Abstract
The presence of increased temperature for gene electrotransfer has largely been considered negative. Many reports have published on the lack of heat from electrotransfer conditions to demonstrate that their effects are from the electrical pulses and not from a rise in temperature. Our hypothesis was to use low levels of maintained heat from an exogenous source to aid in gene electrotransfer. The goal was to increase gene expression and/or reduce electric field. In our study we evaluated high and low electric field conditions from 90 V to 45 V which had been preheated to 40 °C, 43 °C, or 45 °C. Control groups of non-heated as well as DNA only were included for comparison in all experiments. Luciferase gene expression, viability, and percent cell distribution were measured. Our results indicated a 2–4 fold increase in gene expression that is temperature and field dependent. In addition levels of gene expression can be increased without significant decreases in cell death and in the case of high electric fields no additional cell death. Finally, in all conditions percent cell distribution was increased from the application of heat. From these results, we conclude that various methods may be employed depending on the end user’s desired goals. Electric field can be reduced 20–30% while maintaining or slightly increasing gene expression and increasing viability or overall gene expression and percent cell distribution can be increased with low viability.
Keywords: Gene electrotransfer, Heat, Electroporation
1. Introduction
Direct injection of DNA is an easy method for delivering genes. However gene expression is relatively low and requires the use of chemical, electrical, or mechanical enhancers to increase expression levels. Electrical enhancers like gene electrotransfer (GET) have been used increasingly for several gene delivery applications including: cancer vaccines and therapeutics, infectious disease DNA vaccines, and gene replacement for metabolic disorders, and have been translated to the clinic with some success in the areas of infectious disease and cancer vaccines and therapeutics [1–6]. Despite the successes, GET still has its drawbacks. A primary one is the pain and potential cellular damage associated with administration of electrical stimulation.
In addition to the pain of electrical stimulation, insertion of needles into tissue can also add to the pain. However, using this approach, levels of gene expression and distribution can be increased and more tightly controlled. Methods have been employed to counteract some of these issues. Gene expression levels and distribution can be adjusted and regulated based on the type of electrode, pulse number, duration, and amount or concentration of DNA [6–8].
Pain can also be reduced by several of these methods. Non-invasive electrodes have been designed for skin delivery in an effort to reduce discomfort from needle insertion [7,9–11]. However, the drawback has been the need for higher applied voltages to penetrate the stratum corneum. An additional hurdle is the depth within the tissue of gene expression. Newer non-invasive electrode designs have incorporated multiple electrodes. The multi-electrode array allows for a lower applied voltage due to the small gap between electrodes [12,13]. This type of electrode design has been successful in generating immune responses against infectious agents but was ineffective for increasing expression systemically [14–16]. Additionally, while adding electrodes and reducing the gap allows for a reduction in applied voltage it also in turn reduces the penetration of the electric field. Other similar electrodes have been designed, like the MID, that minimally penetrate the tissue, these designs have been successful but again require penetration of the tissue [17,18].
To enhance the applicability of GET it is important to address these issues. It will be necessary to determine if non-invasive GET can be enhanced while still maintaining the safety of low voltages. One possibility is to increase membrane fluidity to allow for greater penetration and uptake of the DNA. One possible approach is to increase the temperature prior to electrotransfer. The idea of heat has been considered a negative factor when associated with electrotransfer. Much work has evaluated whether GET conditions used for gene delivery increase temperature and whether this is a contributing factor for the gene delivery. It has been shown that in fact this is not the case and that the electric field is the cause for gene delivery [19]. However, it is also known that the phospholipid bilayer is designed for 37 °C and when the phospholipid bilayer is heated the phospholipids move apart and the membrane becomes more fluid-like [20]. This effect could be of benefit to non-invasive GET. The application of low to moderate heat may increase the membrane fluidity of the skin allowing the electrical fields to more easily drive gene delivery. Our goal was to evaluate this process under low to moderate heating conditions combined with specific GET conditions. The research was designed to determine if pretreatment with heat could increase gene expression. In addition, the effect on cell viability was evaluated as well as determining if lower voltages could be used when pretreating with heat.
2. Materials and methods
2.1. Cell lines and medium
The human Keratinocyte cell line, HaCaT, was used for all in vitro experimental studies. Cells were cultured in DMEM + pen-Strep and 10% FBS. Cells were harvested from flasks with 0.25% Trypsin-EDTA and resuspended at 5 million cells/ml in complete media.
Plasmids: 5 μl of either Gwizluc or GwizGFP (Aldevron) at 2 mg/ml were used in this study to evaluate gene expression.
2.2. Experimental setup and groups
For these experiments there were 3 experimental setups.
Oil temperature 43–45 °C for 1.5 min for a final media temperature of 43 °C.
Oil temperature 45–48 °C for 2 min for a final media temperature of 45 °C.
Oil temperature 40–43 °C for 1.25 min for a final media temperature of 40 °C.
Untreated controls were included in each experiment. Initial temperature of cells was room temperature ranging between 22–24 °C. Table 1 describes the experiments performed with each temperature.
Table 1.
Shows the experimental setup for each of the laser heated and electrotransfer conditions. All conditions were evaluated by luciferase gene delivery and expression. GFP expression was evaluated with all voltages but only at 43C and No heat.
| 900 V/cm | 750 V/cm | 600 V/cm | 450 V/cm | DNA only | |
|---|---|---|---|---|---|
| Heat 40 °C | Luciferase | Luciferase | Luciferase | Luciferase | Luciferase |
| Heat 43 °C | Luciferase/GFP | Luciferase/GFP | Luciferase/GFP | Luciferase/GFP | Luciferase/GFP |
| Heat 45 °C | Luciferase | Luciferase | Luciferase | Luciferase | Luciferase |
| No heat | Luciferase/GFP | Luciferase/GFP | Luciferase/GFP | Luciferase/GFP | Luciferase/GFP |
2.3. Application of heat
An oil immersion bath was used to heat the HaCaT cells to various temperatures. 150 μl of cell suspension was put into each 1 mm gap electroporation cuvette and placed in the oil immersion bath. Various oil temperatures and times were evaluated to determine the amount of time necessary to heat each sample to our desired temperatures. Oil temperatures ranged from 43–48 °C and time in oil ranged from 1–2 min. The time in heat for each sample was determined by the closest 15 s to the average of all samples. Time in heat was determined to be 1 min 15 s for 40 °C, 1 min 30 s for 43 °C and 2 min for 45 °C.
2.4. Electroporation
150 μl of cells was electroporated at various temperatures and times. Cells were left in the oil immersion bath while pulsing. Electroporation was performed using the BTX ECM 830 at applied voltages of 90, 75,60, or 45 V using 1 mm electroporation cuvettes. Pulse length (t_p), pulse number (p) and frequency (f) remained constant at 5 ms, 1p, and 1 Hz respectively. Untreated cells and non-electroporated cells were used as controls. After treatment, cells were removed from cuvette and 140 μl added to each well of a 12 well cell culture plate.
2.5. in vitro bioluminescent imaging
Luciferase gene expression was measured 24 h after treatment. Complete media was removed from cells and 500 μl of complete media + 150 μg/ml Luciferin was added to each well. Cells were incubated in Luciferin-media mix for 5 mins at 37 °C before imaging. Caliper Life sciences IVIS Spectrum was used to measure luciferase expression. All luciferase data is presented as average total flux in photons/s (p/s).
2.6. Flow cytometry
GFP expression/distribution was measured by flow cytometry 24 h after treatment. Samples were harvested using trypsin and washed and resuspended in 200 μl of DPBS. Propidium Iodide was used to evaluate viability. 10,000 events were collected for each sample and percent GFP events calculated.
2.7. MTT viability
Viability was measured 24 h after treatment using MTT. 10 μl from each treatment was plated for MTT assay in a 96 well cell culture plate. 90 μl of complete media was added to each well. Untreated control and standard curves for cell number were used to measure viability. 25 μl of MTT was added to each well after 24 h and incubated at 37°. After 2 h media + MTT was removed and 100 μl of DMSO (sigma) was added to each well and shaken for 10 min at 100 rpm. Optical density was measured using a plate reader at 540 nm.
3. Results and discussion
3.1. Changes in luciferase gene expression from heat pretreatment GET
The first objective of this study was to determine if increased temperature affected overall gene expression. To test this, we delivered luciferase plasmid by GET at four different applied voltages with each being administered at three elevated temperatures. Luciferase expression was measured after 24 h. Increasing the temperature during the administration of GET can, result in changes in gene expression (Fig. 1). Fig. 1a shows the p/s of luciferase from each electrotransfer condition with and without the addition of heat for each of the three temperatures. Interestingly, different temperature and GET combinations had differing effects on gene expression. This becomes clearer when evaluating the fold changes of heat pretreated GET over GET alone for each condition at each temperature (Fig. 1b). 90 V and 75 V with heat represent the highest fold increases in gene expression; however this is seen at different temperatures (90 V with 40 °C and 75 V with 43 °C). Significant increases in gene expression are not seen between our four voltages at room temperature. However, evaluating each temperature individually shows that with only a slight increase in temperature of 40 °C significant increases in gene expression can be seen. At 40 °C and 90 V there is a significant increase in gene expression over 60 V and 45 V. As voltage is decreased to 75 with the same 40 °C gene expression is significantly increased over 45 V. When temperature was increased to 43 °C the greatest effect was seen with 75 V which was significantly increased over both 60 V and 45 V. Though 60 V was significantly increased, 43 °C also represented its highest levels of gene expression. Additionally at 43 °C, though 90 V was still slightly elevated it had dropped off from 40 °C. At 45 °C there was a drop in gene expression for 60 V and 45 V and only a slight elevation for 75 V and 90 V. This information leads us to believe that there needs to be balance for heat pretreatment with GET. Depending on the desired outcome one may wish to use lower temperatures with higher GET fields or vice versa. Though when using higher temperatures and lower GET conditions, one must be careful to not exceed the threshold. This threshold may be due to cell viability and the number of remaining cells available to express DNA. To answer this question we further evaluated both of these factors.
Fig. 1.
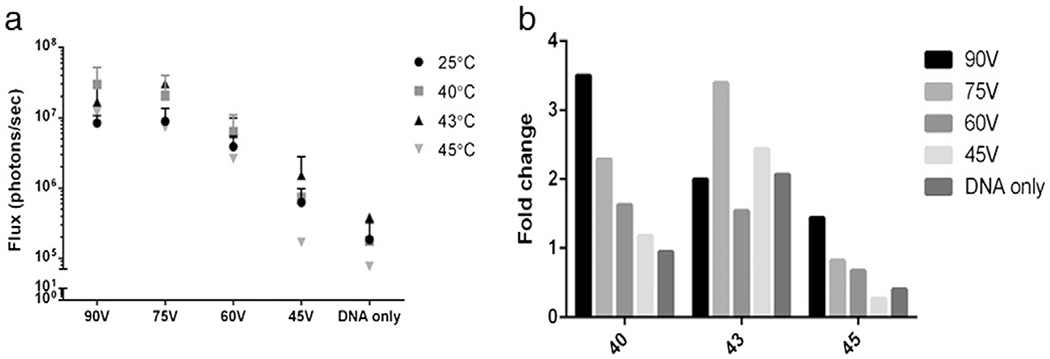
Effect of heat pretreatment on electroporated gene expression. Luciferase expression following plasmid delivery was measured using IVIS spectrum for all conditions. a) Gene expression of heat pretreated and unheated groups; b) average fold changes of the heat pretreatment over electroporation without heat.
3.2. Effect on cell viability from heat pretreated GET
Viability of cells following GET treatment in the presence or absence of elevated temperatures was measured by MTT assay. To reduce experimental variability of cell viability, samples were done in side by side assays on the same day with the same cells. DNA alone samples were used as controls to demonstrate that the addition of DNA did not play a significant role in viability as noted by nearly 100% cell survival in each experiment (Fig. 2). The addition of heat at different temperatures to DNA only samples did have an effect on viability reducing it to about 80% regardless of temperature. Interestingly the least effect on viability was seen in the 90 V and 75 V groups where viability was actually slightly (though not significantly) increased on average. We suspect that the heat effect is negated by GET at those fields.
Fig. 2.
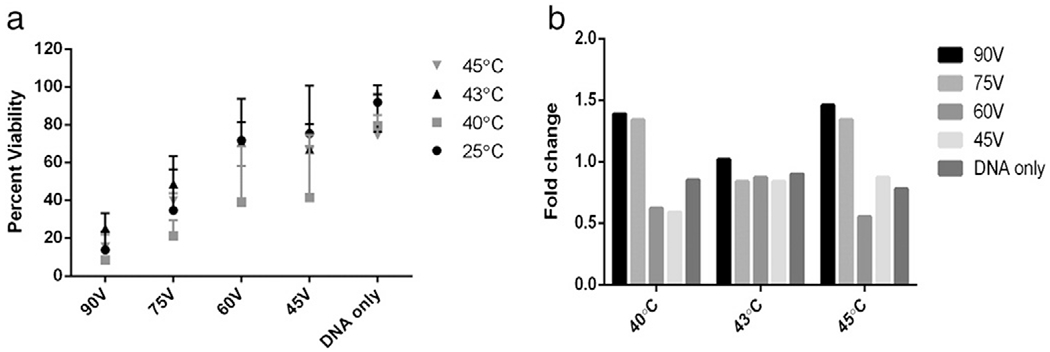
Effect of heat pretreatment on electroporated viability. Viability of cells was measured by MTT assay for all conditions. a) Gene expression of heat pretreated and unheated groups; b) average fold changes of the heat pretreatment over electroporation without heat.
Those cells that are susceptible to death from application of heat are also those cells susceptible to GET and would die regardless of the application of heat. As voltage was decreased to 60 V and 45 V significant differences in viability were noted between heated and non-heated groups. However, the most interesting evaluation of viability was at the conditions that had the highest gene expression from the luciferase experiments and whether the viabilities are different or the same. As shown in Fig. 1, 40 °C with 90 V and 43 °C with 75 V represented the two conditions with the highest gene expression. Viability following delivery with 90 V never exceeded 20% at any temperature (Fig. 3a). With 75 V viability was between 20–50% and for each temperature demonstrating an increase from 10–30% over 90 V (Fig. 3a). While these results are statistically similar, the increase in viability with 75 V at 43 °C could be of benefit for many applications.
Fig. 3.
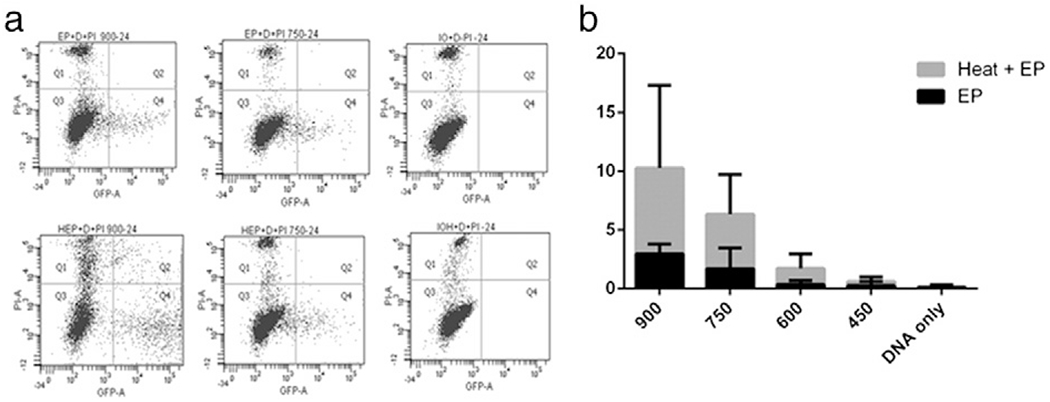
Effect of heat pretreatment on GFP expression and distribution. GFP expression was measured by flow cytometry. a) Representative scatter plots for electroporated controls (top) and for heat pretreated samples at 900 V/cm, 750 V/cm and DNA only; b) increase in GFP expressing events of the heat pretreatment over electroporation.
3.3. Effect on efficiency of expression from heat pretreated GET
In addition to increase in expression, it is also important to determine the efficiency of delivery. For these experiments, only 43 °C was used for heat application. Flow cytometry was used to measure GFP expression from each group. Fig. 3a shows a representative scatter plot of GFP expression in 90 V, 75 V and DNA only samples. There was a declining effect of positive cells for each GET condition with 90 V representing the highest and 45 V the lowest (Fig. 3b). High standard deviation in 90 V is most likely an effect of variation in cell viability. It is surprising given the gene expression results that there would be a difference between 90 V and 75 V. Though again this difference could be related to the viability. There were a large number of cells killed when using 90 V that a large percentage of cells remaining are expressing GFP, however, in the case of 75 V the viability is increased by 30%, therefore total gene expression is similar but there is a larger cell population to express the gene.
4. Conclusions
The study reported here evaluated the effect of the application of heat prior to electrotransfer. The hypothesis was that the addition of heat would aid in increasing overall gene expression. The eventual therapeutic application was to reduce electric field while maintaining gene expression levels. It was clearly demonstrated that heat can in fact assist in increasing gene expression levels. However, this expression is dependant upon the heating temperature and the GET field combined. The “optimal” heating temperatures varied for the different GET conditions. Another important factor to consider was the effect on cell viability. The effect of heat on DNA alone resulted in approximately 20% cell death. However, with the application of electric fields that can result in high levels of gene expression this effect is “masked” by the cell death from electrical pulses. Also in each case the percent of positive cells was increased as seen in the flow cytometry analysis. Based on these results the following three methods should be considered when utilizing increased temperatures with electrotransfer: 1. If the overall goal of the experimentation is to increase gene expression with no concern for the effect on viability then a high electric field with a low application of heat is a possible choice. 2. Taking into consideration both increasing gene expression and an increase in viability one can decrease the GET field by nearly 20% with only a moderate application of heat. 3. However if increasing viability is the primary goal, but high gene expression levels are still important then one can reduce the GET field by 1/3 with only a moderate application of heat while maintaining viability at 70–80%.
Acknowledgments
This research was supported by the Frank Reidy Research Center for Bioelectrics. The authors would like to thank Dr. Malik at ODU Center for Bioelectrics for designing the in vitro electrode used in this study.
References
- [1].Daud AI, et al. , Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma, J. Clin. Oncol. 26 (36) (2008) 5896–5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Heller LC, Heller R, In vivo electroporation for gene therapy, Hum. Gene Ther. 17 (9) (2006) 890–897. [DOI] [PubMed] [Google Scholar]
- [3].Saade F, Petrovsky N, Technologies for enhanced efficacy of DNA vaccines, Expert Rev. Vaccines 11 (2) (2012) 189–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Weiland O, et al. , Therapeutic DNA vaccination using in vivo electroporation followed by standard of care therapy in patients with genotype 1 chronic hepatitis C, Mol. Ther.21 (9) (2013) 1796–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bagarazzi ML, et al. , Immunotherapy against HPV16/18 generates potent TH1 and cytotoxic cellular immune responses, Sci. Transl. Med. 4 (155) (2012) 155ra138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hojman P, Basic principles and clinical advancements of muscle electrotransfer, Curr.Gene Ther.10 (2) (2010) 128–138. [DOI] [PubMed] [Google Scholar]
- [7].Gothelf A and Gehl J, Gene electrotransfer to skin; review of existing literature and clinical perspectives. Curr. Gene Ther. 10(4): p. 287–99. [DOI] [PubMed] [Google Scholar]
- [8].Andre FM, Mir LM, Nucleic acids electrotransfer in vivo: mechanisms and practical aspects, Curr. Gene Ther. 10 (4) (2010) 267–280. [DOI] [PubMed] [Google Scholar]
- [9].Guo S, et al. , Electro-gene transfer to skin using a noninvasive multielectrode array. J Control. Release. 151(3): p. 256–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lin F, et al. , Optimization of electroporation-enhanced intradermal delivery of DNA vaccine using a minimally invasive surface device, Hum. Gene Ther. Methods 23 (3) (2012) 157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Heller LC, et al. , Optimization of cutaneous electrically mediated plasmid DNA delivery using novel electrode, Gene Ther. 14 (3) (2007) 275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ferraro B, et al. , Evaluation of delivery conditions for cutaneous plasmid electrotransfer using a multielectrode array. Gene Ther. 18(5): p. 496–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Heller R, et al. , Electrically mediated delivery of plasmid DNA to the skin, using a multielectrode array. Hum. Gene Ther. 21(3): p. 357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Donate A, Heller R, Assessment of delivery parameters with the multi-electrode array for development of a DNA vaccine against Bacillus anthracis, Bioelectrochemistry 94 (2013) 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Donate A, et al. , Evaluation of a novel non-penetrating electrode for use in DNA vaccination, PLoS One 6 (4) (2011) e19181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Guo S, et al. , Topical gene electrotransfer to the epidermis of hairless guinea pig by non-invasive multielectrode array, PLoS One 8 (8) (2013) e73423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Broderick KE, et al. , Prototype development and preclinical immunogenicity analysis of a novel minimally invasive electroporation device. Gene Ther. 18(3): p. 258–65. [DOI] [PubMed] [Google Scholar]
- [18].Shen X, et al. , Influenza A vaccines using linear expression cassettes delivered via electroporation afford full protection against challenge in a mouse model, Vaccine 30 (48) (2012) 6946–6954. [DOI] [PubMed] [Google Scholar]
- [19].Bhatt DL, Gaylor DC, Lee RC, Rhabdomyolysis due to pulsed electric fields, Plast. Reconstr. Surg. 86 (1) (1990) 1–11. [DOI] [PubMed] [Google Scholar]
- [20].Alberts B, Molecular Biology of the Cell, 4th ed.xxxiv Garland Science, New York, 2002. (1548 pp.). [Google Scholar]


