Abstract
Amblyopic patients are known to have fixation instability, particularly of the amblyopic eye. The stability of the fixation is affected by the presence of nystagmus, the frequency and amplitude of fixational saccades and inter-saccadic drifts. Amblyopic patients without nystagmus have increased amplitude of the fixational saccades with reduced frequency of the physiologic microsaccades and have increased inter-saccadic drifts. Amblyopia patients who have experienced a disruption in binocularity in early infancy develop fusion maldevelopment nystagmus (FMN) previously called latent nystagmus as it is more evident during monocular viewing conditions. We have found that some amblyopic patients can have nystagmus with slow phases that are not directed nasally and without the reversal in direction on ocular occlusion, features seen in patients with FMN. The current mainstay of amblyopia treatment comprises of part-time occlusion therapy of the non- amblyopic eye. The amount of patching treatment is in the range of 2-6 hours/day as determined by the severity of amblyopia. Despite treatment, up to 40% of patients have residual amblyopia. We analyzed the effectiveness of part-time occlusion therapy in amblyopic patients as a function of fixation instability. We categorized amblyopic patients based on their eye movement waveforms obtained during a visual fixation task into those lacking nystagmus, those with FMN and those with nystagmus but no FMN. We did a retrospective chart review to gather information about their clinical characteristics and treatment response. We found that patients with FMN require a more prolonged duration of treatment and have a poorer recovery of stereopsis compared to patients with nystagmus but no FMN and patients lacking nystagmus. This study suggests that eye movement assessment provides valuable information in the management of amblyopia.
Introduction
Fusion Maldevelopment Nystagmus (FMN) is one of the most common subtypes of pathologic nystagmus seen in children. The National Institutes of Health Committee on Eye Movement and Strabismus classification recommended utilizing a new etiologic description from 2001, replacing the term latent nystagmus. This type of nystagmus has initially been called latent because its severity increases, or became evident when an eye is covered. However, it is now known that true latent nystagmus is rare, with the majority of patients have manifest latent nystagmus seen with both eyes uncovered as identified on eye movement recordings. (Abadi and Scallan 2000)) Amblyopia is a neurodevelopmental disorder that occurs due to de-correlated binocular input to the visual cortex. Investigations in non-human primate models have revealed that loss of horizontal binocular connections within area V1 in infancy is the necessary and sufficient cause of FMN. (Tychsen, Richards et al. 2010)) The new terminology describes the strong correlation with a binocular fusion maldevelopment that occurs during the infancy, like strabismus, amblyopia or any monocular vision deprivation. (Tychsen 1992)
Studies by Pediatric Eye Disease Investigator Group (PEDIG) have compared part-time occlusion to full-time occlusion therapy of the non-amblyopic eye and found similar levels of improvement in visual acuity. Thus the current standard of treatment is part-time occlusion ranging from 2-6 hours/eye depending on the severity of amblyopia. (Holmes et al. 2003) The slow phase velocity (SPV) of FMN increases under monocular viewing conditions and therefore in patients with FMN occlusion was believed to be contraindicated because it could enhance the nystagmus intensity or amplitude. (Duke-Elder and Wybar 1973) Subsequently, evidence was provided in a small cohort of patients that a significant improvement of visual acuity was obtained with full-time patching during all waking hours. (von Noorden, Avilla et al. 1987) Similarly, Simonsz demonstrated a decrease in slow phase velocity of nystagmus of the amblyopic eye with full time occlusion over days in 5 patients with latent nystagmus.(Simonsz 1989) Despite good compliance, up to 40% of children treated by occlusion therapy are left with residual amblyopia. Some baseline risk factors that predict the presence of residual amblyopia include severe amblyopia at time of diagnosis and older age at treatment initiation. (PEDIG Group 2011) We asked whether fixation instability could be a contributing factor.
Amblyopes are known to have increased fixation instability. (Gonzalez 2012, Niechwiej-Szwedo, Chandrakumar et al. 2012, Subramanian, Jost et al. 2013) This instability could be due to the presence of FMN. Amblyopic patients without nystagmus have an increase in the amplitude of fixational saccades with increase inter-saccadic drifts that are not unidirectional unlike the slow phases of nystagmus and are frequently disconjugate; these contribute to the instability in both the fellow and amblyopic eye. (Shaikh et al 2016; Shi et al 2012; Chen et al 2018) We have also found increased slow phase velocities in patients with FMN compared to the inter-saccadic drift velocities in amblyopic patients without nystagmus and controls. (Kang et al submitted under review) During occlusion therapy, the amblyopic eye is the viewing eye. Thus we wanted to investigate whether the fast and slow eye movement properties of the amblyopic eye correlate with the presence of residual amblyopia, the treatment duration, and stereopsis at the end of treatment. We hypothesize that the presence of FMN, particularly those patients with greater slow phase velocity, would have poor treatment response. In addition, we hypothesize that in patients without nystagmus, the presence of increased fixational saccade amplitude and inter-saccadic drift would be correlated with poor treatment response. In the current chapter we focus on the different eye movement waveforms seen during fixation in amblyopia patients, and how patients with FMN compare to patients with nystagmus but no FMN and patients lacking nystagmus.
Methods:
The records of 80 amblyopic patients from the practice of FG who had eye movement recordings performed between 2013 to 2019 were reviewed. The Cleveland Clinic Institutional review board approved the experimental protocol and written informed consent was obtained from each participant or parent/legal guardian in accordance with the Declaration of Helsinki. After review, 53 patients, who had at least 12 months of follow up after diagnosis of amblyopia and were prescribed patching treatment were included in the study (Table 1).
Table 1:
Demographic and Clinical Parameters at the time of Diagnosis of Amblyopia
| Patient # | Gender | Category at time of patching | Eye Movement Waveform | Refractive Error Right eye | Refractive Error Left Eye |
Strabismus Near (Prism Diopters) | Strabismus Distance (Prism Diopters) |
|---|---|---|---|---|---|---|---|
| 1 | F | Strabismic Severe |
None | +3.5 +0.75 x 120 | +3.75+0.75 x 60 | ET 45 | ET 45 |
| 2 | F | Strabismic Severe |
None | +6.5 sphere | +6.25 sphere | ET 30 | ET 30 |
| 3 | M | Mixed Severe |
None | +5.0 sphere | +1.0 sphere | ET 35 | ET 35 |
| 4 | F | Mixed Moderate |
None | +3.0+1.25 x 65 | +1.25+0.25 x 115 | E(T) 4-6 | ET 12 |
| 5 | F | Strasbismic Moderate |
None | +4.0 sphere | +4.0 sphere | ET 45 | ET 30 |
| 6 | F | Mixed Moderate |
None | +8.25 +1.75 x 70 | +7.5 +1.5 x 110 | ortho with glasses | ortho with glasses |
| 7 | M | Mixed Moderate |
None | +2.5 sphere | +4.5 sphere | ortho with glasses | ortho with glasses |
| 8 | M | Mixed Moderate |
None | Plano+0.50 x 95 | ∓0.75+3.5 x 85 | XT 20 | XT 30 |
| 9 | M | Anisometropic Moderate |
None | Plano+0.50 x 85 | +5.25+2.0 x 105 | Ortho | Ortho |
| 10 | M | Anisometropic Severe |
None | +7.0+0.50 x 60 | +1.0+0.25 x 50 | Ortho | Ortho |
| 11 | M | Mixed Severe |
None | +6.5 +2.00 x 70 | +0.5+0.5 x 90 | ortho with glasses | ortho with glasses |
| 12 | M | Anisometropic Moderate |
None | Plano +0.75 x 95 | +4.25 +2.0 x 90 | Ortho | Ortho |
| 13 | F | Anisometropic Moderate |
None | +0.25+0.25 x 90 | +5.0+0.5 x 100 | Ortho | Ortho |
| 14 | F | Anisometropic Moderate |
None | +5.0+0.50 x 100 | +3.0+0.50 x 80 | Ortho | Ortho |
| 15 | F | Anisometropic Severe |
None | +7.5 sphere | +5.0+0.50 x 180 | Ortho | Ortho |
| 16 | M | Anisometropic Moderate |
None | +4.0+0.50 x 105 | +0.5+0.5 x 85 | Ortho | Ortho |
| 17 | F | Anisometropic Mild |
None | −0.25+0.5 x 90 | Plano+2.0 x 85 | Ortho | Ortho |
| 18 | F | Anisometropic Moderate |
None | −2.75+4.25 x 95 | +1.5 sphere | Ortho | Ortho |
| 19 | F | Anisometropic Moderate |
None | +0.5+1.0 x 90 | +3.5+1.0 x 90 | Ortho | Ortho |
| 20 | M | Mixed Severe |
None | +5.25+2.0 x 75 | −0.5+0.5 x 95 | ET 10 | ET 10 |
| 21 | F | Anisometropic Severe |
None | −12.0+1.0 x 105 | −0.25+1.25 x 75 | Ortho | Ortho |
| 22 | F | Anisometropic Moderate |
Nystagmus No FMN | +4.25+1.0 x 95 | +1.75+0.25 x 80 | Ortho | Ortho |
| 23 | M | Anisometropic Severe |
Nystagmus No FMN | +0.25+0.5 x 90 | −10.75+ 2.0 x 50 | Ortho | Ortho |
| 24 | M | Anisometropic Moderate |
Nystagmus No FMN | +7.25+1.5 x 90 | +8.25+1.5 x 100 | Ortho | Ortho |
| 25 | F | Mixed Moderate |
Nystagmus No FMN | −1.75+3.0 x 85 | −10.0+3.75 x 85 | ortho with glasses | ortho with glasses |
| 26 | M | Anisometropic Severe |
Nystagmus No FMN | +6.75+3.0 x 90 | +0.5 sphere | Ortho | Ortho |
| 27 | F | Anisometropic Severe |
Nystagmus No FMN | −0.25 sphere | −12.5+3.5 x 120 | Ortho | Ortho |
| 28 | M | Mixed Severe |
Nystagmus No FMN | +4.5+1.00 x 60 | +1.5+0.50 x 120 | ortho with glasses | ortho with glasses |
| 29 | F | Anisometropic Moderate |
Nystagmus No FMN | +4.0+1.25 x 85 | +1.5+0.5 x 85 | Ortho | Ortho |
| 30 | F | Mixed Severe |
Nystagmus No FMN | +2.25+0.75 x 80 | +3.5 +0.5 x 135 | ET 20 | ortho with glasses |
| 31 | M | Mixed Mild |
Nystagmus No FMN | +1.25+0.75 x 90 | +0.25+2.0 x 80 | ortho with glasses | ortho with glasses |
| 32 | F | Mixed Moderate |
Nystagmus No FMN | −11.5 +0.75 x 75 | −6.5 +1.0 x 105 | XT 20 | XT 25 |
| 33 | M | Mixed Mild |
Nystagmus No FMN | +6.0 +2.0 x 90 | +7.0+1.75 x 90 | ortho with glasses | ortho with glasses |
| 34 | M | Mixed Severe |
Nystagmus No FMN | +1.50 sphere | +4.0 sphere | LET 30 | LET 30 |
| 35 | F | Mixed Moderate |
Nystagmus No FMN | +5.5 +1.0 x 100 | +6.5 +1.0 x 80 | ortho with glasses | ortho with glasses |
| 36 | F | Mixed Moderate |
Nystagmus No FMN | +4.50 +2.0 x 90 | +5.5+2.25 x 90 | E(T) 8 | E(T) 10 |
| 37 | M | Strabismic Moderate |
Nystagmus No FMN | +2.75+0.5 x 180 | +2.75+0.50 x 180 | ET 35 | ET 35 |
| 38 | F | Anisometropic Moderate |
Nystagmus No FMN | +1.0+0.5 x 90 | +3.75 sphere | Ortho | Ortho |
| 39 | F | Mixed Severe |
Nystagmus No FMN | +1.50 +0.50 x 70 | +3.5+0.5 x 120 | E(T) 8 | E(T) 8 |
| 40 | F | Mixed Mild |
Nystagmus No FMN | −1.5+0.75 x 90 | −2.5+1.00 x 90 | XT 35 | XT 35 |
| 41 | F | Mixed Moderate |
Nystagmus No FMN | −0.75+0.5 x 75 | +1.5+1.00 x 90 | XT 25 | XT 30 |
| 42 | M | Mixed Moderate |
Nystagmus No FMN | +3.5 +0.50 x 110 | +1.00+0.50 x 90 | 50 RET | 50 RET |
| 43 | M | Anisometropic Severe |
FMN | +5.00+0.50 x 90 | +6.25+1.00 x 95 | Ortho | Ortho |
| 44 | F | Strabismic Severe |
FMN | +3.50+1.75 x 90 | +3.50+1.75 x 90 | XT 8-10 | XT 10 |
| 45 | M | Mixed Moderate |
FMN | −9.5 +2.5 x 165 | plano +0.75 x 45 | ET 4 | ET 4 |
| 46 | M | Strabismic Severe |
FMN | +3.0 sphere | +3.0 sphere | XT >60 | XT >60 |
| 47 | F | Mixed Moderate |
FMN | +5.0+1.5 x 80 | +6 +1.5 x 95 | ortho with glasses | ortho with glasses |
| 48 | M | Mixed Severe |
FMN | −6.75+3.75 x 90 | −9.0+3.75 x 90 | XT 25 | XT 45 |
| 49 | M | Mixed Moderate |
FMN | +4.5 sphere | +3.5 sphere | ET 30 | ET 25 |
| 50 | M | Mixed Severe |
FMN | +4.5+2.75 x 85 | +3.5 +2.75 x 95 | XT 12 | XT 12 |
| 51 | M | Mixed Moderate |
FMN | −0.5+1.00 x 110 | +2.50 +1.50 x 55 | Flick XT | Flick XT |
| 52 | F | Mixed Moderate |
FMN | +4.0 sphere | +2.25 sphere | XT 20 | XT 20 |
| 53 | M | Mixed Severe |
FMN | +8.0+1.5 x 90 | +7.25+0.5 x 90 | ET 6-8 | ET 4 |
ET= esotropia, XT = exotropia, E(T) = intermittent esotropia and X(T)= intermittent exotropia, ortho=orthotropia.
The clinical categorization of amblyopia subtype and severity at the time of diagnosis were based on PEDIG studies (Manh, Holmes et al. 2018). Type of amblyopia: Amblyopia associated with strabismus, anisometropia, or both meeting the following criteria: 1) Strabismic amblyopia: At least one of the following criteria must be met and criteria are not met for combined-mechanism amblyopia: a) Heterotropia at distance and/or near fixation on examination (with or without spectacles) b) History of strabismus surgery c) documented history of strabismus which is no longer present (and which, in the judgment of the investigator, is the cause of amblyopia) 2) Anisometropic amblyopia: At least one of the following criteria must be met: a) ≥0.50 D difference between eyes in spherical equivalent ≥1.50 D difference between eyes in astigmatism in any meridian 3) Mixed mechanism amblyopia: Both of the following criteria must be met: a) criteria for strabismus are met (see above) b) ≥1.00 D difference between eyes in spherical equivalent or ≥1.50 D difference between eyes in astigmatism in any meridian. Severity of amblyopia: Mild amblyopia: if worse eye visual acuity (VA) was <0.30 LogMAR, moderate if ≥ 0.30 and <0.70, severe if ≥0.70; VA of the amblyopic eye at baseline. Visual acuity was measured in each eye using the participant’s optimal spectacle correction with Snellen linear optotype. For patients younger than seven years of age, crowding bars HOTV or Allen pictures were used as per the child’s ability to perform the test if they were unable to do the Snellen linear optotype. There were only four patients that were diagnosed before their ability to perform optotype testing- they all had manifest strabismus with strong fixation preference. They were all assigned as having severe amblyopia at the time of diagnosis.Treatment considered was part-time occlusion (2-6 hours/day), prescribed depending on the severity of amblyopia. Patients with manifest strabismus were treated according to the American Academy of Ophthalmology Preferred Practice Pattern. Investigators judged compliance with patching treatment to be excellent (>75%), good (51%–75%), fair (26%–50%), or poor (≤25%), based on discussions with the parents.
Eye movement recording and analysis:
A high-resolution video-based eye tracker (EyeLink 1000®, SR Research, Ontario, Canada) was used to measure binocular horizontal and vertical eye positions at a temporal resolution of 500 Hz during a fixation task as described previously. Briefly, eye position data was analyzed after removal of blinks and partial blinks. To measure eye velocity, we differentiated the eye position signal using MatlabTM (Mathworks, Natick, MA) diff function. Differential value (velocity signal) was further smoothened with Savitzkey-Golay filter, a function that can be applied to a set of digital data points for the smoothing purpose. (Shaikh, Otero-Millan et al. 2016)
Fixational saccades and quick phases of nystagmus were identified using an unsupervised clustering method. (Otero-Millan, Castro et al. 2014) Drifts were defined as epochs between fixational saccades and blinks. We removed 20 msec data at the beginning and end of each of the drifts to exclude periods of acceleration and deceleration of the eye during fixational saccades and blinks. We characterized fixational eye movements in amblyopic patients based on their waveform characteristics as those without nystagmus, those with nystagmus but without the classic reversal in quick phase of nystagmus and the nasally directed slow phase observed during monocular viewing conditions seen in FMN patients and those with FMN. The form of the slow phase nystagmus appears to be decreasing or linear with dynamic overshoots of quick phases in amblyopia patients with nystagmus unlike the increasing eye velocity waveforms seen in patients with infantile/congenital nystagmus. In addition, patients with nystagmus but no FMN did not have the dissociated vertical deviation frequently seen in FMN patients.
Of the recruited patients, 21 had no nystagmus, 21 had nystagmus without FMN, and 11 had FMN. The subjects were also grouped based on the type of amblyopia (anisometropic = 19, mixed = 28, strabismic = 6). Patients with anisometropia had no nystagmus or had nystagmus no FMN. All three different waveform characteristics were seen in strabismic and mixed amblyopia patients. There was no difference in the follow up time (None: 56 ± 34, Nystagmus without FMN: 71 ± 37, FMN: 75 ± 43, p =0.32), Due to an inadequate number of subjects; we were not able to do subgroup analysis per eye movement waveform within each clinical type of amblyopia. The follow-up duration for amblyopic patients of anisometropic patients was lower than the other two groups (Anisometropic = 46 ± 31, Strabismic = 109 ± 32, Mixed= 71.5 ± 32, p= 0.0001).
Clinical data and Outcome Measures:
The clinical parameters were extracted from a retrospective chart review for all the enrolled subjects (Table 1). The ages at follow up visits, visual acuity of fellow and amblyopic eye, strabismus measurements in the primary position, stereopsis and compliance to treatment were noted. Stereoacuity was measured with the Titmus Stereoacuity Test. Stereoacuity scores in seconds of arc were: 40”, 60”, 100”, 200”, 400”, 800”; 3500” was the value of patients able to see only the fly; subjects with no detectable (nil) stereoacuity were assigned a value of 7000”. For analyses, stereoacuity scores in seconds of arc were converted to log values as follows: 40” (1.60), 60” (1.78), 100” (2.00), 200” (2.30), 400” (2.60), 800” (2.90), 3500” (3.55) and 7000” (3.85). The total duration in months of patching treatment till visual acuity was stabilized with no further improvement or deterioration ≥ 2 consecutive visits ≥ 6 weeks apart was computed for all the patients with at least 50% compliance. The improvement in visual acuity as expressed in arc minutes were calculated as the difference of acuity at the final visit from that of the acuity at the start of treatment. Patients were stratified based on the degree of vision improvement in response to treatment as < 3 arc min, 3-6 arc min and > 6 arc min. In addition, residual amblyopia at the end of treatment was defined as mild<0.30 LogMAR, moderate if ≥ 0.30 and <0.70 and severe if ≥0.70 log MAR scale. Final stereopsis was assessed and patients were classified to have good stereopsis (better than 100 sec arc), some stereopsis (100-400 sec arc) and gross/absent stereopsis (3500 or absent stereo).
Data analysis and statistics: All analyses were performed in Matlab (Mathworks, Natick, MA, USA) and GraphPad Prism 7 (La Jolla, CA, USA). A Kruskal-Wallis analysis of variance test was used to compare the demographics and clinical outcomes across amblyopia subtype. We used one-way ANOVA to compare the clinical and oculomotor parameters across fixation eye movement characteristics. An unpaired t-test was used to analyze clinical/oculomotor parameters between the two groups.
Results:
We investigated the treatment effectiveness of part-time occlusion in amblyopia patients as a function of the fixation instability of the amblyopic eye and the clinical subtype of amblyopia. Besides these, there are several variables such as age at diagnosis, visual acuity at the time of diagnosis and compliance to treatment that could be related to the visual acuity at the end of treatment. The age (in months) when patching treatment was started was similar across eye movement waveforms (No nystagmus = 63 ± 24,Nystagmus no FMN = 56 ± 25, FMN = 57.9 ± 40.5, p = 0.71) and across the subtype of amblyopia (Anisometropic = 76 ± 14, Strabismic = 59 ± 38, Mixed = 65 ± 29, p= 0.19). Similarly, compliance did not correlate with eye movement waveforms (None: 58 ± 21, Nystagmus without FMN: 63 ± 21, FMN: 59 ± 16, p = 0.73) nor did the visual acuity expressed in arc min at time of diagnosis (None: 9.3 ± 15.75, Nystagmus without FMN: 7.78 ± 16.5 and FMN: 4.9 ± 3.3, p = 0.32). The compliance to patching (Anisometropic: 67 ± 11, Strabismic: 51 ± 17 and Mixed: 59 ± 18, p = 0.09) and visual acuity at the time of start of treatment (Anisometropic: 8.1 ± 17.4,Strabismic: 9.0 ± 8.5, Mixed: 6.9 ± 12.9, p = 0.9) was comparable across clinical types.
Treatment outcome measures as a function of the clinical subtype of amblyopia:
Anisometropic patients either did not have nystagmus or had nystagmus without FMN except for only one subject that on initial presentation had anisometropia and FMN. This subject was noted to have intermittent esotropia on subsequent clinical visits. Thus, we categorized this patient as having mixed mechanism amblyopia for statistical analysis. All the patients with strabismic and mixed amblyopia had strabismus surgery and were either orthotropic with glasses or had microstrabismus with glasses. Figure 2 A plots the visual acuity improvement in arc min, which was comparable across all three clinical subtypes (Anisometropic: 6.9 ± 16.9; Strabismic: 6.4 ± 8.4 and Mixed: 4.3 ± 12.3, p = 0.16). The total duration of treatment (Fig 2b) was similar across the three subtypes (Anisometropic: 19.2 ± 22; Strabismic: 27 ± 14 and Mixed: 19.75 ± 20, p = 0.16). Anisometropic amblyopes were more likely to have better stereopsis at the end of treatment compared to the other two groups (Fig 2C- Anisometropic: 1.9 ± 0.51; Strabismic: 3.5 ± 0.69, Mixed: 3.0 ± 0.89, p <0.0001).
Figure 2.
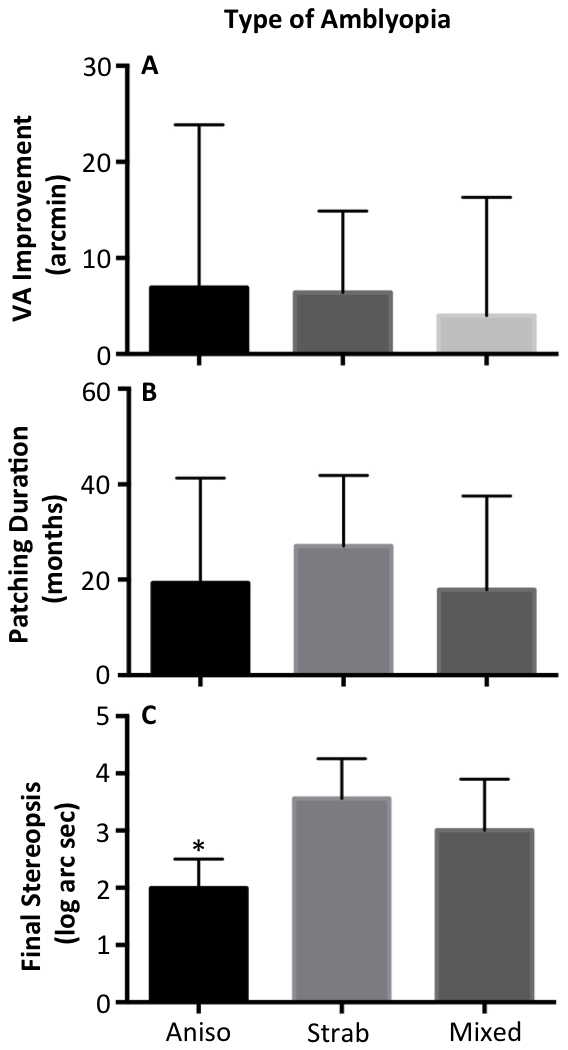
Clinical outcomes sub grouped by the type of amblyopia. Visual acuity improvement and patching duration are not significantly different between types. Final stereopsis is significantly better in anisometropic patients.
Treatment outcome measures as a function of fixation eye movement waveforms:
Patients with FMN had less improvement in visual acuity (Fig 3A) compared to the other groups; however this difference did not reach statistical significance (None: 7.7 ± 14.5, Nystagmus no FMN: 5.0 ± 16, FMN: 3.02 ± 3.4, p =0.2). Patients with FMN had a longer duration of treatment (Fig 3B) compared to the other two groups (No nystagmus: 9.5 ± 6.3, Nystagmus no FMN: 22 ± 22, FMN: 38 ± 19, p = 0.01). The most significant finding is that stereopsis was worse in patients with FMN (Fig 3C) compared to the other two groups (No Nystagmus: 2.4 ± 0.9, Nystagmus no FMN: 2.6 ± 0.9, FMN: 3.3 ± 0.8, p =0.04).
Figure 3.
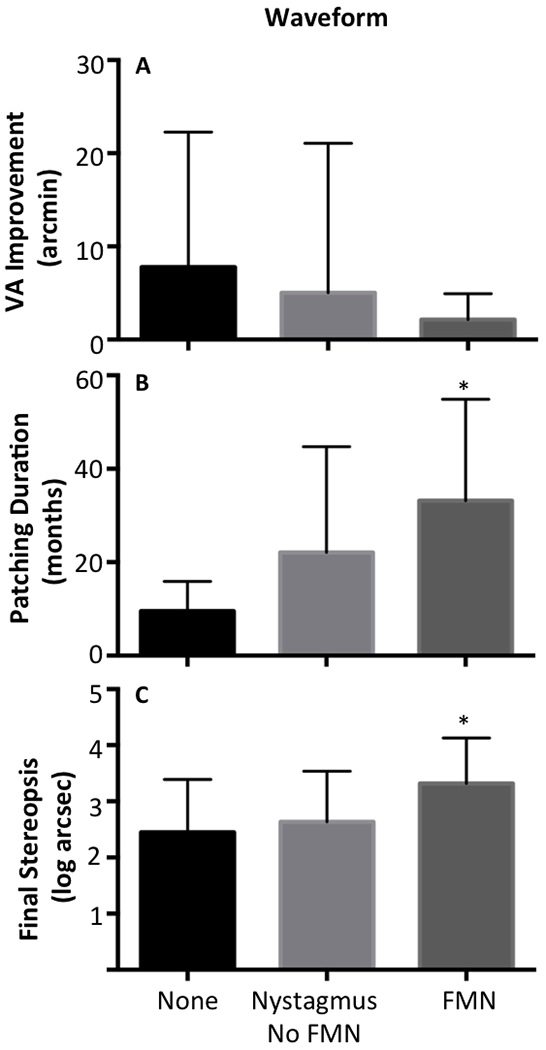
Clinical outcomes sub grouped by the fixation eye movement waveforms. Visual acuity improvement is not significantly different between the waveform groups. However, in FMN patients the duration of patching is significantly longer, and the final stereopsis is significantly worst compared to the other two groups.
Discussion:
The purpose of this study was to identify oculomotor biomarkers that can be used to predict treatment effectiveness of part-time occlusion therapy. In the current manuscript, we characterized fixational eye movements in amblyopia patients. The subjects enrolled in the study had comparable visual acuity at the time of diagnosis and age at initiation of patching across the groups categorized per their eye movement waveforms. We found that rather than clinical subtype (anisometropic, strabismic or mixed), eye movement characteristics were better in predicting treatment outcomes. This is in agreement with previous studies that have shown that baseline visual acuity and younger age at enrollment were associated with the best improvement, but not the cause of amblyopia. (Wallace et al 2015) We found that children with FMN required a longer duration of treatment compared to those without nystagmus. Despite the improvement in visual acuity, the recovery of stereopsis was poor in patients with FMN.
Very few studies to date have examined occlusion therapy effectiveness in amblyopic patients with FMN. Von Noorden et al. (von Noorden et al 1987) was the first to show in 12 patients with FMN noted on the clinical exam that patching during all waking hours was useful in improving VA, while it was previously considered contraindicated. The study had examined the effects of full time patching with no eye movement recordings. Ours is the first study to, our knowledge, measuring the impact of fixation instability on the effectiveness of part-time patching in amblyopia patients. Our results suggest that patients with FMN are at higher risk of regression with part-time occlusion therapy and require a prolonged duration of treatment. They are also less likely to have good stereoacuity at the end of the treatment despite improvement in visual acuity. Amblyopic patients with nystagmus but no FMN had improvement in both visual acuity and stereoacuity but required a longer duration of treatment compared to those without nystagmus.
The analyses were performed independently for different eye movement waveforms and the type of amblyopia. Strabismic patients have an increase in the drift velocity with higher velocities in patients with nystagmus. Our previous study has shown that the drift velocity and variance increase with an increase in the strabismus angle. (Ghasia et al 2018) All of our patients with strabismic and mixed amblyopia had microstrabismus (defined as < 10 prism diopters) at the time of eye movement recordings. In the future, a larger cohort of patients will allow us to independently analyze the effects of eye movement waveforms within each clinical subtype of amblyopia as well as delineate the impact of degree of strabismus.
A significant limitation of the current study is the eye movement recordings were obtained at the end of treatment and the treatment effectiveness was determined based on a retrospective chart review. In addition, only, a small cohort of patients with residual amblyopia was treated with atropine and majority of them had no further improvement in visual acuity. The decision to treat was based on discussions with family and the children with greater deficits of visual acuity were more likely to try an alternative treatment. The mean duration of follow up was greater in our study compared to most amblyopia treatment studies. Thus, we were able to identify regression soon after the treatment was stopped or while it was being tapered for patients who initially had severe amblyopia at diagnosis. The analysis from the current study suggests that eye movement characterization and quantification can play an important role in providing information about prognosis and amblyopia treatment effectiveness. A prospective clinical trial of obtaining eye movement recordings at the time of diagnosis and following the patients longitudinally to determine treatment effectiveness of part-time occlusion will be necessary to confirm the findings of the current observational study. In addition, the study suggests that the timing of amblyopia development seems to play an important role in determining part-time patching treatment effectiveness. Additional prospective studies evaluating alternative treatments such as optical penalization and newer binocular amblyopia treatments in a cohort of amblyopic patients with FMN would help further tailor the treatment.
Figure 1.
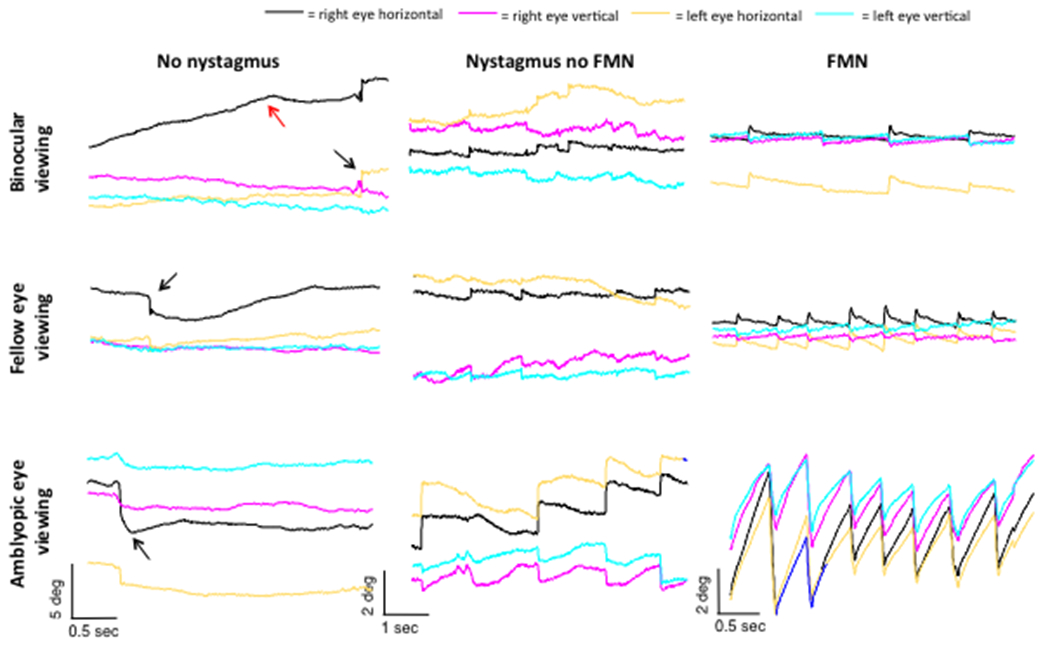
Representative eye position traces obtained during fixation in amblyopia patients without nystagmus, nystagmus no FMN, and FMN. In patients without nystagmus there is an increase in the amplitude of the fixational saccade with an increase in the inter-saccadic drift. In patients with nystagmus no FMN there is no reversal of the quick phase of nystagmus as seen in patients with FMN. In patients with FMN, there is an increase in slow phase velocity of the amblyopic eye during amblyopic eye viewing condition. Of note, in all three patients abnormalities are seen during binocular viewing condition particularly of the amblyopic eye.
Acknowledgments:
Supported by grants from Blind Children’s Center, RPB Unrestricted Grant CCLCM-CWRU, CTSC Pilot Grant Program and Cleveland Clinic RPC Grant (FG) and Departmental NEI T32 grant (JM).
References
- Abadi RV and Scallan CJ (2000). ”Waveform characteristics of manifest latent nystagmus.” Invest Ophthalmol Vis Sci 41(12): 3805–3817. [PubMed] [Google Scholar]
- Chen D, Otero-Millan J, Kumar P, Shaikh AG, Ghasia FF (2018). “Visual Search inAmblyopia: Abnormal Fixational Eye Movements and Suboptimal Sampling Strategies.” Invest Ophthalmol Vis Sci 4;59(11):4506–4517.) [DOI] [PubMed] [Google Scholar]
- Duke-Elder S and Wybar KC (1973). Ocular Motility and Strabismus. System of Ophthalmology, Mosby: 824. [Google Scholar]
- Ghasia FF, Otero-Millan J, Shaikh AG(2018). “Abnormal fixational eye movements in strabismus.” Br J Ophthalmol 102(2):253–259. [DOI] [PubMed] [Google Scholar]
- Gonzalez EG, Wong AM, Niechwiej-Szwedo E, Tarita-Nistor L and Steinbach MJ (2012). “Eye position stability in amblyopia and in normal binocular vision.” Invest Ophthalmol Vis Sci 53(9): 5386–5394. [DOI] [PubMed] [Google Scholar]
- Holmes JM, Kraker RT, Beck RW, Birch EE, Cotter SA, Everett DF, Hertle RW, Quinn GE, Repka MX, Scheiman MM, Wallace DK; Pediatric Eye Disease Investigator Group (2003). “A randomized trial of prescribed patching regimens for treatment of severe amblyopia in children.” Ophthalmology 110(11):2075–87. [DOI] [PubMed] [Google Scholar]
- Kang SL, Otero-Millan JM, Beylergil S, Shaikh AG,Ghasia FF (submitted under review). “Disconjugacy of fixational eye movements in Amblyopia.” [Google Scholar]
- Manh VM, Holmes JM, Lazar EL, Kraker RT, Wallace DK, Kulp MT, Galvin JA, Shah BK, Davis PL and Pediatric G Eye Disease Investigator (2018). “A Randomized Trial of a Binocular iPad Game Versus Part-Time Patching in Children Aged 13 to 16 Years With Amblyopia.” Am J Ophthalmol 186: 104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero-Millan J, Castro JL, Macknik SL and Martinez-Conde S (2014). “Unsupervised clustering method to detect microsaccades.” J Vis 14(2). [DOI] [PubMed] [Google Scholar]
- Pediatric Eye Disease Investigator Group (2002). “A randomized trial of atropine vs. patching for treatment of moderate amblyopia in children.” Arch Ophthalmol 120(3):268–78. [DOI] [PubMed] [Google Scholar]
- Pediatric Eye Disease Investigator Group (2005). “Two-year follow-up of a 6-month randomized trial of atropine vs patching for treatment of moderate amblyopia in children.” Arch Ophthalmol 123:149–57. [DOI] [PubMed] [Google Scholar]
- Pediatric Eye Disease Investigator Group(2008). “A randomized trial of atropine versus patching for treatment of moderate amblyopia: follow-up at age 10 years.” Arch Ophthalmol 126:1039–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pediatric Eye Disease Investigator Group (PEDIG) Writing Committee, Wallace DK, Kraker RT, Beck RW, Cotter SA, Davis PL, Holmes JM, Repka MX, Suh DW (2011). “Randomized trial to evaluate combined patching and atropine for residual amblyopia”. Arch Ophthalmol 129(7):960–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repka MX, Kraker RT, Holmes JM, et al. (2014). “Atropine vs Patching for Treatment of Moderate Amblyopia: Follow-up at 15 Years of Age of a Randomized Clinical Trial.” JAMA Ophthalmology 132(7):799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh AG, Otero-Millan J, Kumar P and Ghasia FF (2016). “Abnormal Fixational Eye Movements in Amblyopia.” PLoS One 11(3): e0149953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi XF, Xu LM, Li Y, Wang T, Zhao KX, Sabel BA (2012). “Fixational saccadic eye movements are altered in anisometropic amblyopia.” Restor Neurol Neurosci 30: 445–462 [DOI] [PubMed] [Google Scholar]
- Simonsz HJ (1989). “The effect of prolonged monocular occlusion on latent nystagmus in the treatment of amblyopia.” Doc Ophthalmol 72(3-4): 375–384. [DOI] [PubMed] [Google Scholar]
- Subramanian V, Jost RM and Birch EE (2013). “A quantitative study of fixation stability in amblyopia.” Invest Ophthalmol Vis Sci 54(3): 1998–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tychsen L (1992). Binocular vision. Adler’s Physiology of the Eye; Clinical Application. St Louis, MO, Mosby. [Google Scholar]
- Tychsen L, Richards M, Wong A, Foeller P, Bradley D and Burkhalter A (2010). “The neural mechanism for Latent (fusion maldevelopment) nystagmus.” J Neuroophthalmol 30(3): 276–283. [DOI] [PubMed] [Google Scholar]
- von Noorden GK, Avilla C, Sidikaro Y and LaRoche R (1987). “Latent nystagmus and strabismic amblyopia.” Am J Ophthalmol 103(1): 87–89. [DOI] [PubMed] [Google Scholar]
- Wallace DK, Lazar EL, Crouch ER 3rd, Hoover DL, Kraker RT, Tamkins SM; Pediatric Eye Disease Investigator Group(2015). “Time course and predictors of amblyopia improvement with 2 hours of daily patching.” JAMA Ophthalmol 133(5):606–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


