Abstract
Persistent postpartum hypertension is a significant cause of maternal morbidity. Our objective was to study the effect of furosemide on postpartum blood pressure recovery in women with hypertensive disorders of pregnancy. We performed a randomized, double-blind, placebo controlled trial of a 5-day course of 20mg oral furosemide versus placebo in women with gestational hypertension and preeclampsia with/without severe features from June 2018 to October 2019. Primary outcomes were persistent hypertension at 7-days postpartum (using generalized linear models to calculate adjusted relative risk) and days to resolution of hypertension (Kaplan Meier curves), stratified by severe/non-severe hypertensive disease. Secondary outcomes included readmissions and need for additional hypertensive medication.We randomized 384 women (192/group). Baseline characteristics were similar except cesarean delivery rate was higher in the furosemide group (29% vs. 20%, p=0.04). In women randomized to furosemide, there was a 60% reduction in the prevalence of persistently elevated blood pressure at 7-days when controlling for cesarean (aRR 0.40, 95%CI 0.20–0.81). The magnitude of reduction was greater in women with non-severe disease (aRR 0.26, 95% CI 0.10–0.67). Days to blood pressure resolution was significantly shorter among women with non-severe disease randomized to furosemide (8.5 vs. 10.5, p=0.001). There were no significant differences in readmissions or need for additional antihypertensive medication postpartum between groups. In this double-blinded randomized trial, a short course of postpartum furosemide significantly improved blood pressure control in women with hypertensive disorders of pregnancy, mostly among women without severe disease.
Keywords: furosemide, hypertensive disorder of pregnancy, hypertension, postpartum, preeclampsia
Graphical Abstract
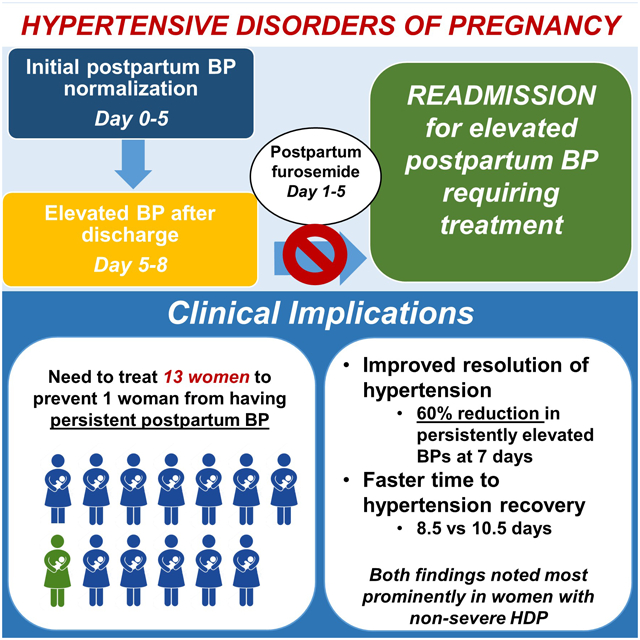
Summary
For every 13 women with HDP, treatment with furosemide would prevent one woman from having persistent postpartum hypertension.
Background
Hypertensive disorders of pregnancy (HDP), including gestational hypertension and preeclampsia, are recognized causes of significant maternal morbidity and mortality, accounting for approximately 18% of maternal deaths worldwide.1 Postpartum hypertension accounts for a significant amount of postpartum maternal morbidity including heart failure and stroke, and is the cause of approximately 27% of readmissions to the hospital, especially within the first 10 days postpartum.2,3 While postpartum hypertension can present de novo following a normotensive pregnancy, most postpartum hypertension complications are in women with persistent hypertension following a pregnancy complicated by preeclampsia or other HDP.4,5
Guidelines from national organizations recognize the growing concern with postpartum hypertension and have put forth specific recommendations to check an ambulatory blood pressure within at least 10 days of delivery among women with HDP.6 However, there are few recommendations on the management of blood pressure during this at-risk time period to improve blood pressure control and prevent the morbidity associated with postpartum hypertension. Therefore, interventions to improve the care of women with postpartum hypertension are urgently needed.
In women with HDP, blood pressure decreases in the first 48 hours postpartum and then increases during days 3–6 postpartum as a result of fluid retention and mobilization of large volumes of sodium into the intravascular compartment.5,7–11 Given these proposed mechanisms, furosemide, a loop diuretic, has been suggested to accelerate blood pressure recovery in the postpartum period. However, no prior studies on postpartum furosemide have examined blood pressure in the days immediately after discharge from the hospital, despite the known increase in BPs occurring days 3–6 after delivery.
Given the prevalence and morbidity of postpartum hypertension and the lack of research focusing on how to manage postpartum hypertension, it is critical to identify strategies to improve postpartum blood pressure which will, in turn, decrease maternal morbidity and hospital readmissions. Our objective, therefore, was to investigate the impact of a 5-day course of furosemide on postpartum blood pressure recovery in women with hypertensive disorders of pregnancy. We hypothesized that a short course of furosemide would improve postpartum blood pressure control.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
We performed a randomized, double-blind, placebo-controlled trial (NCT035556761) of women with HDP at the Hospital of the University of Pennsylvania from June 2018 to October 2019. The study was approved by the Institutional Review Board at the University of Pennsylvania after a convened board review and an Investigational New Drug exemption was obtained.
HDP included gestational hypertension, pre-eclampsia and pre-eclampsia superimposed on chronic hypertension with or without severe features. Per the American College of Obstetricians and Gynecologists (ACOG) guidelines,6 gestational hypertension was defined as two blood pressures (BPs) ≥ 140/90 mmHg at least 4 hours apart with no proteinuria and no other laboratory abnormalities, while pre-eclampsia without severe features has an additional requirement of proteinuria. Pre-eclampsia (or gestational hypertension) with severe features was diagnosed after two BPs ≥160/110 mmHg at least 4 hours apart or any of the following lab abnormalities or symptoms: platelets <100,000/microliter, liver enzymes at least twice the normal concentration, and/or severe right upper quadrant/epigastric pain, serum creatinine > 1.1 mg/dL or a doubling of baseline serum creatinine, pulmonary edema, or new-onset cerebral or visual disturbances (headache, blurry vision). Non-severe HDP disease included women with gestational hypertension, preeclampsia or super-imposed preeclampsia without severe features. Severe HDP included women with severe features in the setting of gestational hypertension, preeclampsia, or super-imposed preeclampsia, consistent with ACOG guidelines.6
Screening and recruitment
We included women diagnosed with HDP within the first day postpartum, who delivered a fetus ≥ 20 weeks gestation, were ≥18 years of age and English-speaking. We excluded women with the following: underlying cardiac disease, rheumatologic disease, advanced diabetes (White class C or higher), elevated creatinine (>1.2 mg/dL), significant hypokalemia (K<3 mEq/L), allergy to furosemide, or those who received diuretics prior to randomization. Eligible women were approached for enrollment by trained research personnel at any time during their labor and delivery course or within the first day postpartum and written consent was obtained.
Randomization
Eligible and consenting women were randomized in blocks of 4 to five days of 20 mg oral furosemide pill vs. placebo. Randomization sequence was prepared by the Penn Investigational Drug Services Pharmacy (IDS) with stratification according to disease severity (non-severe vs. severe HDP) as determined by the clinician placing the order at the time of randomization. Each participant’s supply of study medication (5 pills of furosemide or placebo) was packaged according to the IDS pharmacy and dispensed to the inpatient floor service. The first dose was instructed to be given within 6 to 24 hours postpartum and then every 24 hours thereafter until discharge. Women were discharged with the remaining medication, with study personnel confirming the number of pills remaining prior to discharge.
Postpartum Blood Pressure Treatment Algorithm
At our hospital, all women with HDP are enrolled in the bidirectional text-based program, HeartSafe Motherhood, as part of standard of care.12 Prior to discharge, women were provided with an Omron blood pressure monitor and instructed on its use. As part of the program, women were prompted via text to send in a blood pressure in the morning and afternoon. A physician received an alert message when BPs were ≥160/100 and all other BPs were reviewed daily; clinical discretion was used to assess the patient and begin antihypertensive agents based on blood pressure value. Our institution has a postpartum hypertension guideline created by Cardiology, Obstetrics, and Family Medicine teams. Women were maintained on their previous antihypertensive medication if it was started antepartum or intrapartum at the discretion of their provider. In the setting of no prior antihypertensive medication use, the algorithm includes the following (regardless of severity of HDP diagnosis upon enrollment): for women with persistent BPs ≥150/100, they are started on a calcium channel blocker (5 mg of amlodipine or 30 mg of nifedipine) daily. For BPs ≥160/110, after assessment for symptoms, hospital course, blood pressure trends, and need for potential clinic/hospital visit, women were started on 10 mg of amlodipine or their prior antihypertensive medication dose was adjusted.
Data Collection
Demographic and clinical information including obstetric history, medical history, and labor and delivery information was obtained on detailed chart review. Postpartum BPs included in the analysis were obtained from inpatient BPs prior to discharge along with home BPs that women supplied via a hospital text-based program for 7–10 days after discharge from the hospital. Data was extracted from patients’ charts up to 6 weeks postpartum.
Study Outcomes
The primary outcomes were (1) the prevalence of persistent hypertension 7-days postpartum (defined as at least two consecutive BP readings over 48 hours of systolic BP ≥140 mmHg and diastolic BP ≥90 mmHg) and (2) the number of days required to achieve resolution of hypertension (defined as at least two consecutive BP readings over 48 hours of SBP <140 mmHg and DBP<90 mmHg). Secondary outcomes included percentage of postpartum blood pressures in the severe range (SBP ≥160 mmHg or DBP ≥110 mmHg), the need for any additional antihypertensive medications postpartum (including at time of discharge and post-discharge), postpartum readmissions or emergency room visits, postpartum length of stay, pulmonary edema and severe maternal morbidity (eclampsia, acute kidney injury, disseminated intravascular coagulation, stroke, myocardial infarction, ICU stay). Other outcomes assessed were adverse effects associated with furosemide (hypokalemia, polydipsia, headaches, mental confusion, muscle aches, tetany, muscle weakness, heart rhythm disturbances), neonatal admission to the intensive care unit, and self-reported breastfeeding issues including decreased breast milk production.
Statistical analysis
A 35% prevalence of persistent postpartum hypertension was predicted based on prior studies.6,12 Based on this, we would need 345 women to have 80% power to see a reduction in persistent hypertension by 40% with a two-sided test and alpha of 0.05. We assumed a 10% loss of follow-up with texting, and therefore a final sample size of 384 women was required.Pearson chi-square and Wilcoxon rank sum test were used to assess univariate characteristics. Generalized linear models were used to calculate adjusted relative risk (aRR) for persistent hypertension as well as secondary outcomes. Kaplan Meier curves were used to obtain a hazard ratio (HR) for time (days) to resolution of hypertension. HR >1 represents a faster time to resolution of hypertension and a HR <1 represents a slower time to resolution. Linear mixed effects modeling was used for predicted BP trends. Covariates with a p < 0.1 from the univariate analysis were considered for adjustment. Ultimately all models were adjusted for mode of delivery (cesarean). A sensitivity analysis was performed for those women with incomplete outpatient data who did not text blood pressures after day 5 postpartum. Women with incomplete data were tested both in the positive (imputing all as persistent hypertension), and negative (imputing all as hypertension resolution). Results were stratified by HDP disease severity (severe and non-severe).
A data safety monitoring board was established to independently evaluate the safety of the study. An interim safety analysis was performed for predefined adverse outcomes with recommendations to continue the study without changes.
Results
Characteristics of the participants
There were 1265 women with HDP diagnosed within the first postpartum day and 904 were eligible and approached for enrollment. Of those, 384 were consented and randomized (192 in each arm). 331 women had complete postpartum BP ascertainment via texting (Figure 1).
Figure 1.
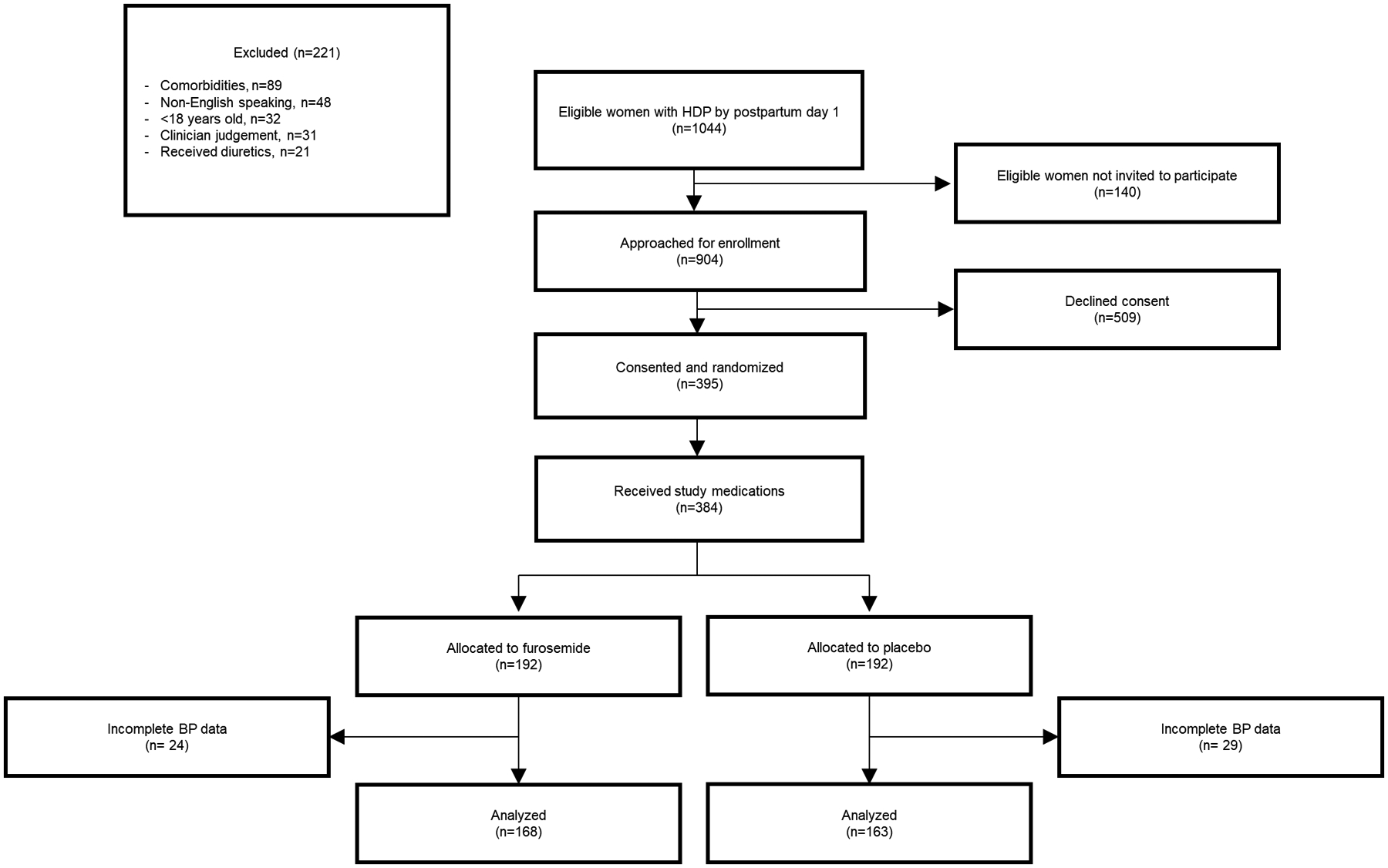
Study schema
HDP: Hypertensive disorder of pregnancy; BP: Blood Pressure
Baseline characteristics were similar between groups although the cesarean delivery rate was higher in the furosemide group (29% vs. 20%, p=0.04), Table 1. Approximately 75% of women in our cohort were Black and the mean BMI was 36.1 kg/m2. The majority of women (66%) had non-severe HDP.
Table 1.
Demographics
| Characteristics | Placebo n=192 | Furosemide n=192 | p-value |
|---|---|---|---|
| Maternal age (years)* | 27 (23–33) | 27 (22–32) | 0.50 |
| Race | 0.07 | ||
| Black | 138 (72) | 151 (79) | |
| White | 47 (24) | 30 (16) | |
| Other | 7 (4) | 11 (6) | |
| Insurance | 0.05 | ||
| Private | 65 (34) | 47 (25) | |
| Medicaid | 126 (66) | 142 (75) | |
| Nulliparous | 101 (53) | 93 (48) | 0.41 |
| Smoking | 18 (37) | 18 (32) | 0.58 |
| Timing of HDP diagnosis | |||
| Antepartum | 114 (59) | 112 (58) | 0.64 |
| Intrapartum | 65 (34) | 62 (32) | |
| Postpartum | 13 (7) | 18 (9) | |
| Severity of HDP | |||
| Non-severe HDP | 133 (69) | 128 (67) | 0.58 |
| Severe HDP | 59 (31) | 64 (33) | |
| Type of HDP | |||
| Gestational hypertension/pre-eclampsia | 181 (94) | 173 (90) | 0.13 |
| Super-imposed preeclampsia | 11 (6) | 19 (10) | |
| Chronic hypertension overall | 11 (6) | 19 (10) | 0.29 |
| Chronic hypertension on no antihypertensive medication | 7 (4) | 13 (7) | |
| Chronic hypertension on antihypertensive medication | 4 (2) | 6 (3) | |
| Gestational diabetes | 10 (6) | 12 (7) | 0.70 |
| Pre-gestational diabetes | 2 (1) | 2 (1) | 1 |
| BMI at delivery (kg/m2)* | 34.6 (29.6–42.1) | 36.5 (30.6–43.6) | 0.21 |
| GA at delivery (weeks)* | 38.4 (27.3–39.7) | 38.6 (37.3–39.7) | 0.91 |
| Method of delivery | 0.04 | ||
| Vaginal | 154 (80) | 137 (71) | |
| Cesarean delivery | 38 (20) | 55 (29) | |
| Creatinine at enrollment (mg/dL)* | 0.58 (0.53–0.70) | 0.59 (0.51–0.67) | 0.63 |
| Use of oral antihypertensive medication prior to delivery | 7 (4) | 10 (5) | 0.47 |
| Furosemide given outside of study meds postpartum | 10 (5) | 11 (6) | 0.81 |
Data presented as n (%) unless otherwise indicated
Median (inter-quartile range)
HDP: Hypertensive disorder of pregnancy; BMI: Body Mass Index; GA: Gestational age
Primary outcomes
There was a 60% reduction in the risk of persistent hypertension at 7-days postpartum in women randomized to furosemide versus placebo (6% vs.14%; aRR 0.40, 95% CI 0.20–0.81), Table 2. When stratified by HDP disease severity, there was a 74% reduction in persistent hypertension in women with non-severe HDP randomized to furosemide (5% vs. 16%, aRR 0.26, 95% CI 0.10–0.67) with no difference in the severe HDP group. A sensitivity analysis was performed including the 53 women with incomplete outpatient blood pressure data. For our sensitivity analysis, when assuming all women had resolution of hypertension by 7-days, results were unchanged. Similarly, results were unchanged under the assumption that all women had persistent hypertension 7-days postpartum.
Table 2.
Primary outcomes
| HDP category | Placebo N=192 | Furosemide N=192 | Relative Risk (RR) | aRR* (95% CI) | p-value |
|---|---|---|---|---|---|
| Persistent hypertension at 7-days postpartum† | |||||
| Overall | 23 (14) | 10 (6) | 0.42 (0.21–0.86) | 0.40 (0.20–0.81) | 0.01 |
| Non-severe | 18 (16) | 5 (5) | 0.28 (0.11–0.72) | 0.26 (0.10–0.67) | 0.006 |
| Severe | 5 (9) | 5 (9) | 0.93 (0.29–3.03) | 0.86 (0.27–2.79) | 0.81 |
| HDP category | Placebo | Furosemide | Hazard Ratio (HR) | aHR* (95% CI) | p-value |
| Number of days required for hypertension resolution‡ | |||||
| Overall | 10.5 (6.5–12.5) | 10 (6.5–12.5) | 1.09 (0.87–1.37) | 1.20 (0.95–1.51) | 0.12 |
| Non-severe | 10.5 (7–12.5) | 8.5 (5.5–12.5) | 1.46 (1.10–1.94) | 1.62 (1.22–2.15) | 0.001 |
| Severe | 10 (6.5–12.5) | 11.5 (7.5–13) | 0.74 (0.50–1.10) | 0.77 (0.52–1.15) | 0.20 |
Adjusted for cesarean delivery
Data presented as n (%);
Data presented as median [interquartile range]
HR: Hazard Ratio: HR >1 represents a faster time to resolution of hypertension; HR <1 represents a slower time to resolution
The average time to resolution of hypertension was 10 days with no difference between the two groups overall (Table 2). However, among women with non-severe HDP, time to resolution was two days shorter in women randomized to furosemide (8.5 vs. 10.5, aHR 1.62, 95% CI 1.22–2.15). No significant difference was noted in women with severe HDP.
Figure 2 displays the postpartum systolic blood pressure trajectories by treatment arm and disease severity. There was a significant difference between all four groups (p<0.0001) however, this was mostly driven by the difference in blood pressure trajectory among women with non-severe HDP when comparing the furosemide to placebo trajectories (p=0.0001). As noted in the figure, peak blood pressure was noted 3–4 days postpartum among women with severe HDP and 6–8 days postpartum among women with non-severe HDP.
Figure 2.
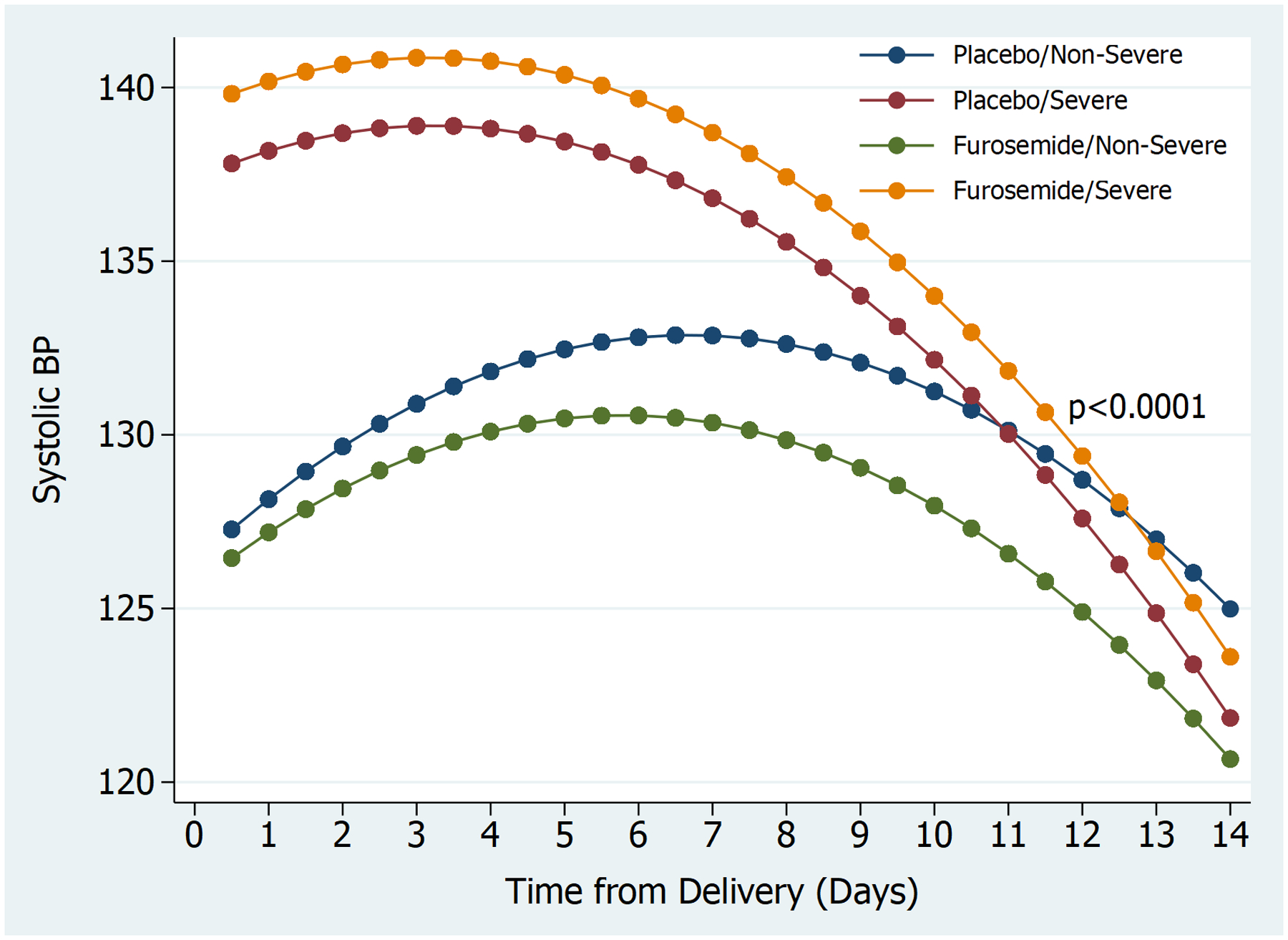
Postpartum systolic blood pressure trajectories by treatment group, stratified by severe and non-severe hypertensive disorder of pregnancy
BP: Blood Pressure
Secondary outcomes (Table 3)
Table 3.
Secondary outcomes
| HDP category | Placebo N=192 | Furosemide N=192 | Relative Risk (RR) | aRR* (95% CI) | p-value |
|---|---|---|---|---|---|
| Hypertension related hospital readmissions/emergency room visits | 16 (8) | 9 (5) | 0.56 (0.25–1.24) | 0.55 (0.25–1.21) | 0.14 |
| Percentage of postpartum blood pressures in the severe range† (%) | 6.8 | 6.6 | 0.86 (0.27–2.71) | 0.81 (0.26–2.57) | 0.36 |
| Overall need for any antihypertensive medication postpartum (at time of discharge or post-discharge) | 62 (32) | 63 (33) | 0.91 | ||
| Discharged on antihypertensive medication | 22 (11) | 40 (21) | 1.82 (1.12–2.94) | 1.67 (1.04–2.68) | 0.04 |
| Required new or additional antihypertensive medication after discharge | 39 (20) | 25 (13) | 0.64 (0.40–1.02) | 0.61 (0.39–0.96) | 0.03 |
| Pulmonary edema | 1 (1) | 3 (2) | 3 (0.32–28.59) | 2.07 (0.22–19.18) | 0.52 |
| Postpartum length of stay (days)‡ | 2 (2–2) | 2 (2–3) | 0.15 (−0.02–0.32) | 0.02 (−0.09–0.13) | 0.76 |
| Adverse effects associated with furosemide | 4 (2) | 0 | -- | -- | -- |
| NICU admission | 0 (0) | 2 (1) | -- | -- | 0.25 |
| Reported breast-feeding issues | 9 (6) | 4 (3) | 0.46 (0.15–1.47) | -- | 0.26 |
| Systolic blood pressure at 6 weeks postpartum (mm Hg)‡ | 120 (112–128) | 120 (110–124) | −1.94 (−4.93 – 1.05) | -- | 0.25 |
| Blood pressure ≥140/90 at 6 weeks postpartum | 13 (13) | 10 (10) | 0.78 (0.36–1.71) | 0.78 (0.36–1.69) | 0.53 |
| Blood pressure ≥130/80 at 6 weeks postpartum | 48 (47) | 41 (41) | 0.87 (0.64–1.19) | 0.87 (0.64–1.19) | 0.38 |
Adjusted for mode of delivery
Frequency of severe hypertension defined as: SBP ≥160 mmHg or DBP ≥110 mmHg
NICU: neonatal intensive care unit
Data are presented as n (%) except where indicated
Median [Inter-quartile range]
There was no difference in the overall need for additional antihypertensives postpartum (32% vs. 33%, p=0.91). Women in the furosemide group were more likely to require antihypertensive medication prior to discharge and women in the placebo group were more likely to require antihypertensive medication post-discharge. There was no statistically significant difference in hypertension related readmissions/ER visits or any other secondary outcomes noted in Table 3. There were no cases of severe maternal morbidity.
Discussion
In this double blind, placebo controlled randomized trial, a 5-day course of postpartum furosemide significantly improved blood pressure control in women with hypertensive disorders of pregnancy, specifically leading to improved resolution of hypertension and faster time to hypertension recovery. These findings were noted most prominently among women with non-severe HDP.
Prior studies of furosemide for the prevention and treatment of postpartum hypertension had mixed results with a recent Cochrane review on postpartum hypertension concluding that more data are needed on substantive outcomes before the practice of postpartum furosemide can be recommended.5 Many of these studies were small, and were predominantly done in women with severe preeclampsia despite the fact that the majority of postpartum hypertension readmissions are in women with non-severe HDP13–16 Furthermore, prior studies were limited to inpatient blood pressures with minimal outpatient ascertainment. No prior study has examined blood pressures after discharge from the hospital, despite the known increase in BPs that occurs 3–6 days after delivery.
A major difference between our results and prior studies is the efficacy of postpartum furosemide in women with non-severe HDP but not severe HDP. Unlike Ascarelli et al. and Veena et al., we did not find any significant differences in hypertension resolution or need for antihypertensives in women with severe HDP.14,15 This may be due to our more frequent use of antihypertensive medications at an earlier stage in the postpartum period among this group of high-risk women at our institution (n=63, 51%). By starting women with severe HDP on antihypertensive medications early in the postpartum course (often while still inpatient), we may be altering the blood pressure trajectory and dampening the effect of blood pressure recovery with furosemide use. An earlier and more aggressive start of antihypertensive medications may also explain the difference in prevalence of postpartum hypertension that we found (14%) compared to what we proposed in our sample size (35%).
A notable strength of this study was that it was a large randomized, double-blinded placebo control trial. Our population included more than 70% self-reported Black women, a population that is known to be at highest risk for HDP and its related morbidity. Therefore, this study is one of the largest trials on postpartum furosemide within a diverse patient population, which lends to the generalizability of our results. The use of mobile technology, as we had in our study, allowed for more accurate and longitudinal assessments of blood pressure trajectories in the two weeks postpartum - the time frame that blood pressures are at their peak. While it was a single center trial, adherence to hospital protocols for postpartum hypertension ensured that differences in outcomes seen between the two groups were due to the actual intervention itself instead of how postpartum hypertension was managed. A limitation of the study is that there was a slightly higher rate of loss to follow-up with texting in blood pressures than expected (14%).12 Importantly, we performed sensitivity analyses analyzing this missing data which did not change our overall results. Lastly, while powered for our primary outcomes, we were underpowered for many of our clinically meaningful secondary outcomes, including readmission rate.
Novelty and significance.
What is new?
We evaluated the impact of furosemide on postpartum blood pressure recovery post-discharge from the hospital in women with hypertensive disorder of pregnancy.
This study includes women with both severe and non-severe hypertensive disorders of pregnancy, is larger than prior studies and has a higher population of Black women.
What is relevant?
In women with a hypertensive disorder of pregnancy, a 5-day course of postpartum furosemide leads to a lower risk of persistent postpartum hypertension and faster resolution of postpartum hypertension.
PERSPECTIVES.
Given the significant maternal morbidity and mortality associated with postpartum hypertension, it is critical to find ways to improve blood pressure control and optimize maternal outcomes. We found that in women with HDP, a 5-day course of postpartum furosemide decreases the risk of persistent hypertension postpartum and leads to a faster resolution of postpartum hypertension most profoundly in women without severe disease. This has important maternal health implications in decreasing the burden of disease related to HDP. Based on our results, for every 13 women with HDP, treatment with furosemide would prevent one woman from having persistent postpartum hypertension. With HDP affecting more than 400,000 deliveries annually in the United States alone, use of furosemide postpartum could have a tremendous impact in improving maternal health. Larger studies are needed in diverse delivery hospital settings to evaluate whether the use of postpartum furosemide leads to reductions in hypertension-related readmissions and maternal morbidity.
Acknowledgements:
We would like to thank all the research, nursing, and physician staff of the Hospital of University of Pennsylvania and the Maternal and Child Health Research Center.
Sources of funding: NHLBI 1R56HL136730
Footnotes
Disclosures: No conflicts of interest.
References
- 1.Khan KS, Wojdyla D, Say L, Gulmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet 2006;367:1066–74. [DOI] [PubMed] [Google Scholar]
- 2.American Hospital Association. Reducing avoidable obstetrical and neonatal readmissions. Available from http://www.aha.org/content/11/Perinatalreadmissionscall1.pdf retrieved December 12, 2017.
- 3.Too G, Wen T, Boehme AK, Miller EC, Leffert LR, Attenello FJ, Mack WJ, D’Alton ME, Friedman AM. Timing and risk factors of postpartum stroke. Obstet Gynecol 2018; 131: 70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sibai BM. Etiology and management of postpartum hypertension-preeclampsia. Am J Obstet Gynecol 2012;206:470–5. [DOI] [PubMed] [Google Scholar]
- 5.Magee L, von Dadelszen P. Prevention and treatment of postpartum hypertension. Cochrane Database Syst Rev 2013;4:CD004351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American College of Obstetricians and Gynecologists; Gestational hypertension and preeclampsia: ACOG practice bulletin. Obstet Gynecol 2020; 135: 1492–1495. [DOI] [PubMed] [Google Scholar]
- 7.Hirshberg A, Levine LD, Srinivas SK. Clinical factors associated with readmission for postpartum hypertension in women with pregnancy-related hypertension: a nested case control study. J Perinatol 2016; 36: 405–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Makkonen N, Harju M, Kirkinen P. Postpartum recovery after severe pre-eclampsia and HELLP-syndrome. J Perinat Med 1996;24:641–9. [DOI] [PubMed] [Google Scholar]
- 9.Stepan H, Nordmeyer AK, Faber R. Proteinuria in hypertensive pregnancy diseases is associated with a longer persistence of hypertension postpartum. J Hum Hypertens 2006;20:125–8. [DOI] [PubMed] [Google Scholar]
- 10.Podymow T, August P. Postpartum course of gestational hypertension and preeclampsia. Hypertens Pregnancy 2010;29:294–300. [DOI] [PubMed] [Google Scholar]
- 11.Walters BN, Walters T. Hypertension in the puerperium. Lancet 1987; 2: 330. [DOI] [PubMed] [Google Scholar]
- 12.Hirshberg A, Downes K, Srinivas S. Comparing standard office-based follow-up with text-based remote monitoring in the management of postpartum hypertension: a randomised clinical trial. BMJ Qual Saf 2018; 27:871–877. [DOI] [PubMed] [Google Scholar]
- 13.Matthews G, Gornall R, Saunders NJ. A randomized placebo controlled trial of loop diuretics in moderate/severe pre-eclampsia, following delivery. J Obstet Gynaecol 1997; 17: 30–2. [DOI] [PubMed] [Google Scholar]
- 14.Ascarelli MH, Johnson V, McCreary H, Cushman J, May Wl, Martin JN Jr. Postpartum preeclampsia management with furosemide: a randomized clinical trial. Obstet Gynecol 2005: 105:29–33. [DOI] [PubMed] [Google Scholar]
- 15.Veena P, Perivela L, Soundara Raghavan S. Furosemide in postpartum management of severe preeclampsia: a randomized controlled trial. Hypertens Pregnancy 2017; 36: 84–89. [DOI] [PubMed] [Google Scholar]
- 16.Viteri OA, Alrais MA, Pedroza C, Hutchinson M, Chauhan SP, Blackwell SC, Sibai BM. Torsemide for prevention of persistent postpartum hypertension in women with preeclampsia: a randomized controlled trial. Obstet Gynecol 2018; 132: 1185–1191. [DOI] [PubMed] [Google Scholar]


