Abstract
Background:
The extent of posttraumatic osteoarthritis (PTOA) in the porcine ACL transection model is dependent on the surgical treatment of that injury. In a previous study, animals treated with bridge-enhanced ACL repair using a tissue-engineered implant developed less PTOA than those treated with ACL reconstruction. Alterations in gait, including asymmetric weight bearing and shorter stance times, have been noted in clinical studies of subjects with osteoarthritis.
Hypothesis:
Animals receiving a surgical treatment that results in less posttraumatic osteoarthritis (i.e., bridge-enhanced ACL repair) would exhibit fewer post-surgical gait asymmetries over a 1-year period when compared to treatments that result in greater PTOA (i.e., ACL reconstruction and ACL transection).
Study Design:
Controlled laboratory study
Methods:
36 Yucatan minipigs underwent ACL transection and were randomized to: 1) no further treatment, 2) ACL reconstruction, or 3) bridge-enhanced ACL repair. Gait analyses were performed pre-operatively, and at 4, 12, 26 and 52 weeks post-operatively. Macroscopic cartilage assessments were performed following euthanasia at 52 weeks.
Results:
Knees treated with bridge-enhanced ACL repair had less macroscopic damage in the medial tibial plateau than those treated with ACL reconstruction or ACL transection (P-adj=.03 for both comparisons). The knees treated with bridge-enhanced ACL repair had greater asymmetry in hindlimb maximum force and impulse loading than the knees treated with ACL transection at 52 weeks (P-adj<.05 for both comparisons). There was evidence to show that knees treated with bridge-enhanced ACL repair also had greater asymmetry in hindlimb maximum force and impulse loading (P-adj<.10 for both comparisons) compared to ACL reconstruction.
Conclusions:
Contrary to our hypothesis, the surgical treatment resulting in the lowest amount of macroscopic cartilage damage exhibited greater asymmetry in load-related gait parameters than the other surgical groups. This finding suggests that increased off-loading of the surgical knee may be associated with a slower rate of PTOA development.
Clinical Relevance:
Less cartilage damage at 52 weeks was found in the surgical group that continued to protect the limb from full body weight during gait. This finding suggests that protection of the knee from maximum stresses may be important in minimizing the development of PTOA in the ACL-injured knee.
Keywords: Anterior cruciate ligament, Gait, ACL reconstruction, bridge-enhanced ACL repair
Introduction
Anterior cruciate ligament (ACL) injured patients develop posttraumatic osteoarthritis (PTOA) even after ACL reconstruction.1 New treatments that would minimize cartilage damage, while providing mechanical stability, would be of significant value. Bridge-enhanced ACL repair is an innovative approach for the treatment of ACL injuries.24–26 In the porcine model, macroscopic cartilage damage of the tibiofemoral joint was shown to be less in knees receiving bridge-enhanced ACL repair at 1 year post-surgery compared to those treated with ACL reconstruction and those who had an untreated ACL transection.23 The mechanism by which bridge-enhanced ACL repair confers chondroprotection has yet to be determined, and may be dependent on the degree of stability conferred by the healing ligament or graft, differences in joint loading during activities such as gait, and/or biological factors. In a previous porcine study, it was found that both bridge-enhanced ACL repair and traditional ACL reconstruction resulted in similar knee stability and ligament/graft biomechanics.23 Likewise, the gene expression profiles between the two groups within the acute phase of healing have been shown to be similar.32,33 To the best of our knowledge, longitudinal gait changes during PTOA development in animals that underwent bridge-enhanced ACL repair or ACL reconstruction have not been compared.
Patients with knee osteoarthritis (OA) reduce joint loading by decreasing the extensor knee moment,14 which can be approximated in the porcine model as a change in maximum vertical ground reaction force normalized to body weight. Patient-reported outcomes in people with knee OA also correlate with gait asymmetries during the single limb support phases of gait.7,8 However, longitudinal studies of gait changes in humans corresponding to direct measures of the cartilage damage have yet to be reported, likely due to the decades of time required to develop end-stage disease and the invasive procedure required to directly visualize the cartilage. Without longitudinal studies, the long-term effects of limb offloading on the injured knee in human patients remains unknown.
The pig provides a relevant model to longitudinally study alterations in gait during PTOA development. We have previously shown that the porcine knee reliably develops PTOA after an ACL transection or an ACL reconstruction within a 1-year period.23 Furthermore, gait assessments, similar to those used in humans and small animal models of PTOA,6 can also be performed in pigs. As bridge-enhanced ACL repair has been shown to result in less PTOA in the porcine model,23 a comparison of these three surgical groups (ACL transection, ACL reconstruction and bridge-enhanced ACL repair) would provide an opportunity to evaluate longitudinal gait changes between groups of animals undergoing varying degrees of disease progression.
The study objective was to compare longitudinal changes in gait, and how those differed between groups of animals developing PTOA at different rates. We hypothesized that animals receiving a surgical treatment (i.e., bridge-enhanced ACL repair) that produces less PTOA, as determined by macroscopic and microscopic assessments, would exhibit less post-surgical gait asymmetries over a 1-year period when compared to those treatments exhibiting more PTOA (i.e., ACL reconstruction or untreated ACL transection).
Methods
Study Design
Institutional Animal Care and Use Committee approvals were acquired prior to beginning the study. Thirty six Yucatan mini-pigs in late adolescence [age (mean±SD): 15.3±1.6 months; weight: 52.1±4.6 kg] underwent ACL transection and were block randomized (using a random number generator) to one of three experimental groups: 1) no treatment (ACLT group), 2) ACL reconstruction with bone-patellar tendon-bone allograft (ACLR group), and 3) bridge-enhanced ACL repair using a scaffold combined with autologous blood (BE-R group).23 Randomization was blocked so that an equal number of male and female animals were represented in each group. Justification of the model and details of the surgical procedures have been previously reported,23 and are summarized in an online supplement (see Supplemental Methods). Detailed information regarding animal husbandry and pain management are available in the online supplement (see Supplemental Methods). The sample size was established by an a priori power analysis based on gait parameters (see Supplemental Methods). Longitudinal gait analyses were performed pre-operatively, and at 4, 12, 26 and 52 weeks after surgery. Macroscopic and microscopic cartilage assessments of the articular cartilage, as well as microscopic evaluation of the synovium, were performed following euthanasia and limb harvest at 52 weeks. No animals were excluded from the assigned treatment or any of the analyses. All post-operative assessments were performed with the animals blinded to the group assignment.
Gait measurement
Gait was evaluated using a pressure mat (HRV6 Walkway System; Tekscan Inc, Boston, MA) with an active sensing area of 292.6 x 44.7 cm.6 Animals were trained to walk on the pressure mat in one direction using food for encouragement. Based on the pressures observed for this cohort, a step calibration was performed for each sensing tile using a 58 kg custom three-legged phantom as recommended by the manufacturer. Data were collected at 104 Hz using the first hoof contact as a trigger until the animal stepped off the mat. Commercial software (Walkway 7.0; Tekscan Inc, Boston, MA) was used for data collection and analysis. Five trials were obtained for each animal at each timepoint (preop, 4, 12, 26 and 52 weeks). For analysis, hoof strikes were automatically identified by the software, and partial strikes were discarded. Gait data were generated using the force, spatial and temporal parameters of the identified foot strikes. Force parameters including maximum force (kg) as a percentage of body weight, impulse (kg-sec) as a percentage of body, and maximum peak pressure (KPa) were calculated for each limb. The spatiotemporal parameters included the stance time, stride time, stride length, and stride velocity. The average of five trials was reported for each outcome as a ratio of surgical and contralateral knees. The ratios at each time point were compared between treatment groups.
Macroscopic Articular Cartilage Analysis
Knee joints were opened using aseptic technique immediately after euthanasia. Macroscopic damage of the articular cartilage surfaces was assessed according to OARSI guidelines for sheep and goat.19 Damage to six articular surfaces, including the medial femoral condyle, medial tibial plateau, lateral femoral condyle, lateral tibial plateau, femoral trochlea, and the patella, was scored from 0 (i.e., normal) to 4 (i.e., large erosions down to subchondral bone). Scores from the four tibiofemoral surfaces were then added to make up the total macroscopic score of the tibiofemoral joint, which ranged from 0 to 16.
Microscopic Articular Cartilage Analysis
Bilateral osteochondral samples were harvested from the medial femoral condyles for histopathological analysis. Central coronal slabs were fixed in 10% neutral buffered formalin and decalcified in 10% formic acid (EMD Millipore, Darmstadt, Germany)/5% formalin solution (Acros Organics, Belgium) before dehydrating and embedding in paraffin. Serial sections were stained with hematoxylin and eosin (H&E), Safranin-O and Fast green (Saf-O), and nuclear fast red (NFR). Microscopic scoring of the articular cartilage was performed according to OARSI guidelines19 by three independent experienced readers (BCF, NPK, BLP), who were blinded to the experimental condition of the samples. Examiner scores were averaged for analysis.
Microscopic Synovium Analysis
Central sections of medial meniscus and the adjacent synovium were fixed in formalin, dehydrated and embedded in paraffin. 6 μm sections were stained with H&E. Scoring was performed by an examiner blinded to treatment group using three individual features of the synovial membrane (synovial cell lining layer, cellular density of the synovial stroma, and inflammatory infiltrate).18 Each feature was graded on a scale of 0-3. The sum of the three individual sub-scores was defined as the synovitis sum score. Knees with total scores of 0 or 1 were classified as having no synovitis, 2-4 as low-grade synovitis, and 5-9 as high-grade synovitis.
Statistical analyses
Data were imported into commercial software (SAS version 9.4; SAS Institute Inc., Cary, NC) for analysis. Generalized estimating equations (GEEs) were used (1) to model the gait parameters as a function of animal within time and experimental group, and (2) to model the macroscopic articular cartilage measures, the microscopic articular cartilage measures, and the synovitis histology measures as a function of knee within animal and experimental group. A linear distribution was assumed for all gait parameters, while a binomial distribution was assumed for all remaining macroscopic, microscopic, and synovitis measures. Classical sandwich estimation was used to adjust for any possible model misspecification. Pairwise comparisons between groups were conducted via orthogonal contrasts. The Holm-test was used to adjust for multiple comparisons to maintain a two-tailed familywise alpha at 0.05. All modeling was completed using PROC GLIMMIX and all orthogonal contrasts were completed using the lsmeans statement. A p-value of 0.05 was used to determine statistical significance.
Results
There was a significant effect of surgical group on the post-operative gait changes (Table 1, Supplemental Table S1). Pre-operatively, no significant differences (P-adj>.05) were observed between treatment groups for any of the gait parameters. Four weeks after surgery, the surgical limb of the BE-R group had a 20% lower mean ratio of maximum force (%BW) of the surgical to the contralateral limb (Table 1, P-adj=.003), a 20% lower mean impulse ratio (P-adj=.003), a 16% lower maximum peak pressure (P-adj=.03) and some evidence for a lower stance time ratio (P-adj = 0.05) than the ACLT group. There were no differences between the BE-R and ACLR groups for gait at 4, 12 or 26 weeks after surgery. The ACLR group at four weeks had a 13% lower mean impulse ratio (P-adj=.03) and 7% lower mean stance time ratio (P-adj=.03), compared to the ACLT group at the same time point.
Table 1.
Mean±standard deviations of the gait parameter ratios (surgical/contralateral) for the three treatment groups over time. ACLT=ACL Transection, ACLR=ACL reconstruction, and BE-R=Bridge-enhanced ACL Repair. Values in bold highlight significant findings. The complete data set related to the gait parameters can be found in Supplemental Table S1.
| Gait Parameter | Time | ACLT | ACLR | BE-R |
|---|---|---|---|---|
| Max force (%BW) | Pre-op | 1.01±0.08 | 1.01±0.06 | 1.03±0.08 |
| 4 weeks | 0.73±0.12 | 0.65±0.14 | 0.55±0.15* | |
| 12 weeks | 0.97±0.13 | 0.88±0.14 | 0.89±0.1 | |
| 26 weeks | 0.98±0.07 | 0.97±0.11 | 0.91±0.09** | |
| 52 weeks | 1.01±0.09 | 1.00±0.12 | 0.89±0.12*, ## | |
| Impulse (%BW) | Pre-op | 1.03±0. 09 | 1.03±0.11 | 1.03±0.11 |
| 4 weeks | 0.72±0.11 | 0.59±0.15* | 0.52±0.19* | |
| 12 weeks | 0.95±0.13 | 0.90±0.13 | 0.93±0.12 | |
| 26 weeks | 1.00±0.07 | 0.96±0.10 | 0.93±0.10 | |
| 52 weeks | 1.03±0.09 | 0.98±0.08 | 0.91±0.10*,## | |
| Max Peak Pressure | Pre-op | 1.02±0.12 | 1.00±0.12 | 1.03±0.07 |
| 4 weeks | 1.05±0.21 | 0.95±0.12 | 0.88±0.11* | |
| 12 weeks | 0.97±0.06 | 0.95±0.12 | 1.00±0.12 | |
| 26 weeks | 1.03±0.14 | 0.97±0.08 | 1.00±0.1 | |
| 52 weeks | 0.99±0.12 | 1.01±0.11 | 1.01±0.09 | |
| Stance time | Pre-op | 1.01±0.06 | 0.98±0.06 | 1.02±0.10 |
| 4 weeks | 0.96±0.06 | 0.89±0.06* | 0.87±0.11** | |
| 12 weeks | 0.98±0.05 | 0.99±0.08 | 1.00±0.08 | |
| 26 weeks | 1.02±0.06 | 0.98±0.08 | 1.01±0.04 | |
| 52 weeks | 1.03±0.05 | 1.00±0.09 | 1.01±0.04 | |
| Stride velocity | Pre-op | 1.01±0.06 | 1.00±0.04 | 1.01±0.04 |
| 4 weeks | 0.98±0.05 | 1.00±0.04 | 1.00±0.05 | |
| 12 weeks | 1.00±0.02 | 0.99±0.02 | 1.01±0.03 | |
| 26 weeks | 1.01±0.03 | 0.99±0.03 | 1.00±0.03 | |
| 52 weeks | 1.00±0.04 | 0.98±0.04 | 1.01±0.04## | |
different from ACLT, P-adj<.0.05 for all comparisons.
different from ACLT, .05< P-adj<.10 for all comparisons.
different from ACLR, .05< P-adj<.10 for all comparisons.
Fifty-two weeks after surgery, the BE-R group had a lower mean maximum force ratio (P-adj=.02) and mean impulse ratio (P-adj=−.01) compared to ACLT and there was some evidence for a lower mean maximum force ratio (P-adj=.07), mean impulse ratio (P-adj=.09), and higher stride velocity (P-adj=.07) compared to ACLR. No significant differences in mean stride time and stride length ratios were observed between groups at any time point (P>.05 for all comparisons).
Macroscopic Articular Cartilage Score
There was a significant effect of surgical treatment on the mean tibiofemoral macroscopic damage scores in the articular cartilage of the surgical knees at 52 weeks (Figure 1; Table 2; Supplemental Table S2). Compared to ACLT and ACLR, the knees treated with BE-R had significantly lower scores in the medial tibial plateau (Table 2; P-adj<.03 for both comparisons). The medial tibial plateau had erosions of cartilage down to the subchondral bone in 3 of the ACL transected knees, 4 of the ACL reconstructed knees and 0 of the BE-R knees. Compared with ACLT, the knees treated with BE-R and ACLR had significantly lower scores in the lateral femoral condyle (Table 2; P-adj<.01 for both comparisons). For the lateral femoral condyle, 5 of the ACL transected knees had erosions down to subchondral bone, 1 of the ACL reconstructed knees had large erosions to bone and 1 of the bridge-enhanced ACL repair knees had a small area of erosion down to bone. There were no significant differences between groups for damage to the medial femoral condyle or lateral tibial plateau, trochlea or patella (Table 2). There were also no significant differences in the contralateral macroscopic damage scores between the groups (Table 2, Supplemental Table S2; P-adj>.05 for all comparisons).
Figure 1.
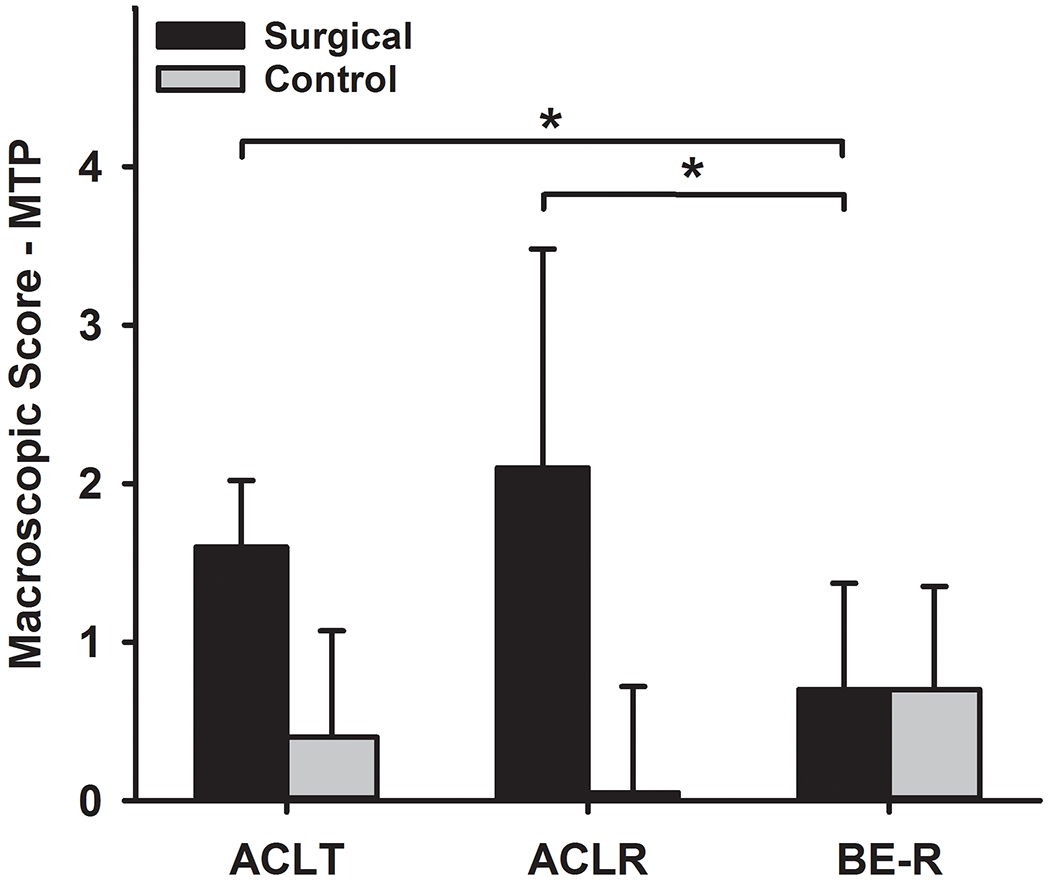
Mean Macroscopic Damage Scores for the medial tibial plateau (MTP) for all groups at 52 weeks. ACLT=ACL Transection, ACLR=ACL reconstruction, and BE-R=Bridge-enhanced ACL Repair. Error bars represent the standard deviation. * Significant differences P-adj=.03. The complete data related to the macroscopic cartilage damage set can be found in Supplemental Table S2.
Table 2.
Macroscopic Damage Scores (mean±standard deviations) for each region for all groups at 52 weeks. ACLT=ACL Transection, ACLR=ACL reconstruction, and BE-R=Bridge-enhanced ACL Repair, TF=tibiofemoral
| ACLT | ACLR | BE-R | ||||
|---|---|---|---|---|---|---|
| Surgical | Contralateral | Surgical | Contralateral | Surgical | Contralateral | |
| Medial Tibial Plateau | 1.6±0.42 | 0.4±0.67 | 2.1±1.38 | 0.5±0.67 | 0.7±0.67*,# | 0.7±0.65 |
| Medial Femoral Condyle | 1.5±1.31 | 1.2±0.83 | 2.0±1.53 | 0.7±0.98 | 1.7±1.44 | 1.0±0.60 |
| Lateral Femoral Condyle | 2.2±1.03 | 0.3±0.45 | 1.1±1.16* | 0.1±0.29 | 0.7±0.99* | 0.2±0.39 |
| Lateral Tibial Plateau | 1.8±0.94 | 0.3±0.45 | 2.1±0.79 | 0.2±0.79 | 1.6±1.08 | 0.1±0.29 |
| Trochlea | 3.2±1.19 | 0 ±0 | 3.0±1.35 | 0.3±0.89 | 2.6±1.68 | 0.6±1.19 |
| Patella | 0.1±0.29 | 0±0 | 0.1±0.28 | 0±0 | 0.4±1.00 | 0.3±0.88 |
| Total TF Macroscopic Score | 7.8±2.79 | 1.8±1.27 | 7.5±2.68 | 1.4±2.68 | 4.8±3.04**,## | 1.9±1.24 |
P-adj<.05 compared to ACLT
P-adj=.06 compared to ACLT
P-adj<.05 compared to ACLR
P-adj =.06 compared to ACLR
Microscopic Articular Cartilage Score
There were no significant differences in the microscopic OARSI scores between the surgical groups for either the surgical knee or the contralateral knee at 52 weeks (P-adj>.09 for all comparisons, Figure 2, Supplemental Table S3). The mean OARSI microscopic score in the surgical knee was twice as high as the score of the contralateral knee in the BE-R group (P-adj=.04) and four times as high for the ACLR group (P-adj<.001), while no difference between the surgical and contralateral knees was noted for the ACLT group (P-adj=.28).
Figure 2.
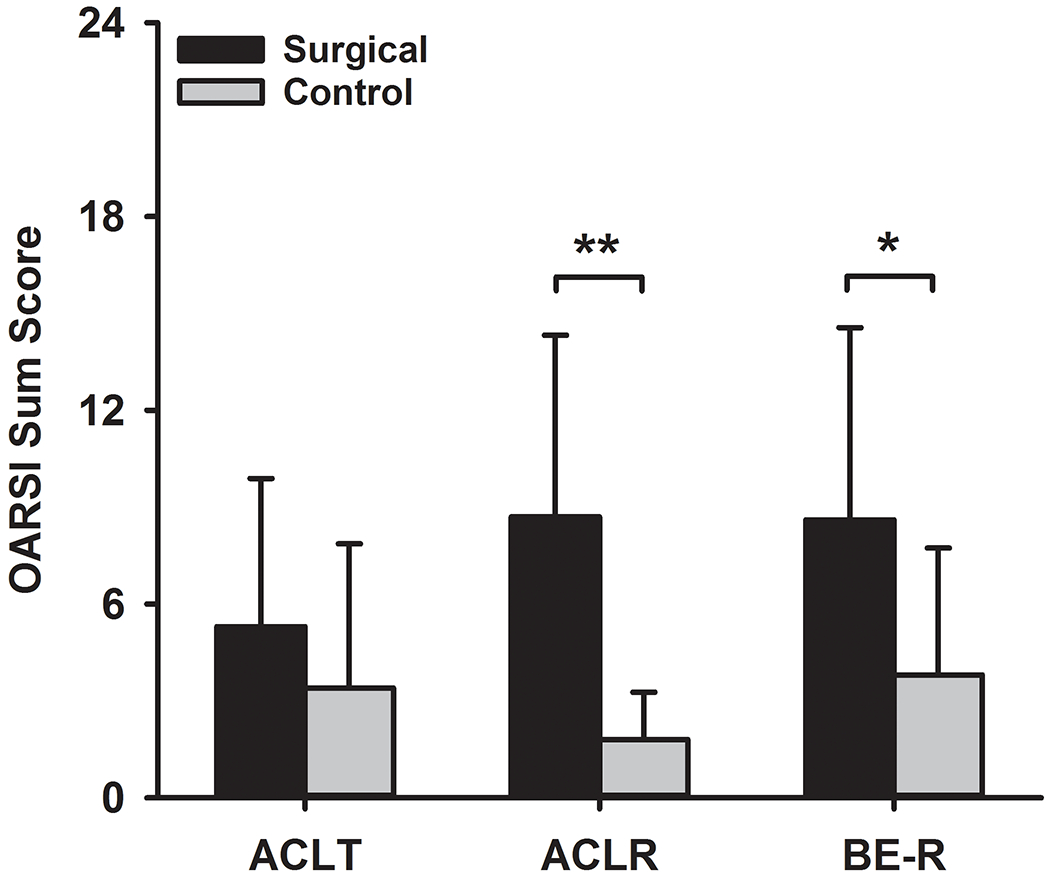
Microscopic OARSI Sum Scores (mean±standard deviations) for all groups at 52 weeks. There were no significant differences between groups in the surgical or contralateral knees. ACLT=ACL Transection, ACLR=ACL reconstruction, and BE-R=Bridge-enhanced ACL Repair. **Significant difference between surgical and contralateral, P-adj<.001. *Significant difference between surgical and contralateral, P-adj<.05. The complete data set related to the microscopic assessment can be found in Supplemental Table S3.
Synovitis Assessment
There were significantly higher mean synovitis sum scores in the surgical knees compared to contralateral knees in all groups at 52 weeks (P-adj<.01 for the ACLT and ACLR groups, P-adj=.06 for the BE-R group; Figure 3, Supplemental Table 4), and the mean synovial cell densities were higher in the surgical knees compared to the contralateral knees in the ACLR and ACLT groups (P-adj<.05 for both comparisons). There was no difference in the surgical or contralateral knee scores between groups (P-adj>.05 for all comparisons; Figure 2, Supplemental Table 4). There was no evidence of a higher inflammatory infiltrate in the surgical knee compared to the contralateral knee in any of the groups p (P-adj=.15 for ACLT and P-adj=1.0 for ACLR and BE-R groups).
Figure 3.
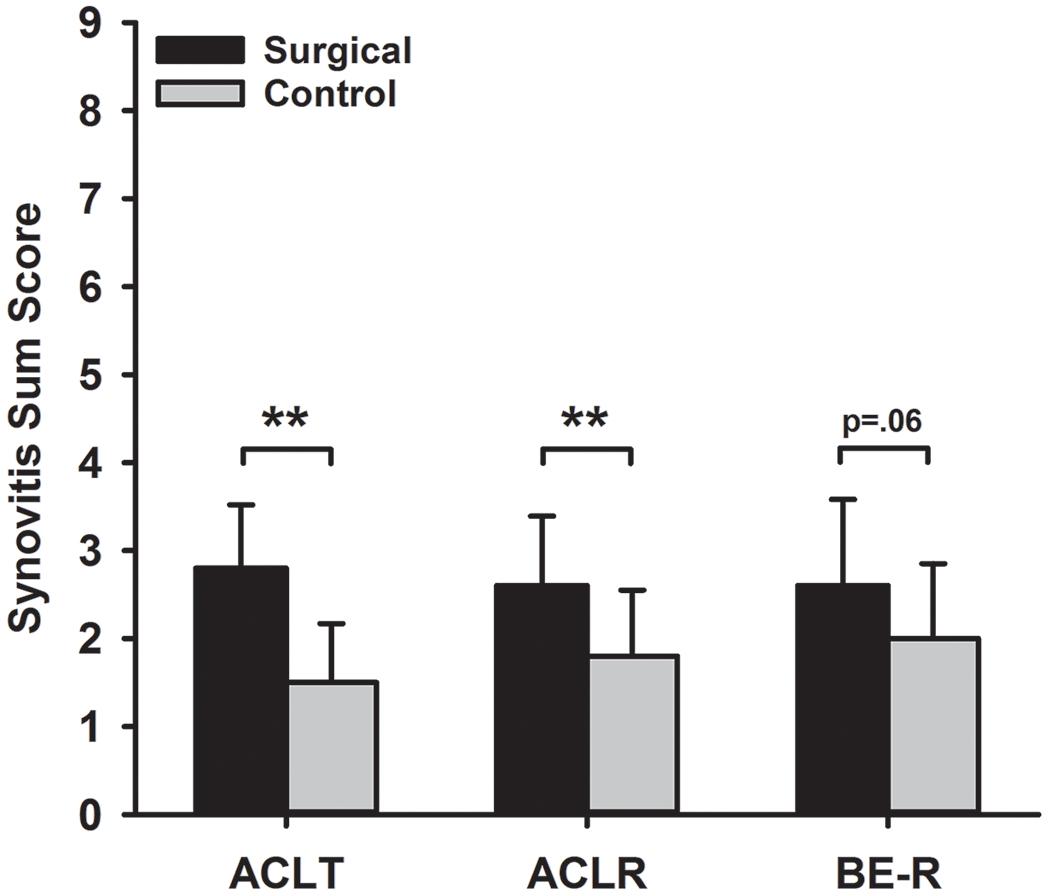
Mean synovitis sum score between three treatment groups at 52 weeks. Sx and Ctrl indicate surgical and contralateral limbs respectively. ACLT=ACL Transection, ACLR=ACL reconstruction, and BE-R=Bridge-enhanced ACL Repair. Error bars represent the standard deviations. **Significant difference between surgical and contralateral, P-adj<.01. The complete data set including the subscores related to the synovitis can be found in Supplemental Table S4.
Discussion
Our hypothesis was that animals receiving a surgical treatment that results in less macroscopic cartilage damage (BE-R) would have smaller alterations in gait during the initial period of osteoarthritis development. To the contrary, we found that the BE-R animals offloaded the surgical limb post-operatively to a greater degree than the other groups, a difference that was statistically significant at 52 weeks, even with a relatively small number of animals in each group. This finding was despite the result that the BE-R knees had significantly less macroscopic cartilage damage than the ACLT or ACLR knees at that same time point. Interestingly, despite having less macroscopic damage, the microscopic cartilage changes were greater in the BE-R group than in the ACLT group, differences that were driven largely by increases in structure and cloning sub-scores in those groups. Finally, there was no difference in microscopic synovitis scores between the groups, suggesting that there was no ongoing synovial reaction to the implanted scaffold in the BE-R group.
The findings of lower maximum force and shorter stance time on the operated knee at the 4-week time point in all groups are consistent with those previously reported in rodent models of PTOA over 6 weeks, where prolonged swing phase, slower swing velocity, and loading asymmetry were observed.11,13,21 An ovine model of bilateral medial menisectomy also documented decreased ground reaction forces at 6 weeks post-surgery persisting over a duration of 20 weeks.5 Using a longitudinal experimental design to evaluate knee joint kinematics, we were able to longitudinally assess gait asymmetries in the porcine model of PTOA over a 1 year period post-injury. We found that while the ACLT group had largely resolved loading asymmetries by the 12-week time point, and the ACLR group resolved them by the 26-week time point, asymmetry in maximum force and impulse loading persisted in the BE-R group at the 52-week time point. Interestingly, while the BE-R group had slower resolution of loading asymmetries, that group also had significantly less macroscopic cartilage damage and a faster stride velocity than the ACLR and ACLT groups at 52 weeks.
Humans with osteoarthritis develop similar gait compensations to those seen in the BE-R group, including lower peak vertical forces.14,22 Recently, less activity, and hence less knee loading, following ACL reconstruction in humans was associated with greater Kellgren-Lawrence scores at 5 years,15,35 and higher T1rho values with MRI at 6 months,27,28 both of which are imaging biomarkers suggesting more joint arthrosis. It is possible, however, that a joint treated with ACL reconstruction (where the ligament is removed and replaced with a tendon graft) responds differently than a joint that is treated with repair of the ligament. From previous studies in the porcine model, it was determined that the biomechanics of the joint following ACL reconstruction or bridge-enhanced ACL repair are similar,23 and that the inflammation of the joint in the acute phase following injury/surgery is also similar for both treatments.32,33 Therefore, other factors may be involved. One could speculate that the mechanoreceptors within the ACL remain intact following ACL repair, which would, in turn, preserve the neuromuscular control required to protect the joint long-term, while evidence suggests that the mechanoreceptors within the graft and the neuromuscular control of the reconstructed joint do not return to normal.17,38 Future studies are required to test this hypothesis. Nonetheless, the current results suggest that limb offloading may be a protective mechanism for the joint, as the group which continued to put less force and impulse through the ACL transected knee also developed less cartilage damage.
Bridge-enhanced ACL repair resulted in less macroscopic cartilage damage than was seen in the ACLT and ACLR groups at 52 weeks, a difference that was most significant in the medial tibial plateau where 3 of the ACLT knees and 4 of the ACLR knees had erosions down to bone, while none of the knees in the BE-R group did. The medial compartment is the primary site of cartilage loss in ACL injured patients presenting with post-surgical osteoarthritis.3,34 A porcine study evaluating the macroscopic cartilage damage of these three treatment groups was previously performed, and the results of the current study independently confirm the macroscopic findings of the previous study.23 The current study was performed to determine if changes in gait were responsible, in part, for the development of the observed damage. The results indicate that this is true macroscopically.
Despite a difference in macroscopic cartilage damage, there were no significant differences noted between the surgical groups for the microscopic scores.19 This may have been due to greater macroscopic damage occurring in locations other than the central coronal section of the knee where the microscopic scores were evaluated. In addition to the microscopic analysis of the cartilage, all treatment groups had low degrees of synovitis in their joints at 1 year, with no significant differences noted between groups. This finding suggests that there was not an additional synovial reaction to the implanted bridge material for the bridge-enhanced ACL repair procedure.30,31 Since none of the surgical knees for the three treatment groups were free of synovitis, the mild, yet persistent, synovial changes suggest that the metabolic activity may be increased in an effort to repair the joint.2,32 Future research is required to determine the long-term effects of these observed synovial changes.
This study has several strengths. The porcine model enabled us to evaluate gait changes at discrete time points following treatment. Gait asymmetries were assessed using a pressure mat system that reliably measures peak pressures and maximum forces in humans39 and other large animal models.10,20 Given that the Yucatan minipig is of similar size and weight to a human, the pressure mat was appropriate to measure the same parameters used in human studies. The porcine model was also well suited for this project given the anatomic and biomechanical similarities between the pig and human.4,29,37 Furthermore, the articular cartilage thickness of the porcine knee is closer to that of humans compared to other animal models of osteoarthritis.16 Furthermore, disease progression is accelerated in the porcine model which limits the time required to complete the study, as it takes years for significant PTOA to develop in humans.23 Finally, the pre-clinical model also allowed us to harvest the joints to directly assess articular cartilage damage, which would not be possible in human patients. Macroscopic examination of the cartilage surface, which was performed using the OARSI guidelines for common large animal models,19 allowed for comparisons to be made of the damage over the entire articulating surfaces of the tibiofemoral joint instead of at discrete locations as was done with the microscopic assessment.
There are several study limitations to consider. The pig is a quadruped and therefore does not fully represent the human condition. However, the model advantages described above may at least in part alleviate this limitation. Although gait asymmetries at the hoof were assessed using a pressure mat, no kinematic measurements of the hindlimb were performed, which could have provided additional insight into functional gait changes inducing PTOA.12,27,36 The number of animals in each group was relatively small. However, significant changes were detected in several of the gait and macroscopic assessment variables. It is possible that additional differences in some of the other outcome measures could have been found with a larger sample size. Finally, microscopic OARSI scoring for the goat and sheep19 was performed using a central coronal section from the medial compartment. Chondral damage existing in other locations of the articulating surfaces between groups would have been missed, as the location of maximum cartilage damage was not always at the center of the articular cartilage surface with ACL disruption, unlike the pattern seen with meniscal destabilization.9 However, the macroscopic scoring system allowed us to consider the entire articulating surfaces of the tibiofemoral joints to assess the extent of cartilage damage.
In conclusion, contrary to our initial hypothesis, the surgical treatment resulting in the lowest amount of cartilage damage (bridge-enhanced ACL repair) exhibited greater asymmetry in load-related gait parameters (i.e., more offloading of the surgical limb) than ACL reconstruction or untreated ACL transection 1-year post-operatively. This finding suggests that increased off-loading of the surgical knee may be associated with a slower rate of development of PTOA after ACL injury and surgery.
Supplementary Material
What is known about the subject:
ACL injury has been reported to increase the risk for PTOA by 5X. The mechanisms behind PTOA development following ACL injury remains a topic of debate and are likely due to a combination of biomechanical, neuromuscular and biochemical factors. In order to understand these mechanisms, long-term, longitudinal clinical studies are required. However, these are difficult to perform due to confounding factors, time required for PTOA development, and the lack of direct methods to quantify PTOA. Thus, a clinically relevant large animal model provides an efficient method to better evaluate factors contributing to PTOA onset and progression.
What this study adds to existing knowledge:
Using the minipig model, we previously demonstrated that different surgical interventions for an ACL injury produce different degrees of PTOA. Thus, the model provides us with an opportunity to evaluate different mechanisms of disease progression. Using this model, we established the relationships between biomechanical gait changes and cartilage damage between animals receiving no treatment, ACL reconstruction, and bridge-enhanced ACL repair following ACL transection.
Acknowledgements
We gratefully acknowledge the support from the National Institutes of Health [NIAMS R01-AR056834, R01-AR065462, NIGMS P30-GM122732 (Bioengineering Core of the COBRE Centre for Skeletal Health and Repair)], and the Lucy Lippitt Endowment. Whole Slide Imaging was performed in the Neurobiology Imaging Facility / HNDC Enhanced NeuroImaging Core (NIFENC) which is supported by NIH-NINDS-P30-NS072030.
We sincerely thank our other team members, Scott McAllister, Kaitlyn Chin, Kimberly Waller and Jillian Beveridge, for assisting with surgical procedures and post-operative care. We also appreciate the support of the Brown University Animal Care Facility (ACF) veterinary technicians, Veronica Bouvier, Roxanne Burrill, and Pamela Norberg, for coordinating and assisting with the animal procedures. We sincerely thank the ACF veterinarians, Dr. James Harper and Dr. Lara Helwig, for their leadership and clinical oversight in carrying out the study.
It should be noted that M.M.M. is a founder, paid consultant, and equity holder, B.C.F. is a founder, and B.L.P. is a paid consultant and equity holder for Miach Orthopaedics, Inc, which was formed to upscale production of a scaffold for ACL repair, and is related to one of the ACL surgical procedures described herein. M.M.M. and B.L.P. maintain a conflict-of-interest management plan that was approved by Boston Children’s Hospital and Harvard Medical School during the conduct of the research. B.C.F. also maintains a conflict of interest management plan with Rhode Island Hospital with similar oversight.
Footnotes
The study was performed at Rhode Island Hospital & Brown University
References
- 1.Ajuied A, Wong F, Smith C, et al. Anterior cruciate ligament injury and radiologic progression of knee osteoarthritis: A systematic review and meta-analysis. Am J Sports Med. 2014;42:2242–2252. [DOI] [PubMed] [Google Scholar]
- 2.Ayturk UM, Sieker JT, Haslauer CM, et al. Proteolysis and cartilage development are activated in the synovium after surgical induction of post traumatic osteoarthritis. PLoS One. 2020;15:e0229449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barenius B, Ponzer S, Shalabi A, Bujak R, Norlen L, Eriksson K. Increased risk of osteoarthritis after anterior cruciate ligament reconstruction: a 14-year follow-up study of a randomized controlled trial. Am J Sports Med. 2014;42:1049–1057. [DOI] [PubMed] [Google Scholar]
- 4.Boguszewski DV, Shearn JT, Wagner CT, Butler DL. Investigating the effects of anterior tibial translation on anterior knee force in the porcine model: Is the porcine knee ACL dependent? J Orthop Res. 2011;29:641–646. [DOI] [PubMed] [Google Scholar]
- 5.Cake M, Read R, Edwards S, et al. Changes in gait after bilateral meniscectomy in sheep: effect of two hyaluronan preparations. J Orthop Sci. 2008;13:514–523. [DOI] [PubMed] [Google Scholar]
- 6.Chin KE, Akelman MR, Karamchedu NP, et al. Evaluation of gait as a tool to assess longitudinal healing of ACL-reconstruction in a porcine model. Trans Orthop Res Soc. 2016;62:1827. [Google Scholar]
- 7.Debi R, Mor A, Segal G, et al. Correlation between single limb support phase and self-evaluation questionnaires in knee osteoarthritis populations. Disabil Rehabil. 2011;33:1103–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elbaz A, Mor A, Segal O, et al. Can single limb support objectively assess the functional severity of knee osteoarthritis? Knee. 2012;19:32–35. [DOI] [PubMed] [Google Scholar]
- 9.Elsaid KA, Zhang L, Waller K, et al. The impact of forced joint exercise on lubricin biosynthesis from articular cartilage following ACL transection and intra-articular lubricin’s effect in exercised joints following ACL transection. Osteoarthritis Cartilage. 2012;20:940–948. [DOI] [PubMed] [Google Scholar]
- 10.Faramarzi B, Nguyen A, Dong F. Changes in hoof kinetics and kinematics at walk in response to hoof trimming: pressure plate assessment. J Vet Sci. 2018;19:557–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu SC, Cheuk YC, Hung LK, Chan KM. Limb Idleness Index (LII): a novel measurement of pain in a rat model of osteoarthritis. Osteoarthritis Cartilage. 2012;20:1409–1416. [DOI] [PubMed] [Google Scholar]
- 12.Heard BJ, Beveridge JE, Atarod M, et al. Analysis of change in gait in the ovine stifle: normal, injured, and anterior cruciate ligament reconstructed. BMC Musculoskelet Disord. 2017;18:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jay GD, Elsaid KA, Kelly KA, et al. Prevention of cartilage degeneration and gait asymmetry by lubricin tribosupplementation in the rat following anterior cruciate ligament transection. Arthritis Rheum. 2012;64:1162–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufman KR, Hughes C, Morrey BF, Morrey M, An KN. Gait characteristics of patients with knee osteoarthritis. J Biomech. 2001;34:907–915. [DOI] [PubMed] [Google Scholar]
- 15.Khandha A, Manal K, Wellsandt E, Capin J, Snyder-Mackler L, Buchanan TS. Gait mechanics in those with/without medial compartment knee osteoarthritis 5 years after anterior cruciate ligament reconstruction. J Orthop Res. 2017;35:625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiapour AM, Shalvoy MR, Murray MM, Fleming BC. Validation of porcine knee as a sex-specific model to study human anterior cruciate ligament disorders. Clin Orthop Relat Res. 2015;473:639–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim HJ, Lee JH, Lee DH. Proprioception in patients with anterior cruciate ligament tears: A meta-analysis comparing injured and uninjured limbs. Am J Sports Med. 2017;45:2916–2922. [DOI] [PubMed] [Google Scholar]
- 18.Krenn V, Morawietz L, Burmester GR, et al. Synovitis score: discrimination between chronic low-grade and high-grade synovitis. Histopathology. 2006;49:358–364. [DOI] [PubMed] [Google Scholar]
- 19.Little CB, Smith MM, Cake MA, Read RA, Murphy MJ, Barry FP. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in sheep and goats. Osteoarthritis Cartilage. 2010;18 Suppl 3:S80–92. [DOI] [PubMed] [Google Scholar]
- 20.Little D, Johnson S, Hash J, et al. Functional outcome measures in a surgical model of hip osteoarthritis in dogs. J Exp Orthop. 2016;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mok SW, Fu SC, Cheuk YC, et al. Intra-articular delivery of quercetin using thermosensitive hydrogel attenuate cartilage degradation in an osteoarthritis rat model. Cartilage. 2018:1947603518796550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mundermann A, Dyrby CO, Andriacchi TP. Secondary gait changes in patients with medial compartment knee osteoarthritis: increased load at the ankle, knee, and hip during walking. Arthritis Rheum. 2005;52:2835–2844. [DOI] [PubMed] [Google Scholar]
- 23.Murray MM, Fleming BC. Use of a bioactive scaffold to stimulate anterior cruciate ligament healing also minimizes posttraumatic osteoarthritis after surgery. Am J Sports Med. 2013;41:1762–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray MM, Fleming BC, Badger GJ, et al. Bridge-enhanced anterior cruciate ligament repair is not inferior to autograft anterior cruciate ligament reconstruction at 2 years: Results of a prospective randomized clinical trial. Am J Sports Med. 2020:363546520913532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murray MM, Kalish LA, Fleming BC, et al. Bridge-Enhanced Anterior Cruciate Ligament Repair: Two-year results of a first-in-human study. Orthop J Sports Med. 2019;7:2325967118824356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perrone GS, Proffen BL, Kiapour AM, Sieker JT, Fleming BC, Murray MM. Bench-to-bedside: Bridge-enhanced anterior cruciate ligament repair. J Orthop Res. 2017;35:2606–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfeiffer S, Harkey MS, Stanley LE, et al. Associations between slower walking speed and T1rho magnetic resonance imaging of femoral cartilage following anterior cruciate ligament reconstruction. Arthritis Care Res (Hoboken). 2018;70:1132–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pietrosimone B, Pfeiffer SJ, Harkey MS, et al. Quadriceps weakness associates with greater T1rho relaxation time in the medial femoral articular cartilage 6 months following anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2019;27:2632–2642. [DOI] [PubMed] [Google Scholar]
- 29.Proffen BL, McElfresh M, Fleming BC, Murray MM. A comparative anatomical study of the human knee and six animal species. Knee. 2012;19:469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Proffen BL, Perrone GS, Fleming BC, et al. Electron beam sterilization does not have a detrimental effect on the ability of extracellular matrix scaffolds to support in vivo ligament healing. J Orthop Res. 2015;33:1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Proffen BL, Perrone GS, Fleming BC, et al. Effect of low-temperature ethylene oxide and electron beam sterilization on the in vitro and in vivo function of reconstituted extracellular matrix-derived scaffolds. J Biomater Appl. 2015;30:435–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sieker JT, Proffen BL, Waller KA, et al. Transcriptional profiling of synovium in a porcine model of early post-traumatic osteoarthritis. J Orthop Res. 2018;36:2128–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sieker JT, Proffen BL, Waller KA, et al. Transcriptional profiling of articular cartilage in a porcine model of early post-traumatic osteoarthritis. J Orthop Res. 2018;36:318–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ushio T, Okazaki K, Osaki K, et al. Degenerative changes in cartilage likely occur in the medial compartment after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2019;27:3567–3574. [DOI] [PubMed] [Google Scholar]
- 35.Wellsandt E, Gardinier ES, Manal K, Axe MJ, Buchanan TS, Snyder-Mackler L. Decreased knee joint loading associated with early knee osteoarthritis after anterior cruciate ligament injury. Am J Sports Med. 2016;44:143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams AA, Titchenal MR, Andriacchi TP, Chu CR. MRI UTE-T2* profile characteristics correlate to walking mechanics and patient reported outcomes 2 years after ACL reconstruction. Osteoarthritis Cartilage. 2018;26:569–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xerogeanes JW, Fox RJ, Takeda Y, et al. A functional comparison of animal anterior cruciate ligament models to the human anterior cruciate ligament. Ann Biomed Engin. 1998;26:345–352. [DOI] [PubMed] [Google Scholar]
- 38.Young SW, Valladares RD, Loi F, Dragoo JL. Mechanoreceptor reinnervation of autografts versus allografts after anterior cruciate ligament reconstruction. Orthop J Sports Med. 2016;4:2325967116668782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zammit GV, Menz HB, Munteanu SE. Reliability of the TekScan MatScan(R) system for the measurement of plantar forces and pressures during barefoot level walking in healthy adults. J Foot Ankle Res. 2010;3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


