Abstract
Pharmacological agents directed to either opioid receptors or peroxisome proliferator-activated receptor gamma (PPARγ) at peripheral tissues reduce behavioral signs of persistent pain. Both receptors are expressed in muscle tissue, but the contribution of PPARγ activation to muscle pain and its modulation by opioid receptors remains unknown. To address this question, we first tested whether the endogenous PPARγ ligand 15d-PGJ2 would decrease mechanical hyperalgesia induced by carrageenan administration into the gastrocnemius muscle of rats. Next, we used receptor antagonists to determine whether the anti-hyperalgesic effect of 15d-PGJ2 was PPARγ- or opioid receptor-dependent. Three hours after carrageenan, muscle hyperalgesia was quantified with the Randall-Selitto test. 15d-PGJ2 prevented carrageenan-induced muscle hyperalgesia in a dose-dependent manner. The anti-hyperalgesic effect of 15d-PGJ2 was dose-dependently inhibited by either the PPARγ antagonist, GW9662, or by the opioid receptor antagonist, naloxone. We conclude that 15d-PGJ2 targets PPARγ and opioid receptors to prevent muscle hyperalgesia. We suggest that local PPARγ receptors are important pharmacological targets for inflammatory muscle pain.
Keywords: PPARγ, 15d-PGJ2, opioid, muscle hyperalgesia
Introduction
Chronic musculoskeletal pain impacts approximately 5% - 33% of the population (including children)1,2 and afflicts one third to one-half of multimorbidity patients particularly in the elderly2. Chronic musculoskeletal pain limits mobility and is the second most common cause of disability worldwide, carrying a significant socio-economic impact1,3 and representing a risk factor for the development of depression, obesity, cardiovascular diseases, and earlier mortality4. Thus, chronic musculoskeletal pain is among the most prevalent examples of persistent pain that can last throughout life.
Peripheral pro-inflammatory cytokines and nociceptor-sensitizing agents, such as neutrophil migration, bradykinin, sympathetic amines and prostanoids mediate acute muscle hyperalgesia5. Intense, long-lasting muscle pain leads to a transition from acute to chronic pain6 due to peripheral nociceptor sensitization, central sensitization of dorsal horn neurons, and/or a pathological reorganization of pain modulatory centers in the brain6,7. Although these mechanisms overlap with other painful conditions, muscle pain has unique characteristics: the quality of muscle pain is often described as aching and cramping; depending on the source of nociceptive input, the clinical presentation of muscle pain is both localized and referred7; nerve innervation to muscle is less than the skin, but greater than the visceral structures6; and descending inhibition of muscle pain is relatively strong as compared to cutaneous pain7.
In this study, we propose the 15-deoxyΔ−12,14-prostaglandin J2 (15d-PGJ2) / peroxisome proliferator-activated receptors gamma (PPARγ) system as a new pharmacological target for the control of muscle hyperalgesia. PPARγ is a nuclear receptor established as a primary lipid sensor and a modulator of lipid metabolism8. 15d-PGJ2 is a potent endogenous ligand of PPARγ receptors and produces anti-nociceptive effects in multiple animal models of inflammatory pain including intraplantar carrageenan, formalin and PGE29, intra-articular formalin10, collagen-induced arthritis11, gout12, among others. PPARγ activation by 15d-PGJ2 induces the local release of opioid peptides from immune cells, leading to a decrease in mechanical hypersensitivity13. However, whether 15d-PGJ2 can also decrease muscle pain remained unknown. Here, we demonstrate for the first time the anti-hyperalgesic effect of 15d-PGJ2 in an animal model of muscle pain. In addition, using a pharmacological receptor blockade strategy, we show that the anti-hyperalgesic actions of 15d-PGJ2 are mediated by PPARγ and /or opioid receptors.
Material and methods
Animals
Male Wistar rats (200–250 g) from CEMIB (Multidisciplinary Center for Biological Research) UNICAMP were used under the approval of the ethics committee for animal use of the State University of Campinas (2986–1) and carried out in accordance with IASP guidelines on using laboratory animals. Laboratory animals were housed in plastic cages (five per cage) with soft bedding with food and water ad libitum. 12h light/dark cycles were set in a room temperature of 23°C. Animals were habituated to the test room for 1 h before testing began. Animals were euthanized with CO2 asphyxiation once experimental procedures were complete.
Drugs
15d-PGJ2 was obtained from (Calbiochem, San Diego, CA, USA). The selective PPARγ antagonist GW9662 (2-Chloro-5-nitro-N-phenylbenzamide), the non-selective opioid antagonist naloxone and the inflammatory agent λ-Carrageenan were obtained from Sigma-Aldrich (St. Louis, MO, USA). Naloxone and λ-Carrageenan were diluted in saline, while 15d-PGJ2 and GW9662 were diluted in 100% dimethyl sulfoxide (DMSO) from Sigma-Aldrich (St. Louis, MO, USA) before dilution in 0.9% NaCl (saline) to a final concentration of 5% DMSO.
Intramuscular injections
All drugs or their vehicles were injected in a total volume of 50 μL using a 30-gauge needle into the left gastrocnemius muscle of briefly restrained, awake rats. As a control for systemic or central actions, 15d-PGJ2 was also administered into the right gastrocnemius.
Carrageenan-induced inflammatory pain model
Muscle hyperalgesia was induced by single administration of carrageenan in a dose of 100 μg/25μl into the left gastrocnemius muscle.
Behavioral testing
Experiments were conducted between 9:00 a.m. to 5:00 p.m. in a quiet room at a controlled temperature of 23°C. Muscle hyperalgesia was evaluated with a Randall–Selitto analgesiometer (Insight, Ribeirao Preto, SP, Brazil) test of mechanical nociceptive threshold. This method applies a linearly increasing force (g) to the gastrocnemius muscle until a reflex withdrawal of the hindlimb was observed. The average of three measures were collected at 5 minutes intervals. Mechanical muscle hyperalgesia, represented by the y-axis, reflects the difference in threshold between measurements collected before (baseline) versus after carrageenan injection. Measurements were taken three hours after carrageenan administration, at the peak of nociceptive behaviors. Time points represent the time elapsed since carrageenan injection and are referred to as post injection.
Statistical analysis
The groups sizes per experiment was determined by a power analysis (α, 0.05; power, 0.99) using the effect size and variance estimated from previous studies or preliminary data. G*Power 3 was used for the power analysis. Data were analyzed with GraphPad Prism 7.0 by one-way ANOVA followed by Tukey’s post hoc test. Statistical significance was set at P < 0.05. Dose-response curves were calculated via nonlinear regression analysis (Y=Bottom + (Top-Bottom)/(1+10^((X-LogED50))). Data are expressed as a decrease in paw-withdrawal threshold in grams (g) and presented as mean ± SEM.
Results
Intra-muscular administration of 15d-PGJ2 inhibits carrageenan-induced mechanical muscle hyperalgesia in a dose-dependent manner
We first tested the hypothesis that local administration of 15d-PGJ2 would reduce carrageenan-induced mechanical muscle hyperalgesia. To this end, animals were pre-treated with 15d-PGJ2 15 minutes before induction of muscle hyperalgesia (Fig 1 A). As illustrated in Fig. 1B, carrageenan (100 μg), but not saline vehicle, induced mechanical hyperalgesia (F=52.31; P < 0.0001; n = 5). Pretreatment with 15d-PGJ2 (100 and 10, but not 1 ng), prevented carrageenan-induced mechanical hyperalgesia when administered in the ipsilateral, but not the contralateral gastrocnemius muscle (F=49.6; P < 0.0001). The anti-hyperalgesic effect of 15d-PGJ2 occurred in a dose-dependent manner with an ED50 of 5.8 ng (fig 1C).
Figure 1.
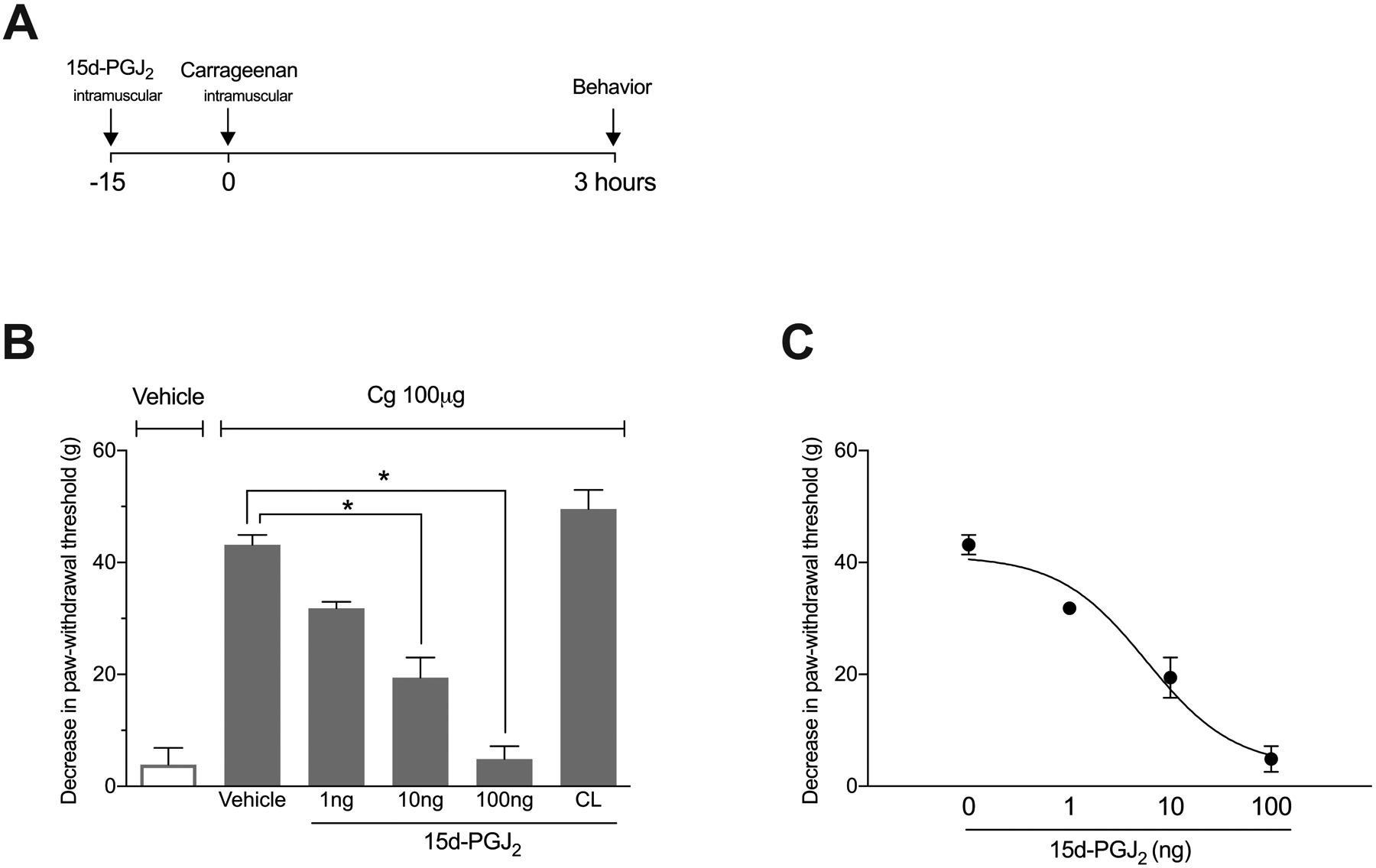
Experimental timeline and the effect of the PPARγ receptor agonist 15d-PGJ2 in carrageenan-induced mechanical muscle hyperalgesia. (A) 15d-PGJ2 was administered 15 minutes before carrageenan injection. Behavior was quantified 3h after carrageenan. (B) Pre-treatment with 15d-PGJ2 (10 and 100, but not 1 ng) 15 min before carrageenan (Cg) injection significantly prevented hyperalgesia when administered into the ipsilateral, but not contralateral (CL) gastrocnemius muscle (Tukey post-tests: * P < 0.05 n = 5). (C) Pre-treatment with 15d-PGJ2 prevented mechanical muscle hyperalgesia in a dose-dependent manner (nonlinear regression analysis; ED50 = 5.8 ng; n = 3–5).
The local anti-hyperalgesic effect of 15d-PGJ2 in the carrageenan model requires PPARγ activation
Next, we evaluated whether the anti-hyperalgesic effect of 15d-PGJ2 on carrageenan-induced mechanical muscle hyperalgesia was dependent on PPARγ receptor activation. To this end, animals were pre-treated 15 minutes before 15d-PGJ2 with the PPARγ antagonist, GW9962 (Fig 2 A). As shown in Fig. 2B, intramuscular administration of GW9662 prevented the anti-hyperalgesic effect of 15d-PGJ2 (F=114.2 P < 0.0001 in a dose-dependent manner with an ED50 of 4.1 ng (fig 2C). Administration of GW9662 had no effect by itself (p>0.05).
Figure 2.

The anti-nociceptive effect of 15d-PGJ2 in carrageenan-induced mechanical muscle hyperalgesia is PPARγ receptor-dependent. (A) PPARγ receptor antagonist, GW9662 was administered 15 minutes before 15d-PGJ2 and behavior was quantified 3h after carrageenan (B) Pre-treatment with GW9662 (3 and 9, but not 1 ng or vehicle) significantly prevented the anti-nociceptive effect of 15d-PGJ2, when tested 3h after carrageenan (Cg) injection (Tukey post-tests: * P < 0.05 n = 5). (C) Pre-treatment with GW9662 prevented the anti-hyperalgesic effect of 15d-PGJ2 in a dose-dependent manner (nonlinear regression analysis, p<0.05; ED50 = 4.1ng n = 4–5).
The local anti-hyperalgesic effect of 15d-PGJ2 in the carrageenan model requires opioid receptor activation
To evaluate whether the anti-hyperalgesic effect of 15d-PGJ2 requires opioid receptor activation, the animals were pre-treated 15 minutes before 15d-PGJ2 with naloxone (Fig 3 A). As illustrated in Fig. 3B, intramuscular naloxone prevented the anti-hyperalgesic effect of 15d-PGJ2 (fig 3B, F=55.06; P < 0.0001; n = 5) in a dose-dependent manner with an ED50 of 0.2 μg (fig 3C).
Figure 3.

The anti-nociceptive effect of 15d-PGJ2 in carrageenan-induced mechanical muscle hyperalgesia is opioid receptor-dependent. (A) The non-specific opioid receptor antagonist, Naloxone was administered 15 minutes before 15d-PGJ2 and behavior was quantified 3h after carrageenan (B) Pre-treatment with naloxone (0.1 and 1, but not 0.01 μg or vehicle) significantly prevented the anti-hyperalgesic effect of 15d-PGJ2 when tested 3h after carrageenan (Cg) (Tukey post-tests: * P < 0.05 n = 5). (C) Naloxone prevented the anti-hyperalgesia effect of 15d-PGJ2 in a dose-dependent manner (nonlinear regression analysis, p<0.05; ED50 = 0.2μg n = 4–5).
Discussion
In the present study, we show for the first time that intra-muscular administration of 15d-PGJ2 prevents mechanical hyperalgesia in an inflammatory model of muscle pain. Moreover, the selective PPARγ receptor antagonist GW9662 and the non-selective opioid receptor antagonist naloxone prevented the anti-hyperalgesic effect of the 15d-PGJ2, indicating a PPARγ- and opioid receptor-dependent mechanism.
Relying on muscle hyperalgesia models to test a potential analgesic is important due to their unique characteristics that differs from other types of pain regarding the neural mechanisms6. Different muscle skeletal pain conditions are related to markers of inflammation such as cytokines and prostaglandins that can lead to peripheral and central sensitization7. For example, carrageenan-induced muscle hyperalgesia is associated with the local release of pro-inflammatory cytokines such as IL-6, CINC-1 and IL-1β14.
The potent antinociceptive effect of 15d-PGJ2 is at least in part due to downregulation of proinflammatory cytokines15. 15d-PGJ2 can inhibit NF-κB12 first leading to downregulation of TNFα11, IL-6, IL-12, IL-18 and/or the chemokine CINC-115, and then leading decreases in nociceptive neuronal excitability and hypersensitivity9. Furthermore, chronic administration of 15d-PGJ2 decreases mechanical hypersensitivity by inhibition of T cells11, while single injection of 15d-PGJ2 decreases mechanical hypersensitivity in multiple models including PGE2-induced hyperalgesia and hyperalgesia induced by MSU crystals (gout pain model) by decreasing local neutrophil and leucocyte migration, respectively10,12. Although we did not measure the expression of pro-nociceptive signaling molecules or cell types involved in the anti-hyperalgesic effect of 15d-PGJ2, given that muscle hyperalgesia is modulated by polymorphonuclear cells5, we hypothesize that 15d-PGJ2 prevented carrageenan-induced muscle hyperalgesia due its ability to modulate peripheral immune cells leading to the downregulation of pro-nociceptive mediators.
The majority of the studies using inflammatory pain models have described a potent antinociceptive effect of 15d-PGJ2 when locally administered9,13. Intraplantar administration of 15d-PGJ2 decreased mechanical hypersensitivity induced by the plantar injection of either carrageenan or prostaglandin E29. This effect lasted for 3 hours; a time frame similar with the present study. A rapid effect of 15d-PGJ2 can be also found in neuropathic pain models: 15d-PGJ2 dose-dependently decreased mechanical hypersensitivity within 45 minutes, a time course that suggests a transcription-independent mechanism of action10. In fact, PPARγ agonists can inhibit hyperalgesia by both genomic and non-genomic receptor-dependent mechanisms16. Therefore, considering the very short half-life of 15d-PGJ2, the antihyperalgesic effect 3 hours after carrageenan administration, as shown in the present study, may indicate a receptor-dependent genomic or translation mechanism.
We used a receptor antagonist strategy to ask whether the inhibitory effect of 15d-PGJ2 on muscle hyperalgesia was dependent on PPARγ receptors. We found that the selective PPARγ antagonist GW9662 prevented the antihyperalgesic effect of 15d-PGJ2 in a dose dependent manner, suggesting a local receptor dependent-mechanism. 15d-PGJ2 is endogenously synthesized and therefore might activate PPARγ and thus tonically inhibit pain. To test this hypothesis, we asked whether GW9662 given by itself would increase carrageenan-induced mechanical hyperalgesia. We found that GW9662 did not change hyperalgesia. This result is consistent with the literature indicating that baseline or inflammation-evoked concentrations of 15d-PGJ2 are insufficient to tonically activate PPARγ receptors8. Furthermore, lipopolysaccharide-induced COX activation in vitro by LPS did not increase the biosynthesis of 15d-PGJ2, and the same study reported similar 15d-PGJ2 concentrations in the joint fluid of patients with or without arthritis17. It is likely that a 1000 -fold increase in 15d-PGJ2 concentrations is required to sufficiently activate PPARγ so as to downregulate NF-κB signaling17,18.
In the present study, the non-selective opioid receptor antagonist, naloxone, prevented the anti-hyperalgesic effect of 15d-PGJ2 in a dose dependent manner. These results are consistent with previous data showing that the anti-hyperalgesic effect of 15d-PGJ2 was prevented by naloxone in the intra-plantar PGE2 model of hyperalgesia9. Further studies using selective opioid antagonists showed that kappa (κ) and delta (δ), but not mu (μ) receptors10, were involved in the anti-hyperalgesic effect of 15d-PGJ2 via a peripheral site of action13. Other evidence for opioid-dependent effects of PPARγ agonists include studies of morphine tolerance. For example, pioglitazone, a PPARγ receptors agonist, negatively modulated morphine tolerance, while a PPARγ antagonist facilitated signs of opioid withdrawal19.
Based on published preclinical and clinical studies, we do not believe that pharmacological inhibition of peripheral or central opioid receptors with naloxone would increase muscle hyperalgesia in our carrageenan model. Although tonic descending inhibition of dorsal horn nociceptive neurons requires opioidergic signaling at the supraspinal level20, pre-treatment with systemic naloxone does not further increase mechanical or thermal hyperalgesia induced by intraplantar carrageenan21. Naloxone does not to increase hyperalgesia in other models of pain either. For example, neither naloxone (crosses blood–brain barrier), nor the peripherally restricted opioid antagonist naloxone methiodide, changed the intensity of nociceptive responses induced by a 5% hypertonic saline injection in the gastrocnemius, triceps or masseter muscles of rats22. Also, continuous intravenous infusion of naloxone did not change intraplantar formalin-evoked nociceptive responses23. Furthermore, naloxone does not change muscle pain induced by maximum voluntary contraction and post-exercise muscle ischemia in humans24. However, it must be kept in mind that we cannot completely exclude the possibility that intra-muscular carrageenan produces a latent sensitization that is tonically inhibited by mu opioid receptor constitutive activity, whereby naloxone would unmask hyperalgesia25.
15d-PGJ2-induced activation of PPARγ can increase the expression and likely the release of β-endorphin and dynorphin in leucocytes. Subsequent binding to κ and δ opioid receptors in primary sensory neurons may then lead to anti-nociception13. We conclude that 15d-PGJ2 -mediated anti-hyperalgesia engages endogenous opioid systems. If this occurs without producing adverse effects such as withdrawal or tolerance (as occurs with opioid receptors agonists), then 15d-PGJ2-like compounds may be useful as a new class of analgesics for muscle pain.
Therefore, we conclude that intramuscular administration of 15d-PGJ2 prevented mechanical muscle hyperalgesia induced by carrageenan. This effect is mediated by both PPARγ and opioid receptors. We propose future investigations to clarify the mechanism of action, aiming to establish 15d-PGJ2 activation of PPARγ as an important pharmacological strategy for the treatment of muscle pain.
Acknowledgments
We thank Andrew H. Cooper and Tyler S. Nelson for proofreading this manuscript.
Funding: This work was supported in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001, by the Sao Paulo Research Foundation (FAPESP) [grant number 2011/11064-4]. National Institutes of Health R01NS62306, R01DA037621 and R01NS045954 to BKT
Footnotes
Conflict of interest: none declared.
References
- 1.Brennan-Olsen SL, Cook S, Leech MT, Bowe SJ, Kowal P, Naidoo N, et al. Prevalence of arthritis according to age, sex and socioeconomic status in six low and middle income countries: analysis of data from the World Health Organization study on global AGEing and adult health (SAGE) Wave 1. BMC Musculoskelet Disord 2017; 18 (1):271–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Briggs AM, Woolf AD, Dreinhöfer K, Homb N, Hoy DG, Kopansky-Giles D, et al. Reducing the global burden of musculoskeletal conditions. Bull World Health Organ 2018; 96 (5):366–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet 2018; 392 (10159):1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nüesch E, Dieppe P, Reichenbach S, Williams S, Iff S, Jüni P. All cause and disease specific mortality in patients with knee or hip osteoarthritis: population based cohort study. BMJ (Clinical research ed) 2011; 342:d1165–d1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santos DF, Melo Aquino B, Jorge CO, Azambuja G, Schiavuzzo JG, Krimon S, et al. Muscle pain induced by static contraction in rats is modulated by peripheral inflammatory mechanisms. Neuroscience 2017; 358:58–69. [DOI] [PubMed] [Google Scholar]
- 6.Mense S Nociception from skeletal muscle in relation to clinical muscle pain. Pain 1993; 54 (3):241–289. [DOI] [PubMed] [Google Scholar]
- 7.Mense S Muscle pain: mechanisms and clinical significance. Deutsches Arzteblatt international 2008; 105 (12):214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Guo C, Wu J. 15-Deoxy-Δ-(12,14)-Prostaglandin J2 (15d-PGJ2), an Endogenous Ligand of PPAR-γ: Function and Mechanism. PPAR research 2019; 2019:7242030–7242030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Napimoga MH, Souza GR, Cunha TM, Ferrari LF, Clemente-Napimoga JT, Parada CA, et al. 15d-prostaglandin J2 inhibits inflammatory hypernociception: involvement of peripheral opioid receptor. J Pharmacol Exp Ther 2008; 324 (1):313–321. [DOI] [PubMed] [Google Scholar]
- 10.Pena-Dos-Santos DR, Severino FP, Pereira SAL, Rodrigues DBR, Cunha FQ, Vieira SM, et al. Activation of peripheral [kappa]/[delta] opioid receptors mediates 15-deoxy-[Delta]12,14-prostaglandin J2 induced-antinociception in rat temporomandibular joint. Neuroscience 2009; 163 (4):1211–1219. [DOI] [PubMed] [Google Scholar]
- 11.Carregaro V, Napimoga MH, Peres RS, Benevides L, Sacramento LA, Pinto LG, et al. Therapeutic Treatment of Arthritic Mice with 15-Deoxy Delta(12,14)-Prostaglandin J2 (15d-PGJ2) Ameliorates Disease through the Suppression of Th17 Cells and the Induction of CD4(+)CD25(−)FOXP3(+) Cells. Mediators of inflammation 2016; 2016:9626427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruiz-Miyazawa KW, Staurengo-Ferrari L, Pinho-Ribeiro FA, Fattori V, Zaninelli TH, Badaro-Garcia S, et al. 15d-PGJ2-loaded nanocapsules ameliorate experimental gout arthritis by reducing pain and inflammation in a PPAR-gamma-sensitive manner in mice. Sci Rep 2018; 8 (1):13979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macedo CG, Napimoga MH, Rocha-Neto LM, Abdalla HB, Clemente-Napimoga JT. The role of endogenous opioid peptides in the antinociceptive effect of 15-deoxy(Delta12,14)-prostaglandin J2 in the temporomandibular joint. Prostaglandins, leukotrienes, and essential fatty acids 2016; 110:27–34. [DOI] [PubMed] [Google Scholar]
- 14.Loram LC, Fuller A, Fick LG, Cartmell T, Poole S, Mitchell D. Cytokine profiles during carrageenan-induced inflammatory hyperalgesia in rat muscle and hind paw. J Pain 2007; 8 (2):127–136. [DOI] [PubMed] [Google Scholar]
- 15.Silva Quinteiro M, Henrique Napimoga M, Gomes Macedo C, Furtado Freitas F, Balassini Abdalla H, Bonfante R, et al. 15-deoxy-Delta12,14-prostaglandin J2 reduces albumin-induced arthritis in temporomandibular joint of rats. Eur J Pharmacol 2014; 740:58–65. [DOI] [PubMed] [Google Scholar]
- 16.Griggs RB, Donahue RR, Morgenweck J, Grace PM, Sutton A, Watkins LR, et al. Pioglitazone rapidly reduces neuropathic pain through astrocyte and nongenomic PPARgamma mechanisms. Pain 2015; 156 (3):469–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bell-Parikh LC, Ide T, Lawson JA, McNamara P, Reilly M, FitzGerald GA. Biosynthesis of 15-deoxy-delta12,14-PGJ2 and the ligation of PPARgamma. J Clin Invest 2003; 112 (6):945–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Narumiya S, FitzGerald GA. Genetic and pharmacological analysis of prostanoid receptor function. J Clin Invest 2001; 108 (1):25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Guglielmo G, Kallupi M, Scuppa G, Stopponi S, Demopulos G, Gaitanaris G, et al. Analgesic tolerance to morphine is regulated by PPARgamma. Br J Pharmacol 2014; 171 (23):5407–5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu XM, Hua M, Mense S. The effects of intracerebroventricular injection of naloxone, phentolamine and methysergide on the transmission of nociceptive signals in rat dorsal horn neurons with convergent cutaneous-deep input. Neuroscience 1991; 44 (3):715–723. [DOI] [PubMed] [Google Scholar]
- 21.Khalid S, Ullah MZ, Khan AU, Afridi R, Rasheed H, Khan A, et al. Antihyperalgesic Properties of Honokiol in Inflammatory Pain Models by Targeting of NF-κB and Nrf2 Signaling. Front Pharmacol 2018; 9:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanchez EM, Bagues A, Martin MI. Contributions of peripheral and central opioid receptors to antinociception in rat muscle pain models. Pharmacol Biochem Behav 2010; 96 (4):488–495. [DOI] [PubMed] [Google Scholar]
- 23.Taylor BK, Peterson MA, Basbaum AI. Continuous intravenous infusion of naloxone does not change behavioral, cardiovascular, or inflammatory responses to subcutaneous formalin in the rat. Pain 1997; 69 (1–2):171–177. [DOI] [PubMed] [Google Scholar]
- 24.Ray CA, Carter JR. Central modulation of exercise-induced muscle pain in humans. J Physiol 2007; 585 (Pt 1):287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corder G, Doolen S, Donahue RR, Winter MK, Jutras BL, He Y, et al. Constitutive mu-opioid receptor activity leads to long-term endogenous analgesia and dependence. Science 2013; 341 (6152):1394–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]


