Abstract
Extensive work has characterized endoplasmic reticulum (ER) and mitochondrial stress responses. In contrast, very little has been published about stress responses in lysosomes; subcellular acidic organelles that are physiologically important and are of pathological relevance. The greater lysosomal system is dynamic and is comprised of endosomes, lysosomes, multivesicular bodies, autophagosomes, and autophagolysosomes. They are important regulators of cellular physiology, they represent about 5% of the total cellular volume, they are heterogeneous in their sizes and distribution patterns, they are electron dense, and their subcellular positioning within cells varies in response to stimuli, insults and pH. These organelles are also integral to the pathogenesis of lysosomal storage diseases and it is increasingly recognized that lysosomes play important roles in the pathogenesis of such diverse conditions as neurodegenerative disorders and cancer. The purpose of this review is to focus attention on lysosomal stress responses (LSR), compare LSR with better characterized stress responses in ER and mitochondria, and form a framework for future characterizations of LSR. Here, we present the concept of LSR such that the definition of LSR can be modified as new knowledge is added and specific therapeutics are developed.
Keywords: endosomes, lysosomes, inter-organellar signaling, endoplasmic reticulum stress, mitochondrial stress
INTRODUCTION
Lysosomes were described in 1955 by Christian de Duve and since that time it has become increasingly obvious that these acidic organelles are physiologically important as well as pathologically relevant (de Duve, 2005). Decades ago, lysosomes were simply thought to be cellular waste bins and endpoints of cellular degradation events. Now, however, increasingly appreciated are the many cellular functions homeostatically regulated by lysosomes (Settembre et al., 2013; Xu and Ren, 2015; Pu et al., 2016). They represent about 5% of the total cellular volume, they are heterogeneous in their sizes and distribution patterns, they are electron dense, and their subcellular positioning in the cytoplasm varies in response to stimuli, insults and pH (Holtzman, 1989; Wartosch et al., 2015; Pu et al., 2016).
Lysosomes are integral to the greater lysosomal system, which is also comprised of endosomes, multivesicular bodies (MVB), autophagosomes, and autophagolysosomes (Huotari and Helenius, 2011). The importance and the dynamic nature of endosomes and lysosomes, henceforth referred to as endolysosomes, continues to be recognized in many ways including the awarding of Nobel Prizes for the discovery of lysosomes themselves, for receptor-mediated endocytosis, and for autophagy. These organelles are also integral to the pathogenesis of lysosomal storage diseases (LSD) and it is increasingly recognized that lysosomes play important roles in the pathogenesis of a variety of diseases including neurodegenerative disorders and cancer. However, when considered in the same vein as other subcellular organelles it could be argued that lysosomes do not receive the same degree of recognition as do other intracellular organelles. While endoplasmic reticulum (ER) and mitochondria have well known and clearly described stress responses (Martinus et al., 1996; Zhao et al., 2002; Huotari and Helenius, 2011; Oakes and Papa, 2015; Valera-Alberni and Canto, 2018), lysosomal stress responses (LSR) are ill-defined and have been referred to in the literature only sparingly.
The purpose of this review is to focus attention on LSR and initiate a framework for its existence, its characterization, its physiological importance, and its pathological relevance. First, we provide an overview of lysosome biogenesis, function and dysfunction. Second, we discuss ER and mitochondrial stress responses in order to provide a context for LSR. Third, we discuss the characterization of LSR as well as review briefly ER stress and mitochondrial stress responses. Ultimately, a better understanding of stress responses in lysosomes may enhance our understanding of cellular physiology and disease pathogenesis and promote the discovery of new treatments for diseases.
OVERVIEW OF LYSOSOME BIOGENESIS, FUNCTION AND DYSFUNCTION
Lysosome biogenesis:
Lysosome biogenesis includes the formation of lysosomes through the maturation of endocytic vesicles as well as the delivery of newly synthesized lysosomal enzymes and membrane proteins from the trans-Golgi network (Kornfeld and Mellman, 1989; Saftig and Klumperman, 2009). The maturation process of endosomes has been extensively reviewed elsewhere (Kornfeld and Mellman; Saftig and Klumperman; Huotari and Helenius, 2011; Luzio et al., 2014; Bajaj et al.), but to provide context we briefly outline this process here. When extracellular cargo is endocytosed, it is trafficked into early endosomes and from there it is either recycled back to the plasma membrane or alternatively it is degraded in lysosomes (Saftig and Klumperman, 2009; Huotari and Helenius, 2011). Cargo that is destined for degradation stays contained in endosomes as they mature from early endosomes to late endosomes, and during this maturation endosomes undergo changes in size and morphology, become more acidic, traffic perinuclearly, and form intraluminal vesicles (ILVs); late endosomes containing ILVs are termed MVBs (Huotari and Helenius, 2011). MVBs can fuse with existing MVBs or late endosomes to form late endosomes. Late endosomes can fuse with existing lysosomes to form endolysosomes where degradation of cargo takes place. After fusion, endolysosomes are converted into lysosomes where acidic hydrolases and lysosomal membrane proteins are stored (Huotari and Helenius, 2011).
The biosynthesis and delivery of lysosomal enzymes and membrane proteins are critical for the development and proper functioning of lysosomes. It is well understood that most newly synthesized lysosomal proteins are post-translationally modified in the Golgi complex (Kornfeld and Mellman; Braulke and Bonifacino, 2009; Saftig and Klumperman; Luzio et al., 2014). Lysosomal hydrolases are inserted into the ER lumen where they receive signal sequence cleavage and core glycosylation followed by trafficking to the cis-Golgi network (CGN). In the CGN, lysosomal enzymes are unmasked revealing a mannose-6-phosphate (M6P) signal (Kornfeld and Mellman, 1989). Once these proteins reach the trans-Golgi network (TGN), they are recognized by M6P receptors (M6PR) (Kornfeld and Mellman, 1989; Saftig and Klumperman, 2009). The binding of M6P tagged proteins to M6PR leads to receptor-dependent vesicle transport of these proteins to endolysosomes via transport vesicles derived from the TGN. When lysosomal proteins reach their destination in the acidic environment of endolysosomes there is a dissociation between M6PRs and M6P-tagged proteins; M6PRs are recycled back to the TGN (Kornfeld and Mellman, 1989; Saftig and Klumperman, 2009).
Lysosome biogenesis (Figure 1) is coordinated by transcription factor EB (TFEB) (Sardiello et al., 2009). TFEB is a member of the microphthalmia-transcription factor E (MiT/TFE) family of proteins, which are involved in responding and adapting to intracellular stresses (Rehli et al., 1999; Steingrimsson et al., 2004; La Spina et al., 2020). The subcellular localization of TFEB is modified by kinases and phosphatases; phosphorylated TFEB targets the cytosol and de-phosphorylated TFEB targets the nucleus. The serine/threonine kinase mammalian target of rapamycin complex 1 (mTORC1) phosphorylates TFEB on serine residues 211 and 122, which results in binding by 14-3-3 proteins. Other serine/threonine kinases such as mitogen-activated protein kinases 1 (MAPK1), protein kinase B (Akt), glycogen synthase kinase 3ß (GSK3ß), and mitogen-activated protein kinase kinase kinase kinase 3 phosphorylate TFEB at Ser142, Ser467, Ser134/138, and Ser3, respectively (Settembre et al., 2011; Li et al., 2016b; Palmieri et al., 2017; Hsu et al., 2018). Phosphorylation of TFEB results in its cytosolic sequestration and an inhibition of its ability to upregulate lysosome related genes (Martina et al.; Roczniak-Ferguson et al., 2012; Vega-Rubin-de-Celis et al., 2017). Genes that encode for lysosome function contain a coordinated lysosomal expression and regulation (CLEAR) binding element in their promoter region. TFEB is dephosphorylated by the phosphatases calcineurin and protein phosphatase 2 (Sardiello et al.; Medina et al., 2015; Chen et al., 2018; Martina and Puertollano, 2018). Dephosphorylated TFEB translocates to the nucleus where it binds to CLEAR elements and promotes an increase of lysosomal-, autophagy-, and mitochondrial-gene expression (Sardiello et al., 2009; Settembre et al., 2011). Recently, a mechanistic study revealed how TFEB activity is regulated. STIP1 homology and u-box containing protein 1 (STUB1) increases TFEB activity by clearing ubiquitinated and phosphorylated TFEB for proteasomal degradation (Sha et al., 2017); mTORC1 phosphorylates TFEB which is proposed to form heterodimers with non-phosphorylated TFEB thus sequestering TFEB to the cytosol and preventing the promotion of lysosome biogenesis (Sha et al., 2017).
Fig 1.
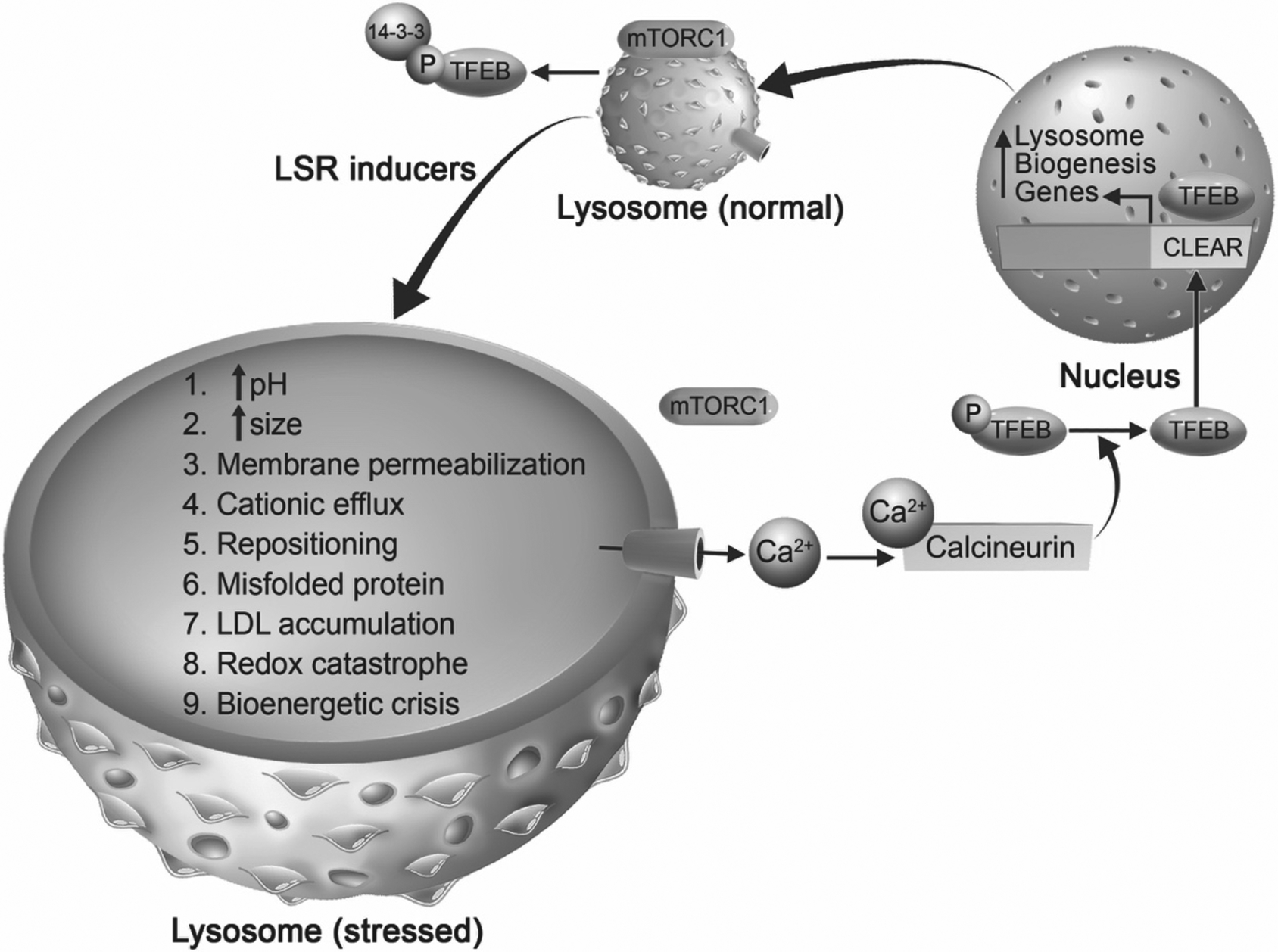
Involvement of mTOR-TFEB in mediating lysosomal stress responses (LSR). Under unstressed conditions, mammalian target of rapamycin complex 1 (mTORC1) is localized on lysosome membranes and phosphorylates transcription factor EB (TFEB), which is a master regulator of lysosome biogenesis. Phosphorylated TFEB is bound by 14-3-3 protein promoting the sequestration of TFEB in the cytoplasm. Under stressful conditions, which can be caused by any drug, pathogen, or material that enters the endolysosome system (termed LSR inducers), lysosomes undergo lysosomal stress responses (labeled 1–9). We posit that during LSR, stressed lysosomes can use various signals, one of which being mTOR-TFEB signaling pathway to promote lysosome biogenesis. When lysosomes are stressed, mTORC1 is released from lysosome membranes, calcium is released through calcium permeable channels (depicted by blue cylinder) into the cytosol where it activates the cytosolic phosphatase calcineurin. Activated calcineurin dephosphorylates TFEB allowing it to translocate into the nucleus where it binds at the coordinated lysosomal expression and regulation (CLEAR) element of lysosome biogenesis genes. Subsequently, there is an increase of lysosome-related genes that lead to the possible restoration of stressed lysosomes. Thus, LSR may use lysosome biogenesis as one of the pathways to restore lysosome function.
Lysosome function:
Lysosomes are single membrane acidic organelles that function to degrade intracellular cargo (Holtzman, 1989). These organelles are heterogeneous in size (range 0.5 to 1.5 nm), have intraluminal pH values ranging from 4.5 to 6.0, and contain over 60 different acidic hydrolase enzymes (Xu and Ren, 2015). The intraluminal pH of lysosomes is maintained by membrane-resident ion channels and vacuolar-ATPases (Steinberg et al., 2010; Huotari and Helenius, 2011; Mindell, 2012; Colacurcio and Nixon, 2016). The acidic lumen of lysosomes provides an environment that is conducive for optimal activity levels of resident hydrolase enzymes which catalyze the degradation of cargo that is received via endocytic or autophagic pathways (Kornfeld and Mellman, 1989). Lysosomes also contain readily releasable stores of biologically important divalent cations including iron and calcium (Xu and Ren, 2015; Lakpa et al., 2020). Intracellularly, the positioning of endolysosomes is dynamic and their positioning in the cytoplasm changes markedly according to many factors including cell type and intraluminal pH; highly acidic endolysosomes are perinuclearly positioned while de-acidified endolysosomes are re-positioned more towards the periphery of cells near plasma membranes (Johnson et al., 2016; Pu et al., 2016).
As mentioned above, lysosomes can receive cargo from endocytic or autophagic pathways. Once cargo enters the endolysosomal system it has two fates, it can either be trafficked toward lysosomes to be degraded or it can be recycled to the plasma membrane. Along the maturation path of endosomes to lysosomes, intraluminal vesicles begin to form in endosomes thus creating MVBs (van Niel et al., 2018). Cargo within MVBs can be trafficked toward the plasma membrane to release contents into the extracellular space; these secreted ILVs are termed exosomes; a type of extracellular vesicles. The release of exosomes can potentially serve as intercellular signals, therapeutic delivery-vesicles, and biomarkers for various pathologies (Lener et al., 2015; Fais et al., 2016; Torrano et al., 2016; Maia et al., 2018).
Autophagy is the means by which cytoplasmic material is degraded. There are different forms of autophagy such as macro-autophagy (which will be referred to as autophagy), micro-autophagy (selective autophagy), and chaperone-mediated autophagy (Ravikumar et al., 2010; Galluzzi et al., 2017). Intracellular cargo is engulfed by double membrane vesicles called autophagosomes; autophagosome formation, elongation, and maturation have been reviewed elsewhere (Ravikumar et al., 2010). Important to the clearance of damaged proteins and dysfunctional organelles via autophagy is the fusion of autophagosomes with lysosomes forming autolysosomes (Ravikumar et al., 2010; Galluzzi et al., 2017; Yim and Mizushima, 2020). In post-mitotic cells, such as neurons, the autophagic-lysosome system is important for the maintenance of neuronal health (Kuijpers et al., 2020).
Apart from their degradative roles, lysosomes also participate in intracellular nutrient sensing (Settembre et al., 2013; Xu and Ren, 2015; Perera and Zoncu, 2016). Lysosomes use mTOR and TFEB to sense and regulate intracellular nutrient conditions, and the roles of mTOR and TFEB in lysosome nutrient sensing and cellular function were reviewed extensively elsewhere (Settembre et al., 2013; Perera and Zoncu, 2016; Rabanal-Ruiz and Korolchuk, 2018). mTOR is part of a larger protein complex that interacts with lysosome membranes and senses cytosolic nutrient levels (Rabanal-Ruiz and Korolchuk, 2018). During high nutrient conditions, mTOR is recruited to lysosome membranes where it is activated and initiates downstream effectors that promote cell growth and proliferation. Conversely, during low nutrient conditions mTOR is inactive, disassociates from lysosome membranes, and is no longer able to phosphorylate TFEB; dephosphorylated TFEB promotes the production of lysosome genes (Sardiello et al., 2009; Napolitano and Ballabio, 2016; Rabanal-Ruiz and Korolchuk, 2018).
Lysosome dysfunction:
Central to lysosome dysfunction appears to be an increase in intraluminal pH - lysosome deacidification. Lysosome de-acidification causes many changes to the biology of cells including endolysosome positioning in the cytoplasm, endolysosome sizes and volumes, intraluminal and cytosolic levels of divalent cations, expression levels and activity levels of acid hydrolases, and the aggregation of various proteins. And, as we will expand on later, these changes are also central to the concept of LSR.
The intracellular distribution (positioning) of endolysosomes in the cytoplasm is affected by endolysosome de-acidification; an increase in endolysosome pH causes redistribution of lysosomes away from the nucleus and more toward plasma membranes (Heuser, 1989; Parton et al., 1991; Johnson et al., 2016). The cytosolic pH which is influenced by H+ ions originating from endolysosomes also affects endolysosome positioning (Gottschling and Nystrom, 2017); cytosolic acidification promotes the localization of lysosomes towards plasma membranes and cytosolic alkalinization leads to perinuclear localization of lysosomes (Heuser, 1989; Parton et al., 1991; Halcrow et al., 2019). Although the biological significance of the pH-dependent re-positioning of endolysosomes in cells is unclear, some have shown that the location of endolysosomes dictates their pH and not that their pH dictates the location (Johnson et al., 2016). Microtubules and motor proteins also contribute to the translocation and clustering of endolysosomes (Matteoni and Kreis, 1987; Cabukusta and Neefjes, 2018).
Morphologically, endolysosome de-acidification leads to increased endolysosome size and an accumulation of undigested intracellular cargo (Ohkuma and Poole, 1981; Myers et al., 1991; Yoshimori et al., 1991). Endolysosome de-acidification also leads to impaired fusion between autophagosomes and lysosomes, and such changes have been implicated in cell death (Doherty and Baehrecke, 2018; Mauthe et al., 2018).
Endolysosomes contain readily releasable stores of biologically important divalent cations including calcium, iron, copper and zinc. De-acidification of endolysosomes induces the efflux of calcium and iron (Christensen et al., 2002; Kiselyov et al., 2011; Kurz et al., 2011; Hui et al., 2015; Lee et al., 2015; Xu and Ren, 2015; Fernández et al., 2016) such that accumulations are measurable in cytosol, mitochondria, and ER. As a result of decreased levels of cations in endolysosomes, downstream events can occur including ER stress as well as mitochondrial redox catastrophe and bioenergetic crisis (Uchiyama et al., 2008; Baixauli et al., 2015; Martina et al., 2016; Lv and Shang, 2018; Wang et al., 2018; Nakashima et al., 2019).
Lysosome dysfunction in neurodegenerative diseases:
Dysfunctional lysosomes have been implicated in several neurodegenerative disorders including lysosomal storage diseases (LSD), Alzheimer’s disease (AD), Parkinson’s disease (PD), and Huntington’s disease (HD) (Martini-Stoica et al., 2016; Audano et al., 2018; Bonam et al., 2019; Nguyen et al., 2019; Wallings et al., 2019). Neurological complications are observed in people living with LSD, which are characterized by intra-lysosomal aggregation of substrates due to mutations in lysosome hydrolases (Platt, 2018; Sun, 2018). The inhibition of autophagosome-lysosome fusion and/or the accumulation of autophagosomes in LSDs could be a potential mechanism underlying the neuropathogenesis of LSDs (Tanaka et al., 2000; Koike et al., 2005; Cao et al., 2006; Fukuda et al., 2006; Settembre et al.). Further observed in LSD are impaired autophagy of damage mitochondria (mitophagy), increased production of reactive oxygen species, and bioenergetic crisis.
Amyloid plaques and hyperphosphorylated tau are hallmarks of AD and in AD there is lysosome dysfunction and an impairment of autophagic flux. Endolysosomes are the sites where amyloidogenic processing of amyloid precursor protein occurs; the result is accumulation of amyloid beta (Aß) proteins Aß40 and Aß42 (Oikawa and Walter, 2019). Neurofibrillary tangles are made from hyperphosphorylated tau protein and lysosomal enzymes (cathepsin B, D, and E) generate and digest both Aß and tau (Siman et al., 1993). Lysosome dysfunction leads to an accumulation of Aß42 in neurons, inhibition of lysosomal cathepsins B and E, increases expression of cathepsin D, increases lysosomal pH, impairs autophagy, and changes permeability of lysosomal membranes (Cataldo et al., 1991; Cataldo et al., 1995; Bi et al., 2000; Wolfe et al., 2013). Furthermore, defective autophagy and mitophagy are present in AD (Reddy and Oliver, 2019).
The hallmark of PD are Lewy bodies which are made up of alpha-synuclein aggregates in neurons. Genetic mutations in GBA1 (encoding for lysosomal hydrolase glucocerebrosidase), which is the most common genetic factor of PD is linked to Lewy body formation (Wallings et al., 2019). Extracellular alpha-synuclein can be internalized into endolysosomes via clathrin-mediated endocytosis leading to changes in lysosome morphology, decreased cathepsin D activity, increased lysosome pH, and impaired fusion with autophagosomes (Winslow et al., 2010; Hoffmann et al., 2019).
HD is a rare autosomal-dominant neurodegenerative disease that is caused by a mutation in the HTT gene leading to HTT mutants (mHTT) prone to aggregation. mHTT localizes in lysosomes, impairs autophagy, inhibits trafficking of non-mutant HTT to lysosomes, and changes cathepsin activity (Ravikumar et al., 2002; Ravikumar et al., 2004; Rubinsztein, 2006; del Toro et al., 2009; Liang et al., 2011; Ratovitski et al., 2011; Trajkovic et al., 2017; Wallings et al., 2019). Interestingly, one study shows that mHTT induces perinuclear localization of lysosomes, reduces lysosome mobility, and promotes premature fusion of lysosomes with autophagosomes (Erie et al., 2015).
Impairment of the autophagic-lysosomal pathway can also promote the release of toxic intracellular protein aggregates in exosomes; such observations have been made for AD, PD, and HD (Danzer et al., 2012; Perez-Gonzalez et al., 2012; Trajkovic et al., 2017; Miranda et al., 2018; Sardar Sinha et al., 2018; Guix, 2020). An increase in the release of undigested substrates and stored material also occurs in LSDs (Tancini et al., 2019)
ER AND MITOCHONDRAL STRESS RESPONSES
Stress is a holistic concept that affects organisms at every conceivable level; minor stressors can promote resilience while major stressors can induce cell death (Figure 3). Cellular stressors include, but are not limited to, hypoxia, heat shock, nutrient depletion, and genetic defects (Deus et al., 2020). Thus, organelles have developed stress responses to counter the potential detrimental effects of stress. It happens with ER, it happens with mitochondria, and, as we will later describe, it happens with lysosomes where stress responses attempt to maintain and/or restore organellar function. In order to contextualize the concept of LSR, we will first discuss ER and mitochondria stress responses.
Fig. 3.
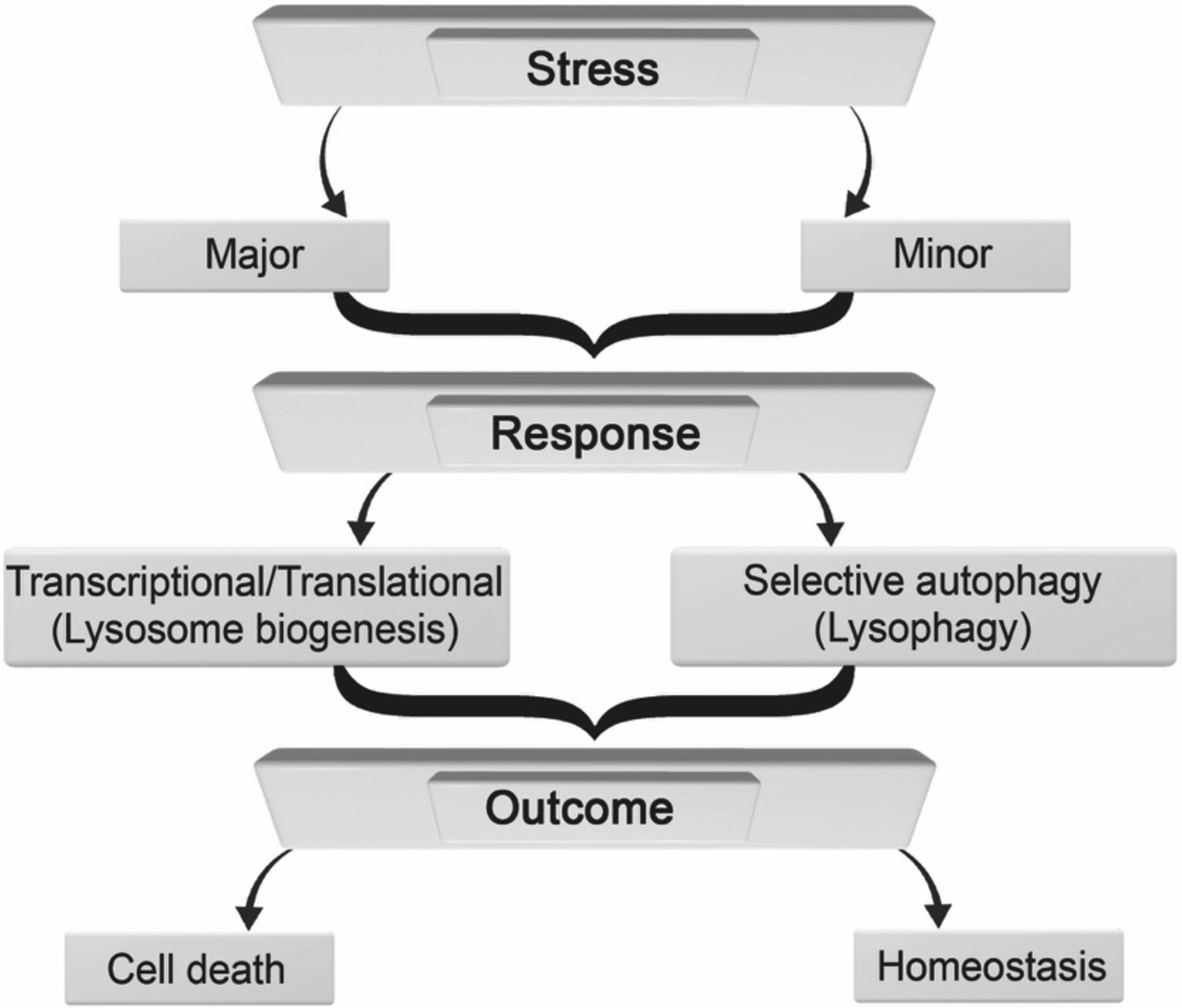
Flow diagram depicting responses and outcomes resulting from lysosomal stress. High levels of lysosomal stress (major) can lead to transcriptional/translational changes including increases in lysosome biogenesis. Lower levels of lysosomal stress (minor) can lead to selective autophagy and/or lysophagy. In either case, outcomes can include cell life or death
ER stress response:
ER helps to maintain calcium homeostasis and lipid metabolism, and are sites for synthesis and proper folding of secretory and transmembrane proteins. Molecular chaperones in ER bind newly synthesized proteins and facilitate the acquisition of three-dimensional structures. Proteins are then delivered to Golgi apparatus where they undergo post-translational modifications. In the case of misfolded proteins, proteins are released from ER into the cytosol where they are targeted for proteasomal degradation; ER associated degradation (ERAD) (Yanagawa et al.; Ruggiano et al., 2014). When levels of misfolded proteins exceed ERAD capacity, ER stress is triggered and accumulation of misfolded and/or unfolded proteins occurs in the lumen of ER (Bravo et al., 2013; Mori, 2015). In response to ER stress, the unfolded protein response (UPR) is activated, and this results in halting the translation of and increasing gene expression of ER folding proteins (Kohno et al., 1993; Partaledis and Berlin, 1993; Bravo et al., 2013; Mori, 2015). UPR was first discovered in 1988 for glucose deprivation initiating the accumulation of misfolded proteins within the ER lumen, which then leads to a subsequent transcriptional response to increase ER-resident molecular chaperones (Kozutsumi et al., 1988; Mori, 2015).
ER uses three resident transmembrane proteins as sensors of ER stress and initiators of the UPR signaling pathways; inositol requiring protein 1 alpha (IRE1α), activating transcription factor 6 (ATF6), and protein kinase RNA-like ER kinase (PERK) (Hetz, 2012; Sasaki and Yoshida, 2015). IRE1α senses ER stress luminally, is activated via oligomerization and phosphorylation of its cytosolic domain, and once activated splices immature X box protein 1 (XBP1) mRNA into mature XBP1 mRNA. After protein translation, XBP1 is translocated into the nucleus where it acts as a transcription factor to promote the expression of ERAD genes (Lee et al., 2003; Yoshida et al., 2003). PERK is an ER transmembrane protein (Harding et al., 1999; Harding et al., 2000a) and similar to IRE1α, PERK is activated by oligomerization and phosphorylation when it senses ER stress luminally (Harding et al., 2000b; Harding et al., 2000a). Once activated, PERK phosphorylates eukaryotic translational initiation factor 2 alpha (eIF2α), which reduces protein translation and increases translation of activating transcription factor 4 (ATF4); ATF4 is a transcription factor that increases gene expression of anti-oxidation and pro-apoptotic genes (Harding et al., 2000a; Harding et al., 2003). The ER transmembrane protein ATF6 senses ER stress and once translocated to Golgi apparatus, it is cleaved by proteases (Shen et al., 2002). Then, the cytosolic domain of ATF6 is translocated to the nucleus to increase the expression of ER chaperone genes (Ye et al., 2000).
Mitochondrial stress response:
Mitochondria produce energy in the form of adenosine triphosphate (ATP) via oxidative phosphorylation, serve as sinks for cations such as calcium and iron, and help maintain redox balance. Although mitochondria contain their own genome, the majority of mitochondrial proteins are nuclear-encoded and those proteins are translated in the cytoplasm (Sasaki and Yoshida, 2015). Unfolded proteins are transported from the cytoplasm into mitochondria via heat shock protein 70 (HSP70); a molecular chaperone (Ryan and Hoogenraad, 2007). Once translocated into the inner membrane space of mitochondria, the proper folding of proteins is assisted by mitochondrial HSP70 (mtHSP70) and mtHSP60 (Endo et al., 2011; Sasaki and Yoshida, 2015).
Mitochondrial stress is characterized by redox catastrophe and bioenergetic crisis caused by dysfunctional oxidative phosphorylation, increased levels of ROS, and/or accumulation of misfolded proteins (Hill et al., 2018). Mitochondrial UPR (UPRMT) was first described in 2004 (Yoneda et al., 2004); it helps restore mitochondrial function (Martinus et al., 1996; Zhao et al., 2002; Manoli et al., 2007; Huang et al., 2019) via transcriptional signaling pathways (Sasaki and Yoshida, 2015) and removing the mitochondrial genome or mutating matrix proteins (Martinus et al., 1996; Zhao et al., 2002).
LYSOSOME STRESS RESPONSE (LSR)
Lysosome stress:
The term “lysosomal stress” appears to have been first mentioned in 1968 in the context of a freeze-thaw method used to rupture lysosomes (Persellin, 1969). However, it appeared to take another 50 years before “lysosome stress” was described as “any disturbance in lysosomal membrane integrity, enzyme function, or internal pH that leads to cell damage or even diseases” (Lin et al., 2016). We posit that in addition to membrane integrity, enzyme function and pH, more recent reports from others and us strongly suggests that additional criteria be added including size, efflux of cations, organelle repositioning within cells, protein aggregation, LDL cholesterol accumulation, and increases in reactive oxygen species (Hui et al., 2012a; Hui et al., 2012b; Bae et al., 2014; Gowrishankar et al., 2015; Lin et al., 2016; Papadopoulos et al., 2017; Yogalingam et al., 2017; He et al., 2018; Li et al., 2019; Pan et al., 2019). These criteria are consistent with findings from the study of LSDs; mutations in lysosomal acid hydrolases lead to aggregation of substrates, elevated luminal pH, altered morphology and subcellular distribution patterns, and lowered luminal concentrations of biologically important metal ions (Holopainen et al., 2001; Fossale et al., 2004; Raben et al., 2007; Yanagawa et al., 2007; Lloyd-Evans et al., 2008; Ballabio and Gieselmann, 2009; Lee et al., 2011; Zigdon et al., 2017; Platt, 2018; Sun, 2018; Marques and Saftig, 2019).
Characterization of lysosome stress response (LSR):
In this section we provide a framework for characterizing LSR. The greater lysosomal system is complex and so too is the nomenclature used to describe lysosome function, dysfunction and stress. Although there is likely overlap between the concepts of lysosome stress, LSR and lysosome dysfunction, there are also differences. Lysosome dysfunction is the progressive accumulation of undegraded substrates within lysosomes (Settembre and Ballabio, 2014) and we posit that lysosome stress is a state that lysosomes enter into due to internal or external stimuli; the lysosome responses to those stimuli characterize LSR. Currently, we propose nine criteria with which to characterize LSR; (1) increased intralysosomal pH, (2) increased lysosome size, (3) membrane permeabilization, (4) cationic efflux, (5) repositioning intracellularly, (6) misfolded protein aggregation, (7) LDL cholesterol accumulation, (8) redox catastrophe, and (9) bioenergetic crisis (see Figure 2). Principal among these 9 criteria might be lysosome de-acidification because lysosome de-acidification can trigger each of the other 8 criteria involved in LSR. LSR might be distinct from lysosome dysfunction because LSR is the means by which lysosomes attempt to adapt to environmental cues. The concept of LSR is built on the foundation of lysosome adaptation, which is the ability of lysosomes to respond to metabolic changes (Settembre and Ballabio, 2014). Here, we are broadening the idea of lysosome adaptation; LSR is lysosomal adaptation to stimuli such as pathogens, drugs, molecules or other material that could affect the greater endolysosome system. LSR utilizes selective autophagy and transcriptional response machinery in an attempt to restore lysosome function. Thus, the term – LSR.
Fig. 2.
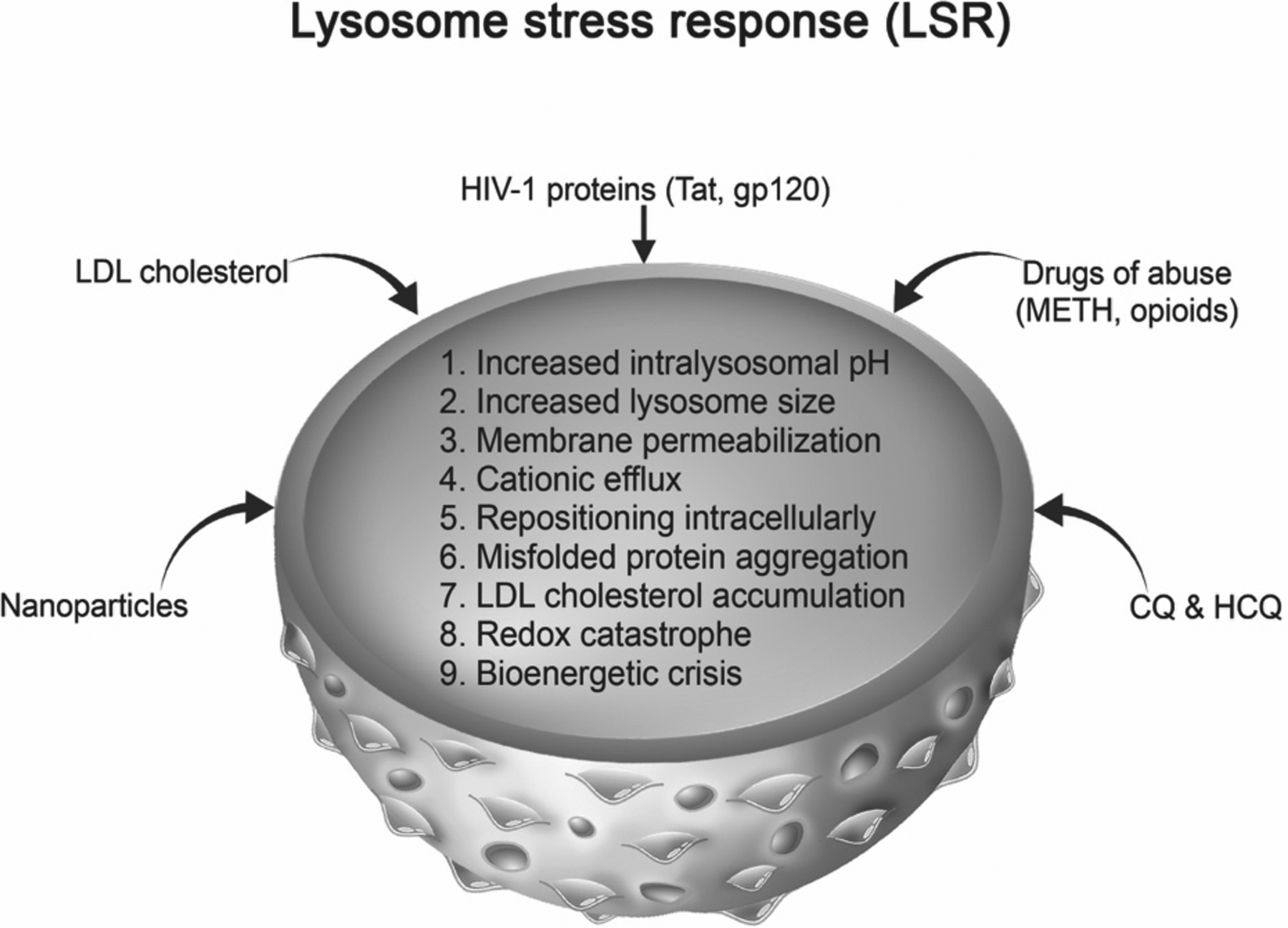
Lysosome stress response characteristics and examples of stimuli that cause LSR. Lysosomal stress responses can be characterized by (1) increased lysosome pH, (2) increased lysosome size, (3) lysosomal membrane permeabilization, (4) cationic efflux, (5) intracellular repositioning, (6) intraluminal protein aggregation, (7) intraluminal accumulation of LDL cholesterol, (8) redox catastrophe, and (9) bioenergetic crisis. Endogenous and exogenous stimuli such as the HIV-1 proteins Tat and gp120, drugs of abuse including methamphetamine and opioids, nanoparticles, LDL cholesterol, and chloroquine (CQ) and hydroxychloroquine (HCQ) can induce lysosome stress
For both ER and mitochondrial stress, selective autophagy works to remove damaged organelles (Kubli and Gustafsson, 2012; Galluzzi et al., 2017; Chino and Mizushima, 2020; Liang et al., 2020). When lysosomes are damaged, they are cleared through selective autophagy; lysophagy (Galluzzi et al., 2017; Yu et al., 2018). Lysophagy detects and clears membrane-permeabilized endolysosomes (Papadopoulos and Meyer, 2017). Permeabilization of lysosome membranes leads to the binding of galectins to sugars on the inner leaflet of lysosome membranes; this initiates ubiquitination and autophagy of damaged endolysosomes (Papadopoulos et al., 2017; Papadopoulos and Meyer, 2017; Koerver et al., 2019). Relatedly, the term “endo-lysosomal damage response” was used to describe autophagic clearance of damaged lysosomes via p97 and other cofactors (Papadopoulos et al., 2017). Lysophagy may also help with homeostatically-regulated responses.
Although, ER and mitochondrial stress responses have defined sensors of stress the sensors for LSR are unclear. It is possible that an increase in lysosome pH can trigger a host of changes, described above, that can act as stress signals. For example, lysosome biogenesis can be promoted by the release of cations from endolysosomes. Endolysosomes contain levels of calcium approaching those found in ER (Feng and Yang, 2016) and when de-acidified, readily releasable pools of calcium are released from endolysosomes and initiate transcriptional responses to increase lysosome biogenesis via TFEB (Christensen et al., 2002; Settembre et al., 2013; Hoglinger et al., 2015; Medina et al., 2015; Lu et al., 2017). One potential LSR stress sensor could be the endolysosome resident transient receptor mucolipin 1 channel (TRPML1). TRPML1 is a divalent cation efflux channel and is implicated in sensing elevated reactive oxygen species (Zhang et al., 2016). Upon activation, TRPML1 can release calcium, and this calcium can activate calcineurin and lead to TFEB dephosphorylation (Medina et al., 2015) (Figure 1).
The status of TFEB during LSR might depend on the magnitude and duration of the stressor. Under minor stress conditions, which might occur physiologically, TFEB is phosphorylated by mTORC1 or other kinases, and localized in the cytosol bound to the phospho-binding 14-3-3 protein (Settembre et al., 2013; Medina et al., 2015). Under major stress conditions, which might occur pathologically, TFEB is dephosphorylated and translocates into the nucleus, activates the CLEAR gene network and leads to an up-regulation of genes involved in lysosome functioning (Martini-Stoica et al., 2016; Lu et al., 2017). These responses mirror those of the unfolded protein response (UPR) in the ER, and those in mitochondria (Bravo et al., 2013; Sasaki and Yoshida, 2015) (Figure 3). Although speculative, such responses may play important roles in LSR. Therefore, LSR can be posited to be a homeostatic control mechanism that functions to protect against and/or rescue stressed lysosomes.
In the context of therapeutics, it seems that targeting components of the mTOR-TFEB signaling pathway restores cellular functions in in vitro and in vivo models of LSD. For example, TFEB nuclear translocation has been suggested as a potential therapeutic target against LSDs. In Gaucher disease (GD), the most prevalent LSD, TFEB overexpression and the restoration of glucocerebrosidase (GCase) restored lysosome function, at least in GD-derived pluripotent stem cells (Awad et al., 2015). Similarly, in a drosophila model of GD, mTOR inhibitor rapamycin is able to partly recover the oxidative stress, locomotor, starvation and stress phenotypes (Kinghorn et al., 2016). In another study, increased expression levels of TFEB rescued GCase activity as well as the activity of β -hexosaminidase, both of which are proteins mutated in Tay-Sachs disease (Song et al., 2013). Moreover, TFEB overexpression in in vitro and in vivo models of Pompe disease caused a decrease in lysosome size, a reduction of aggregated glycogen, and an increase in autophagy (Spampanato et al., 2013). Re-acidification of damaged lysosomes is another potential therapeutic target for LSDs; lysosomal de-acidification could be a central mechanism to target in various LSDs and re-acidification of lysosomes could be a therapeutic target (Folts et al., 2016). Furthermore, re-acidification of lysosomes was found to have clinical benefits including motor improvement, weight gain, and prolonged survival (Folts et al., 2016).
INDUCERS OF LSR
In this section, we have selected to highlight 5 inducers of LSR based on the literature as well as the work we have completed in our laboratory. We acknowledge that these are not the only LSR inducers, but they are examples of how different molecules induce LSR.
Chloroquine and hydroxychloroquine:
Weak-base compounds following protonation accumulate and are trapped in lysosomes (MacIntyre and Cutler, 1988; Zhitomirsky and Assaraf, 2015); such actions may initiate LSR. Chloroquine (CQ) and hydroxychloroquine (HCQ) are both weak base FDA-approved drugs that appear to induce LSR. However, many other commonly used lysosomotropic agents may also promote LSR (Yoshimori et al., 1991; Chen et al., 2011; Settembre et al., 2012; Redmann et al., 2017; Jia et al., 2018; Zhitomirsky et al., 2018). CQ increases lysosome pH, lysosome size and lysosome membrane permeabilization, as well as inhibits protein degradation (Wibo and Poole, 1974; Lu et al., 2017; Redmann et al., 2017; Khan et al., 2019b; Khan et al., 2020b). CQ impairment of autophagosome and lysosome fusion results in bioenergetic dysfunction (Redmann et al., 2017). Further, CQ and HCQ inhibit autophagosome-lysosome fusion and increase lysosome size (Mauthe et al., 2018). In an in vivo model for age-related macular degeneration, CQ induced lysosome dysfunction; lysosomes size increased and lipid accumulation was observed (Chen et al., 2011). Moreover, CQ and other lysosomotropic drugs activate TFEB translocation to the nucleus through the inhibition of mTOR (Settembre et al., 2012; Lu et al., 2017; Zhitomirsky et al., 2018).
HIV-1 proteins:
The HIV-1 proteins, transactivator of transcription (Tat) and glycoprotein 120 (gp120) affect endolysosomes and appear to induce LSR. Tat and gp120 de-acidify endolysosomes, alter their positioning intracellularly, increase lysosome permeabilization, and promote vacuolation (Pietrella et al., 1998; Hui et al., 2012b; Bae et al., 2014; Datta et al., 2019); those effects help explain findings that Tat and gp120 increase the release of divalent cations including calcium and iron from endolysosomes (Nath et al., 1995; Nath et al., 2000; Khan et al., 2020a; Khan et al., 2020b). In the context of LSR, the damaging effects of HIV-1 proteins may be due at least in part to lysosome de-acidification, and the subsequent release of divalent cations from endolysosomes because acidification of lysosomes via activation of endolysosome-resident transient receptor mucolipin 1 (TRPML1) channel protected against gp120-induced changes to lysosomes (Bae et al., 2014). TRPML1 is also a reactive oxygen species (ROS)-sensor and calcium efflux channel, which promotes TFEB translocation (Bae et al., 2014; Zhang et al., 2016). In the context of HIV-1 replication, we studied the escape of HIV-1 Tat from endolysosomes and showed that HIV-1 Tat increases LTR activation only in the presence of CQ (Khan et al., 2018). These findings were interpreted as evidence that CQ-induced de-acidification and lysosome membrane permeabilization precipitated the escape of HIV-1 Tat from lysosomes (Khan et al., 2018; Khan et al., 2020b). The conclusion that lysosome pH was centrally involved in that response was supported by findings that lysosome acidification reduced HIV-1 Tat-induced LTR activation (Khan et al., 2019b).
In addition to HIV-1 proteins, antiretroviral therapeutic (ART) agents used to treat people living with HIV-1 can also affect endolysosomes. Antiretrovirals are a class of drugs that inhibit different stages of HIV-1 infection (Pau and George, 2014) and ARTs have been shown to affect endolysosomes (Festa et al., 2019; Hui et al., 2019). Weak-base ARTs de-acidified endolysosomes and promoted amyloidogenesis (Hui et al., 2019). These effects might contribute to the pathogenesis of HIV-1 associated neurocognitive disorder and Alzheimer’s disease-like pathology in people living with HIV-1 (Festa et al., 2019; Hui et al., 2019; Khan et al., 2019a; Afghah et al., 2020).
Low density lipoprotein (LDL) cholesterol:
Cells internalize exogenous cholesterol through receptor-mediated-endocytosis and this cholesterol traffics through the endolysosome system. For example, low-density lipoprotein receptor-related protein 1 (LRP1), part of the low-density lipoprotein receptor family, mediates endocytosis of LDL cholesterol (Lillis et al., 2015). Knockdown of LRP1 in mice increases LDL serum levels and increases cholesterol levels in isolated macrophages. Additionally, LRP1 is linked to amyloid beta clearance and is shown to be involved in multiple pathways that are associated with AD (reviewed by Kanekiyo and Bu, 2014). In the context of LSR, de-acidification of lysosomes promoted amyloidogenesis (Chen et al., 2013; Hui et al., 2019). An interesting question would be to determine the surface levels of LRP1 in in vitro and in vivo models of AD. Moreover, it might be the case that LRP1 surface level expression is reduced resulting in the extracellular accumulation of senile plaques. Although unexplored, a better understanding of LSR might reveal novel mechanisms underlying AD pathogenesis.
Late endosomes and lysosomes can export cholesterol through lysosomal membrane localized export channels; Niemann Pick C1 and 2 (NPC1/2) (Davies and Ioannou, 2000; Friedland et al., 2003). Endolysosome enlargement and reduction in luminal lysosome calcium levels are evident in Niemann Pick-type C disease, a LSD, where lysosomes are unable to export cholesterol resulting in an accumulation of cholesterol (Jin et al., 2004; Liao et al., 2007; Lloyd-Evans et al., 2008). LDL cholesterol treatment can induce LSR-type responses both in vitro and in vivo; lysosomes were enlarged in skeletal muscles of rabbits fed a cholesterol-rich diet (Chen et al., 2008). Further, endolysosomes in primary cortical neurons treated with apolipoprotein B-containing LDL were enlarged, de-acidified, and contained increased levels of cholesterol and amyloid beta (Hui et al., 2012a). Mechanistically, inactivating mTOR and increasing lysosome activity can reduce cholesterol aggregation in NPC cells (Wang et al., 2015).
Nanomaterials:
Nanomaterials can traffic into cells via endocytosis (Oh and Park, 2014) and affect the morphology and function of endolysosomes (Jia et al., 2018; Manshian et al., 2018; Ye et al., 2019). While some nanomaterials have been found to be harmful others have protective properties. Silica nanoparticles de-acidified endolysosomes, increased amyloid-beta peptide secretion, and reduced levels of calcium in endolysosomes (Ye et al., 2019). They have also been found to inhibit mTOR activity and increase nuclear TFEB localization (Jia et al., 2018). Silica nanoparticles also increased lysosome activity and degraded more readily compared to gold nanoparticles (Manshian et al., 2018). Gold nanoparticles also seem to cause dysfunction of endolysosomes; they promoted lysosome enlargement and alkalinization, inactivated mTOR, and inhibited fusion of lysosomes with autophagosomes (Ma et al., 2011). Conversely, beneficial effects of acidic nanoparticles on endolysosome function have been observed; carboxyl-modified polystyrene nanoparticles activated the TFEB-mTOR pathway and inhibited ferroptosis (Li et al., 2019). Poly (DL-lactide-co-glycolide) (PLGA) acidic nanoparticles (aNP) were found to accumulate in endolysosomes, re-acidify endolysosomes, and restore lysosome functionality (Baltazar et al., 2012). In models of Parkinson’s disease, PGLA-aNP re-acidified endolysosomes, decreased lysosome membrane permeabilization, and protected against PD-related neuronal degeneration (Bourdenx et al., 2016). Similarly, in presenilin 1 knock out cells, PGLA-aNP attenuated de-acidification, lysosomal calcium efflux, and autophagy (Lee et al., 2015).
Drugs of abuse (morphine and methamphetamine):
Several drugs of abuse including opioids and amphetamines may cause LSR; they are up-taken into endolysosomes, increase luminal pH, cause vacuolization, permeabilize membranes, and disrupt iron homeostasis (Liesse et al., 1976; Cubells et al., 1994; Patierno et al., 2011; Funakoshi-Hirose et al., 2013; Xu et al., 2018; Nash et al., 2019). Methamphetamine increased lysosome size, inhibited autophagosome-lysosome fusion (Nara et al., 2012; Funakoshi-Hirose et al., 2013), de-acidified lysosomes, impaired phagocytosis, inhibited antigen presentation, and inhibited macrophage functions during immune responses (Talloczy et al., 2008). Similarly, the opioid morphine damaged lysosomes; morphine alone and in combination with HIV-1 increased lysosome pH, and inhibited fusion between autophagosomes and lysosomes (El-Hage et al., 2015), induced iron efflux from endolysosomes (Nash et al., 2019), and increased lysosome pH through mu-opioid receptor-mediated mechanisms (Nash et al., 2019).
Many clinically used drugs share the physicochemical property of being weakly or strongly basic. As such and similar to what has been demonstrated for opioids and methamphetamine, these basic drugs accumulate in acidic endolysosomes where they would become increasingly charged and where they would be expected to de-acidify endolysosomes. And recent evidence supports that some of the following drugs may induce lysosome stress and illicit what we would characterize as LSR. Commonly used basic drugs (listed in alphabetical order) include; antipyrine (analgesic), barbiturates (sedative/hypnotics), bupivacaine (local anesthetic), chlorpromazine and olanzapine (antipsychotics), diazepam (anxiolytic), pentazocine (opioid agonist/antagonist), phenylbutazone (NSAID), propranolol (beta blocker), pyrimethamine (anti-parasitic), quinidine (antiarrhythmic), sildenafil citrate (erective dysfunction), telmisartan (anti-hypertensive), tolbutamide (sulfonylurea), valproic acid and phenytoin (anticonvulsants), warfarin (anticoagulant) (Staneva-Stoicheva et al., 1977; Cramb, 1986; Pourahmad et al., 2012; Hamaguchi et al., 2014; Jang et al., 2016; Kozako et al., 2016; Boz et al., 2020).
Thus, the possibility exists that any drug, pathogen, or material that enters into the greater endolysosomal system may induce, to varying degrees, lysosome stress and LSR (Figure 2). Of course, the definition of LSR will likely change as new knowledge is added and specific therapeutics are developed, and it is unclear the extent to which LSR might be protective or destructive. Likely, LSR occurs in combination with other intracellular stress responses including those mediated through ER and mitochondria.
INTER-ORGANELLAR SIGNALING
In this era of modern cell biology, there is ever-growing appreciation of the complexities of inter-organellar signaling in health and disease (Gottschling and Nystrom, 2017; Khan et al., 2019a; Afghah et al., 2020). Organelles are highly mobile in cells and dynamically form contacts between organelles; as such extensive communications between organelles exists (Soto-Heredero et al., 2017; Deus et al., 2020). Inter-organellar signaling helps maintain cellular functions (Gottschling and Nystrom, 2017; Deus et al., 2020).
Cell stress can cause disrupted crosstalk between organelles (Soto-Heredero et al., 2017; Torres et al., 2017; Guerra et al., 2019; Deus et al., 2020). For example, mitochondria, ER, and endolysosome dysfunction are implicated in multiple diseases and disorders (Demers-Lamarche et al., 2016; Plotegher and Duchen, 2017; Soto-Heredero et al., 2017; Torres et al., 2017; Annunziata et al., 2018; Audano et al., 2018). Cellular stress signals converge on one common adaptive pathway, termed the integrated stress response (ISR) (Pakos-Zebrucka et al., 2016). The integration point of stress stimuli occurs at the phosphorylation of eukaryotic translation initiation factor 2 alpha (eIF2α) and subsequent synthesis of activating transcription factor 4 (ATF4), which is a transcription factor that upregulates genes that promote cellular survival and recovery (Pakos-Zebrucka et al., 2016). ISR can stimulate autophagy pathways and is suggested to promote regulation of cellular fate (Kroemer et al., 2010; Humeau et al., 2020). The type and duration of a stressor plays an important factor in the outcomes of the ISR. Stress signals from both ER and mitochondrial participate in ISR, although, it remains unclear the degree to which LSR contributes to ISR.
LSR observed in ER- and mitochondrial-stress responses:
Endolysosomes dynamically interact physically and chemically with the nucleus, ER and mitochondria (Settembre et al., 2013; Deus et al., 2020; Lee and Blackstone, 2020) and such inter-organellar signaling might affect organellar stress responses. Indeed, ER and mitochondrial stress can induce LSR (Table 1). ER stress de-acidifies lysosomes, increases lysosome size, induces lysosome membrane permeabilization, and leads to lysosome repositioning (Elfrink et al., 2013; Kim and Cunningham, 2015; Dauer et al., 2017; Bae et al., 2019; Nakashima et al., 2019). Moreover, it inhibits autophagic flux (Nakashima et al., 2019). Pharmacological induction of ER stress with tunicamycin causes nuclear translocation of the transcription factors TFEB and TFE3 (Martina et al., 2016). Further, the lysosome resident protein mTOR contributes to ER stress, and cell life and death by regulating expression levels of ER stress response genes (Appenzeller-Herzog and Hall, 2012; Dong et al., 2015). However, there is evidence of mTOR-independent nuclear translocation of TFEB/3 (Martina et al., 2016).
Table 1.
Comparison of lysosome, mitochondria and ER stress responses.
| Comparison of Organellar Stress Responses | |||
|---|---|---|---|
| Lysosome stress responses | Mitochondria | Endoplasmic reticulum stress | References |
| Increased intralysosomal pH | + | + | Lu et al., 2017; khan et al., 2020; Demers-Lamarche et al.. 2016; Nakashima et al., 2019 |
| Increased lysosome size | + | + | Ohkuma & Poole, 1981; Hui et al., 2012; Demers-Lamarche et al. 2016; Elfrink et al., 2013 |
| Membrane permeabilization | + | + | Khan et al., 2020; Hwang et al., 2008; Kim & Cunningham, 2015; Dauer et al., 2017 |
| Cationic efflux | + | + | Christensen et al., 2002; Hui et al., 2015; Zhang etal, 2016 |
| Repositioning intracellularly | − | + | Johnson et al.. 2016; Bae et al., 2019; Elfrink etal., 2013 |
| Misfolded protein aggregation | + | + | Hui & Ye et al., 2019; Bae et al., 2019; Demers-Lamarche et al., 2016; Baixauli et al., 2015 |
| LDL cholesterol accumulation | − | − | Liao etal., 2007 |
| Redox catastrophe | + | − | Uchiyama et al., 2008; Zhang et al., 2016 |
| Bioenergetic crisis | + | − | Redman et al., 2017; Baixauli et al., 2015 |
This table highlights the absence or presence of evidence for the observation of LSR in mitochondrial- and ER-stress responses. (+) indicate evidence for LSR, while (−) indicate a lack of evidence for LSR.
Similar to ER, LSR is observed in mitochondrial stress such as lysosome membrane permeabilization, de-acidification, changes in morphology, LDL cholesterol accumulation, and misfolded protein aggregation (Butler and Bahr, 2006; Hwang et al., 2008; Schieber and Chandel, 2014; Baixauli et al., 2015; Demers-Lamarche et al., 2016; Zhang et al., 2016). De-acidification of endolysosomes promotes efflux of redox active iron into the cytosol which can then generate ROS through the Fenton reaction in the cytosol and within mitochondria (Uchiyama et al., 2008; Yambire et al., 2019). H2O2-induced oxidative stress suppressed TFEB nuclear translocation and promoted apoptosis (Su et al., 2018) and TFEB-overexpression was protective; it reduced glucose-mediated oxidative stress by increasing the expression of antioxidant enzymes and mitochondrial biogenesis genes (Kang et al., 2019), and increased autophagy (Li et al., 2016a).
BROADER IMPLICATIONS
In comparison to ER and mitochondrial stress responses, relatively little is known about LSR. PubMed and Google Scholar searches for the keywords “ER stress”, “unfolded protein response”, “mitochondrial stress”, “mitochondrial redox catastrophe”, and “mitochondrial bioenergetic crisis” generate citations to thousands of manuscripts. Conversely, a search for the keywords “lysosomal stress” or “lysosome stress response” yields less than one dozen manuscript citations; only a few attempted to define lysosome stress. Similar to ER and mitochondria, when presented with excess cargo, lysosomes can undergo stress responses implicated in multiple pathologies (Reuser and Drost, 2006; Davidson and Vander Heiden, 2017; Lie and Nixon, 2019). Thus, establishing a framework for defining LSR is of physiological and pathological importance. Central to the criteria described above for LSR are lysosome de-acidification and lysosome membrane permeabilization. Any drug, protein, virus or insult that affects the endocytic or autophagic pathways may cause lysosome stress and trigger LSR.
CONCLUSIONS
Lysosomes are integral to the dynamic endolysosome system and are important for maintaining cellular homeostasis through intracellular degradation of cargo, cytosolic nutrient sensing, and cation homeostasis. Under stressful conditions all organelles including lysosomes, ER and mitochondria exhibit stress responses as they attempt to restore organellar function or promote cell death pathways when stress responses are too great. Few have provided characterizations of LSR and here a framework is presented upon which we can build on the physiological roles and pathological implications for LSR. Through a better understanding of LSR, new and potentially impactful therapeutic interventions might be discovered. In the meantime, LSR might find its place in the vernacular of modern cell biology and might lead to a wave of investigations focused on inter-organellar signaling (Figure 4).
Fig. 4.
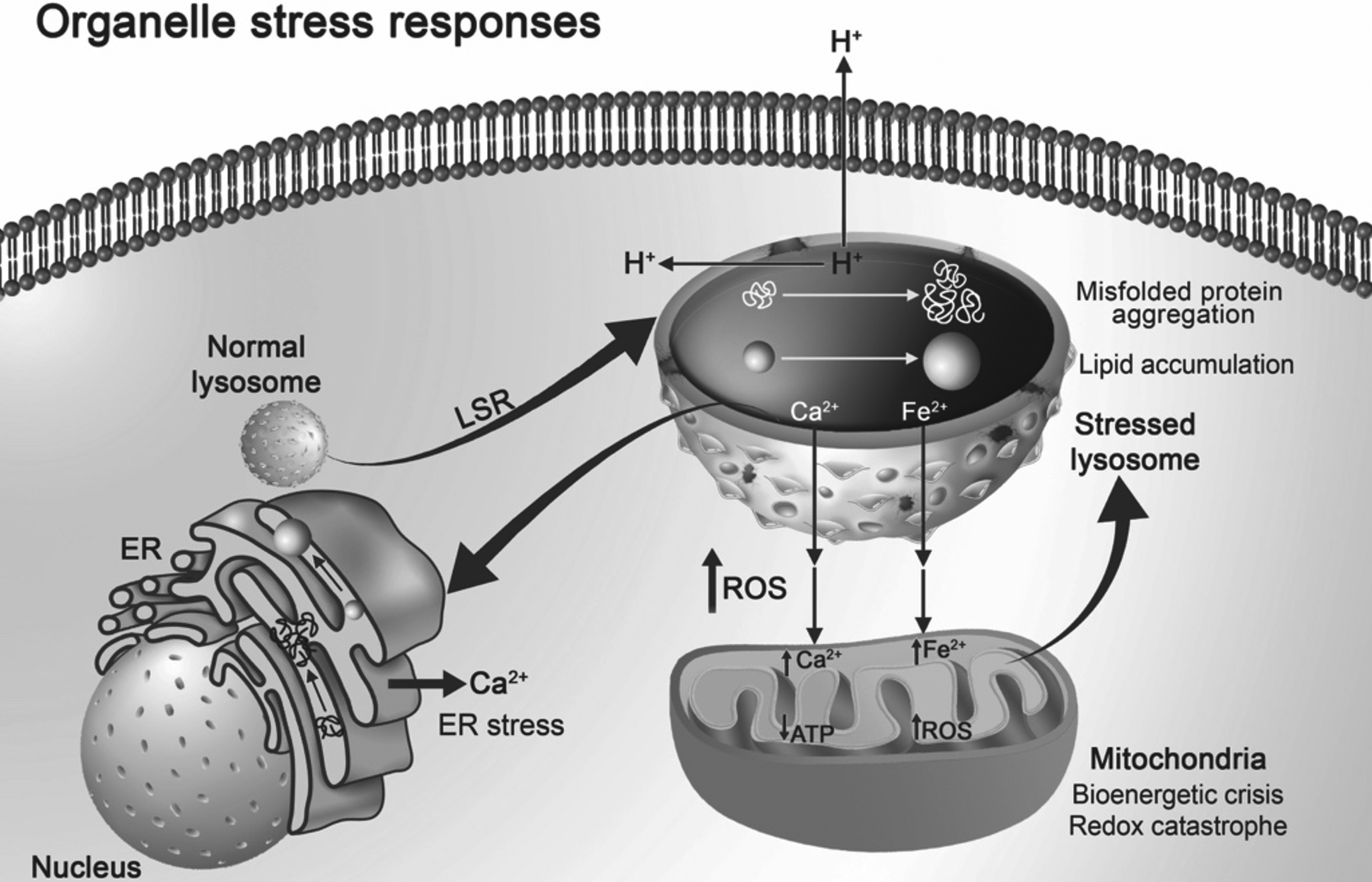
Organellar stress responses. Resulting from acute and chronic stimuli and stressors, lysosomes can exhibit lysosome stress responses (LSR). LSR as characterized in the text and in Figure 2 is characterized by perinuclear to peripheral repositioning of lysosomes, increases in lysosome size, reduction in intralysosomal proton concentrations and decreases in cytosolic and extracellular pH, intraluminal accumulation of LDL cholesterol, increased misfolded protein aggregation, lysosomal membrane permeabilization, and efflux of calcium and iron from lysosomes. Endoplasmic reticulum (ER) and mitochondria exhibit their own stress responses, and these might be triggered by LSR. Mitochondria in response to increases in cytosolic iron and calcium act as cation sinks; when excessive results include increases in reactive oxygen species (ROS) leading to redox catastrophe and decreases in adenosine triphosphate (ATP) promoting bioenergetic crisis. A vicious cycle can result from LSR including (1) increases in ROS can cause further lysosome stress, (2) increases in cytosolic calcium can trigger calcium-induced calcium release from ER, and (3) increases in lipid accumulation and protein aggregation in ER can lead to ER stress. Therefore, LSR might be an early and upstream event that participates in inter-organellar stress responses.
Funding:
We gratefully acknowledge research support from the National Institute of General Medical Sciences under award numbers P30GM100329 and U54GM115458, the National Institute of Mental Health under award numbers R01MH100972 and R01MH105329, the National Institute of Neurological Diseases and Stroke (NINDS) under award number R01NS065957, and the National Institute of Drug Abuse under award number R01DA032444. The authors would like to thank Parinaz Ghanbari for designing illustrations contained in this manuscript.
Footnotes
Conflict of interest/Competing Interest: The authors declare that this manuscript was written in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Afghah Z, Chen X, Geiger JD (2020) Role of endolysosomes and inter-organellar signaling in brain disease. Neurobiol Dis 134:104670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziata I, Sano R, d’Azzo A (2018) Mitochondria-associated ER membranes (MAMs) and lysosomal storage diseases. Cell Death Dis 9:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appenzeller-Herzog C, Hall MN (2012) Bidirectional crosstalk between endoplasmic reticulum stress and mTOR signaling. Trends Cell Biol 22:274–282. [DOI] [PubMed] [Google Scholar]
- Audano M, Schneider A, Mitro N (2018) Mitochondria, lysosomes, and dysfunction: their meaning in neurodegeneration. J Neurochem 147:291–309. [DOI] [PubMed] [Google Scholar]
- Awad O, Sarkar C, Panicker LM, Miller D, Zeng X, Sgambato JA, Lipinski MM, Feldman RA (2015) Altered TFEB-mediated lysosomal biogenesis in Gaucher disease iPSC-derived neuronal cells. Hum Mol Genet 24:5775–5788. [DOI] [PubMed] [Google Scholar]
- Bae D, Moore KA, Mella JM, Hayashi SY, Hollien J (2019) Degradation of Blos1 mRNA by IRE1 repositions lysosomes and protects cells from stress. J Cell Biol 218:1118–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae M, Patel N, Xu H, Lee M, Tominaga-Yamanaka K, Nath A, Geiger J, Gorospe M, Mattson MP, Haughey NJ (2014) Activation of TRPML1 clears intraneuronal Abeta in preclinical models of HIV infection. The Journal of neuroscience : the official journal of the Society for Neuroscience 34:11485–11503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baixauli F, Acin-Perez R, Villarroya-Beltri C, Mazzeo C, Nunez-Andrade N, Gabande-Rodriguez E, Ledesma MD, Blazquez A, Martin MA, Falcon-Perez JM, Redondo JM, Enriquez JA, Mittelbrunn M (2015) Mitochondrial Respiration Controls Lysosomal Function during Inflammatory T Cell Responses. Cell Metab 22:485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj L, Lotfi P, Pal R, Ronza AD, Sharma J, Sardiello M (2019) Lysosome biogenesis in health and disease. J Neurochem 148:573–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballabio A, Gieselmann V (2009) Lysosomal disorders: from storage to cellular damage. Biochim Biophys Acta 1793:684–696. [DOI] [PubMed] [Google Scholar]
- Baltazar GC, Guha S, Lu W, Lim J, Boesze-Battaglia K, Laties AM, Tyagi P, Kompella UB, Mitchell CH (2012) Acidic nanoparticles are trafficked to lysosomes and restore an acidic lysosomal pH and degradative function to compromised ARPE-19 cells. PLoS One 7:e49635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi X, Haque TS, Zhou J, Skillman AG, Lin B, Lee CE, Kuntz ID, Ellman JA, Lynch G (2000) Novel cathepsin D inhibitors block the formation of hyperphosphorylated tau fragments in hippocampus. J Neurochem 74:1469–1477. [DOI] [PubMed] [Google Scholar]
- Bonam SR, Wang F, Muller S (2019) Lysosomes as a therapeutic target. Nat Rev Drug Discov 18:923–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdenx M, Daniel J, Genin E, Soria FN, Blanchard-Desce M, Bezard E, Dehay B (2016) Nanoparticles restore lysosomal acidification defects: Implications for Parkinson and other lysosomal-related diseases. Autophagy 12:472–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boz Z, Hu M, Yu Y, Huang XF (2020) N-acetylcysteine prevents olanzapine-induced oxidative stress in mHypoA-59 hypothalamic neurons. Sci Rep 10:19185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braulke T, Bonifacino JS (2009) Sorting of lysosomal proteins. Biochim Biophys Acta 1793:605–614. [DOI] [PubMed] [Google Scholar]
- Bravo R, Parra V, Gatica D, Rodriguez AE, Torrealba N, Paredes F, Wang ZV, Zorzano A, Hill JA, Jaimovich E, Quest AF, Lavandero S (2013) Endoplasmic reticulum and the unfolded protein response: dynamics and metabolic integration. Int Rev Cell Mol Biol 301:215–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler D, Bahr BA (2006) Oxidative stress and lysosomes: CNS-related consequences and implications for lysosomal enhancement strategies and induction of autophagy. Antioxid Redox Signal 8:185–196. [DOI] [PubMed] [Google Scholar]
- Cabukusta B, Neefjes J (2018) Mechanisms of lysosomal positioning and movement. Traffic 19:761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Espinola JA, Fossale E, Massey AC, Cuervo AM, MacDonald ME, Cotman SL (2006) Autophagy is disrupted in a knock-in mouse model of juvenile neuronal ceroid lipofuscinosis. J Biol Chem 281:20483–20493. [DOI] [PubMed] [Google Scholar]
- Cataldo AM, Paskevich PA, Kominami E, Nixon RA (1991) Lysosomal hydrolases of different classes are abnormally distributed in brains of patients with Alzheimer disease. Proc Natl Acad Sci U S A 88:10998–11002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo AM, Barnett JL, Berman SA, Li J, Quarless S, Bursztajn S, Lippa C, Nixon RA (1995) Gene expression and cellular content of cathepsin D in Alzheimer’s disease brain: evidence for early up-regulation of the endosomal-lysosomal system. Neuron 14:671–680. [DOI] [PubMed] [Google Scholar]
- Chen D, Xie J, Fiskesund R, Dong W, Liang X, Lv J, Jin X, Liu J, Mo S, Zhang T, Cheng F, Zhou Y, Zhang H, Tang K, Ma J, Liu Y, Huang B (2018) Chloroquine modulates antitumor immune response by resetting tumor-associated macrophages toward M1 phenotype. Nat Commun 9:873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PM, Gombart ZJ, Chen JW (2011) Chloroquine treatment of ARPE-19 cells leads to lysosome dilation and intracellular lipid accumulation: possible implications of lysosomal dysfunction in macular degeneration. Cell Biosci 1:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Ghribi O, Geiger JD (2008) Rabbits fed cholesterol-enriched diets exhibit pathological features of inclusion body myositis. Am J Physiol Regul Integr Comp Physiol 294:R829–835. [DOI] [PubMed] [Google Scholar]
- Chen X, Hui L, Geiger NH, Haughey NJ, Geiger JD (2013) Endolysosome involvement in HIV-1 transactivator protein-induced neuronal amyloid beta production. Neurobiol Aging 34:2370–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chino H, Mizushima N (2020) ER-Phagy: Quality Control and Turnover of Endoplasmic Reticulum. Trends Cell Biol 30:384–398. [DOI] [PubMed] [Google Scholar]
- Christensen KA, Myers JT, Swanson JA (2002) pH-dependent regulation of lysosomal calcium in macrophages. J Cell Sci 115:599–607. [DOI] [PubMed] [Google Scholar]
- Colacurcio DJ, Nixon RA (2016) Disorders of lysosomal acidification-The emerging role of v-ATPase in aging and neurodegenerative disease. Ageing Res Rev 32:75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramb G (1986) Selective lysosomal uptake and accumulation of the beta-adrenergic antagonist propranolol in cultured and isolated cell systems. Biochem Pharmacol 35:1365–1372. [DOI] [PubMed] [Google Scholar]
- Cubells JF, Rayport S, Rajendran G, Sulzer D (1994) Methamphetamine neurotoxicity involves vacuolation of endocytic organelles and dopamine-dependent intracellular oxidative stress. The Journal of neuroscience : the official journal of the Society for Neuroscience 14:2260–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzer KM, Kranich LR, Ruf WP, Cagsal-Getkin O, Winslow AR, Zhu L, Vanderburg CR, McLean PJ (2012) Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol Neurodegener 7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta G, Miller NM, Afghah Z, Geiger JD, Chen X (2019) HIV-1 gp120 Promotes Lysosomal Exocytosis in Human Schwann Cells. Front Cell Neurosci 13:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer P, Gupta VK, McGinn O, Nomura A, Sharma NS, Arora N, Giri B, Dudeja V, Saluja AK, Banerjee S (2017) Inhibition of Sp1 prevents ER homeostasis and causes cell death by lysosomal membrane permeabilization in pancreatic cancer. Sci Rep 7:1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson SM, Vander Heiden MG (2017) Critical Functions of the Lysosome in Cancer Biology. Annu Rev Pharmacol Toxicol 57:481–507. [DOI] [PubMed] [Google Scholar]
- Davies JP, Ioannou YA (2000) Topological analysis of Niemann-Pick C1 protein reveals that the membrane orientation of the putative sterol-sensing domain is identical to those of 3-hydroxy-3-methylglutaryl-CoA reductase and sterol regulatory element binding protein cleavage-activating protein. J Biol Chem 275:24367–24374. [DOI] [PubMed] [Google Scholar]
- de Duve C (2005) The lysosome turns fifty. Nat Cell Biol 7:847–849. [DOI] [PubMed] [Google Scholar]
- del Toro D, Alberch J, Lazaro-Dieguez F, Martin-Ibanez R, Xifro X, Egea G, Canals JM (2009) Mutant huntingtin impairs post-Golgi trafficking to lysosomes by delocalizing optineurin/Rab8 complex from the Golgi apparatus. Mol Biol Cell 20:1478–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demers-Lamarche J, Guillebaud G, Tlili M, Todkar K, Belanger N, Grondin M, Nguyen AP, Michel J, Germain M (2016) Loss of Mitochondrial Function Impairs Lysosomes. J Biol Chem 291:10263–10276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deus CM, Yambire KF, Oliveira PJ, Raimundo N (2020) Mitochondria-Lysosome Crosstalk: From Physiology to Neurodegeneration. Trends Mol Med 26:71–88. [DOI] [PubMed] [Google Scholar]
- Doherty J, Baehrecke EH (2018) Life, death and autophagy. Nat Cell Biol 20:1110–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong G, Liu Y, Zhang L, Huang S, Ding HF, Dong Z (2015) mTOR contributes to ER stress and associated apoptosis in renal tubular cells. Am J Physiol Renal Physiol 308:F267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N, Rodriguez M, Dever SM, Masvekar RR, Gewirtz DA, Shacka JJ (2015) HIV-1 and morphine regulation of autophagy in microglia: limited interactions in the context of HIV-1 infection and opioid abuse. J Virol 89:1024–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfrink HL, Zwart R, Baas F, Scheper W (2013) Inhibition of endoplasmic reticulum associated degradation reduces endoplasmic reticulum stress and alters lysosomal morphology and distribution. Mol Cells 35:291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T, Yamano K, Kawano S (2011) Structural insight into the mitochondrial protein import system. Biochim Biophys Acta 1808:955–970. [DOI] [PubMed] [Google Scholar]
- Erie C, Sacino M, Houle L, Lu ML, Wei J (2015) Altered lysosomal positioning affects lysosomal functions in a cellular model of Huntington’s disease. Eur J Neurosci 42:1941–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fais S et al. (2016) Evidence-Based Clinical Use of Nanoscale Extracellular Vesicles in Nanomedicine. ACS Nano 10:3886–3899. [DOI] [PubMed] [Google Scholar]
- Feng X, Yang J (2016) Lysosomal Calcium in Neurodegeneration. Messenger (Los Angel) 5:56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández B, Fdez E, Gómez-Suaga P, Gil F, Molina-Villalba I, Ferrer I, Patel S, Churchill GC, Hilfiker S (2016) Iron overload causes endolysosomal deficits modulated by NAADP-regulated 2-pore channels and RAB7A. In: Autophagy, pp 1487–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa L, Roth LM, B KJ, Geiger JD, Jordan-Sciutto KL, Grinspan JB (2019) Protease Inhibitors, Saquinavir and Darunavir, Inhibit Oligodendrocyte Maturation: Implications for Lysosomal Stress. J Neuroimmune Pharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folts CJ, Scott-Hewitt N, Proschel C, Mayer-Proschel M, Noble M (2016) Lysosomal Re-acidification Prevents Lysosphingolipid-Induced Lysosomal Impairment and Cellular Toxicity. PLoS Biol 14:e1002583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossale E, Wolf P, Espinola JA, Lubicz-Nawrocka T, Teed AM, Gao H, Rigamonti D, Cattaneo E, MacDonald ME, Cotman SL (2004) Membrane trafficking and mitochondrial abnormalities precede subunit c deposition in a cerebellar cell model of juvenile neuronal ceroid lipofuscinosis. BMC Neurosci 5:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedland N, Liou HL, Lobel P, Stock AM (2003) Structure of a cholesterol-binding protein deficient in Niemann-Pick type C2 disease. Proc Natl Acad Sci U S A 100:2512–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T, Ahearn M, Roberts A, Mattaliano RJ, Zaal K, Ralston E, Plotz PH, Raben N (2006) Autophagy and mistargeting of therapeutic enzyme in skeletal muscle in Pompe disease. Mol Ther 14:831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi-Hirose I, Aki T, Unuma K, Funakoshi T, Noritake K, Uemura K (2013) Distinct effects of methamphetamine on autophagy-lysosome and ubiquitin-proteasome systems in HL-1 cultured mouse atrial cardiomyocytes. Toxicology 312:74–82. [DOI] [PubMed] [Google Scholar]
- Galluzzi L et al. (2017) Molecular definitions of autophagy and related processes. EMBO J 36:1811–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschling DE, Nystrom T (2017) The Upsides and Downsides of Organelle Interconnectivity. Cell 169:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowrishankar S, Yuan P, Wu Y, Schrag M, Paradise S, Grutzendler J, De Camilli P, Ferguson SM (2015) Massive accumulation of luminal protease-deficient axonal lysosomes at Alzheimer’s disease amyloid plaques. Proc Natl Acad Sci U S A 112:E3699–3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra F, Girolimetti G, Beli R, Mitruccio M, Pacelli C, Ferretta A, Gasparre G, Cocco T, Bucci C (2019) Synergistic Effect of Mitochondrial and Lysosomal Dysfunction in Parkinson’s Disease. Cells 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guix FX (2020) The interplay between aging-associated loss of protein homeostasis and extracellular vesicles in neurodegeneration. J Neurosci Res 98:262–283. [DOI] [PubMed] [Google Scholar]
- Halcrow P, Khan N, Datta G, Ohm JE, Chen X, Geiger JD (2019) Importance of measuring endolysosome, cytosolic, and extracellular pH in understanding the pathogenesis of and possible treatments for glioblastoma multiforme. Cancer Rep 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi R, Haginaka J, Tanimoto T, Kuroda Y (2014) Maintenance of luminal pH and protease activity in lysosomes/late endosomes by vacuolar ATPase in chlorpromazine-treated RAW264 cells accumulating phospholipids. Cell Biol Toxicol 30:67–77. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Ron D (1999) Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397:271–274. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D (2000a) Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell 5:897–904. [DOI] [PubMed] [Google Scholar]
- Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D (2000b) Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell 6:1099–1108. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D (2003) An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11:619–633. [DOI] [PubMed] [Google Scholar]
- He B, Shi Y, Liang Y, Yang A, Fan Z, Yuan L, Zou X, Chang X, Zhang H, Wang X, Dai W, Wang Y, Zhang Q (2018) Single-walled carbon-nanohorns improve biocompatibility over nanotubes by triggering less protein-initiated pyroptosis and apoptosis in macrophages. Nat Commun 9:2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz C (2012) The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 13:89–102. [DOI] [PubMed] [Google Scholar]
- Heuser J (1989) Changes in lysosome shape and distribution correlated with changes in cytoplasmic pH. J Cell Biol 108:855–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill S, Sataranatarajan K, Van Remmen H (2018) Role of Signaling Molecules in Mitochondrial Stress Response. Front Genet 9:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann AC, Minakaki G, Menges S, Salvi R, Savitskiy S, Kazman A, Vicente Miranda H, Mielenz D, Klucken J, Winkler J, Xiang W (2019) Extracellular aggregated alpha synuclein primarily triggers lysosomal dysfunction in neural cells prevented by trehalose. Sci Rep 9:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoglinger D, Haberkant P, Aguilera-Romero A, Riezman H, Porter FD, Platt FM, Galione A, Schultz C (2015) Intracellular sphingosine releases calcium from lysosomes. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holopainen JM, Saarikoski J, Kinnunen PK, Jarvela I (2001) Elevated lysosomal pH in neuronal ceroid lipofuscinoses (NCLs). Eur J Biochem 268:5851–5856. [DOI] [PubMed] [Google Scholar]
- Holtzman E (1989) Lysosomes. New York: Plenum Press [Google Scholar]
- Hsu CL, Lee EX, Gordon KL, Paz EA, Shen WC, Ohnishi K, Meisenhelder J, Hunter T, La Spada AR (2018) MAP4K3 mediates amino acid-dependent regulation of autophagy via phosphorylation of TFEB. Nat Commun 9:942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ML, Chiang S, Kalinowski DS, Bae DH, Sahni S, Richardson DR (2019) The Role of the Antioxidant Response in Mitochondrial Dysfunction in Degenerative Diseases: Cross-Talk between Antioxidant Defense, Autophagy, and Apoptosis. Oxid Med Cell Longev 2019:6392763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui L, Chen X, Geiger JD (2012a) Endolysosome involvement in LDL cholesterol-induced Alzheimer’s disease-like pathology in primary cultured neurons. Life Sci 91:1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui L, Chen X, Haughey NJ, Geiger JD (2012b) Role of endolysosomes in HIV-1 Tat-induced neurotoxicity. ASN Neuro 4:243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui L, Geiger NH, Bloor-Young D, Churchill GC, Geiger JD, Chen X (2015) Release of calcium from endolysosomes increases calcium influx through N-type calcium channels: Evidence for acidic store-operated calcium entry in neurons. Cell Calcium 58:617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui L, Ye Y, Soliman ML, Lakpa KL, Miller NM, Afghah Z, Geiger JD, Chen X (2019) Antiretroviral Drugs Promote Amyloidogenesis by De-Acidifying Endolysosomes. J Neuroimmune Pharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humeau J, Leduc M, Cerrato G, Loos F, Kepp O, Kroemer G (2020) Phosphorylation of eukaryotic initiation factor-2alpha (eIF2alpha) in autophagy. Cell Death Dis 11:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huotari J, Helenius A (2011) Endosome maturation. EMBO J 30:3481–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JJ, Lee SJ, Kim TY, Cho JH, Koh JY (2008) Zinc and 4-hydroxy-2-nonenal mediate lysosomal membrane permeabilization induced by H2O2 in cultured hippocampal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience 28:3114–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang JW, Song Y, Kim KM, Kim JS, Choi EK, Kim J, Seo H (2016) Hepatocellular carcinoma-targeted drug discovery through image-based phenotypic screening in co-cultures of HCC cells with hepatocytes. BMC Cancer 16:810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J, Abudu YP, Claude-Taupin A, Gu Y, Kumar S, Choi SW, Peters R, Mudd MH, Allers L, Salemi M, Phinney B, Johansen T, Deretic V (2018) Galectins Control mTOR in Response to Endomembrane Damage. Mol Cell 70:120–135 e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin LW, Shie FS, Maezawa I, Vincent I, Bird T (2004) Intracellular accumulation of amyloidogenic fragments of amyloid-beta precursor protein in neurons with Niemann-Pick type C defects is associated with endosomal abnormalities. Am J Pathol 164:975–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DE, Ostrowski P, Jaumouille V, Grinstein S (2016) The position of lysosomes within the cell determines their luminal pH. J Cell Biol 212:677–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanekiyo T, Bu G (2014) The low-density lipoprotein receptor-related protein 1 and amyloid-beta clearance in Alzheimer’s disease. Front Aging Neurosci 6:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Li Y, Zhang T, Chi Y, Liu M (2019) Effects of transcription factor EB on oxidative stress and apoptosis induced by high glucose in podocytes. Int J Mol Med 44:447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N, Chen X, Geiger JD (2020a) Role of Divalent Cations in HIV-1 Replication and Pathogenicity. Viruses 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N, Datta G, Geiger JD, Chen X (2018) Apolipoprotein E isoform dependently affects Tat-mediated HIV-1 LTR transactivation. J Neuroinflammation 15:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N, Haughey NJ, Nath A, Geiger JD (2019a) Involvement of organelles and inter-organellar signaling in the pathogenesis of HIV-1 associated neurocognitive disorder and Alzheimer’s disease. Brain Res 1722:146389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N, Lakpa KL, Halcrow PW, Afghah Z, Miller NM, Geiger JD, Chen X (2019b) BK channels regulate extracellular Tat-mediated HIV-1 LTR transactivation. Sci Rep 9:12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N, Halcrow PW, Lakpa KL, Afghah Z, Miller NM, Dowdy SF, Geiger JD, Chen X (2020b) Two-pore channels regulate Tat endolysosome escape and Tat-mediated HIV-1 LTR transactivation. FASEB J 34:4147–4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A, Cunningham KW (2015) A LAPF/phafin1-like protein regulates TORC1 and lysosomal membrane permeabilization in response to endoplasmic reticulum membrane stress. Mol Biol Cell 26:4631–4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinghorn KJ, Gronke S, Castillo-Quan JI, Woodling NS, Li L, Sirka E, Gegg M, Mills K, Hardy J, Bjedov I, Partridge L (2016) A Drosophila Model of Neuronopathic Gaucher Disease Demonstrates Lysosomal-Autophagic Defects and Altered mTOR Signalling and Is Functionally Rescued by Rapamycin. The Journal of neuroscience : the official journal of the Society for Neuroscience 36:11654–11670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselyov K, Colletti GA, Terwilliger A, Ketchum K, Lyons CW, Quinn J, Muallem S (2011) TRPML: transporters of metals in lysosomes essential for cell survival? Cell Calcium 50:288–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerver L, Papadopoulos C, Liu B, Kravic B, Rota G, Brecht L, Veenendaal T, Polajnar M, Bluemke A, Ehrmann M, Klumperman J, Jaattela M, Behrends C, Meyer H (2019) The ubiquitin-conjugating enzyme UBE2QL1 coordinates lysophagy in response to endolysosomal damage. EMBO Rep 20:e48014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno K, Normington K, Sambrook J, Gething MJ, Mori K (1993) The promoter region of the yeast KAR2 (BiP) gene contains a regulatory domain that responds to the presence of unfolded proteins in the endoplasmic reticulum. Mol Cell Biol 13:877–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike M, Shibata M, Waguri S, Yoshimura K, Tanida I, Kominami E, Gotow T, Peters C, von Figura K, Mizushima N, Saftig P, Uchiyama Y (2005) Participation of autophagy in storage of lysosomes in neurons from mouse models of neuronal ceroid-lipofuscinoses (Batten disease). Am J Pathol 167:1713–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld S, Mellman I (1989) The biogenesis of lysosomes. Annu Rev Cell Biol 5:483–525. [DOI] [PubMed] [Google Scholar]
- Kozako T, Soeda S, Yoshimitsu M, Arima N, Kuroki A, Hirata S, Tanaka H, Imakyure O, Tone N, Honda S, Soeda S (2016) Angiotensin II type 1 receptor blocker telmisartan induces apoptosis and autophagy in adult T-cell leukemia cells. FEBS Open Bio 6:442–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozutsumi Y, Segal M, Normington K, Gething MJ, Sambrook J (1988) The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature 332:462–464. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Marino G, Levine B (2010) Autophagy and the integrated stress response. Mol Cell 40:280–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubli DA, Gustafsson AB (2012) Mitochondria and mitophagy: the yin and yang of cell death control. Circ Res 111:1208–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers M, Azarnia Tehran D, Haucke V, Soykan T (2020) The axonal endo-lysosomal and autophagic systems. J Neurochem. [DOI] [PubMed] [Google Scholar]
- Kurz T, Eaton JW, Brunk UT (2011) The role of lysosomes in iron metabolism and recycling. Int J Biochem Cell Biol 43:1686–1697. [DOI] [PubMed] [Google Scholar]
- La Spina M, Contreras PS, Rissone A, Meena NK, Jeong E, Martina JA (2020) MiT/TFE Family of Transcription Factors: An Evolutionary Perspective. Front Cell Dev Biol 8:609683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakpa KL, Halcrow PW, Chen X, Geiger JD (2020) Readily Releasable Stores of Calcium in Neuronal Endolysosomes: Physiological and Pathophysiological Relevance. Adv Exp Med Biol 1131:681–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AH, Iwakoshi NN, Glimcher LH (2003) XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol 23:7448–7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CA, Blackstone C (2020) ER morphology and endo-lysosomal crosstalk: Functions and disease implications. Biochim Biophys Acta Mol Cell Biol Lipids 1865:158544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, McBrayer MK, Wolfe DM, Haslett LJ, Kumar A, Sato Y, Lie PP, Mohan P, Coffey EE, Kompella U, Mitchell CH, Lloyd-Evans E, Nixon RA (2015) Presenilin 1 Maintains Lysosomal Ca(2+) Homeostasis via TRPML1 by Regulating vATPase-Mediated Lysosome Acidification. Cell Rep 12:1430–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Sato Y, Nixon RA (2011) Lysosomal proteolysis inhibition selectively disrupts axonal transport of degradative organelles and causes an Alzheimer’s-like axonal dystrophy. The Journal of neuroscience : the official journal of the Society for Neuroscience 31:7817–7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lener T et al. (2015) Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. J Extracell Vesicles 4:30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Lang F, Zhang H, Xu L, Wang Y, Hao E (2016a) Role of TFEB Mediated Autophagy, Oxidative Stress, Inflammation, and Cell Death in Endotoxin Induced Myocardial Toxicity of Young and Aged Mice. Oxid Med Cell Longev 2016:5380319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Sun S, Tan L, Wang Y, Wang L, Zhang Z, Zhang L (2019) Polystyrene Nanoparticles Reduced ROS and Inhibited Ferroptosis by Triggering Lysosome Stress and TFEB Nucleus Translocation in a Size-Dependent Manner. Nano Lett 19:7781–7792. [DOI] [PubMed] [Google Scholar]
- Li Y et al. (2016b) Protein kinase C controls lysosome biogenesis independently of mTORC1. Nat Cell Biol 18:1065–1077. [DOI] [PubMed] [Google Scholar]
- Liang JR, Lingeman E, Luong T, Ahmed S, Muhar M, Nguyen T, Olzmann JA, Corn JE (2020) A Genome-wide ER-phagy Screen Highlights Key Roles of Mitochondrial Metabolism and ER-Resident UFMylation. Cell 180:1160–1177 e1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Q, Ouyang X, Schneider L, Zhang J (2011) Reduction of mutant huntingtin accumulation and toxicity by lysosomal cathepsins D and B in neurons. Mol Neurodegener 6:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao G, Yao Y, Liu J, Yu Z, Cheung S, Xie A, Liang X, Bi X (2007) Cholesterol accumulation is associated with lysosomal dysfunction and autophagic stress in Npc1 −/− mouse brain. Am J Pathol 171:962–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie PPY, Nixon RA (2019) Lysosome trafficking and signaling in health and neurodegenerative diseases. Neurobiol Dis 122:94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesse M, Lhoest G, Trouet A, Tulkens P (1976) Uptake and intracellular localization of morphine in lysosomes and cell sap of cultured fibroblasts. Arch Int Physiol Biochim 84:638–639. [PubMed] [Google Scholar]
- Lillis AP, Muratoglu SC, Au DT, Migliorini M, Lee MJ, Fried SK, Mikhailenko I, Strickland DK (2015) LDL Receptor-Related Protein-1 (LRP1) Regulates Cholesterol Accumulation in Macrophages. PLoS One 10:e0128903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Shi SS, Zhang JQ, Zhang YJ, Zhang L, Liu Y, Jin PP, Wei PF, Shi RH, Zhou W, Wen LP (2016) Giant Cellular Vacuoles Induced by Rare Earth Oxide Nanoparticles are Abnormally Enlarged Endo/Lysosomes and Promote mTOR-Dependent TFEB Nucleus Translocation. Small 12:5759–5768. [DOI] [PubMed] [Google Scholar]
- Lloyd-Evans E, Morgan AJ, He X, Smith DA, Elliot-Smith E, Sillence DJ, Churchill GC, Schuchman EH, Galione A, Platt FM (2008) Niemann-Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nat Med 14:1247–1255. [DOI] [PubMed] [Google Scholar]
- Lu S, Sung T, Lin N, Abraham RT, Jessen BA (2017) Lysosomal adaptation: How cells respond to lysosomotropic compounds. PLoS One 12:e0173771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzio JP, Hackmann Y, Dieckmann NM, Griffiths GM (2014) The biogenesis of lysosomes and lysosome-related organelles. Cold Spring Harb Perspect Biol 6:a016840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv H, Shang P (2018) The significance, trafficking and determination of labile iron in cytosol, mitochondria and lysosomes. Metallomics 10:899–916. [DOI] [PubMed] [Google Scholar]
- Ma X, Wu Y, Jin S, Tian Y, Zhang X, Zhao Y, Yu L, Liang XJ (2011) Gold nanoparticles induce autophagosome accumulation through size-dependent nanoparticle uptake and lysosome impairment. ACS Nano 5:8629–8639. [DOI] [PubMed] [Google Scholar]
- MacIntyre AC, Cutler DJ (1988) The potential role of lysosomes in tissue distribution of weak bases. Biopharm Drug Dispos 9:513–526. [DOI] [PubMed] [Google Scholar]
- Maia J, Caja S, Strano Moraes MC, Couto N, Costa-Silva B (2018) Exosome-Based Cell-Cell Communication in the Tumor Microenvironment. Front Cell Dev Biol 6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoli I, Alesci S, Blackman MR, Su YA, Rennert OM, Chrousos GP (2007) Mitochondria as key components of the stress response. Trends Endocrinol Metab 18:190–198. [DOI] [PubMed] [Google Scholar]
- Manshian BB, Pokhrel S, Madler L, Soenen SJ (2018) The impact of nanoparticle-driven lysosomal alkalinization on cellular functionality. J Nanobiotechnology 16:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques ARA, Saftig P (2019) Lysosomal storage disorders - challenges, concepts and avenues for therapy: beyond rare diseases. J Cell Sci 132. [DOI] [PubMed] [Google Scholar]
- Martina JA, Puertollano R (2018) Protein phosphatase 2A stimulates activation of TFEB and TFE3 transcription factors in response to oxidative stress. J Biol Chem 293:12525–12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina JA, Chen Y, Gucek M, Puertollano R (2012) MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy 8:903–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina JA, Diab HI, Brady OA, Puertollano R (2016) TFEB and TFE3 are novel components of the integrated stress response. EMBO J 35:479–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini-Stoica H, Xu Y, Ballabio A, Zheng H (2016) The Autophagy-Lysosomal Pathway in Neurodegeneration: A TFEB Perspective. Trends Neurosci 39:221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinus RD, Garth GP, Webster TL, Cartwright P, Naylor DJ, Hoj PB, Hoogenraad NJ (1996) Selective induction of mitochondrial chaperones in response to loss of the mitochondrial genome. Eur J Biochem 240:98–103. [DOI] [PubMed] [Google Scholar]
- Matteoni R, Kreis TE (1987) Translocation and clustering of endosomes and lysosomes depends on microtubules. J Cell Biol 105:1253–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauthe M, Orhon I, Rocchi C, Zhou X, Luhr M, Hijlkema KJ, Coppes RP, Engedal N, Mari M, Reggiori F (2018) Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 14:1435–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina DL, Di Paola S, Peluso I, Armani A, De Stefani D, Venditti R, Montefusco S, Scotto-Rosato A, Prezioso C, Forrester A, Settembre C, Wang W, Gao Q, Xu H, Sandri M, Rizzuto R, De Matteis MA, Ballabio A (2015) Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat Cell Biol 17:288–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindell JA (2012) Lysosomal acidification mechanisms. Annu Rev Physiol 74:69–86. [DOI] [PubMed] [Google Scholar]
- Miranda AM, Lasiecka ZM, Xu Y, Neufeld J, Shahriar S, Simoes S, Chan RB, Oliveira TG, Small SA, Di Paolo G (2018) Neuronal lysosomal dysfunction releases exosomes harboring APP C-terminal fragments and unique lipid signatures. Nat Commun 9:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K (2015) The unfolded protein response: the dawn of a new field. Proc Jpn Acad Ser B Phys Biol Sci 91:469–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers BM, Prendergast FG, Holman R, Kuntz SM, LaRusso NF (1991) Alterations in the structure, physicochemical properties, and pH of hepatocyte lysosomes in experimental iron overload. J Clin Invest 88:1207–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima A, Cheng SB, Kusabiraki T, Motomura K, Aoki A, Ushijima A, Ono Y, Tsuda S, Shima T, Yoshino O, Sago H, Matsumoto K, Sharma S, Saito S (2019) Endoplasmic reticulum stress disrupts lysosomal homeostasis and induces blockade of autophagic flux in human trophoblasts. Sci Rep 9:11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napolitano G, Ballabio A (2016) TFEB at a glance. J Cell Sci 129:2475–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nara A, Aki T, Funakoshi T, Unuma K, Uemura K (2012) Hyperstimulation of macropinocytosis leads to lysosomal dysfunction during exposure to methamphetamine in SH-SY5Y cells. Brain Res 1466:1–14. [DOI] [PubMed] [Google Scholar]
- Nash B, Tarn K, Irollo E, Luchetta J, Festa L, Halcrow P, Datta G, Geiger JD, Meucci O (2019) Morphine-Induced Modulation of Endolysosomal Iron Mediates Upregulation of Ferritin Heavy Chain in Cortical Neurons. eNeuro 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A, Padua RA, Geiger JD (1995) HIV-1 coat protein gp120-induced increases in levels of intrasynaptosomal calcium. Brain Res 678:200–206. [DOI] [PubMed] [Google Scholar]
- Nath A, Haughey NJ, Jones M, Anderson C, Bell JE, Geiger JD (2000) Synergistic neurotoxicity by human immunodeficiency virus proteins Tat and gp120: protection by memantine. Ann Neurol 47:186–194. [PubMed] [Google Scholar]
- Nguyen M, Wong YC, Ysselstein D, Severino A, Krainc D (2019) Synaptic, Mitochondrial, and Lysosomal Dysfunction in Parkinson’s Disease. Trends Neurosci 42:140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes SA, Papa FR (2015) The role of endoplasmic reticulum stress in human pathology. Annu Rev Pathol 10:173–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh N, Park JH (2014) Endocytosis and exocytosis of nanoparticles in mammalian cells. Int J Nanomedicine 9 Suppl 1:51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuma S, Poole B (1981) Cytoplasmic vacuolation of mouse peritoneal macrophages and the uptake into lysosomes of weakly basic substances. J Cell Biol 90:656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikawa N, Walter J (2019) Presenilins and gamma-Secretase in Membrane Proteostasis. Cells 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakos-Zebrucka K, Koryga I, Mnich K, Ljujic M, Samali A, Gorman AM (2016) The integrated stress response. EMBO Rep 17:1374–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri M, Pal R, Nelvagal HR, Lotfi P, Stinnett GR, Seymour ML, Chaudhury A, Bajaj L, Bondar VV, Bremner L, Saleem U, Tse DY, Sanagasetti D, Wu SM, Neilson JR, Pereira FA, Pautler RG, Rodney GG, Cooper JD, Sardiello M (2017) mTORC1-independent TFEB activation via Akt inhibition promotes cellular clearance in neurodegenerative storage diseases. Nat Commun 8:14338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan HY, Alamri AH, Valapala M (2019) Nutrient deprivation and lysosomal stress induce activation of TFEB in retinal pigment epithelial cells. Cell Mol Biol Lett 24:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos C, Meyer H (2017) Detection and Clearance of Damaged Lysosomes by the Endo-Lysosomal Damage Response and Lysophagy. Curr Biol 27:R1330–R1341. [DOI] [PubMed] [Google Scholar]
- Papadopoulos C, Kirchner P, Bug M, Grum D, Koerver L, Schulze N, Poehler R, Dressler A, Fengler S, Arhzaouy K, Lux V, Ehrmann M, Weihl CC, Meyer H (2017) VCP/p97 cooperates with YOD1, UBXD1 and PLAA to drive clearance of ruptured lysosomes by autophagy. EMBO J 36:135–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partaledis JA, Berlin V (1993) The FKB2 gene of Saccharomyces cerevisiae, encoding the immunosuppressant-binding protein FKBP-13, is regulated in response to accumulation of unfolded proteins in the endoplasmic reticulum. Proc Natl Acad Sci U S A 90:5450–5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton RG, Dotti CG, Bacallao R, Kurtz I, Simons K, Prydz K (1991) pH-induced microtubule-dependent redistribution of late endosomes in neuronal and epithelial cells. J Cell Biol 113:261–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patierno S, Anselmi L, Jaramillo I, Scott D, Garcia R, Sternini C (2011) Morphine induces mu opioid receptor endocytosis in guinea pig enteric neurons following prolonged receptor activation. Gastroenterology 140:618–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pau AK, George JM (2014) Antiretroviral therapy: current drugs. Infect Dis Clin North Am 28:371–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera RM, Zoncu R (2016) The Lysosome as a Regulatory Hub. Annu Rev Cell Dev Biol 32:223–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Gonzalez R, Gauthier SA, Kumar A, Levy E (2012) The exosome secretory pathway transports amyloid precursor protein carboxyl-terminal fragments from the cell into the brain extracellular space. J Biol Chem 287:43108–43115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persellin RH (1969) Lysosome stabilization by leukocyte granule membrane antiserum. J Immunol 103:29–44. [PubMed] [Google Scholar]
- Pietrella D, Monari C, Retini C, Palazzetti B, Bistoni F, Vecchiarelli A (1998) Human immunodeficiency virus type 1 envelope protein gp120 impairs intracellular antifungal mechanisms in human monocytes. J Infect Dis 177:347–354. [DOI] [PubMed] [Google Scholar]
- Platt FM (2018) Emptying the stores: lysosomal diseases and therapeutic strategies. Nat Rev Drug Discov 17:133–150. [DOI] [PubMed] [Google Scholar]
- Plotegher N, Duchen MR (2017) Mitochondrial Dysfunction and Neurodegeneration in Lysosomal Storage Disorders. Trends Mol Med 23:116–134. [DOI] [PubMed] [Google Scholar]
- Pourahmad J, Eskandari MR, Kaghazi A, Shaki F, Shahraki J, Fard JK (2012) A new approach on valproic acid induced hepatotoxicity: involvement of lysosomal membrane leakiness and cellular proteolysis. Toxicol In Vitro 26:545–551. [DOI] [PubMed] [Google Scholar]
- Pu J, Guardia CM, Keren-Kaplan T, Bonifacino JS (2016) Mechanisms and functions of lysosome positioning. J Cell Sci 129:4329–4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabanal-Ruiz Y, Korolchuk VI (2018) mTORC1 and Nutrient Homeostasis: The Central Role of the Lysosome. Int J Mol Sci 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raben N, Roberts A, Plotz PH (2007) Role of autophagy in the pathogenesis of Pompe disease. Acta Myol 26:45–48. [PMC free article] [PubMed] [Google Scholar]
- Ratovitski T, Chighladze E, Waldron E, Hirschhorn RR, Ross CA (2011) Cysteine proteases bleomycin hydrolase and cathepsin Z mediate N-terminal proteolysis and toxicity of mutant huntingtin. J Biol Chem 286:12578–12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravikumar B, Duden R, Rubinsztein DC (2002) Aggregate-prone proteins with polyglutamine and polyalanine expansions are degraded by autophagy. Hum Mol Genet 11:1107–1117. [DOI] [PubMed] [Google Scholar]
- Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, Scaravilli F, Easton DF, Duden R, O’Kane CJ, Rubinsztein DC (2004) Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet 36:585–595. [DOI] [PubMed] [Google Scholar]
- Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M, Korolchuk VI, Lichtenberg M, Luo S, Massey DC, Menzies FM, Moreau K, Narayanan U, Renna M, Siddiqi FH, Underwood BR, Winslow AR, Rubinsztein DC (2010) Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev 90:1383–1435. [DOI] [PubMed] [Google Scholar]
- Reddy PH, Oliver DM (2019) Amyloid Beta and Phosphorylated Tau-Induced Defective Autophagy and Mitophagy in Alzheimer’s Disease. Cells 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmann M, Benavides GA, Berryhill TF, Wani WY, Ouyang X, Johnson MS, Ravi S, Barnes S, Darley-Usmar VM, Zhang J (2017) Inhibition of autophagy with bafilomycin and chloroquine decreases mitochondrial quality and bioenergetic function in primary neurons. Redox Biol 11:73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehli M, Den Elzen N, Cassady AI, Ostrowski MC, Hume DA (1999) Cloning and characterization of the murine genes for bHLH-ZIP transcription factors TFEC and TFEB reveal a common gene organization for all MiT subfamily members. Genomics 56:111–120. [DOI] [PubMed] [Google Scholar]
- Reuser AJ, Drost MR (2006) Lysosomal dysfunction, cellular pathology and clinical symptoms: basic principles. Acta Paediatr Suppl 95:77–82. [DOI] [PubMed] [Google Scholar]
- Roczniak-Ferguson A, Petit CS, Froehlich F, Qian S, Ky J, Angarola B, Walther TC, Ferguson SM (2012) The transcription factor TFEB links mTORC1 signaling to transcriptional control of lysosome homeostasis. Sci Signal 5:ra42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsztein DC (2006) The roles of intracellular protein-degradation pathways in neurodegeneration. Nature 443:780–786. [DOI] [PubMed] [Google Scholar]
- Ruggiano A, Foresti O, Carvalho P (2014) Quality control: ER-associated degradation: protein quality control and beyond. J Cell Biol 204:869–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MT, Hoogenraad NJ (2007) Mitochondrial-nuclear communications. Annu Rev Biochem 76:701–722. [DOI] [PubMed] [Google Scholar]
- Saftig P, Klumperman J (2009) Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat Rev Mol Cell Biol 10:623–635. [DOI] [PubMed] [Google Scholar]
- Sardar Sinha M, Ansell-Schultz A, Civitelli L, Hildesjo C, Larsson M, Lannfelt L, Ingelsson M, Hallbeck M (2018) Alzheimer’s disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol 136:41–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardiello M, Palmieri M, di Ronza A, Medina DL, Valenza M, Gennarino VA, Di Malta C, Donaudy F, Embrione V, Polishchuk RS, Banfi S, Parenti G, Cattaneo E, Ballabio A (2009) A gene network regulating lysosomal biogenesis and function. Science 325:473–477. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Yoshida H (2015) Organelle autoregulation-stress responses in the ER, Golgi, mitochondria and lysosome. J Biochem 157:185–195. [DOI] [PubMed] [Google Scholar]
- Schieber M, Chandel NS (2014) ROS function in redox signaling and oxidative stress. Curr Biol 24:R453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C, Ballabio A (2014) Lysosomal adaptation: how the lysosome responds to external cues. Cold Spring Harb Perspect Biol 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C, Fraldi A, Medina DL, Ballabio A (2013) Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Nat Rev Mol Cell Biol 14:283–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C, Fraldi A, Jahreiss L, Spampanato C, Venturi C, Medina D, de Pablo R, Tacchetti C, Rubinsztein DC, Ballabio A (2008) A block of autophagy in lysosomal storage disorders. Hum Mol Genet 17:119–129. [DOI] [PubMed] [Google Scholar]
- Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D, Colella P, Sardiello M, Rubinsztein DC, Ballabio A (2011) TFEB links autophagy to lysosomal biogenesis. Science 332:1429–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C, Zoncu R, Medina DL, Vetrini F, Erdin S, Erdin S, Huynh T, Ferron M, Karsenty G, Vellard MC, Facchinetti V, Sabatini DM, Ballabio A (2012) A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J 31:1095–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha Y, Rao L, Settembre C, Ballabio A, Eissa NT (2017) STUB1 regulates TFEB-induced autophagy-lysosome pathway. EMBO J 36:2544–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Chen X, Hendershot L, Prywes R (2002) ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev Cell 3:99–111. [DOI] [PubMed] [Google Scholar]
- Siman R, Mistretta S, Durkin JT, Savage MJ, Loh T, Trusko S, Scott RW (1993) Processing of the beta-amyloid precursor. Multiple proteases generate and degrade potentially amyloidogenic fragments. J Biol Chem 268:16602–16609. [PubMed] [Google Scholar]
- Song W, Wang F, Savini M, Ake A, di Ronza A, Sardiello M, Segatori L (2013) TFEB regulates lysosomal proteostasis. Hum Mol Genet 22:1994–2009. [DOI] [PubMed] [Google Scholar]
- Soto-Heredero G, Baixauli F, Mittelbrunn M (2017) Interorganelle Communication between Mitochondria and the Endolysosomal System. Front Cell Dev Biol 5:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spampanato C, Feeney E, Li L, Cardone M, Lim JA, Annunziata F, Zare H, Polishchuk R, Puertollano R, Parenti G, Ballabio A, Raben N (2013) Transcription factor EB (TFEB) is a new therapeutic target for Pompe disease. EMBO Mol Med 5:691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staneva-Stoicheva D, Krustev L, Kitova E (1977) [Morphological changes in the liver of rats treated with phenobarbital, methylphenobarbital and their N-substituted morpholinoethyl derivatives]. Eksp Med Morfol 16:90–96. [PubMed] [Google Scholar]
- Steinberg BE, Huynh KK, Brodovitch A, Jabs S, Stauber T, Jentsch TJ, Grinstein S (2010) A cation counterflux supports lysosomal acidification. J Cell Biol 189:1171–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steingrimsson E, Copeland NG, Jenkins NA (2004) Melanocytes and the microphthalmia transcription factor network. Annu Rev Genet 38:365–411. [DOI] [PubMed] [Google Scholar]
- Su Q, Zheng B, Wang CY, Yang YZ, Luo WW, Ma SM, Zhang XH, Ma D, Sun Y, Yang Z, Wen JK, Liu ZX (2018) Oxidative Stress Induces Neuronal Apoptosis Through Suppressing Transcription Factor EB Phosphorylation at Ser467. Cell Physiol Biochem 46:1536–1554. [DOI] [PubMed] [Google Scholar]
- Sun A (2018) Lysosomal storage disease overview. Ann Transl Med 6:476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talloczy Z, Martinez J, Joset D, Ray Y, Gacser A, Toussi S, Mizushima N, Nosanchuk JD, Goldstein H, Loike J, Sulzer D, Santambrogio L (2008) Methamphetamine inhibits antigen processing, presentation, and phagocytosis. PLoS Pathog 4:e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Guhde G, Suter A, Eskelinen EL, Hartmann D, Lullmann-Rauch R, Janssen PM, Blanz J, von Figura K, Saftig P (2000) Accumulation of autophagic vacuoles and cardiomyopathy in LAMP-2-deficient mice. Nature 406:902–906. [DOI] [PubMed] [Google Scholar]
- Tancini B, Buratta S, Sagini K, Costanzi E, Delo F, Urbanelli L, Emiliani C (2019) Insight into the Role of Extracellular Vesicles in Lysosomal Storage Disorders. Genes (Basel) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrano V, Royo F, Peinado H, Loizaga-Iriarte A, Unda M, Falcon-Perez JM, Carracedo A (2016) Vesicle-MaNiA: extracellular vesicles in liquid biopsy and cancer. Curr Opin Pharmacol 29:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres S, Balboa E, Zanlungo S, Enrich C, Garcia-Ruiz C, Fernandez-Checa JC (2017) Lysosomal and Mitochondrial Liaisons in Niemann-Pick Disease. Front Physiol 8:982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trajkovic K, Jeong H, Krainc D (2017) Mutant Huntingtin Is Secreted via a Late Endosomal/Lysosomal Unconventional Secretory Pathway. The Journal of neuroscience : the official journal of the Society for Neuroscience 37:9000–9012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama A, Kim JS, Kon K, Jaeschke H, Ikejima K, Watanabe S, Lemasters JJ (2008) Translocation of iron from lysosomes into mitochondria is a key event during oxidative stress-induced hepatocellular injury. Hepatology 48:1644–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera-Alberni M, Canto C (2018) Mitochondrial stress management: a dynamic journey. Cell Stress 2:253–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Niel G, D’Angelo G, Raposo G (2018) Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 19:213–228. [DOI] [PubMed] [Google Scholar]
- Vega-Rubin-de-Celis S, Pena-Llopis S, Konda M, Brugarolas J (2017) Multistep regulation of TFEB by MTORC1. Autophagy 13:464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallings RL, Humble SW, Ward ME, Wade-Martins R (2019) Lysosomal Dysfunction at the Centre of Parkinson’s Disease and Frontotemporal Dementia/Amyotrophic Lateral Sclerosis. Trends Neurosci 42:899–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Gomez-Sintes R, Boya P (2018) Lysosomal membrane permeabilization and cell death. Traffic 19:918–931. [DOI] [PubMed] [Google Scholar]
- Wang W, Gao Q, Yang M, Zhang X, Yu L, Lawas M, Li X, Bryant-Genevier M, Southall NT, Marugan J, Ferrer M, Xu H (2015) Up-regulation of lysosomal TRPML1 channels is essential for lysosomal adaptation to nutrient starvation. Proc Natl Acad Sci U S A 112:E1373–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wartosch L, Bright NA, Luzio JP (2015) Lysosomes. Curr Biol 25:R315–316. [DOI] [PubMed] [Google Scholar]
- Wibo M, Poole B (1974) Protein degradation in cultured cells. II. The uptake of chloroquine by rat fibroblasts and the inhibition of cellular protein degradation and cathepsin B1. J Cell Biol 63:430–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow AR, Chen CW, Corrochano S, Acevedo-Arozena A, Gordon DE, Peden AA, Lichtenberg M, Menzies FM, Ravikumar B, Imarisio S, Brown S, O’Kane CJ, Rubinsztein DC (2010) alpha-Synuclein impairs macroautophagy: implications for Parkinson’s disease. J Cell Biol 190:1023–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe DM, Lee JH, Kumar A, Lee S, Orenstein SJ, Nixon RA (2013) Autophagy failure in Alzheimer’s disease and the role of defective lysosomal acidification. Eur J Neurosci 37:1949–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu E, Liu J, Liu H, Wang X, Xiong H (2018) Inflammasome Activation by Methamphetamine Potentiates Lipopolysaccharide Stimulation of IL-1beta Production in Microglia. J Neuroimmune Pharmacol 13:237–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Ren D (2015) Lysosomal physiology. Annu Rev Physiol 77:57–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yambire KF, Rostosky C, Watanabe T, Pacheu-Grau D, Torres-Odio S, Sanchez-Guerrero A, Senderovich O, Meyron-Holtz EG, Milosevic I, Frahm J, West AP, Raimundo N (2019) Impaired lysosomal acidification triggers iron deficiency and inflammation in vivo. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagawa M, Tsukuba T, Nishioku T, Okamoto Y, Okamoto K, Takii R, Terada Y, Nakayama KI, Kadowaki T, Yamamoto K (2007) Cathepsin E deficiency induces a novel form of lysosomal storage disorder showing the accumulation of lysosomal membrane sialoglycoproteins and the elevation of lysosomal pH in macrophages. J Biol Chem 282:1851–1862. [DOI] [PubMed] [Google Scholar]
- Ye J, Rawson RB, Komuro R, Chen X, Dave UP, Prywes R, Brown MS, Goldstein JL (2000) ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell 6:1355–1364. [DOI] [PubMed] [Google Scholar]
- Ye Y, Hui L, Lakpa KL, Xing Y, Wollenzien H, Chen X, Zhao JX, Geiger JD (2019) Effects of silica nanoparticles on endolysosome function in primary cultured neurons (1). Can J Physiol Pharmacol 97:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim WW, Mizushima N (2020) Lysosome biology in autophagy. Cell Discov 6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogalingam G, Lee AR, Mackenzie DS, Maures TJ, Rafalko A, Prill H, Berguig GY, Hague C, Christianson T, Bell SM, LeBowitz JH (2017) Cellular Uptake and Delivery of Myeloperoxidase to Lysosomes Promote Lipofuscin Degradation and Lysosomal Stress in Retinal Cells. J Biol Chem 292:4255–4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda T, Benedetti C, Urano F, Clark SG, Harding HP, Ron D (2004) Compartment-specific perturbation of protein handling activates genes encoding mitochondrial chaperones. J Cell Sci 117:4055–4066. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Matsui T, Hosokawa N, Kaufman RJ, Nagata K, Mori K (2003) A time-dependent phase shift in the mammalian unfolded protein response. Dev Cell 4:265–271. [DOI] [PubMed] [Google Scholar]
- Yoshimori T, Yamamoto A, Moriyama Y, Futai M, Tashiro Y (1991) Bafilomycin A1, a specific inhibitor of vacuolar-type H(+)-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J Biol Chem 266:17707–17712. [PubMed] [Google Scholar]
- Yu L, Chen Y, Tooze SA (2018) Autophagy pathway: Cellular and molecular mechanisms. Autophagy 14:207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Cheng X, Yu L, Yang J, Calvo R, Patnaik S, Hu X, Gao Q, Yang M, Lawas M, Delling M, Marugan J, Ferrer M, Xu H (2016) MCOLN1 is a ROS sensor in lysosomes that regulates autophagy. Nat Commun 7:12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Wang J, Levichkin IV, Stasinopoulos S, Ryan MT, Hoogenraad NJ (2002) A mitochondrial specific stress response in mammalian cells. EMBO J 21:4411–4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhitomirsky B, Assaraf YG (2015) Lysosomal sequestration of hydrophobic weak base chemotherapeutics triggers lysosomal biogenesis and lysosome-dependent cancer multidrug resistance. Oncotarget 6:1143–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhitomirsky B, Yunaev A, Kreiserman R, Kaplan A, Stark M, Assaraf YG (2018) Lysosomotropic drugs activate TFEB via lysosomal membrane fluidization and consequent inhibition of mTORC1 activity. Cell Death Dis 9:1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigdon H, Meshcheriakova A, Farfel-Becker T, Volpert G, Sabanay H, Futerman AH (2017) Altered lysosome distribution is an early neuropathological event in neurological forms of Gaucher disease. FEBS letters 591:774–783. [DOI] [PubMed] [Google Scholar]


