Abstract
Neuromyelitis optica spectrum disorder (NMOSD) is an uncommon antibody-mediated disease of the central nervous system, often associated with aquaporin-4 antibodies (AQP4-Ab). NMOSD may present as a subacute myelopathy, progressing over days with MRI revealing a contiguous inflammatory lesion of the spinal cord, ≥3 vertebral segments, a longitudinally extensive transverse myelitis. We describe an unusual paraneoplastic form of AQP4-Ab NMOSD that developed in a patient with an advanced diffuse large B-cell lymphoma. The patient had an unusual hyperacute onset, reaching a clinical nadir within hours.
Keywords: haematology (drugs and medicines), neurology (drugs and medicines), radiology
Background
Neuromyelitis optica spectrum disorder (NMOSD) with aquaporin-4 antibodies (AQP4-Ab) cause a range of neurological problems, including optic neuritis, area postrema, acute brainstem and thalamic or hypothalamic syndromes but a longitudinally extensive transverse myeliti (LETM) is most specific.1–3 There is a female predominance; median age at presentation is 39 years.3 It is usually an idiopathic autoimmune condition, but rare instances of paraneoplastic form of AQP4-Ab NMOSD are described.4 Most paraneoplastic syndromes associated with solid tumours develop prior to tumour discovery but lymphoproliferative disorders are more frequently advanced when complicated by paraneoplastic syndromes.5–10 If a LETM develops in patients with advanced lymphoproliferative disorders, investigations should include AQP4-Ab, as the differential diagnosis of LETM is wide and AQP4-Ab are pathogenic and a highly specific diagnostic biomarker for NMOSD.1–3 It is important to be aware that AQP4-Ab can develop in patients with lymphoproliferative disorders even though they may have received rituximab as part of their chemotherapy.
Case presentation
A 55-year-old man with diffuse large B-cell lymphoma (DLBCL) presented with a complete transverse cord syndrome that developed over a few hours. He presented with back pain and had no flicker of movement in his legs, an atonic bladder requiring urinary catheter and complete loss of all sensation with a sensory loss to T4. He was areflexic in his lower limbs, with mute plantars.
He was alert with no meningism, his cranial and upper limb examination were normal, in particular, visual acuities were 6/6 bilaterally with normal pupillary light responses and colour vision.
He was febrile (38°C) with rigours, tachycardic, but normotensive. He had no rash. He had some mild tenderness on abdominal examination.
He received broad-spectrum antibiotics and acyclovir.
DLBCL was diagnosed 18 months prior to his current presentation. He had been treated with chemotherapy but his lymphoma had relapsed and he had begun R-ESHAP chemotherapy (cisplatin, cytarabine, etoposide, methylprednisolone and rituximab). He received two cycles of R-ESHAP chemotherapy 6 weeks and 2 weeks prior to his presentation with his myelitis.
Investigations
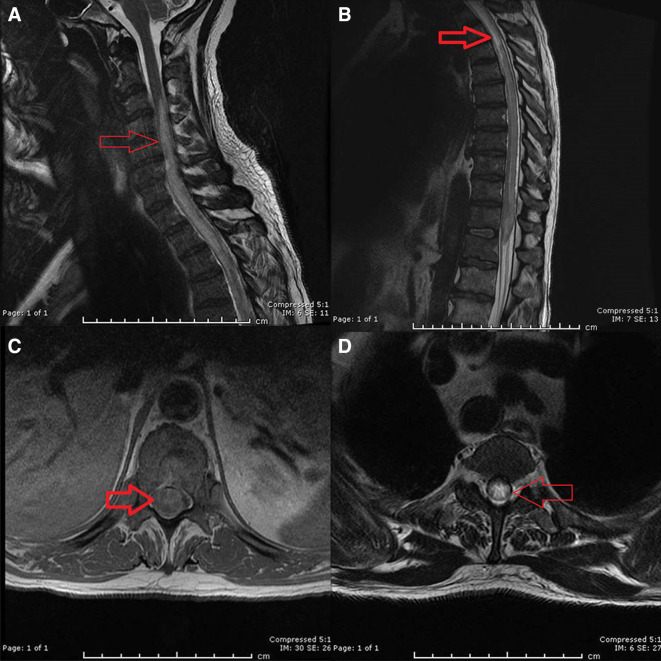
MRI spine revealed a LETM that enhanced from C3 to conus with holospine involvement (see figure 1). There were no lesions on his brain MRI.
Figure 1.
(A) Sagittal T2 MRI of the cervical spine showing the involvement of myelitis (area of increase signal within the cervical spine (red arrow), from the mid C3 spine and downwards). (B) Sagittal T2 MRI spine. The red arrow shows an area with increase signal in the upper thoracic spinal cord which can also be seen in the conus. Also, note heterogenous signal within different areas of the spinal cord. (C) Axial T1 MRI spine showing swelling and contrast enhancement of holocord (red arrow). (D) Axial T2 MRI spine revealing again the holocord involvement of the myelitis (red arrow).
Cerebro-spinal fluid (CSF) showed 200 white cells/μL (80% polymorph, reference range 0–5 cells/µL), no red cells, with raised protein (3.02 g/L, reference range 0.15–0.45 g/L), CSF glucose 3.5 mmol/L and blood glucose 6.0 mmol/L. CSF cytology showed no malignant cells and the flow cytometric immunophenotyping revealed no evidence of lymphoma cells. CSF gram stain was negative. Viral PCR for herpes simplex type 1 and 2, enteroviruses and varicella zoster virus (VZV) were negative. Anti-VZV IgG antibody in CSF was negative. Oligoclonal bands were negative.
Blood cultures were negative and echocardiogram was normal. A CT abdomen and pelvis revealed a small bowel fistula communicating with intra-abdominal DLBCL.
Lyme serology, syphilis and HIV tests were negative. Standard blood markers for vasculitic conditions, B12, folate, copper, caeruloplasmin were normal or negative. Antibodies against myelin oligodendrocyte glycoprotein were negative.
His AQP4-Ab were positive.
An 18F-fluorodeoxyglucose positron emission tomography/CT showed no spinal uptake.
Differential diagnosis
Differential diagnoses are shown in table 1.
Table 1.
Differential diagnoses that may show longitudinal transverse myelitis on MRI
| Differential diagnoses | MRI features |
| NMOSD* AQP4 antibody and MOG antibody–associated disease. |
Central grey or holocord affected axially. Gadolinium enhancement—one-third ring enhancing. Spinal cord swelling. MOG antibody–associated disease involves conus more frequently than AQP4.18 (Note: 10%–15% NMOSD AQP4-Ab present with short lesions.) |
| Spinal cord infarction* | Most often anterior spinal artery territory infarctions—radiologically and clinically sparing the dorsal columns.† Pencil-like T2 hyperintensity over multiple segments, anterior horn cells most vulnerable to ischaemia (axially, ‘owl’s or snake eyes’). No contrast enhancement acutely but sometimes appears subacutely. Restricted diffusion in the first week but not invariably found.18 |
| Malignancy |
Primary intramedullary spinal cord tumour, most commonly an ependymoma in adults. Cord enlargement with variable contrast enhancement. Intramedullary metastasis, are enhancing lesions, sometimes with adjacent oedema resembling a LETM. Fludeoxyglucose uptake on positron emission tomography (FDG) PET-CT. Paraneoplastic*. Acute necrotic myelitis, described by Mancall and Rosales in 1964,19 clinically and radiologically resembles NMOSD. Paraneoplastic myelitis is more commonly progresses over weeks with symmetrical lateral tract LETM sometimes with enhancement. Imaging can be normal.18 |
| Infective and postinfective transverse myelitis. | Can be short or LETM. Enterovirus and poliovirus—central grey matter anterior horn affinity. HIV—sometimes dorsal T2 hyperintensity resembles metabolic. |
| Connective tissue disorders, for example, rheumatoid arthritis, systemic lupus erythematosus and Sjögren’s syndrome | Identical appearance to NMOSD, AQP4-Ab positive in 75% of LETM cases in connective tissue disorders. |
| Sarcoid | Imaging features are variable but include subpial enhancement with central canal enhancement, ‘trident sign’ on axial images, often with leptomeningeal enhancement.18 |
| Behcets | ‘Bagel Sign’ pattern: a central lesion with hypointense core and hyperintense rim with or without contrast enhancement.18 |
| Drugs and toxins, for example, Heroin*, intrathecal methotrexate, radiation and nitrous oxide | Heroin-related myelopathy can resemble NMOSD with hyperacute onset typically after taking heroin following a period of abstinence.30 |
| MS | Lesions usually peripheral, less than half cross sectional area and less than two vertebral lengths rostrocaudally. |
| Dural arteriovenous fistula—direct communication between radiculomedullary artery and vein, frequently in a dural sleeve of a nerve root | Venous hypertension swells the cord with breakdown in the blood brain barrier breaks. An enhancing LETM often with conus involvement; sometimes with a pathognomonic, ‘missing piece sign’. Dorsal dilated perimedullary vessels enhance with contrast and flow voids on T2 images.31 32 |
| Nutritional—B12, copper deficiency | T2 hyperintensity posterior and anterolateral columns. Rarely enhance. Copper deficiency similar with more central cord involvement. |
*Denotes conditions that may present hyperacutely with clinical nadir reached in minutes to hours (<12 hours); remainder subacute with onset over days to 6 weeks or chronic.
†Other ischaemic cord syndromes include hemicord, posterior spinal artery and central cord syndromes as well as mixed patterns.
AQP4-Ab, aquaporin-4 antibodies; LETM, longitudinally extensive transverse myelitis; MOG, myelin oligodendrocyte glycoprotein; MS, multiple sclerosis; NMOSD, neuromyelitis optica spectrum disorder.
Treatment and outcome
Typically, acute treatment of an NMOSD is high-dose steroid and if no improvement is seen within days, plasma exchange should be commenced.
Our patient received high-dose steroids but not plasma exchange. This was in part because of concerns about his intercurrent illness, particularly his sepsis that recurred and uncertainty about the cause of his myelopathy while investigations were pending. When the AQP4-Ab antibody result was known, he was once again septic with intermittent symptoms of bowel obstruction and plasma exchange was not performed.
His legs did not recover. He did not develop any upper limb or brainstem problems that may include respiratory failure, a concern in NMOSD.1
The surgeons advised a conservative treatment for his abdomen.
He commenced mini-BEAM (carmustine, etoposide, cytarabine and melphalan) therapy prior to consideration of a stem cell transplant but sadly this was complicated by further bowel obstruction and neutropaenic sepsis and he died.
Discussion
Our patient reminds us that NMOSD AQP4-Ab may be paraneoplastic, NMOSD can rarely present in a stroke-like fashion with hyperacute onset and that despite rituximab and steroid therapy patients may still develop autoimmune complications.4 11 12
In paraneoplastic disorders, an antigen is shared between the tumour and the nervous system, resulting in an onconeural antibody.13 According to Posner’s classification, NMOSD is a non-classical paraneoplastic syndrome.14 Non-classical paraneoplastic syndromes are sometimes associated with cancer, but more often are not.13 The AQP4-Ab is the onconeural antibody, it is pathogenic, targets a neuronal surface antigen and can respond to immunotherapy.13
Paraneoplastic syndromes tend to precede solid tumour diagnosis in most patients ranging from weeks up to several years.7–10 13 In contrast, patients with lymphoproliferative disorders tend to develop paraneoplastic complications when their lymphoproliferative disease is advanced, as observed in our case.5 6
Lymphoproliferative disorders can be complicated by autoimmune diseases, most commonly haematological, for instance, haemolytic anaemia.15 16 Inherited and somatic genetic mutations within the inhibitory pathways preventing uncontrolled B-cell proliferation are found in both autoimmune and lymphoproliferative diseases.17 Paraneoplastic autoimmunity might, therefore, provide an alternative explanation to the appearance of an AQP4-Ab in our patient.
Our case highlights that myelopathy in NMOSD can rarely present hyperacutely with back pain; features more commonly observed with spinal stroke.11 A complete transverse cord syndrome is, however, uncommon in cord infarction.18 Differences in CSF and imaging also help distinguish spinal stroke from NMOSD presenting hyperacutely11 18 (see table 1).
Paraneoplastic myelitis generally has a far more indolent onset although the necrotising paraneoplastic myelitis described by Mancall and Rosales in 1964 may also present hyperacutely. Its relationship to AQP4-Ab status is uncertain.18 19
Our patient developed NMOSD despite receiving rituximab for treatment of his DLBCL. Rituximab, a B-cell depleting monoclonal antibody (anti-CD20), has been used in NMOSD, reducing relapse rates by up to 88.2%.3 20
Patients with NMOSD can relapse shortly after beginning rituximab therapy, reflecting inadequate time for immunosuppressive effects to develop, although circulating CD20+ B cells undergo rapid depletion in the peripheral blood by 24–72 hours and remain depleted for at least 2–3 months, following administration of rituximab.12 21 22 No specific B-cell markers were measured in our patient although he was established on rituximab, receiving two cycles 6 weeks and 2 weeks prior to onset of his myelitis.
Some treatment regimens measure CD19 and/or CD27 markers to help guide the timing of repeat rituximab infusions.22 Despite adequate suppression of these B-cell markers, patients may still suffer relapses while on rituximab.
Reasons may include the lack of CD20 antigen on plasmablasts and plasma cells that continue to produce the AQP4 antibodies.23 24 There can be a rebound cytokine release following rituximab or emergence of neutralising anti–human chimeric antibody or a down modulation of CD20 on reconstituted memory B-cells; all factors potentially modifying the pharmacodynamic effects of rituximab.25–27 Some literature implicates rituximab in paradoxically provoking autoimmune disease, since a reconstitution of B-cells follows their depletion and a new autoimmune disease might therefore develop, a similar phenomenon can follow treatment with alemtuzamab.28 29
We do not know which of these mechanisms was responsible in our patient; they remain speculations. It is important, however, that as clinicians we remain aware that treatment with rituximab does not preclude an autoimmune condition or in our case a non-classical paraneoplastic syndrome from emerging with devastating consequences.
Learning points.
Neuromyelitis optica spectrum disorder (NMOSD) can rarely be a paraneoplastic disorder.
NMOSD myelitis can rarely present hyperacutely and with back pain more typical of spinal stroke.
Paraneoplastic disorders in lymphoproliferative disease develop with late advanced disease unlike paraneoplastic disorders seen with solid tumours that tend to precede cancer diagnosis.
Patients remain at risk of developing autoimmune neurological complications even after treatment with rituximab.
Footnotes
Contributors: MG: participated directly in the patient’s clinical care, and the construction, image formatting and editing of this manuscript. RW: the patient was under his care as he is a consultant neurologist. He participated in the construction and editing of this manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Wingerchuk DM, Lennon VA, Lucchinetti CF, et al. The spectrum of neuromyelitis optica. Lancet Neurol 2007;6:805–15. 10.1016/S1474-4422(07)70216-8 [DOI] [PubMed] [Google Scholar]
- 2. Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015;85:177–89. 10.1212/WNL.0000000000001729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huda S, Whittam D, Bhojak M, et al. Neuromyelitis optica spectrum disorders. Clin Med 2019;19:169–76. 10.7861/clinmedicine.19-2-169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sepúlveda M, Sola-Valls N, Escudero D, et al. Clinical profile of patients with paraneoplastic neuromyelitis optica spectrum disorder and aquaporin-4 antibodies. Mult Scler 2018;24:1753–9. 10.1177/1352458517731914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Graus F, Ariño H, Dalmau J. Paraneoplastic neurological syndromes in Hodgkin and non-Hodgkin lymphomas. Blood 2014;123:3230–8. 10.1182/blood-2014-03-537506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grant R. What the general neurologist needs to know about the paraneoplastic syndromes. Pract Neurol 2002;2:318–27. 10.1046/j.1474-7766.2002.00096.x [DOI] [Google Scholar]
- 7. Lucchinetti CF, Kimmel DW, Lennon VA. Paraneoplastic and oncologic profiles of patients seropositive for type 1 antineuronal nuclear autoantibodies. Neurology 1998;50:652–7. 10.1212/WNL.50.3.652 [DOI] [PubMed] [Google Scholar]
- 8. Peterson K, Rosenblum MK, Kotanides H, et al. Paraneoplastic cerebellar degeneration. I. A clinical analysis of 55 anti-Yo antibody-positive patients. Neurology 1992;42:1931–7. 10.1212/wnl.42.10.1931 [DOI] [PubMed] [Google Scholar]
- 9. Graus F, Keime-Guibert F, Reñe R, et al. Anti-Hu-Associated paraneoplastic encephalomyelitis: analysis of 200 patients. Brain 2001;124:1138–48. 10.1093/brain/124.6.1138 [DOI] [PubMed] [Google Scholar]
- 10. Candler PM, Hart PE, Barnett M, et al. A follow up study of patients with paraneoplastic neurological disease in the United Kingdom. J Neurol Neurosurg Psychiatry 2004;75:1411–5. 10.1136/jnnp.2003.025171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brownlee WJ, Anderson NE. An elderly woman with leg weakness. Pract Neurol 2014;14:119–22. 10.1136/practneurol-2013-000778 [DOI] [PubMed] [Google Scholar]
- 12. Palace J, Leite I, Jacob A. A practical guide to the treatment of neuromyelitis optica. Pract Neurol 2010;10:260–70. [DOI] [PubMed] [Google Scholar]
- 13. Gozzard P, Maddison P. Which antibody and which cancer in which paraneoplastic syndromes? Pract Neurol 2010;10:260–70. 10.1136/jnnp.2010.224105 [DOI] [PubMed] [Google Scholar]
- 14. De Angelis LM, Posner JB. Neurologic complications of cancer. 2nd edn. Oxford: Oxford University Press, 2009: 577–608. [Google Scholar]
- 15. Miller EB. Autoimmunity and lymphoma: a brief review. J Rheum Dis Treat;4:062. [Google Scholar]
- 16. Goldin LR, Landgren O. Autoimmunity and lymphomagenesis. Int J Cancer 2009;124:1497–502. 10.1002/ijc.24141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goodnow CC. Multistep pathogenesis of autoimmune disease. Cell 2007;130:25–35. 10.1016/j.cell.2007.06.033 [DOI] [PubMed] [Google Scholar]
- 18. Mariano R, Flanagan EP, Weinshenker BG, et al. A practical approach to the diagnosis of spinal cord lesions. Pract Neurol 2018;18:187–200. 10.1136/practneurol-2017-001845 [DOI] [PubMed] [Google Scholar]
- 19. Mancall EL, Rosales RK. Necrotizing myelopathy associated with visceral carcinoma. Brain 1964;87:639–56. 10.1093/brain/87.4.639 [DOI] [PubMed] [Google Scholar]
- 20. Kim S-H, Huh S-Y, Lee SJ, et al. A 5-year follow-up of rituximab treatment in patients with neuromyelitis optica spectrum disorder. JAMA Neurol 2013;70:1110–7. 10.1001/jamaneurol.2013.3071 [DOI] [PubMed] [Google Scholar]
- 21. Maloney DG, Liles TM, Czerwinski DK, et al. Phase I clinical trial using escalating single-dose infusion of chimeric anti-CD20 monoclonal antibody (IDEC-C2B8) in patients with recurrent B-cell lymphoma. Blood 1994;84:2457–66. 10.1182/blood.V84.8.2457.2457 [DOI] [PubMed] [Google Scholar]
- 22. Lebrun C, Cohen M, Rosenthal-Allieri MA, et al. Only follow-up of memory B cells helps monitor rituximab administration to patients with neuromyelitis optica spectrum disorders. Neurol Ther 2018;7:373–83. 10.1007/s40120-018-0101-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Edwards JCW, Cambridge G. Prospects for B-cell-targeted therapy in autoimmune disease. Rheumatology 2005;44:151–6. 10.1093/rheumatology/keh446 [DOI] [PubMed] [Google Scholar]
- 24. Hoyer BF, Manz RA, Radbruch A, et al. Long-Lived plasma cells and their contribution to autoimmunity. Ann N Y Acad Sci 2005;1050:124–33. 10.1196/annals.1313.014 [DOI] [PubMed] [Google Scholar]
- 25. Valgardsdottir R, Cattaneo I, Klein C, et al. Human neutrophils mediate trogocytosis rather than phagocytosis of CLL B cells opsonized with anti-CD20 antibodies. Blood 2017;129:2636–44. 10.1182/blood-2016-08-735605 [DOI] [PubMed] [Google Scholar]
- 26. Jones JD, Hamilton BJ, Skopelja S, et al. Induction of interleukin-6 production by rituximab in human B cells. Arthritis Rheumatol 2014;66:2938–46. 10.1002/art.38798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nakashima I, Takahashi T, Cree BAC, et al. Transient increases in anti-aquaporin-4 antibody titers following rituximab treatment in neuromyelitis optica, in association with elevated serum BAFF levels. J Clin Neurosci 2011;18:997–8. 10.1016/j.jocn.2010.12.011 [DOI] [PubMed] [Google Scholar]
- 28. Kersh AE, Feldman RJ. Autoimmune sequelae following rituximab therapy: a review of the literature and potential immunologic mechanisms. J Clin Rheumatol 2018;24:427–35. 10.1097/RHU.0000000000000756 [DOI] [PubMed] [Google Scholar]
- 29. Jones JL, Phuah C-L, Cox AL, et al. Il-21 drives secondary autoimmunity in patients with multiple sclerosis, following therapeutic lymphocyte depletion with alemtuzumab (Campath-1H). J Clin Invest 2009;119:2052–61. 10.1172/JCI37878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sveinsson O, Herrman L, Hietala MA. Heroin-induced acute myelopathy with extreme high levels of CSF glial fibrillar acidic protein indicating a toxic effect on astrocytes. BMJ Case Rep 2017;2017. 10.1136/bcr-2017-219903. [Epub ahead of print: 28 Jun 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Howard RS. Spinal vascular disease: a neglected cause of myelopathy. Pract Neurol 2019;19:184–6. 10.1136/practneurol-2019-002194 [DOI] [PubMed] [Google Scholar]
- 32. Zalewski NL, Rabinstein AA, Brinjikji W, et al. Unique gadolinium enhancement pattern in spinal dural arteriovenous fistulas. JAMA Neurol 2018;75:1542–5. 10.1001/jamaneurol.2018.2605 [DOI] [PMC free article] [PubMed] [Google Scholar]