cardiac arrhythmic risk in children with Covid-19 infection. Pediatr Cardiol 2021;42:264–8. 10.1007/s00246-020-02474-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Godfred-Cato S, Bryant B, Leung J, et al. COVID-19-Associated Multisystem Inflammatory Syndrome in Children - United States, March-July 2020. MMWR Morb Mortal Wkly Rep 2020;69:1074–80. 10.15585/mmwr.mm6932e2 [DOI] [PMC free article] [PubMed] [Google Scholar]