Abstract
Chronic lymphocytic leukemia (CLL) is the most common leukemia in adults. Emerging data suggest that CLL-cells efficiently evade immunosurveillance. T-cell deficiencies in CLL include immuno(metabolic) exhaustion that is achieved by inhibitory molecules, with programmed cell death 1/programmed cell death ligand 1 (PD-L1) signaling emerging as a major underlying mechanism. Moreover, CLL-cells are characterized by a close and recurrent interaction with their stromal niches in the bone marrow and lymph nodes. Here, they receive nurturing signals within a well-protected environment. We could previously show that the interaction of CLL-cells with stroma leads to c-Myc activation that is followed by metabolic adaptations. Recent data indicate that c-Myc also controls expression of the immune checkpoint molecule PD-L1. Therefore, we sought out to determine the role of stromal contact for the CLL-cells’ PD-L1 expression and thus their immuno-evasive phenotype.
To do so, we analyzed PD-L1 expression on CLL cell (subsets) in untreated patients and on healthy donor-derived B-cells. Impact of stromal contact on PD-L1 expression on CLL-cells and the underlying signaling pathways were assessed in well-established in vitro niche models. Ex vivo and in vitro findings were validated in the Eµ-TCL1 transgenic CLL mouse model.
We found increased PD-L1 expression on CLL-cells as compared with B-cells that was further enhanced in a cell-to-cell contact-dependent manner by stromal cells. In fact, circulating recent stromal-niche emigrants displayed higher PD-L1 levels than long-time circulating CLL-cells. Using our in vitro niche model, we show that a novel Notch-c-Myc-enhancer of zeste homolog 2 (EZH2) signaling axis controls PD-L1 upregulation. Ultimately, elevated PD-L1 levels conferred increased resistance towards activated autologous T-cells.
In summary, our findings support the notion that the CLL microenvironment contributes to immune escape variants. In addition, several targetable molecules (eg, Notch or EZH2) could be exploited in view of improving immune responses in patients with CLL, which warrants further in-depth investigation.
Keywords: B-lymphocytes, hematologic neoplasms, immunomodulation, programmed cell death 1 receptor, tumor microenvironment
Introduction
Chronic lymphocytic leukemia (CLL) is the leukemia with the highest incidence among adults in the Western world. Clonally expanded CD5+ B-lymphocytes accumulate in the peripheral blood (PB), lymph nodes (LN) and bone marrow (BM).1 The disease is associated with immune defects that lead to an increased susceptibility towards infectious complications and prevent mounting of an efficient antitumor immunity.2 Those dysfunctions mainly affect the T-cell compartment and coincide with an increased expression of the programmed cell death ligand 1 (PD-L1) on CLL-cells and of its cognate receptor programmed cell death 1 (PD-1) on CLL-derived T-cells.3 Blockade of PD-1/PD-L1 ligation restored T-cell function and yielded superior disease control in the Eµ-TCL1 transgenic CLL model.4 Current studies across various malignant entities have unveiled mechanisms of cellular PD-L1 control that include oncogenic signaling, transcriptional regulation and post-translational modifications (as reviewed in Shen et al 5). However, information regarding PD-L1 control in CLL remains rather limited, with two recent reports pointing towards an involvement of the BCR-STAT3 and CD84-Akt-mTOR signaling axes.6 7
CLL-cells are highly dependent on a recurrent interaction with their microenvironment. Stromal crosstalk can protect CLL-cells from spontaneous and drug-induced apoptosis and shifts their bioenergetic phenotype towards aerobic glycolysis by triggering among others the Notch pathway.8 9 However, it remains unknown whether stromal contact promotes immune escape variants of CLL-cells, especially in view of several recently identified oncogenes such as c-Myc (that are responsive towards microenvironmental activation)9 as regulators of PD-L1.10 Therefore, we sought out to investigate whether stromal cells control PD-L1 expression in CLL-cells and to identify the underlying signaling axes.
Here, we report for the first time that stromal contact drives oncogenic (Notch-c-Myc-EZH2) signaling in CLL-cells, which among other effects controls expression of the immunological checkpoint PD-L1. Our findings are in accordance with the emerging concept of oncogenic drivers as promoters of an immunosuppressive environment and could be therapeutically exploited.
Materials and methods
Patient samples
Cells
Blood samples from untreated patients (online supplemental table 1) and healthy donors were collected and peripheral blood mononuclear cells (PBMCs) were obtained using Ficoll-Paque (GE Healthcare). The HS-5 human bone marrow stromal cell line was purchased from American Type Culture Collection (Manassas, Virginia, USA). CLL cell lines Mec-1 and Eheb were purchased from DSMZ (Braunschweig, Germany). Mesenchymal stromal cells (MSCs) were isolated from iliac crest BM aspirates taken from healthy donors and expanded as previously detailed while fulfilling uniformly minimal MSC criteria.11
jitc-2020-001889supp001.pdf (2.1MB, pdf)
Antibodies and flow cytometry
Cells were stained according to the manufacturer’s recommendations using fluorochrome-coupled antibodies (online supplemental table 2). Samples were analyzed using a FACSCanto II cytometer (BD Biosciences) and the FlowJo V.9.5 and V.10 software (FlowJo). Cell sorting was performed on MoFlo Astrios EQ devices (Beckman Coulter) by the Core Unit of the Friedrich-Alexander-University Erlangen-Nuremberg.
Statistical analyses
Outliers were determined using the ROUT (robust regression with outlier removal) test. Differences in means were evaluated with parametric (paired/unpaired t-test, one-way analysis of variance) or non-parametric (unpaired Mann-Whitney U test, paired Wilcoxon, unpaired Kruskal-Wallis) tests based on the number of comparisons (two or more than two) and distribution levels (as determined by the Shapiro-Wilk and Kolmogorov-Smirnov test). All statistical analyses were performed using GraphPad Prism V.7 or V.8 (GraphPad Prism Software) at a significance level of p<0.05.
For more details, see the online supplemental information.
Results
Stromal cells promote PD-L1 expression on CLL-cells
First, we analyzed PD-L1 expression of circulating CLL-cells retrieved from untreated patients by FACS. In line with previous studies evaluating clinical3 and preclinical4 CLL samples, we measured significantly higher PD-L1 levels on malignant cells as compared with healthy donor-derived B-cells (figure 1A). This observation could be recapitulated in the murine Eµ-TCL1 CLL model (online supplemental figure 1).
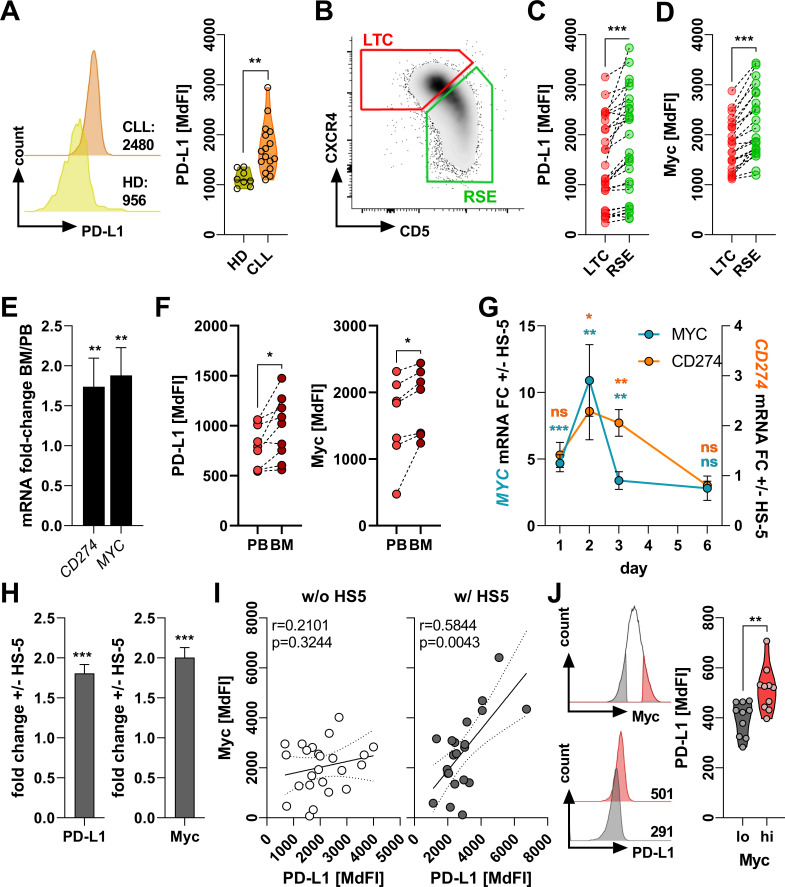
Figure 1.
Stroma cells promote programmed cell death ligand 1 (PD-L1) expression in chronic lymphocytic leukemia (CLL)-cells. (A) Peripheral blood mononuclear cells (PBMCs) from healthy donors (HD) and patients with CLL were analyzed ex vivo for the expression of PD-L1 on the surface of CD19+ lymphocytes. The left histogram shows one representative example and the right graph summarizes the values of HD (n=8) and CLL (n=19) cases. (B) Representative density plot showing the ex vivo CD5/CXCR4 distribution on human PB-derived CLL-cells defining recent stromal-niche emigrants (RSE, green) and long-term circulating (LTC, red) subsets. (C, D) Protein levels of surface PD-L1 (C, n=28) and intracellular c-Myc (D, n=23) were semi-quantified ex vivo in LTCs and RSEs by flow cytometry. (E) Total RNA was prepared from CD19+ lymphocytes isolated from matched-pairs of human CLL peripheral blood (PB) and bone marrow (BM) (n=7). Relative gene expression of CD274 and MYC were quantified by real-time PCR and are depicted as the fold-change (FC) between BM and PB. (F) Surface expression of PD-L1 (left, n=9) and intracellular levels of c-Myc (right, n=7) were analyzed by flow cytometry in CD19+ lymphocytes in matched-pairs of PB and BM from patients with CLL. (G) CLL PBMCs were cultured over 6 days on a confluent layer of the human BM stroma cell line HS-5. CLL-cells were repurified, RNA was prepared, and gene expression of MYC (n=9–10) and CD274 (n=5–10) was quantified by real-time PCR. Relative expression is depicted as the FC in absence (−) or presence (+) of HS-5. (H) CLL PBMCs were cultured in absence/presence of HS-5 for 3 days. Protein levels of surface PD-L1 (left, n=40) and intracellular Myc (right, n=20) were analyzed by flow cytometry. Values are depicted as the FC between presence (+) and absence (−) of stroma. (I) Protein levels of PD-L1 and Myc as determined by flow cytometry (based on the median fluorescence intensity (MdFI)) were subjected to a Pearson’s correlation analysis of CLL-cells cultured in absence (w/o, white circles, n=24) or presence (w/, gray circles, n=22) HS-5 stroma cells for 3 days. (J) CLL PBMCs were analyzed ex vivo for their Myc content in CD19+ lymphocytes by flow cytometry. Samples were gated on the 15% lowest (black) and 15% highest (red) Myc expressing cells (as shown on the upper left). Within these gates, surface levels of PD-L1 were semi-quantified as representatively shown on the lower left (numbers indicate the MdFI) and summarized on the right (n=10). Error bars depict the SEM. *p<0.05, **p<0.01, ***p<0.001; ns, not significant.
Studies on compartmental trafficking suggest that CLL-cells that proliferate within the BM-niche/LN-niche upregulate CD5 and internalize CXCR4, thereby forming a CD5hiCXCR4lo recent stromal-niche emigrant (RSE) subset. After migrating into the PB, CLL-cells eventually turn quiescent and reduce proliferative activity (online supplemental figure 2). Moreover, they downregulate CD5 and renew the CXCR4 expression as CD5loCXCR4hi long-term circulating cells (LTCs).12 Analyzing PD-L1 expression on RSEs and LTCs, respectively, revealed a significantly stronger PD-L1 density on RSEs that had been recently exposed to microenvironmental stimuli (figure 1B, C). As activation of the c-Myc oncoprotein within the CLL microenvironment has been previously reported, while recent data advocate its involvement in the transcriptional and translational control of PD-L1, we compared c-Myc expression in LTCs and RSEs. As anticipated, PD-L1hi RSEs displayed a superior c-Myc expression (figure 1D). The notion that stromal contact might promote both c-Myc and PD-L1 in CLL-cells was further corroborated when analyzing matched-paired PB and BM samples from patients with CLL. In fact, PD-L1/CD274 and c-Myc where found significantly increased on mRNA and protein level in the BM-derived CLL-cells (figure 1E, F). We confirmed those observations when we comparatively assessed PB-derived and BM-derived CLL-cells from the murine Eµ-TCL1 CLL model (online supplemental figure 3). To demonstrate the impact of PD-1/PD-L1 interaction, we stimulated T-cells using anti-CD2/anti-CD3/anti-CD28 beads in presence of autologous CLL-cells while co-applying an anti-PD-L1 blocking antibody. Interfering with the PD-1/PD-L1 binding led to a significantly enhanced upregulation of several T-cell activation markers and cytokines. Physiologically, T-cell receptor (TCR) engagement yields a metabolic switch towards aerobic glycolysis, which is found compromised in CLL-T-cells. Anti-PD-L1 treatment re-established glycolytic activity in CLL-T-cells as indicated by an enhanced mobilization of glucose transporter 1 to the cell surface together with an increased glucose uptake following TCR triggering (online supplemental figure 4A). To test if the anti-PD-L1 blocking approach was potentially affected by a self-inhibiting loop of T-cells expressing both PD-1 and PD-L1, we repeated the experimental setup using magnetic bead-isolated CLL patient-derived T-cells in absence of CLL-cells. To this end, we could not detect any significant effect by blocking the PD-1/PD-L1 axis on T-cells, further corroborating the notion that CLL-cells represent the primary source of PD-L1-mediated T-cell suppression (online supplemental figure 4B).
To mimic the BM-niche, we cultured patient-derived CLL-cells on a monolayer of HS-5 cells, a human BM-derived stromal cell line, and of primary BM-MSCs. CLL-cells co-cultured with stromal cells displayed a pronounced PD-L1/CD274 expression (at the protein and mRNA level), which was paralleled by rising c-Myc (figure 1G, H, online supplemental figure 5). Equivalent results were obtained when co-culturing murine CLL-cells with a murine BM-derived stromal cell line (online supplemental figure 6). The impact of the stromal component was highlighted by the fact that in vitro we could only detect a significant correlation between the c-Myc and PD-L1 levels, when the CLL-cells were cultured on HS-5 cells (figure 1I).
In line with our observations, re-assessing publicly available transcriptome data13 by gene set enrichment analyses revealed a significant enrichment of c-Myc-regulated genes in the BM-derived as compared with PB-derived CLL-cells (online supplemental figure 7). The positive interrelation between PD-L1/CD274 and c-Myc was further confirmed in a larger cohort of PB-derived CLL-cells14 (online supplemental figure 8), which we validated when comparing PD-L1 expression in c-Mychi and c-Myclo circulating CLL-cells (figure 1J).
Stroma cell-educated CLL-cells harbor enhanced T-cell-suppressive properties
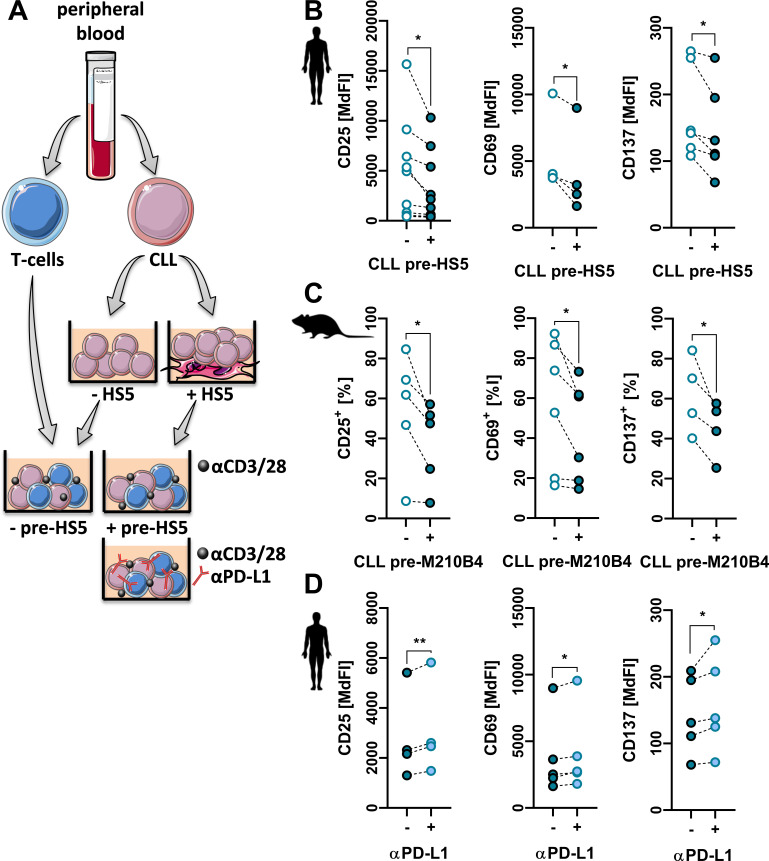
Next, we were interested in whether contact to stromal cells (leading to PD-L1 upregulation) endows CLL-cells with enhanced T-cell suppressive capabilities. To do so, we isolated patient-derived T-cells and CLL-cells. Primary CLL-cells were cultured in presence or absence of HS-5 cells, and on repurification co-cultured with autologous T-cells stimulated by anti-CD2/anti-CD3/anti-CD28 beads (figure 2A). In line with their enhanced PD-L1 expression, suppression of T-cell activation and of T-cell proliferation by HS-5-educated CLL-cells was stronger. At the same time, HS-5-educated CLL-cells exhibited an increased persistence under T-cell-mediated immunological pressure suggesting the stromal promotion of an immunoevasive CLL-phenotype (figure 2B, online supplemental figure 9A). Similarly, inhibition of T-cell activation as well as persistence of CLL-cells was stronger with stroma-educated as compared with non-educated CLL-cells of the murine Eµ-TCL1 model (figure 2C, online supplemental figure 9B). Moreover, blocking PD-L1 in a co-culture of patient-derived CLL T-cells with stroma-educated autologous CLL-cells partially but significantly restored T-cell activation and attenuated CLL-cell persistence (figure 2D, online supplemental figure 9C).
Figure 2.
Stroma-educated chronic lymphocytic leukemia (CLL)-cells harbor enhanced T-cell suppressive properties via programmed cell death ligand 1 (PD-L1). (A) T-cells and CLL-cells were isolated from CLL peripheral blood (PB). CLL-cells were cultured in absence (−HS5) or presence (+HS5) of human BM stroma for 3 days and repurified by magnetic beads. T-cells were then co-cultured with repurified CLL-cells in an autologous setup at a ratio of 1:1 in presence of anti-CD2/anti-CD3/anti-CD28-coated microbeads including an anti-PD-L1 antibody in selected cases for 48 hours. (B) T-cells were then analyzed for their activation based on CD25 (n=10), CD69 (n=4) and CD137 (n=6) surface expression. (C) Similarly, murine CLL-cells isolated from spleens of Eµ-Tcl1 transgenic mice were cultured for 3 days in absence or presence of M2-10B4 stroma cells and, after repurification, co-cultured with autologous lymph node-derived T-cells. T-cell activation was assessed by the frequency of CD25+ (n=5), CD69+ (n=6) and CD137+ (n=4) T-cells. (D) Additionally, human T-cells co-cultured with HS-5 pre-educated CLL-cells were maintained in absence (−) or presence (+) of a PD-L1 blocking antibody and surface levels of CD25, CD69 and CD137 were measured (n=4–5). Error bars depict the SEM. *p<0.05, **p<0.01.
Notch-c-Myc signaling axis controls stroma-mediated PD-L1 induction
Intercellular communication involves cell contact-dependent as well as contact-independent mechanisms. Therefore, we tested promotion of PD-L1 and c-Myc by HS-5-conditioned medium and in co-cultures with a semipermeable membrane separating CLL-cells from HS-5 cells. In fact, cell contact was indispensable for both PD-L1 and c-Myc upregulation (online supplemental figure 10).
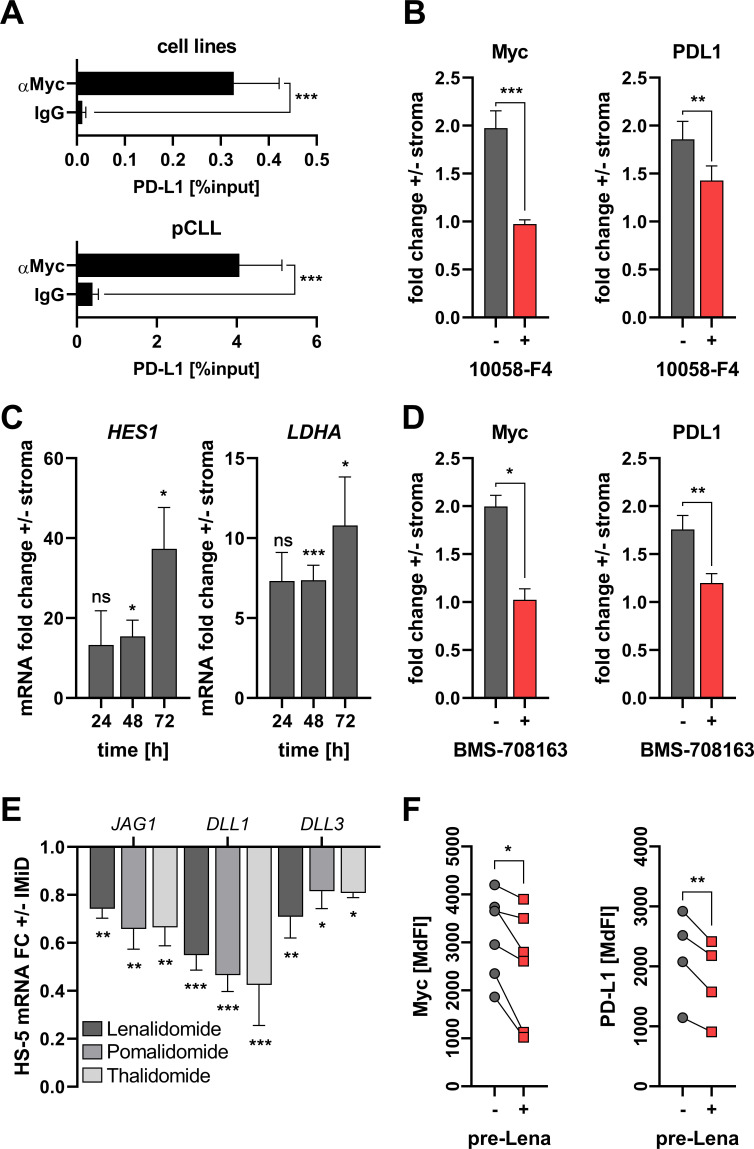
Based on our observations (figure 1C, D) and current literature,10 we reasoned that c-Myc might be directly involved in stroma-mediated PD-L1 upregulation in CLL-cells. Therefore, we tested the occupancy of the PD-L1 promoter region for presence of c-Myc using chromatin immuno-precipitation in both CLL-cell lines and primary CLL-cells. In fact, we found a clear signal for PD-L1 DNA when precipitating with an anti-c-Myc antibody (figure 3A). As anticipated, blocking c-Myc in CLL-cells by 10058-F4 abolished both c-Myc and PD-L1 upregulation on stromal contact (figure 3B).
Figure 3.
Stromal programmed cell death ligand 1 (PD-L1) induction in chronic lymphocytic leukemia (CLL)-cells is mediated by Notch-c-Myc signaling. (A) CLL cell lines (Mec-1/Eheb, top, n=16) and primary human CLL-cells (bottom, n=11) were subjected to chromatin immunoprecipitation using either an anti-Myc antibody (αMyc) or control immunoglobulin (IgG). Precipitated DNA was analyzed for sequences at the PD-L1 promoter using specific primer pairs and quantitative real-time PCR. (B) CLL peripheral blood mononuclear cells (PBMCs) were cultured on stroma (HS-5) for 3 days in absence (gray) or presence (red) of the Myc inhibitor 10058-F4 (50 µM). Protein levels of intracellular Myc (left, n=9) and surface PD-L1 (right, n=12) were analyzed by flow cytometry and are depicted as the fold-change±stroma. (C) Total RNA was extracted from CLL-cells cultured in absence/presence of HS-5 stroma cells after indicated times and quantified for HES1 (n=3–6) and LDHA (n=3–6) gene expression by real-time PCR. Relative gene expression values are depicted as the fold-change±stroma. (D) CLL PBMCs were cultured on stroma (HS-5) for 3 days in absence (gray) or presence (red) of the γ-secretase inhibitor BMS-708163 (8 µM). Protein levels of intracellular Myc (left, n=5) and surface PD-L1 (right, n=11) were analyzed by flow cytometry and are depicted as the fold-change±stroma. (E) The human BM stroma cell line HS-5 was cultured for 24 hours in absence or presence of immunomodulatory drugs (IMiD as indicated, 5 µM each inhibitor). Total RNA was extracted and quantified for the gene expression of Notch ligands JAG1 (n=3–10), DLL1 (n=3–13) and DLL3 (n=3–9). Relative gene expression values are depicted as the fold-change (FC)±IMiD. (F) CLL PBMCs were cultured on HS-5 BM stroma that was either untreated (−) or pretreated (+) for 24 hours with 5 µM lenalidomide (Lena). Protein levels of intracellular Myc (left, n=6) and surface PD-L1 (right, n=4) were analyzed by flow cytometry and are depicted as median fluorescence intensity (MdFI). Error bars depict the SEM. *p<0.05, **p<0.01, ***p<0.001; ns, not significant.
In line with our previous observations that stromal cells activate via cell-to-cell contact the Notch-c-Myc axis (together with PD-L1 expression) in CLL-cells,9 we detected upregulation of both Notch (HES1) and c-Myc (LDHA, CCNB1, HK2) target genes in CLL-cells following stromal crosstalk (figure 3C and online supplemental figure 11). In order to confirm the emerging notion that stroma promotes Notch-c-Myc signaling, which in turn positively regulates PD-L1, we blocked the intracellular Notch pathway using the pharmacological γ-secretase inhibitor BMS-708163. Indeed, BMS-708163 fully abolished the induction of c-Myc and PD-L1 by stromal cells (figure 3D).
Treatment of patient-derived CLL-cells with the recombinant human Notch ligands Jagged-1, Jagged-2, DLL1 and DLL4, respectively lead to a moderate but significant induction of both c-Myc and PD-L1 in all cases additionally confirming the existence of a Notch/c-Myc/PD-L1 axis (online supplemental figure 12).
Immunemodulatory drugs (IMiDs) such as lenalidomide display clinical efficacy in patients with CLL and hold the potential to restore immune dysfunctions. Furthermore, recent studies suggest that lenalidomide suppresses expression of Notch ligands on BM-derived mesenchymal stromal cells.15 Accordingly, treating HS-5 cells with different types of IMiDs in non-cytotoxic dosages (online supplemental figure 13) resulted in a significant downregulation of the tested Notch ligands Jagged 1 (JAG1) and delta-like canonical notch ligand (DLL1) 1 and 3 (figure 3E). Next, we pretreated HS-5 cells with lenalidomide for 24 hours, washed them thoroughly, and co-cultured them with CLL-cells. On co-culture with lenalidomide-pretreated HS-5 cells we documented less c-Myc activation and less PD-L1 upregulation in CLL-cells (figure 3F), thus revealing a potentially novel IMiD-mechanism of interfering with stromal triggering of oncogene-driven PD-L1 expression in CLL.
Notch-c-Myc-facilitated PD-L1 induction requires EZH2
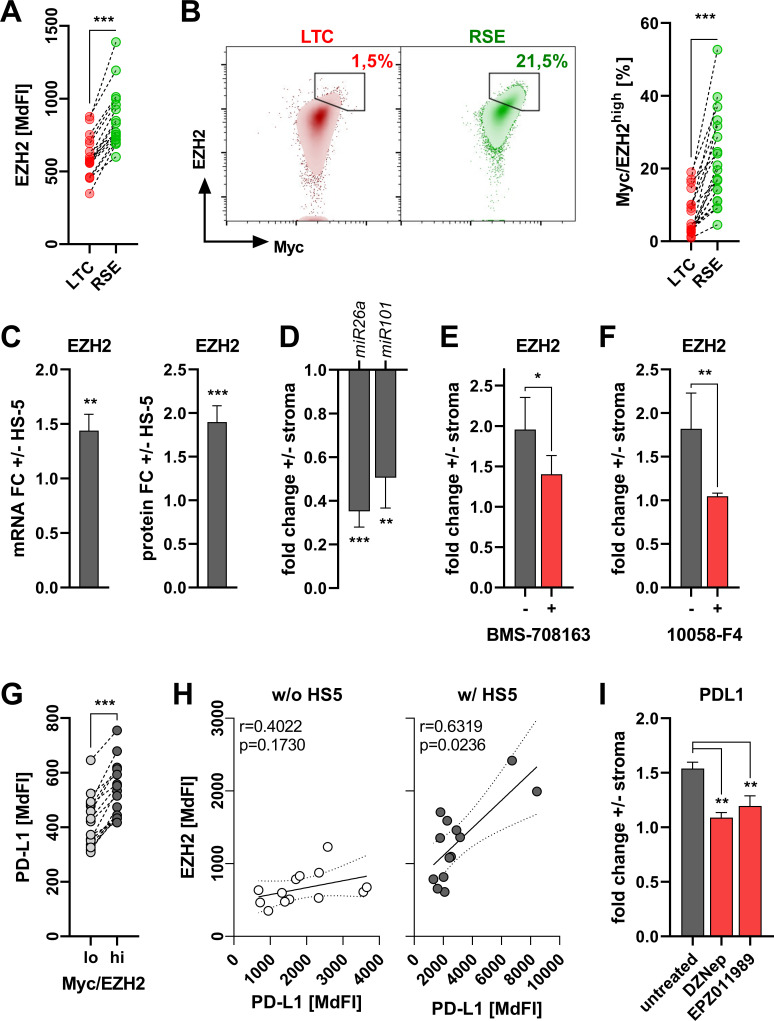
When we further analyzed compartmental differences in oncogenic pathways using publicly available transcriptome data (GSE2102913), we detected an enrichment of target genes of the histone methyltransferase enhancer of zeste homolog 2 (EZH2) within the BM-derived CLL-cells (online supplemental figure 14). We also found an increased EZH2 gene and protein expression in patient CLL-cells from the BM as compared with PB in matched-pair samples (online supplemental figure 15). Remarkably, EZH2 is linked to a more aggressive CLL phenotype,16 is positively regulated by c-Myc17 and correlates with PD-L1 expression in lung cancer.18 Previous work already points towards an EZH2 induction by stromal contact.19 Ex vivo we detected significantly higher EZH2 levels in circulating RSEs as compared with LTCs (figure 4A). Appropriately, RSEs were over-represented in a c-Mychigh/EZH2high subset of CLL-cells (figure 4B). Accordingly, this c-Mychigh/EZH2high population was found increased in BM-derived CLL-cells as compared with PB in ex vivo analyses (online supplemental figure 16). Co-culturing human or murine CLL-cells together with the stromal cell lines increased their EZH2 transcription and translation (figure 4C, online supplemental figure 17). Again, the role of the stromal component was highlighted by the fact that in vitro we could only detect a significant correlation between c-Myc and EZH2, when the CLL-cells were cultured on HS-5 cells (online supplemental figure 18).
Figure 4.
Notch-c-Myc-induced programmed cell death ligand 1 (PD-L1) upregulation is enhancer of zeste homolog 2 (EZH2)-dependent. (A) EZH2 protein levels were determined ex vivo in long-term circulating (LTC) and recent stromal-niche emigrant (RSE) subsets (see figure 1B) of chronic lymphocytic leukemia (CLL) peripheral blood mononuclear cells (PBMCs) (n=17). (B) Frequencies of Myc/EZH2high expressing CD19+ lymphocytes in LTC and RSE subsets as representatively shown on the left were semi-quantified by flow cytometry in 19 human CLL PBMC specimen ex vivo (right). (C) EZH2 mRNA (left, n=8) and protein (right, n=20) level were determined in CLL-cells cultured in absence/presence of HS-5 BM stroma for 3 days by quantitative real-time PCR and flow cytometry, respectively. Values are depicted as the fold-change (FC)±stroma. (D) Total small RNAs were extracted from human CLL-cells cultured in absence/presence of HS-5 BM stroma for 3 days after repurification. Relative abundance of miR26a and miR101 was analyzed by quantitative real-time PCR and is depicted as the fold-change±stroma. (E, F) CLL PBMCs were cultured on HS-5 BM stroma for 3 days in absence (gray) or presence (red) of the γ-secretase inhibitor BMS-708163 (E, n=7, 8 µM) or the Myc inhibitor 10058-F4 (F, n=6, 50 µM). Protein levels of intracellular EZH2 were analyzed by flow cytometry and are depicted as the fold-change±stroma. (G) CLL PBMCs were analyzed ex vivo for the surface expression on CD19+ lymphocytes comparing Myc/EZH2lo (light gray) and Myc/EZH2hi (dark gray) subset based on the median fluorescence intensity (n=15). (H) Protein levels of PD-L1 and EZH2 as determined by flow cytometry (based on the median fluorescence intensity (MdFI)) were subjected to a Pearson’s correlation analysis of CLL-cells cultured in absence (w/o, white circles, n=13) or presence (w/, gray circles, n=13) HS-5 BM stroma cells for 3 days. (I) CLL PBMCs were cultured on HS-5 BM stroma for 3 days in absence (gray) or presence (red) of the EZH2 inhibitors DZNep (10 µM) and EPZ011989 (50 µM). Protein levels of surface PD-L1 were analyzed by flow cytometry and are depicted as the fold-change±stroma. Error bars depict the SEM. *P<0.05, **p<0.01, ***p<0.001.
Previous studies have shown that c-Myc promotes EZH2 expression by inhibiting two EZH2-repressing micro-RNAs miR26a20 and miR101.21 Moreover, EZH2 content was highest in the c-Mychigh population of circulating CLL-cells (online supplemental figure 19). When co-culturing CLL-cells in presence of HS-5 cells, we observed a significant downregulation of both miR26a and miR101 (figure 4D). EZH2 can be very well integrated into the Notch-c-Myc signaling cascade, as both inhibition of Notch (by BMS-708163) or c-Myc (by 10058-F4), diminished EZH2 induction triggered by stromal contact (figure 4E, F).
Finally, PD-L1 expression was superior in c-Mychigh/EZH2high PB CLL-cells (figure 4G). Similar to our previous findings (figure 1I, online supplemental figure 18), we could only detect a significant correlation between EZH2 and PD-L1 in in vitro cultured CLL-cells, when CLL-cells were maintained on a stromal monolayer (figure 4H). Ultimately, pharmacological interference with EZH2 in CLL-cells using DZNep or EPZ011989 prevented stroma-mediated PD-L1 induction (figure 4I, online supplemental figure 20). Notably, EZH2 was recently shown to be capable to augment c-Myc transcriptional activity regardless of its methyl transferase activity.22 In fact, analyzing public data (GSE11577222) and datasets from the ENCODE database we also found a clear signal of EZH2 in the PD-L1 promoter region but without the characteristic trimethylated lysine 27 on histone 3 (H3K27me3) as well as signals for c-Myc in the same areas (online supplemental figure 21) suggesting a cooperative regulation of PD-L1 expression by c-Myc and EZH2 that does not involve the methyltransferase activity of the latter one and which warrants further investigations.
Taken together, we show for the first time in the context of CLL that the stromal niche contributes to a PD-L1high immunoevasive phenotype by triggering an oncogenic Notch-c-Myc-EZH2 loop.
Discussion
The immune system plays a key role in recognizing and eradicating malignant cells. Furthermore, immune-based approaches such as chimeric antigen receptor-carrying T-cells have heralded a new era in cancer treatment. However, tumor cells employ a plethora of mechanisms to evade immunosurveillance including expression of immune checkpoint inhibitors. PD-L1 represents a bona fide example for inhibitory molecules and interferes with function of innate and adaptive immunity cells by binding to its cognate receptor PD-1. Blocking the PD-L1/PD-1 interaction has been successful in preclinical CLL models but has failed to produce meaningful results in clinical studies.3 4 6 Therefore, it is inevitable to better understand mechanisms that control PD-L1 expression and that might contribute to primary and/or secondary resistance towards immunotherapies.
CLL-cells are characterized by a recurrent close interaction with their stromal niches within the BM and LNs.8 9 Here, we show for the first time that the stromal compartment provides nurturing signals and protection from chemotherapy-induced cell death and promotes a PD-L1high CLL-cell phenotype with superior T-cell regulatory properties. Interfering with the crosstalk between CLL-cells and the stroma might assist in preventing the emergence of immune escape variants, thus further improving the efficacy of immune based therapies. We identified binding of stromal Notch ligands on their respective receptors on CLL-cells as the mechanism responsible for PD-L1 upregulation. In fact, developing agents that interfere with the ligand-receptor binding and/or the Notch downstream signaling has been a great impetus, particularly for Notch mutated patients with CLL. As of to date, none of the Notch-targeted approaches has been clinically approved and several roadblocks associated with, for example, off-target toxicities need to be overcome. Interestingly, we observed that IMiDs that exert pleiotropic (partially not fully understood) effects and that are already preclinically and clinically exploited23 in CLL downmodulate the expression of Notch ligands on stroma cells.
Downstream of Notch-signaling c-Myc and EZH2 facilitated the upregulation of PD-L1 on CLL-cells. One of the common features of Notch, c-Myc and EZH2 is their (context-dependent) function as oncogenes.16 22 An enhanced activity of their pathways is linked to a more aggressive disease biology in CLL. This finding is in line with the current concept of tumor-intrinsic signaling playing a key role in regulating a tolerogenic tumor microenvironment and tumor immune escape.24 Cancer is caused by oncogenic mutations that lead to uncontrolled proliferation and consecutively to progressive disease. A critical step for developing malignancies is the escape from the immunological control. Emerging evidence suggests that aberrant oncogenic signaling in cancer cells may limit host immunity by among others regulating immune checkpoints. Indeed, it could be demonstrated that targeting oncogenic signaling can foster the efficacy of immunotherapies in preclinical models and clinical studies.24 In addition to this cell-autonomous process, we describe how stromal cells contribute oncogenic signals in CLL-cells that in turn mediate immune escape. In this case, three oncogenic pathways are triggered by stromal contact cooperatively leading to a PD-L1 upregulation. Furthermore, they represent potential targetable sites within the regulatory framework of PD-L1 and are currently investigated, although not primarily within the immunotherapeutical context, which we present here.22 25
Taken together, our study reveals that the stromal microenvironment controls PD-L1 expression in CLL-cells by activating oncogenic Notch-c-Myc-EZH2 signaling. Interfering with those cooperating pathways could enhance the efficacy of immunotherapy against CLL and needs to be further investigated.
jitc-2020-001889supp002.pdf (1.5MB, pdf)
Footnotes
Contributors: MB designed the study, performed research, analyzed data and wrote the manuscript. HB, JL, EC, NP, KM and MB-H performed research. HB, SV, TZ, MH, WH, PG, KS and AM provided essential intellectual input and feedback. TZ and PG provided essential patient material. DM designed the study, analyzed data and wrote the manuscript.
Funding: MB and DM were supported by a Gilead research grant and the Erich und Gertrud Roggenbuck Stiftung. DM was supported by the Deutsche Forschungsgemeinschaft (DFG, MO 1939/4-1). MH was supported by Deutsche Krebshilfe (70172788). TZ was supported by the Swiss Cancer Research Foundation (KFS-4439-02-2018), the 'Monique-Dornonville de la Cour' Stiftung, the CRC and KFSP (Hematology Oncology). PG was supported by Fondazione AIRC under 5 per Mille 2018 (21198).
Competing interests: HB was supported with a research grant from Celgene. MB and DM were supported with a research grant from Gilead. Beyond this, the authors have no potential conflict of interest to declare.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Blood samples from untreated patients and healthy donors were obtained on patients’ informed consent in accordance with the Declaration of Helsinki (approval numbers: 291_14B, 289_16B, 31/13.09.2012, 2009-0062/1). Mesenchymal stromal cells were isolated from iliac crest BM aspirates taken from healthy donors (approval number: 200_12). All experiments involving human or animal material were performed on approval and according to local regulations.
References
- 1. Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med 2005;352:804–15. 10.1056/NEJMra041720 [DOI] [PubMed] [Google Scholar]
- 2. Forconi F, Moss P. Perturbation of the normal immune system in patients with CLL. Blood 2015;126:573–81. 10.1182/blood-2015-03-567388 [DOI] [PubMed] [Google Scholar]
- 3. Brusa D, Serra S, Coscia M, et al. The PD-1/PD-L1 axis contributes to T-cell dysfunction in chronic lymphocytic leukemia. Haematologica 2013;98:953–63. 10.3324/haematol.2012.077537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McClanahan F, Hanna B, Miller S, et al. Pd-L1 checkpoint blockade prevents immune dysfunction and leukemia development in a mouse model of chronic lymphocytic leukemia. Blood 2015;126:203–11. 10.1182/blood-2015-01-622936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shen X, Zhang L, Li J, et al. Recent findings in the regulation of programmed death ligand 1 expression. Front Immunol 2019;10. 10.3389/fimmu.2019.01337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kondo K, Shaim H, Thompson PA, et al. Ibrutinib modulates the immunosuppressive CLL microenvironment through STAT3-mediated suppression of regulatory B-cell function and inhibition of the PD-1/PD-L1 pathway. Leukemia 2018;32:960–70. 10.1038/leu.2017.304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lewinsky H, Barak AF, Huber V, et al. Cd84 regulates PD-1/PD-L1 expression and function in chronic lymphocytic leukemia. J Clin Invest 2018;128:5465–78. 10.1172/JCI96610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kurtova AV, Balakrishnan K, Chen R, et al. Diverse marrow stromal cells protect CLL cells from spontaneous and drug-induced apoptosis: development of a reliable and reproducible system to assess stromal cell adhesion-mediated drug resistance. Blood 2009;114:4441–50. 10.1182/blood-2009-07-233718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jitschin R, Braun M, Qorraj M, et al. Stromal cell-mediated glycolytic switch in CLL cells involves Notch-c-Myc signaling. Blood 2015;125:3432–6. 10.1182/blood-2014-10-607036 [DOI] [PubMed] [Google Scholar]
- 10. Casey SC, Tong L, Li Y, et al. Myc regulates the antitumor immune response through CD47 and PD-L1. Science 2016;352:227–31. 10.1126/science.aac9935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Krampera M, Galipeau J, Shi Y, et al. Immunological characterization of multipotent mesenchymal stromal cells--The International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy 2013;15:1054–61. 10.1016/j.jcyt.2013.02.010 [DOI] [PubMed] [Google Scholar]
- 12. Calissano C, Damle RN, Marsilio S, et al. Intraclonal complexity in chronic lymphocytic leukemia: fractions enriched in recently born/divided and older/quiescent cells. Mol Med 2011;17:1374–82. 10.2119/molmed.2011.00360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Herishanu Y, Pérez-Galán P, Liu D, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood 2011;117:563–74. 10.1182/blood-2010-05-284984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lu J, Böttcher M, Walther T, et al. Energy metabolism is co-determined by genetic variants in chronic lymphocytic leukemia and influences drug sensitivity. Haematologica 2019;104:1830–40. 10.3324/haematol.2018.203067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guo J, Fei C, Zhao Y, et al. Lenalidomide restores the osteogenic differentiation of bone marrow mesenchymal stem cells from multiple myeloma patients via deactivating Notch signaling pathway. Oncotarget 2017;8:55405–21. 10.18632/oncotarget.19265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Papakonstantinou N, Ntoufa S, Chartomatsidou E, et al. The histone methyltransferase EZH2 as a novel prosurvival factor in clinically aggressive chronic lymphocytic leukemia. Oncotarget 2016;7:35946–59. 10.18632/oncotarget.9371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koh CM, Iwata T, Zheng Q, et al. Myc enforces overexpression of EZH2 in early prostatic neoplasia via transcriptional and post-transcriptional mechanisms. Oncotarget 2011;2:669–83. 10.18632/oncotarget.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Toyokawa G, Takada K, Tagawa T, et al. A positive correlation between the EZH2 and PD-L1 expression in resected lung adenocarcinomas. Ann Thorac Surg 2019;107:393–400. 10.1016/j.athoracsur.2018.08.056 [DOI] [PubMed] [Google Scholar]
- 19. Chartomatsidou E, Ntoufa S, Kotta K, et al. Inhibition of EZH2 and immune signaling exerts synergistic antitumor effects in chronic lymphocytic leukemia. Blood Adv 2019;3:1891–6. 10.1182/bloodadvances.2018030262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sander S, Bullinger L, Klapproth K, et al. Myc stimulates EZH2 expression by repression of its negative regulator miR-26a. Blood 2008;112:4202–12. 10.1182/blood-2008-03-147645 [DOI] [PubMed] [Google Scholar]
- 21. Yan J, Ng S-B, Tay JL-S, et al. Ezh2 overexpression in natural killer/T-cell lymphoma confers growth advantage independently of histone methyltransferase activity. Blood 2013;121:4512–20. 10.1182/blood-2012-08-450494 [DOI] [PubMed] [Google Scholar]
- 22. Kosalai ST, Morsy MHA, Papakonstantinou N, et al. Ezh2 upregulates the PI3K/Akt pathway through IGF1R and Myc in clinically aggressive chronic lymphocytic leukaemia. Epigenetics 2019;14:1125–40. 10.1080/15592294.2019.1633867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen CI, Paul H, Snitzler S, et al. A phase 2 study of lenalidomide and dexamethasone in previously untreated patients with chronic lymphocytic leukemia (CLL). Leuk Lymphoma 2019;60:980–9. 10.1080/10428194.2018.1508669 [DOI] [PubMed] [Google Scholar]
- 24. Kobayashi Y, Lim S-O, Yamaguchi H. Oncogenic signaling pathways associated with immune evasion and resistance to immune checkpoint inhibitors in cancer. Semin Cancer Biol 2020;65:51–64. 10.1016/j.semcancer.2019.11.011 [DOI] [PubMed] [Google Scholar]
- 25. Del Papa B, Baldoni S, Dorillo E, et al. Decreased Notch1 activation correlates with response to ibrutinib in chronic lymphocytic leukemia. Clin Cancer Res 2019;25:7540–53. 10.1158/1078-0432.CCR-19-1009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2020-001889supp001.pdf (2.1MB, pdf)
jitc-2020-001889supp002.pdf (1.5MB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.