Abstract
Background
Cognitive dispersion, or inconsistencies in performance across cognitive domains, has been posited as a cost-effective tool to predict conversion to dementia in older adults. However, there is a dearth of studies exploring cognitive dispersion in the oldest-old (>80 years) and its relationship to dementia incidence.
Objective
The main aim of this study was to examine whether higher cognitive dispersion at baseline was associated with dementia incidence within an 8-year follow-up of very old adults, while controlling for established risk factors and suggested protective factors for dementia.
Methods
Participants (n = 468) were from the Origins of Variance in the Old-Old: Octogenarian Twins study, based on the Swedish Twin Registry. Cox regression analyses were performed to assess the association between baseline cognitive dispersion scores and dementia incidence, while controlling for sociodemographic variables, ApoEe4 carrier status, co-morbidities, zygosity and lifestyle engagement scores. An additional model included a composite of average cognitive performance.
Results
Cognitive dispersion and ApoEe4 were significantly associated with dementia diagnosis. These variables remained statistically significant when global cognitive performance was entered into the model. Likelihood ratio tests revealed that cognitive dispersion and cognitive composite scores entered together in the same model was superior to either predictor alone in the full model.
Conclusions
The study underscores the usefulness of cognitive dispersion metrics for dementia prediction in the oldest-old and highlights the influence of ApoEe4 on cognition in very late age. Our findings concur with others suggesting that health and lifestyle factors pose little impact upon cognition in very advanced age.
Keywords: cognitive dispersion, ApoE4, Alzheimer’s disease, dementia risks, dementia prediction, older people
Key points
Cognitive dispersion, a measure of inconsistency across cognitive performance, has gained research attention as a possible marker for cognitive impairment and disease pathology.
Cognitive dispersion in the oldest-old predicts functional disability and cognitive decline, but to date, no study has explored whether it can predict dementia incidence in the oldest-old.
In our cohort of oldest-old, cognitive dispersion and ApoEe4 predicted dementia incidence within an 8-year follow-up.
Cognitive dispersion is a useful adjunct metric for dementia prediction.
Introduction
Alzheimer’s disease (AD) is now accepted to have a protracted nonclinical period whereby elevations in AD-related pathologies are present years before the emergence of mild cognitive symptoms and a later dementia syndrome [1]. Similarly, cognitive changes are detectable years prior to a diagnosis of vascular dementia [2]. There remains little consensus on the best method to capture and quantify these more subtle changes in cognitive performance. Traditionally, in more progressed stages, cognitive impairment has been determined by comparing performance against cut off scores from normative data or reference group means, but these methods show limited sensitivity to the cognitive changes emerging in preclinical AD [3]. Recently, there has been a shift towards monitoring individual variation in cognitive performance either between cognitive domains (cognitive dispersion) or within repeated trials of a neuropsychological test (intra-individual variability) at one or several time points. Here, we will use the term cognitive dispersion to denote all approaches of variation assessment.
In older adults, dispersion estimates have been associated with reduced white matter volumes in frontal and parietal regions and the corpus callosum [see 4 for review], accelerated atrophy in entorhinal and hippocampal regions [5], as well as levels of amyloid beta [6,7] and neurofibrillary tangles [8], across healthy individuals and those with mild cognitive impairment (MCI) and AD dementia. Furthermore, dispersion estimates vary with severity of cognitive impairment [9], and baseline dispersion scores are associated with subjective memory complaints [10] as well as increased risk and shorter conversion times for dementia in longitudinal studies [11,12]. Holtzer et al. [13] demonstrated a significant association between incident dementia and baseline dispersion scores even after adjusting for education, gender, comorbidities and baseline cognitive performance. More recently, other cohort studies have included biological risk factors into their models, finding that dispersion estimates performed comparably with ApoEe4 genotype and hippocampal volume [11] and independently improved model fit compared with cerebrospinal fluid (CSF) analytes [14] in predicting AD. Only one cross-sectional study has explored the influence of protective factors, finding that an engaged lifestyle reduced the likelihood of being classified as MCI but not AD in models including a dispersion index that itself independently predicted AD but not MCI classification [15]. The influence of lifestyle factors, such as engagement in physical and cognitive stimulating activities, alongside cognitive dispersion metrics to predict dementia incidence longitudinally is yet to be examined.
Our understanding of cognitive dispersion in older adults is still vague, given that some studies demonstrate that cognitive dispersion increases with advancing age [16], whereas other studies show a decrease [17]. These heterogenous findings may be a function of study sample age, since participants aged 65–80 years show increased dispersion with age, whereas those older than 80 years at baseline show reduced dispersion towards terminal decline [18].
There is a dearth of studies exploring cognitive dispersion in oldest-old individuals and consequently of its subsequent importance. Cross-sectional analyses found that cognitive dispersion predicted functional disability in an oldest-old sample (>80 years) of nursing home and community-dwelling residents [17] and baseline dispersion scores across domain subscales of a global cognitive measure predicted 18-month cognitive decline from baseline in a centenarian sample [19].
To our knowledge, no study has so far assessed whether cognitive dispersion might be useful in the prediction of incident dementia in the very old. Similarly, findings regarding the influences of biological, health and lifestyle factors upon cognition and dementia incidence in this age group are inconsistent [20]. Therefore, the aims of the current work were to assess the relative predictive value of cognitive dispersion, alongside other dementia risk and protective factors, as well as existing comorbidities, for the development of dementia in an 8-year cohort of the oldest-old who were free of dementia at the time of enrolment.
Methods
Study design, participants and cohort information for The Origins of Variance in the Old-Old: Octogenarian Twins (OCTO-Twin study) is found in Supplementary Material A1. The study received approval from the ethics committee at the Karolinska Institute in Stockholm and from the Swedish Data Inspection Authority in Sweden.
Dementia diagnosis information is shown in Supplementary Material A1. Neuropsychological tests are reported in Supplementary Material A1 and described elsewhere [21]. A dispersion score was created according to previous criteria [13]. Raw scores of each test were z-transformed on the basis of the distribution of scores from the sample and the variability between scores was calculated across the eight tests within the full assessment. A global cognitive composite (GCC) was also created by summing the z-scores of each task and dividing by the number of tasks. See Supplementary Material A1 for formulae.
Statistical analyses
To assess the relationship between cognitive dispersion and subsequent dementia, we performed generalised Cox regression analyses using the Weibull distribution, with study time as the time scale for all analyses. Detailed information on the models building procedures as well as the assessment of model fit is provided in Supplementary Material A1. We excluded participants with missing values in the predictor variables, as well as those with a Mini-Mental State Examination score ≤24, leaving a total sample size of 421 participants.
Results
Baseline characteristics of the full cohort are shown in Table 1. The number of participants receiving a dementia diagnosis within the follow-up period was 87 (20.7%). During the study 382 participants died, of whom 86 developed dementia.
Table 1.
Cohort baseline characteristics
| Full cohort (N = 421) | |
|---|---|
| Age (SD) | 82.25 (2.57) |
| Years of education (SD) | 7.28 (2.24) |
| MMSE (SD) | 27.97 (1.66) |
| BMI (SD) | 24.57 (3.68) |
| Gender (%) | |
| Male | 145 (34.44) |
| Female | 276 (65.56) |
| SES (%) | |
| High | 232 (55.11) |
| Low | 189 (44.89) |
| ApoEe4 (%) | 102 (24.23) |
| Cognitively inactive (%) | 259 (61.52) |
| Physically inactive (%) | 143 (33.97) |
| Stroke ever (%) | 69 (16.39) |
| Diabetes ever (%) | 51 (12.11) |
| High BP ever (%) | 188 (44.66) |
| CES-D (SD) | 7.82 (7.3) (n = 407) |
BMI, body mass index; BP, blood pressure; CES-D, Center for Epidemiologic Studies Depression Scale; MMSE, Mini-Mental State Examination; SES, socioeconomic status.
The model indicated significant positive associations between baseline cognitive dispersion scores and ApoEe4 carrier status with dementia diagnosis. No other factors contributed a significant association with this outcome (Table 2). The estimated log hazards changed with increasing cognitive dispersion and for each time point (Figure 1). For further ease of interpretation, Figure 2 shows the predicted risk for cognitive dispersion for each year in the study.
Table 2.
Regression coefficients derived from generalised Cox regression for final model
| Variable | Coefficient (SE) | P-Value |
|---|---|---|
| Baseline Hazard 1 | −11.20 (3.95) | 0.01 |
| Baseline Hazard 2 | −7.88 (3.82) | 0.04 |
| Baseline Hazard 3 | −7.595 (3.83) | 0.05 |
| NPH (cogd, time):1 | −1.597 (1.93) | 0.41 |
| NPH (cogd, time):2 | 2.53 (0.70) | <0.001 |
| NPH (cogd, time):3 | −3.28 (1.03) | 0.002 |
| NPH (cogd, time):4 | 2.04 (0.97) | 0.04 |
| Cognitively inactive (yes vs. no) | 0.29 (0.23) | 0.21 |
| Physically inactive (yes vs. no) | −0.10 (0.24) | 0.68 |
| BMI | 0.02 (0.03) | 0.55 |
| SES (Low vs. High) | 0.15 (0.24) | 0.52 |
| Gender (Female vs. Male) | −0.33 (0.23) | 0.15 |
| Years of education | −0.03 (0.06) | 0.64 |
| Age at baseline | 0.06 (0.05) | 0.19 |
| Stroke ever (yes vs. no) | 0.02 (0.32) | 0.96 |
| High BP ever (yes vs. no) | −0.04 (0.23) | 0.86 |
| Diabetes ever (yes vs. no) | 0.34 (0.31) | 0.27 |
| Zygosity (yes vs. no) | −0.12 (0.22) | 0.59 |
| ApoEe4 (yes vs. no) | 0.62 (0.24) | 0.01 |
Note: NPH- nonproportional hazards; cogd – cognitive dispersion; BMI – body mass index; SES – social economic status; cogd was modelled with an NPH spline with one knot positioned at 4 (study time). Statistically significant values are shown in bold.
Figure 1.
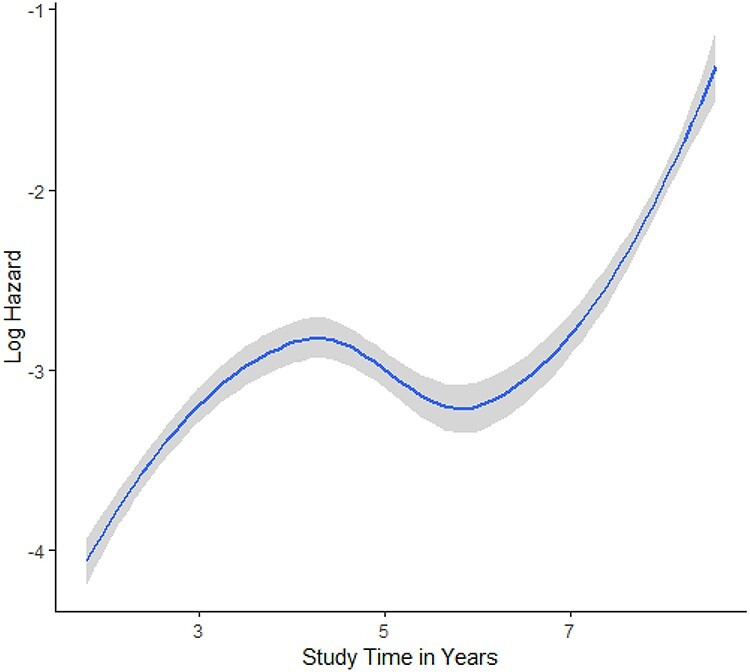
Estimated log hazards over time from full model.
Figure 2.
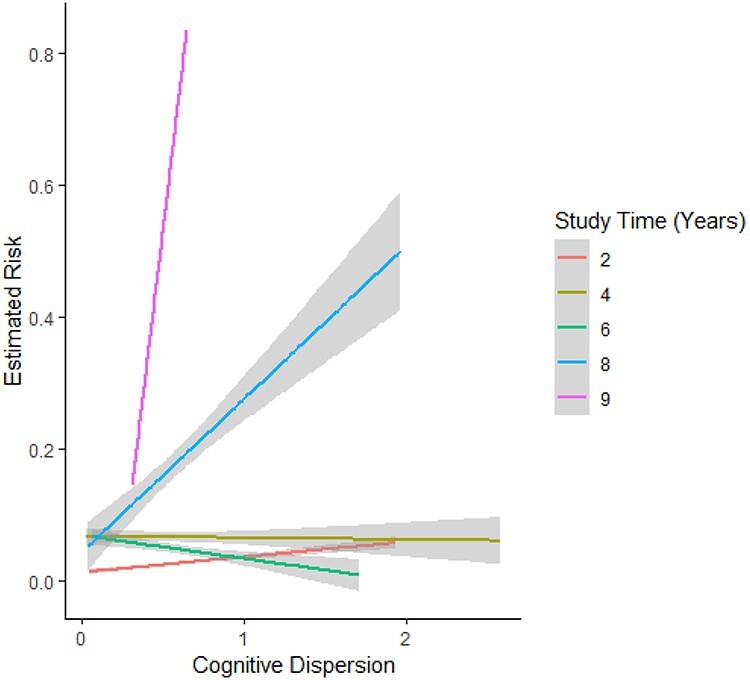
Predicted risk for cognitive dispersion for each year in the study from full model.
Additional analyses revealed that cognitive dispersion and ApoEe4 remained significantly associated with dementia diagnosis, even after entering the GCC into the model (Table 3). Predicted risk over time by cognitive dispersion values are shown in Supplementary Figure A1. The different models (a) with only cognitive dispersion, (b) with only GCC and (c) with both cognitive dispersion and GCC were compared. Information criteria for separate models are shown in Supplementary Table A1. The Akaike information criterion and Bayesian information criterion values were slightly lower for the GCC-only model compared with the cognitive dispersion-only model. However, likelihood ratio tests revealed that cognitive dispersion and GCC entered together in the same model was superior to either predictor entered into the full model alone (all P < 0.001). The correlation between GCC scores and cognitive dispersion indices was small (r = −0.25).
Table 3.
Regression coefficients derived from generalised Cox regression in OCTO (including the global composite score)
| Variable | Coefficient (SE) | P Value |
|---|---|---|
| Baseline Hazard 1 | −9.45 (4.04) | 0.02 |
| Baseline Hazard 2 | −6.09 (3.91) | 0.12 |
| Baseline Hazard 3 | −5.69 (3.93) | 0.15 |
| NPH (cogd, time):1 | −1.78 (1.96) | 0.36 |
| NPH (cogd, time):2 | 2.37 (0.72) | 0.001 |
| NPH (cogd, time):3 | −3.54 (1.05) | <0.001 |
| NPH (cogd, time):4 | 1.63 (0.98) | 0.096 |
| Cognitively inactive (yes vs. no) | 0.14 (0.24) | 0.56 |
| Physically inactive (yes vs. no) | −0.14 (0.24) | 0.57 |
| BMI | 0.01 (0.03) | 0.78 |
| SES (Low vs. High) | 0.07 (0.24) | 0.77 |
| Gender (Female vs. Male) | −0.30 (0.23) | 0.195 |
| Years of education | 0.03 (0.06) | 0.65 |
| Age at baseline | 0.04 (0.05) | 0.41 |
| Stroke ever (yes vs. no) | −0.02 (0.31) | 0.96 |
| High BP ever (yes vs. no) | −0.04 (0.23) | 0.87 |
| Diabetes ever (yes vs. no) | 0.36 (0.31) | 0.25 |
| Zygosity (yes vs. no) | −0.01 (0.23) | 0.97 |
| ApoEe4 (yes vs. no) | 0.47 (0.24) | 0.05 |
| Global Composite Score | −0.64 (0.197) | 0.001 |
Note: NPH- nonproportional hazards; cogd – cognitive dispersion; BMI – body mass index; SES – social economic status; cogd was modelled with a NPH spline with one knot positioned at 4 (study time). Statistically significant values are shown in bold.
A sensitivity analysis, with Center for Epidemiologic Studies Depression Scale (CES-D) scores entered into the model (n = 407 due to missing CES-D data), revealed that the significant positive associations between baseline cognitive dispersion scores and ApoEe4 carrier status with dementia diagnosis persisted (shown in Supplementary Table A2).
Discussion
In the present study, we investigated the predictive value of cognitive dispersion scores, in relation to established AD risk factors, comorbidities and protective lifestyle indicators, for the incidence of dementia in a longitudinal cohort of older adults who were free of dementia symptoms at baseline.
Our main finding is that cognitive dispersion, along with ApoEe4, was the strongest predictor of receiving a dementia diagnosis within 8 years of follow-up. To our knowledge, this is the first longitudinal study of the oldest-old that examined the role of cognitive dispersion as a predictor of dementia risk.
Our results indicate that in very advanced aged, at least, cognitive dispersion and ApoEe4 genotype are more valuable predictors of incident dementia than other AD risk factors, medical illness and lifestyle habits, such as engagement with physical exercise and cognitively stimulating activities. In a slightly younger cohort [mean age (SD), 78.6 (5.3)], cognitive dispersion was significantly associated with incident dementia, after controlling for a range of variables similar to our own such as gender, education and a medical illness index [13]. In another younger cohort [mean age (SD), 73.66 (7.01)], cognitive dispersion at baseline predicted incident MCI and AD and higher cognitive dispersion was associated with shorter conversion times to these clinical categories, even after controlling for ApoEe4 and hippocampal atrophy [11]. In the same cohort, cognitive dispersion independently improved the prediction model fit for incident AD when compared against other CSF analytes [14]. Our model did not include imaging or CSF biomarkers as these were not available as part of the study procedures. Compared with neural imaging and CSF ascertainment, genotyping from serum or saliva samples is comparatively less invasive procedure and does not require repeated evaluations, making it possibly more feasible in oldest-old age groups. A previous analysis using the same cohort here found that ApoEe4 status predicted level of memory performance and steeper rates in memory decline when controlling for age, sex, education and medical illness, but that these associations disappeared when dementia diagnosis was entered as a covariate, indicating that the negative effect of ApoEe4 on cognition is strongly related to dementia incidence [22]. We found that our cognitive dispersion metric remained significantly associated with dementia diagnosis in our model even when including ApoeEe4 status, suggesting that a cognitive dispersion metric alone might be sufficient in identifying individuals at greater risk for the development of dementia in this age group. Nonetheless, much like Praetorius et al.’s [22] study, our finding that ApoEe4 carrier status is predictive of dementia in our oldest-old cohort contradicts several studies proposing that ApoEe4 carrier risk for dementia decreases with very advanced age [23] and suggests that its relative importance in dementia prediction models for this age group should be re-assessed.
Age at baseline, gender, education, socioeconomic status and several comorbidities, such as vascular (e.g. stroke, hypertension) or metabolic abnormalities (diabetes), did not influence the outcome. The age range for the cohort is naturally limited relative to younger cohorts and this might have restricted the predictive value of these variables in our study. Although the underlying processes are not fully characterised, associations of health-related risk factors with dementia do appear to differ according to life stage, and these may pose their principal influences on the development of later-life dementia during mid-life [24].
We also did not find an association between lifestyle indicators, such as engagement with physical exercise and cognitively stimulating activities, with dementia risk. This agrees with previous research conducted in the oldest-old [25], although some other studies have reported that participation in cognitively stimulating activities and physical exercise in very old age is found to be associated with lower dementia risk at this life stage [20,26]. Our lifestyle indicators were based on simple yes/no responses to a simple question in each category and possess only face validity. A more detailed lifestyle interview may have found some protective associations with our outcome. Alternatively, our results might reflect the possibility that underlying neural pathologies may have accumulated to such an extent in the participants or that other unknown resilience factors associated with extreme longevity may have mitigated the predictive value of lifestyle factors at this late age.
We computed a GCC to estimate average cognitive performance at baseline. When these latter scores were entered into the model, both cognitive dispersion and ApoEe4 remained significantly associated with dementia diagnosis, suggesting that cognitive dispersion metrics may possess incremental validity over mean test performance in predicting future cognitive impairment in the very old. Sensitivity analyses revealed that when both cognitive dispersion scores and GCC scores were entered into the full model, this combination performed better than models in which either predictor was entered alone. We also found that cognitive dispersion scores correlated poorly with GCC scores, suggesting that these indices might capture distinct phenomena of ageing or cognitive decline. Recently, there has been a strong interest in the use of global composite measures to detect and track cognitive decline in clinical trials [27]. Our findings imply that cognitive dispersion, which itself is a composite of average variations in cognitive performance, might represent an alternative or adjunct measure more suitable for these clinical research efforts.
This study in not without shortcomings. Although the dementia diagnosis did make use of clinical criteria, we did not differentiate between dementia types (e.g. AD, vascular dementia or mixed) and thus we cannot comment on whether our dispersion index is less or more sensitive to dementia with distinct pathological and cognitive profiles, given the set of neuropsychological tests available here. Holtzer et al. [13] found that dispersion was sensitive to both AD and vascular dementia subtypes in their sample, suggesting that dispersion may index the sum of disease processes to various brain regions or networks rather than a specific isolated region. Clinically, this may mean that dispersion, at a baseline assessment at least, is useful as a screening tool to differentiate healthy individuals from those with pending dementia diagnoses, but limited in terms of its specificity or ability to differentiate between dementia subgroups. Future work including other dementia subtypes and pathologies is needed to realise the metric’s differential diagnostic potential. Better characterisation of oldest-old cohorts, at the biological and cognitive level, could become possible with the advent of existing middle-older-adult cohorts (above 60 years). This will also offer the opportunity to explore lifelong cognitive, medical and lifestyle factors on dementia development in very advanced age. Finally, the study did not consider death as a competing risk within our models. Cognitive change in later adulthood may be driven by an individual’s proximity to their death and is observed through a steeper decline in cognitive abilities a few years before this event [28]. Greater baseline dispersion scores were associated with increased risk of death 5–8 years later in one study of cognitively normal community-dwelling elders (60–94 years old). However, this relationship was partially explained by cardiovascular factors, indicating that dispersion may be a proxy for health status [10]. Although our study was focussed on dementia outcome, and not mortality, and we did control for medical illnesses such as diabetes, stroke and high blood pressure, it is possible that our study failed to capture terminal decline and/or other comorbidities that may have influenced the observed associations. Finally, the dispersion metric adopted in the current study is the most widely used in dementia research [8,13,29]. Nonetheless, other metrics, such as coefficient of variation (CoV, a ratio of the intra-individual standard deviation and intra-individual mean performance), are available. The CoV is usually applied upon reaction time data within tasks assessing this over several trials; it is distinct from our metric, in that it accounts for overall performance, thereby mitigating confounds associated with the mean. However, the CoV has been criticised for obscuring underlying contributions towards any observed effects that might be explained by mean performance [30].
In conclusion, our findings underscore the usefulness of cognitive dispersion for predicting dementia diagnoses in the very old. They also highlight the influence of ApoEe4 in dementia development and concur with previous studies’ [25] findings that health and lifestyle factors pose little impact upon this outcome in very advanced age. Future work examining cognitive dispersion in adults from middle, older and oldest-old age groups and its relation to biological and modifiable factors will further delineate its role over the lifespan as well as its value as a marker of insidious disease onset.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge funds provided by National Institute of Aging (NIA) AG08861 for funding of the OCTO-Twin study. The authors also wish to thank the participants for their valuable contribution towards brain research as well as the various research and professional staff who recruited, collected data and co-ordinated research visits.
Contributor Information
Tam Watermeyer, Edinburgh Dementia Prevention, Centre for Clinical Brain Sciences, University of Edinburgh, Edinburgh, UK; Faculty of Health and Life Sciences, Department of Psychology, Northumbria University, Newcastle, UK.
Jantje Goerdten, Edinburgh Dementia Prevention, Centre for Clinical Brain Sciences, University of Edinburgh, Edinburgh, UK; Department of Epidemiological Methods and Etiological Research, Leibniz Institute for Prevention Research and Epidemiology–BIPS, Bremen, Germany.
Boo Johansson, Department of Psychology, Centre for Ageing and Health (AgeCap), University of Gothenburg, Gothenburg, Sweden.
Graciela Muniz-Terrera, Edinburgh Dementia Prevention, Centre for Clinical Brain Sciences, University of Edinburgh, Edinburgh, UK.
Declaration of Conflicts of Interest
None.
Declaration of Sources of Funding
The present analyses did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors. However, the OCTO-Twin study was previously funded by the National Institute of Aging (NIA AG08861).
References
- 1. Sperling RA, Aisen PS, Beckett LAet al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7: 280–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lambert C, Zeestraten E, Williams Oet al. Identifying preclinical vascular dementia in symptomatic small vessel disease using MRI. NeuroImage Clin 2018; 19: 925–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sperling RA, Karlawish J, Johnson KA. Preclinical Alzheimer disease—the challenges ahead. Nat Rev Neurol 2013; 9: 54–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. MacDonald SWS, Li SC, Bäckman L. Neural underpinnings of within-person variability in cognitive functioning. Psychol Aging 2009; 24: 792–808. [DOI] [PubMed] [Google Scholar]
- 5. Bangen KJ, Weigand AJ, Thomas KRet al. Cognitive dispersion is a sensitive marker for early neurodegenerative changes and functional decline in nondemented older adults. Neuropsychology 2019; 33: 599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Van PR, Fagan AM, Kaufman DAS. Differential cued-Stroop performance in cognitively asymptomatic older adults with biomarker-identified risk for Alzheimer’s disease: a pilot study. Curr Alzheimer Res 2018; 15: 820–7. [DOI] [PubMed] [Google Scholar]
- 7. Duchek JM, Balota DA, Tse C-Set al. The utility of intraindividual variability in selective attention tasks as an early marker for Alzheimer’s disease. Neuropsychology 2009; 23: 746–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Malek-Ahmadi M, Lu S, Chan Yet al. Cognitive domain dispersion association with Alzheimer’s disease pathology. J Alzheimers Dis 2017; 58: 575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hultsch DF, MacDonald SWS, Hunter MAet al. Intraindividual variability in cognitive performance in older adults: comparison of adults with mild dementia, adults with arthritis, and healthy adults. Neuropsychology 2000; 14: 588–98. [DOI] [PubMed] [Google Scholar]
- 10. Thaler NS, Hill BD, Duff Ket al. Repeatable battery for the assessment of neuropsychological status (RBANS) intraindividual variability in older adults: associations with disease and mortality. J Clin Exp Neuropsychol 2015; 37: 622–9. [DOI] [PubMed] [Google Scholar]
- 11. Anderson ED, Wahoske M, Huber Met al. Cognitive variability-a marker for incident MCI and AD: an analysis for the Alzheimer’s Disease Neuroimaging Initiative. Alzheimers Dement Diagnosis Assess Dis Monit 2016; 4: 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bielak AAM, Hultsch DF, Strauss Eet al. Intraindividual variability in reaction time predicts cognitive outcomes 5 years later. Neuropsychology 2010; 24: 731–41. [DOI] [PubMed] [Google Scholar]
- 13. Holtzer R, Verghese J, Wang Cet al. Within-person across-neuropsychological test variability and incident dementia. JAMA 2008; 300: 823–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gleason CE, Norton D, Anderson EDet al. Cognitive variability predicts incident Alzheimer’s disease and mild cognitive impairment comparable to a cerebrospinal fluid biomarker. J Alzheimers Dis 2018; 61: 79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Halliday D, Stawski R, Cerino Eet al. Intraindividual variability across neuropsychological tests: dispersion and disengaged lifestyle increase risk for Alzheimer’s disease. J Intell 2018; 6: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hilborn JV, Strauss E, Hultsch DFet al. Intraindividual variability across cognitive domains: investigation of dispersion levels and performance profiles in older adults. J Clin Exp Neuropsychol 2009; 31: 412–24. [DOI] [PubMed] [Google Scholar]
- 17. Rapp MA, Schnaider-Beeri M, Sano Met al. Cross-domain variability of cognitive performance in very old nursing home residents and community dwellers: relationship to functional status. Gerontology 2005; 51: 206–12. [DOI] [PubMed] [Google Scholar]
- 18. Hultsch DF, MacDonald SWS, Dixon RA. Variability in reaction time performance of younger and older adults. J Gerontol B Psychol Sci Soc Sci 2002; 57: 101–15. [DOI] [PubMed] [Google Scholar]
- 19. Kliegel M, Sliwinski M. MMSE cross-domain variability predicts cognitive decline in centenarians. Gerontology 2004; 50: 39–43. [DOI] [PubMed] [Google Scholar]
- 20. Paganini-Hill A, Kawas CH, Corrada MM. Lifestyle factors and dementia in the oldest-old: the 90+ study. Alzheimer Dis Assoc Disord 2016; 30: 21–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bendayan R, Piccinin AM, Hofer SMet al. Decline in memory, visuospatial ability, and crystalized cognitive abilities in older adults: normative aging or terminal decline? J Aging Res 2017; 2017: 6210105. doi: 10.1155/2017/6210105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Praetorius M, Thorvaldsson V, Hassing LBet al. Substantial effects of apolipoprotein E epsilon4 on memory decline in very old age: longitudinal findings from a population-based sample. Neurobiol Aging 2013; 34: 2734–9. [DOI] [PubMed] [Google Scholar]
- 23. Juva K, Verkkoniemi A, Viramo Pet al. APOE ε4 does not predict mortality, cognitive decline, or dementia in the oldest old. Neurology 2000; 54: 412–5. [DOI] [PubMed] [Google Scholar]
- 24. Lafortune L, Martin S, Kelly Set al. Behavioural risk factors in mid-life associated with successful ageing, disability, dementia and frailty in later life: a rapid systematic review. PLoS One 2016; 11: e0144405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Deckers K, Köhler S, Boxtel Met al. Lack of associations between modifiable risk factors and dementia in the very old: findings from the Cambridge City over-75s cohort study. Aging Ment Health 2018; 22: 1272–8. [DOI] [PubMed] [Google Scholar]
- 26. Sumic A, Michael YL, Carlson NEet al. Physical activity and the risk of dementia in oldest old. J Aging Health 2007; 19: 242–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Donohue MC, Sperling RA, Salmon DPet al. The preclinical Alzheimer cognitive composite: measuring amyloid-related decline. JAMA Neurol 2014; 71: 961–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Muniz-Terrera G, Hout A, Piccinin AMet al. Investigating terminal decline: results from a UK population-based study of aging. Psychol Aging 2013; 28: 377–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kälin AM, Pflüger M, Gietl AFet al. Intraindividual variability across cognitive tasks as a potential marker for prodromal Alzheimer’s disease. Front Aging Neurosci 2014; 6: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stawski RS, MacDonald SWS, Brewster PWHet al. A comprehensive comparison of quantifications of intraindividual variability in response times: a measurement burst approach. J Gerontol B Psychol Sci Soc Sci 2019; 74: 397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


