Abstract
Background:
Within the multidisciplinary management of breast cancer, variations exist in the reconstructive options offered and care provided. This study aimed to evaluate plastic surgeon perspectives on important issues related to breast cancer management and reconstruction and provide some insight into factors that influence these perspectives.
Methods:
Women diagnosed with early stage breast cancer (stages 0-II) between July 2013 and September 2014 were identified through the Georgia and Los Angeles SEER registries. These women were surveyed and identified their treating plastic surgeons. Surveys were sent to the identified plastic surgeons to collect data on specific reconstruction practices.
Results:
Responses from 134 plastic surgeons (74.4% response rate) were received. Immediate reconstructions (79.7%) was the most common approach to timing, and expander/implant reconstructions (72.6%) was the most common technique reported. Nearly a third (32.1%) of respondents reported that reimbursement influenced the proportion of autologous reconstructions performed. Most (82.8%) reported that discussions about contralateral prophylactic mastectomy were initiated by patients. Most surgeons (81.3%-84.3%) felt that good symmetry is achieved with unilateral autologous reconstruction with contralateral symmetry procedures in patients with small or large breasts; a less pronounced majority (62.7%) favored unilateral implant reconstructions in patients with large breasts. In patients requiring post-mastectomy radiation, a quarter of the surgeons (27.6%) reported that they seldom recommend delayed reconstruction, and 64.9% reported recommending immediate expander/implant reconstructions.
Conclusions:
Reconstructive practices in a modern cohort of plastic surgeons suggests that immediate and implant reconstructions are performed preferentially. Respondents perceived a number of factors, including surgeon training, time spent in the operating room and insurance reimbursement, to negatively influence the performance of autologous reconstruction.
INTRODUCTION
The growing evidence for oncologic safety of immediate reconstruction and advances in reconstructive techniques have stimulated increased use of post-mastectomy reconstruction and greater acceptance of reconstruction as part of the overall care of the breast cancer patient (1, 2). Reconstruction rates increased from 46% to 63% between 1998 and 2007, and even more growth appears to have occurred in the intervening decade (1, 3). With growth in the number of women receiving reconstruction and a rapid evolution in the oncologic care delivered, plastic surgeons have had to adapt to provide reconstruction that complements treatment and optimizes outcomes in the long term.
The extant literature suggests growing challenges for clinicians with regard to navigating breast reconstruction with patients. Outcomes appear to vary depending on treatment option and additional patient factors. For instance, in a large national multicenter study of women undergoing immediate breast reconstruction, at one year after surgery, those who had undergone autologous reconstruction reported greater satisfaction with their breasts and greater psychosocial and sexual well-being compared to others who had undergone implant reconstructions (4). Yet additional analyses in this multicenter cohort revealed higher risk for complications with multiple forms of autologous reconstruction relative to expander/implant reconstructions (5).
The interplay between such factors as patient comorbidities, postoperative complications, and satisfaction highlight the complex considerations that should impact decision-making for breast reconstruction. Postoperative outcomes notwithstanding, actual clinical practice may also be influenced by surgeon factors that are challenging to capture. For example, some have speculated that use of autologous reconstruction is impeded by financial considerations, operating room time, or lack of expertise (6). However, little exists in the literature that examines plastic surgeon perspectives and their influences on reconstruction decisions: in particular what plastic surgeons may say to patients that may influence their broader oncologic treatment decisions related to removal of a contralateral unaffected breast or post-mastectomy radiotherapy. As such, this study aims to assess plastic surgeon perspectives and document surgeons’ perceptions of the factors that influence reconstruction in the context of practical patient and treatment variables.
METHODS
Surgeon Sample
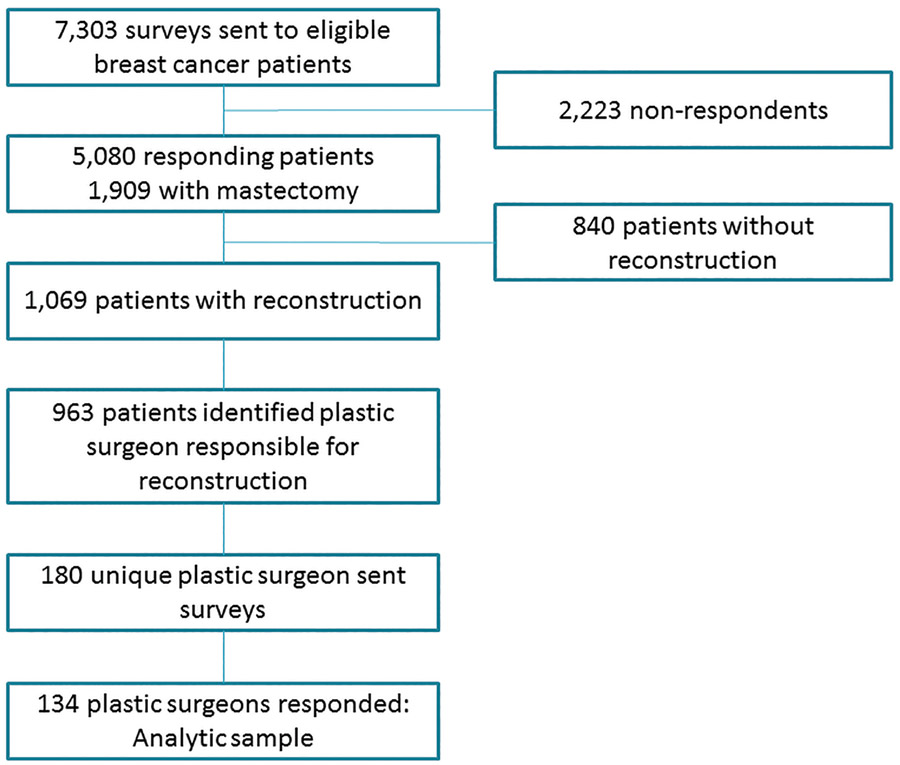
Women with early stage breast cancer (stage 0 to II), identified through the Georgia and Los Angeles County Surveillance, Epidemiology, and End Results (SEER) registries between July 2013 and September 2014 were identified as part of the iCanCare study (7). The Individualized Cancer Care (iCanCare) Study is a population-based survey study of women with early-stage breast cancer and their providers. Participants were women with new breast cancer reported to the SEER registries of Georgia and Los Angeles County. Women were deemed ineligible if they had Stage III or IV disease or could not complete a questionnaire in Spanish or English. Surveys were mailed to 7,303 women and responses received from 69.6% (N=5,080), of whom 1,909 eligible respondents had undergone mastectomies (Figure 1). Of these, 1,069 women underwent mastectomy with reconstruction and 963 (90.0%) identified the plastic surgeon responsible for their reconstruction. Surveys were sent to these plastic surgeons toward the end of the patient data collection period (N=180). 134 surgeons completed and returned the surveys (response rate, 74.4%).
Figure 1:
Study Flow Diagram
Measures
Survey content was assessed for validity using standard techniques, including systematic review by design experts and cognitive pretesting and pilot testing with surgeons outside of the study areas. Surgeon variables included sociodemographic characteristics (age, gender, race), training characteristics (independent pathway: full general surgery training + plastic surgery fellowship, integrated/combined plastic surgery training, additional subspecialty fellowship training) and practice characteristics (years in practice, practice setting with or without trainees, annual volume of new breast cancer patients, annual volume of breast reconstruction procedures, and volume of care performed with tumor board involvement). Reconstructive preferences included: 1) timing (immediate versus delayed reconstruction), and 2) method (expander/implant, autologous, combined autologous and expander/implant reconstruction).
Factors influencing breast reconstruction preferences (training, reimbursement, operating room time, patient BMI) were assessed using a 5-point Likert-type item, ranging from never or not at all (score= 1) to always or very much (score= 5). Responses were categorized as high (score ≥3) and low (score<3). Perspectives on achieving symmetry with unilateral and bilateral breast reconstruction in patients with small and large breasts were assessed with respect to implant and autologous breast reconstruction. Communication variables related to CPM (oncologic outcomes, risk of complications, indications for CPM, sensation with reconstruction, recovery and cosmetic outcomes) were also assessed. Perspectives on achieving symmetry and communication variables related to CPM were assessed using a 4-point scale ranging from definitely no to definitely yes; scores were categorized as often (definitely/probably yes, score ≥3) versus seldom (definitely/probably no). Reconstructive practices including recommendations on type and timing of reconstruction were also evaluated for the subset of patients requiring PMRT. A 5-point Likert-type item, ranging from never or not at all (score= 1) to always or a lot (score= 5) was used to assess these practices. Responses were categorized as often (score ≥3) and seldom (score<3). Finally, perspectives about reconstruction and communication with other providers within the multidisciplinary team and with patients were evaluated.
Simple descriptive statistics are reported for the entire population and by SEER registry; percentages and means are reported for categorical and continuous response data, respectively.
RESULTS
Demographic and Practice Characteristics of Respondents
The majority of the respondents were male (79.9%) and their average age was 50 years (range: 35-76). Seventy percent of the surgeons were white and 17% were Asian (Table 1). Additional subspecialty fellowship training in either microsurgery or a fellowship that included some microsurgery was reported by 39.6% of the surgeons. On average, surgeons had been in practice for a period of 15.8 years (range: 1-37) and one in four (25.4%) practiced in settings with residents and/ or fellows. Over a 12-month period, breast cancer patients represented an average of 28% of the new patients seen by surgeons in the cohort, with 34% of surgeons performing over 50 breast reconstructive procedures over the same period. A quarter of the surgeons reported that greater than 50% of newly diagnosed breast cancer patients seen in their practices were discussed in multidisciplinary tumor boards.
Table 1:
Surgeon Demographic and Practice Characteristics
| Characteristic | Total Sample | Los Angeles County | State of Georgia |
|---|---|---|---|
| Gender: N (%) | |||
| Male | 107 (79.85) | 58 (81.69) | 49 (77.78) |
| Female | 27 (20.15) | 13 (18.31) | 14 (22.22) |
| Age | |||
| Mean (SD) [Minimum - Maximum] | 50.31 (9.32) [35.00 - 76.00] | 50.97 (9.07) [35.00 - 69.00] | 49.54 (9.63) [35.00 - 76.00] |
| Race: N (%) | |||
| Non-Hispanic white | 95 (70.90) | 59 (83.10) | 36 (57.14) |
| Hispanic white | 4 (2.99) | 2 (2.82) | 2 (3.17) |
| African-American | 7 (5.22) | 5 (7.04) | 2 (3.17) |
| South Asian | 17 (12.69) | 3 (4.23) | 14 (22.22) |
| East Asian | 5 (3.73) | 1 (1.41) | 4 (6.35) |
| Other/Not reported | 6 (4.48) | 1 (1.41) | 5 (7.93) |
| Surgical training: N (%) | |||
| Plastic surgery + full general surgery | 95 (70.90) | 53 (74.65) | 42 (66.67) |
| Integrated/combined plastic surgery | 34 (25.37) | 14 (19.72) | 20 (31.75) |
| Not reported | 5 (3.73) | 4 (5.63) | 1 (1.59) |
| Fellowship training: N (%) | |||
| Microsurgery | 27 (20.15) | 8 (11.27) | 19 (30.16) |
| Fellowship that included some microsurgery | 26 (19.40) | 14 (19.72) | 12 (19.05) |
| Other fellowship | 22 (16.42) | 12 (16.90) | 10 (15.87) |
| Not reported | 55 (44.03) | 38 (52.11) | 19 (34.92) |
| Years in practice since completing residency or fellowship | |||
| Mean (SD) [Minimum - Maximum] | 15.75 (9.07) [1.00 - 37.00] | 16.76 (8.81) [1.00 - 36.00] | 14.6 (9.29) [1.00 - 37.00] |
| Residents or Fellows at primary practice: N (%) | |||
| Yes | 34 (25.37) | 10 (14.08) | 24 (38.10) |
| No | 96 (71.64) | 61 (85.92) | 35 (55.56) |
| Not reported | 4 (2.99) | 0 | 4 (6.35) |
| How many practice locations to you see patients: N (%) | |||
| One | 66 (49.25) | 35 (49.30) | 31 (49.21) |
| Two | 44 (32.84) | 24 (33.80) | 20 (31.75) |
| Three or more | 17 (12.69) | 9 (12.68) | 8 (12.70) |
| Not reported | 7 (5.22) | 3 (4.23) | 4 (6.35) |
| In the past 12 months… | |||
| Patients seen with newly diagnosed with breast cancer: N (%) | |||
| 0 – 10 | 16 (11.94) | 10 (14.08) | 6 (9.35) |
| 11 – 20 | 22 (16.42) | 13 (18.31) | 9 (14.29) |
| 21 – 50 | 40 (29.85) | 25 (35.21) | 15 (23.81) |
| 51 – 100 | 31 (23.13) | 13 (18.31) | 18 (28.57) |
| > 100 | 17 (12.69) | 6 (8.45) | 11 (17.46) |
| Not reported | 8 (5.97) | 4 (5.63) | 4 (6.35) |
| How many breast cancer patients did you perform breast: N (%) | |||
| <25 | 44 (32.84) | 30 (42.25) | 14 (22.22) |
| 25 – 50 | 41 (30.60) | 22 (30.99) | 19 (30.16) |
| 51 – 100 | 27 (20.15) | 11 (15.49) | 16 (25.40) |
| >100 | 18 (13.43) | 8 (11.27) | 10 (15.87) |
| Not reported | 4 (2.99) | 0 | 4 (6.35) |
| Percentage of newly diagnosis breast cancer patients discussed in a multidisciplinary meeting (i.e. tumor board) for input on treatment plan: N (%) | |||
| None (0%) | 35 (26.12) | 18 (25.35) | 17 (26.98) |
| 1 – 9% | 15 (11.19) | 10 (14.08) | 5 (7.94) |
| 10 – 25 | 30 (22.39) | 15 (21.13) | 15 (23.81) |
| 26 – 50% | 17 (12.69) | 9 (12.68) | 8 (12.70) |
| >50% | 33 (24.63) | 18 (25.35) | 15 (23.81) |
| Not reported | 4 (2.99) | 1 (1.41) | 3 (4.76) |
| Weekly work hours devoted to patient care (including surgery): N(%) | |||
| 30 or less | 6 (4.48) | 4 (5.63) | 2 (3.17) |
| 31 – 40 | 14 (10.45) | 6 (8.45) | 8 (12.70) |
| 41 – 60 | 60 (44.78) | 33 (46.48) | 27 (42.86) |
| >60 | 47 (35.07) | 25 (35.21) | 22 (34.92) |
| Not reported | 7 (5.22) | 3 (4.23) | 4 (6.35) |
| How often does someone in your primary practice discuss with the patient the financial burden of reconstruction: N (%) | |||
| Never | 13 (9.70) | 6 (8.45) | 7 (11.11) |
| Rarely | 34 (25.37) | 23 (32.39) | 11 (17.46) |
| Sometimes | 29 (21.64) | 16 (22.54) | 13 (20.63) |
| Often | 22 (16.42) | 9 (12.68) | 13 (20.63) |
| Always | 28 (20.90) | 14 (19.72) | 14 (22.22) |
| No reported | 8 (5.97) | 0 | 1 (1.59) |
| Does your primary practice offer out-of-pocket payment arrangements for patients to supplement insurance payments? | |||
| Yes | 68 (50.75) | 41 (57.75) | 27 (42.86) |
| No | 46 (34.33) | 21 (29.58) | 25 (39.68) |
| Don’t know | 13 (9.70) | 6 (8.45) | 7 (11.11) |
| Not reported | 7 (5.22) | 3 (4.23) | 4 (6.35) |
SD- Standard Deviation
Approach to Reconstruction and Factors Perceived to Influence Timing and Method
Respondents reported that most of their patients who underwent reconstruction received it immediately (mean 79.7%). Expander/implant reconstructions were the predominant form of reconstruction performed: Respondents reported a mean of 72.6% of their patients who had expander/implant reconstructions. Purely autologous and combined autologous and expander/implant reconstructions were uncommon with a mean of 14.8% and 12.6% of the reconstructions performed by the respondents, respectively (Table 2).
Table 2:
Surgeon Reconstruction Technique Preferences
| Characteristic | Total Sample | Los Angeles County | State of Georgia |
|---|---|---|---|
| In the past 12 months… | |||
| Percentage of breast reconstruction surgeries performed with immediate timing | |||
| Mean (SD) [Minimum – Maximum] | 79.66 (23.23) [0.00 - 100.00] | 76.93 (26.69) [0.00 - 100.00] | 82.9 (18.00) [0.00 - 100.00] |
| Percentage of breast reconstruction surgeries performed with delayed timing | |||
| Mean (SD) [Minimum – Maximum] | 20.45 (23.28) [0.00 - 100.00] | 23.33 (26.79) [0.00 - 100.00] | 17.1 (18.00) [0.00 - 100.00] |
| Percentage of breast reconstruction procedures performed with exclusively autologous techniques | |||
| Mean (SD) [Minimum – Maximum] | 14.81 (21.55) [0.00 - 96.00] | 12.58 (18.70) [0.00 - 80.00] | 17.38 (24.33) [0.00 - 96.00] |
| Percentage of breast reconstruction surgeries performed using expander/implant techniques | |||
| Mean (SD) [Minimum – Maximum] | 72.58 (24.83) [2.00 - 100.00] | 73.23 (22.85) [10.00 - 100.00] | 71.83 (27.11) [2.00 - 100.00] |
| Percentage of breast reconstruction surgeries performed using combined autologous and expander/implant techniques | |||
| Mean (SD) [Minimum – Maximum] | 12.61 (16.87) [0.00 - 98.00] | 14.2 (16.24) [0.00 - 90.00] | 10.78 (17.52) [0.00 - 98.00] |
SD- Standard Deviation
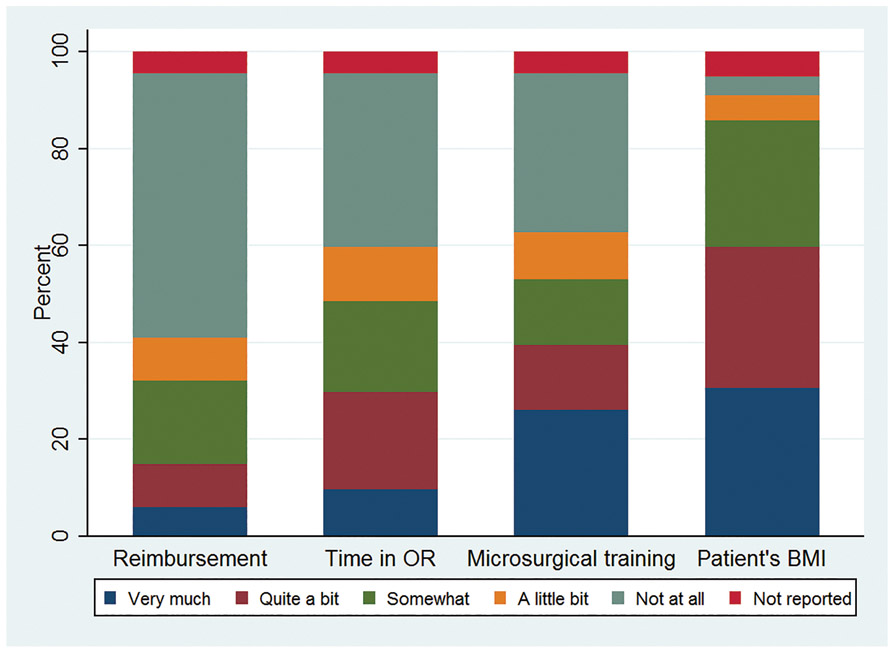
About one third (32.1%) reported that reimbursement rates highly influence the proportion of autologous reconstructions performed (Figure 2). The amount of time spent in the operating room and microsurgical training were also perceived to strongly influence the proportion of autologous reconstructions performed, by 48.5% and 52.9% of responding surgeons, respectively. Eighty-six percent of surgeons reported that patient BMI highly influenced the proportion of autologous reconstructions they performed. About a fifth (21.6%) of surgeons reported delaying reconstructions “often” to “always” in patients with a high BMI.
Figure 2:
Factors perceived to influence the proportion of autologous reconstructions performed
Communication Regarding Contralateral Prophylactic Mastectomy
In a scenario in which the surgeon consulted about breast reconstruction in patients with unilateral breast cancer and no increased risk for contralateral breast cancer, the vast majority (82.8%) of respondents reported that discussions about contralateral prophylactic mastectomy were initiated by patients. Most plastic surgeons (87.3%) indicated that they often referred patients back to the oncologic surgeon for discussions on oncologic aspects of CPM. Three quarters (75.6%) indicated that they seldom specifically inform patients that CPM is not medically indicated; 40% reported often engaging in discussions on oncologic outcomes of CPM.
In cases where a patient with unilateral breast cancer asks about bilateral mastectomy with reconstruction, a majority of responding plastic surgeons report often spending time discussing reconstruction-related topics on alternatives for symmetry (94.8%), the risk of complications for unilateral versus bilateral mastectomy with reconstruction (90.4%), the loss of breast sensation (90.4%), surgical duration (83.7%), differences in duration of recovery with varying techniques (94.8%) and cosmetic outcomes (96.3%).
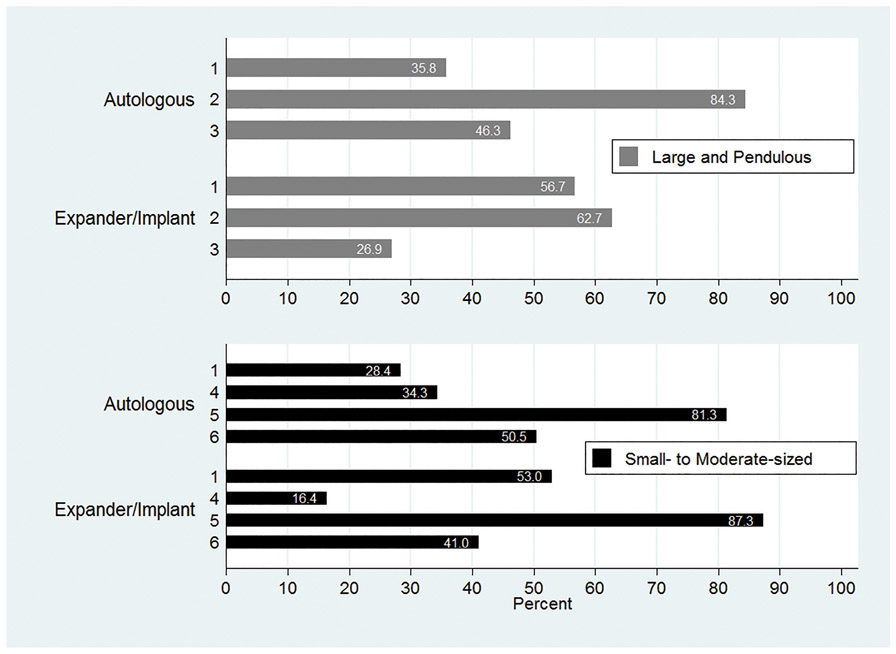
Most of the surveyed plastic surgeons reported that they would communicate that good symmetry is often achieved with unilateral autologous reconstruction (81.3%) or unilateral implant reconstruction (87.3%) with contralateral augmentation and or mastopexy in patients with small to moderate sized breasts considering unilateral mastectomy. A majority would also communicate that good symmetry is often achieved with a unilateral autologous reconstruction with contralateral reduction in patients with large breasts (84.3%). Fewer surgeons (62.7%) often inform patients with large breasts that a unilateral implant reconstruction with contralateral reduction or mastopexy produces good symmetry (Figure 3).
Figure 3:
Surgeon communication with patients on reconstruction approaches to achieve symmetry in small and large breasts
1: Bilateral mastectomy with reconstruction† is the best option to provide good symmetry
2: Reduction and/or mastopexy of the contralateral breast can produce good symmetry
3: Bilateral mastectomy with reconstruction† and unilateral mastectomy with reconstruction† with contralateral reduction and/or mastopexy result in equivalent symmetry.
4: Contralateral surgery is rarely needed for good symmetry.
5: Unilateral mastectomy with reconstruction† and contralateral augmentation and/or mastopexy can produce good symmetry
6: Bilateral mastectomy with reconstruction† and unilateral mastectomy with contralateral augmentation and/or mastopexy result in equivalent symmetry.
†Type of reconstruction (autologous or expander/implant) consistent with scenario (autologous or expander/implant).
Approach to the Patient Considering Post-Mastectomy Radiotherapy
Most respondents indicated that the possibility of post-mastectomy radiation therapy (PMRT) often influenced their recommendations on the timing (88.9%) and type (93.3%) of reconstruction. The possibility of radiation often influenced discussions regarding the risks of complications (97.8%) and anticipated cosmetic outcomes (97%) for nearly all respondents. A quarter of the respondents (27.6%) indicated that they rarely or never recommend delayed reconstruction in patients known to require PMRT. When performing immediate reconstructions in patients requiring PMRT, most plastic surgeons recommend implant reconstructions as opposed to autologous reconstruction (64.9% vs 18.7%). A small subset of surgeons (13.4%) never perform immediate reconstruction in these patients. When performing delayed reconstructions in patients who have received PMRT, respondents indicated performing expander/implant reconstruction for a mean of 23.0% of their patients, combination of autologous/implant for a mean of 37.9%, and purely autologous reconstructions for 39.6%.
Communication, Coordination of Care, and Financial Considerations
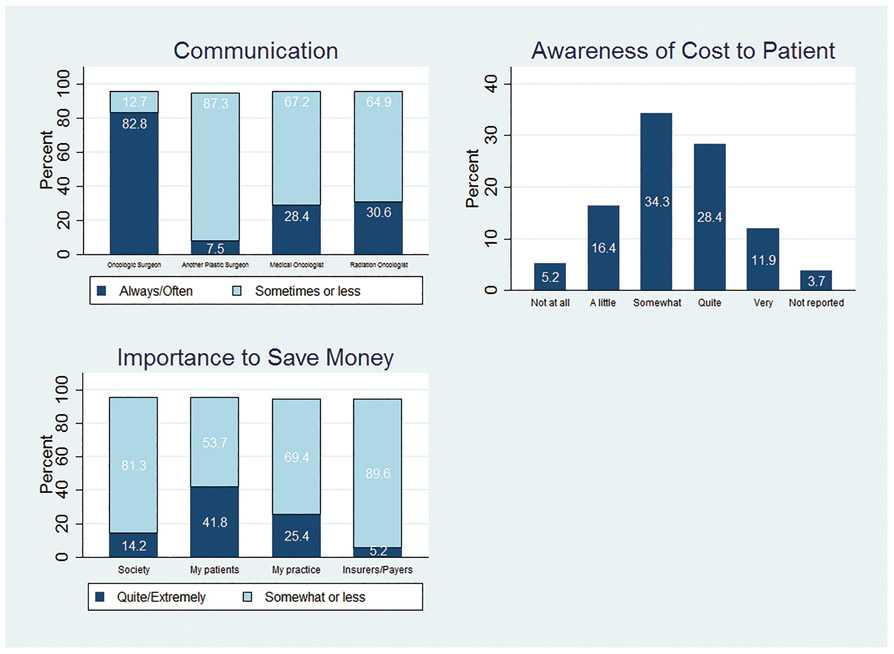
About half of respondents (53.7%) reported that ease of coordination of care with oncologic surgeons rarely or never affected recommendations for reconstruction. The vast majority “always” or “often” discuss treatment plans with oncologic surgeons (82.8%), but few do so with medical oncologists (28.4%), radiation oncologists (30.6%), or other plastic surgeons (7.5%) (Figure 4A).
Figure 4:
Coordination of Care between Plastic Surgeon and Other Providers (A), Awareness of Cost to Patients (B), and Importance to Save Money (C).
When considering the financial implications of treatment options, 41.8% of plastic surgeons felt it was quite or extremely important to save patients money, 25.4% felt it was similarly important to save their practices money, and 14.2% felt it was important to save society money. Few felt it important to save insurers or payers money (5.2%) (Figure 4B). About half of plastic surgeons (56%) indicated that they were only somewhat to not at all aware of the out-of-pocket costs of tests and treatments recommended (Figure 4C). The financial burden of breast reconstruction was discussed with patients 58.9% (sometimes to always) of the time in the primary practices of the respondents. About half (50.8%) offer out-of-pocket payment arrangements for patients to supplement insurance payments.
DISCUSSION
In this survey of a cohort of plastic surgeons providing care to women diagnosed with breast cancer in two areas of the United States in a recent time period, implant/expander was reported as the most common type of reconstruction by a majority of surgeons. Immediate reconstruction was the most common timing for reconstruction. The type and timing of reconstruction offered to patients particularly in the context of anticipated post-mastectomy radiation therapy continue to be subjects of some debate (8). On one hand are questions on how the prevailing approaches to reconstruction strike a balance on postoperative morbidity, patient quality of life and use of resources which include surgeon specific resources such as time and effort. Also debated within oncologic and reconstructive communities is the role plastic surgeons play in encouraging contralateral prophylactic mastectomies based on the need for symmetry with reconstruction (9); this study uniquely explored the plastic surgeon’s views on the subject. Our study findings provide insights into factors that influence current reconstructive practices in ways that may not be apparent in other datasets.
Radiation continues to influence the delivery of post-mastectomy breast reconstruction with some changes in the timing and method of reconstruction offered at present time. Surgeons in this study reported that PMRT influenced their recommendations on the timing and type of reconstruction, but not necessarily in ways that might have been expected. One in four surgeons in this survey seldom recommend delayed reconstruction in patients who require PMRT, consistent with findings from previous studies that have documented an increase in immediate breast reconstructions amongst radiated patients (10). When immediate reconstructions are performed, implant reconstructions are predominant for this cohort. This is in spite of evidence that implant reconstructions in radiated patients results in significantly increased odds for complications relative to autologous reconstructions in similar patients (8). Present approaches to immediate reconstruction of the breast needing radiation include immediate placement of a tissue expander that is subsequently radiated and methods that transition from tissue expanders to a full implant prior to radiation—approaches that have yielded mixed results (11, 12, 13). Potential problems with these approaches include a relatively high explantation rate or suboptimal aesthetic results from contracture (12, 13). A little surprising was our finding that approximately a quarter (23%) of delayed reconstructions performed by surgeons in the context of post-mastectomy radiation therapy were expander/implant reconstructions. Given soft tissue changes following radiation and the increased difficulty for successful tissue expansion, this approach is fraught with high complication and failure rates (14). Acknowledging environments where expertise may be limited for complex autologous reconstructions, the use of the pedicled latissimus dorsi flap in combination with an implant is typically a reliable and effective option (15).
Contralateral prophylactic mastectomies with bilateral breast reconstructions are commonplace at present in the United States, and the influence of consultations with plastic surgeons on the ultimate decision patients make for mastectomy has been somewhat ambiguous. Interestingly, we found that respondents perceived that discussions on the topic of CPM were primarily initiated by patients but that most plastic surgeons do not dissuade patients from considering CPM by informing them that it is not medically indicated. This latter point represents more of a neutral or passive position on the choice for CPM, which contrasts with the position taken by those in the oncologic community who favor active discouragement of CPM in patients who are not known carriers of genetic mutations (16, 17, 18). Position statements from national surgical oncology societies have been published attempting to provide guidance on specific clinical indications for which CPM would be acceptable, in addition to providing resources to help with counseling and decision making (17, 18). There likely would be a benefit to consistent messaging to patients about CPM provided by plastic surgeons that reinforces information provided by other members of the multidisciplinary team; this, in fact, could potentially help decrease or stem the tide of unnecessary CPM procedures (19).
Most plastic surgeons in this study felt that unilateral implant or autologous breast reconstructions with contralateral symmetry procedures in the form of mastopexies or reductions were appropriate to achieve good symmetry. The specific type of reconstruction favored for unilateral breast reconstruction varied based on body habitus as may be expected, given that achieving symmetry in patients with smaller breasts tends to be less challenging with implant or autologous techniques. In patients with larger breasts, autologous reconstructions are better suited to match the large native contralateral breast. In these patients with large breasts and often a high BMI, the largest available gel or saline implants are many times inadequate to provide a desired breast size that is congruent with their habitus. In the context of achieving symmetry following bilateral mastectomies, improved satisfaction with breast was observed in patients with bilateral implant reconstructions relative to patients with similar unilateral procedures (20). These differences based on unilateral or bilateral reconstruction did not exist with autologous procedures (20). As such, it seems reasonable that a lower proportion of surgeons (62.7%) favored unilateral implant reconstruction with a contralateral reduction in patients with large breasts to achieve good symmetry. Also, given the findings of this study, it seems unlikely that a majority of plastic surgeons would proactively advocate for CPM for reasons of superior aesthetic outcomes related to symmetry in patients who are not seeking this surgical intervention for other reasons.
The financial implications of performing breast reconstruction and variations in payments for reconstructive services have been explored in the plastic surgery literature (6, 21, 22). Establishing the impact of these financial realities on surgeon practices and the services offered to patients has been a bit more challenging (23). This study provides some insights, with close to half of the surveyed surgeons indicating that reimbursement rates do influence the proportion of autologous reconstructions performed. Autologous reconstructions are known to offer a number of benefits to women undergoing mastectomy, including sustained quality of life benefits in the long term (24). These tissue-based reconstruction techniques, perforator flaps in particular, are resource and time intensive, and have caused surgeons to consider their feasibility within the financial realities of running a clinical practice. In the context of an academic practice, we previously showed that perforator flap based breast reconstructions reimburse significantly less per hour spent in the operating room; this was also true when relative value units (RVUs) were considered (21, 25). In addition to less reimbursement for flap reconstructions, providing access to reconstruction for as many patients as possible is also a concern. Consequently, improving efficiency has been one solution to this problem with some advocating for centers of excellence where more complex procedures are performed more routinely in high volumes with the potential for improved clinical outcomes (26). Matros and colleagues have suggested that referrals to a limited number of centers may in fact decrease the number of autologous reconstructions performed relative to implants in the US by introducing barriers such as a travel burden (27).
In recent years many centers have begun employing a two-microsurgeon approach, especially for longer bilateral procedures and have demonstrated shorter operative times without compromising care (28, 29). Though the two microsurgeon approach shortens time in the OR and allows for decreased surgeon fatigue, it may not be the most efficient use of each individual surgeon’s time. Others have sought to address the financial imbalance relative to the work required by advocating for higher reimbursements from third party payers (23, 30). Half of the surgeons in this study cohort offered out-of-pocket payment arrangements to supplement payment from insurance companies. This approach though financially sound, is problematic from a societal perspective as it exacerbates disparities in access to reconstructive care for patients of lower socioeconomic status. Without placing a direct financial burden on individual patients, some surgeons and institutions in various parts of the United states negotiate for special payment arrangements or carve outs directly with insurance carriers for additional compensation for perforator flap reconstructions. A recent evaluation of physician payments for breast reconstruction showed a wide range in payments made by insurance companies to physicians for similar autologous reconstruction procedures suggesting that there is room for negotiations with insurance providers, not only to improve insurance payments but also to create more equity and consistency in reimbursement for reconstructive services provided (30).
Aspects of the study merit comment. Women initially surveyed represent a diverse large population-based patient sample and a majority identified their reconstructive surgeons. Data obtained from the surgeons are very pertinent to practice and the response rate was very high. The generalizability of the findings is limited to patient and surgeon experiences in 2 large regions of the United States, our findings might not be generalizable to practices in all locations of the country.
CONCLUSIONS
Perspectives from plastic surgeons in this modern cohort suggest that immediate and implant based breast reconstruction are performed preferentially in patients undergoing mastectomy, including in those who require post-mastectomy radiation therapy. Though associated with long term quality of life benefits, autologous reconstruction techniques are less utilized for multiple reasons including financial considerations. Furthermore, in consultations for reconstruction, discussions between plastic surgeons and patients on their choice for CPM when not clinically indicated appear to be passive. These findings indicate that there may be a role for the implementation policies that encourage reconstructive practices known to result in improved clinical and patient reported outcomes. Also in efforts to help limit poorly informed decisions for CPM, plastic surgeons alongside other members of the multidisciplinary cancer care team, should engage in evidence based discussions with their patients on the subject when the prophylactic procedure is being considered.
ACKNOWLEDGEMENTS
We acknowledge the work of our project staff (Mackenzie Crawford, M.P.H. and Kiyana Perrino, M.P.H. from the Georgia Cancer Registry; Jennifer Zelaya, Pamela Lee, Maria Gaeta, Virginia Parker, B.A. and Renee Bickerstaff-Magee from USC; Rebecca Morrison, M.P.H., Alexandra Jeanpierre, M.P.H., Stefanie Goodell, B.S., Irina Bondarenko, M.S., Paul Abrahamse, M.A., and Rose Juhasz, Ph.D. from the University of Michigan).
This work was funded by grant P01 CA163233 to the University of Michigan from the National Cancer Institute. The collection of Los Angeles County cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries, under cooperative agreement 5NU58DP003862-04/DP003862; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute. Cancer incidence data collection in Georgia was supported by contract HHSN261201300015I, Task Order HHSN26100006 from the NCI and cooperative agreement 5NU58DP003875-04-00 from the CDC.
REFERENCES
- 1.Jagsi R, Jiang J, Momoh AO, et al. Trends and variations in use of breast reconstruction in patients with breast cancer undergoing mastectomy in the United States. J Clin Oncol. 2014;32:919–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albornoz CR, Bach PB, Mehrara BJ, et al. A paradigm shift in U.S. Breast reconstruction: increasing implant rates. Plast Reconstr Surg. 2013;131:15–23. [DOI] [PubMed] [Google Scholar]
- 3.Kamali P, Zettervall SL, Wu W, et al. Differences in the reporting of racial and socioeconomic disparities among three large national databases for breast reconstruction. Plast Reconstr Surg. 2017;139:795–807. [DOI] [PubMed] [Google Scholar]
- 4.Pusic AL, Matros E, Fine N, et al. Patient-Reported Outcomes 1 Year After Immediate Breast Reconstruction: Results of the Mastectomy Reconstruction Outcomes Consortium Study. J Clin Oncol. 2017;35:2499–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilkins EG, Hamill JB, Kim HM, et al. Complications in Postmastectomy Breast Reconstruction: One-year Outcomes of the Mastectomy Reconstruction Outcomes Consortium (MROC) Study. Ann Surg. 2018;267:164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alderman AK, Storey AF, Nair NS, Chung KC. Financial impact of breast reconstruction on an academic surgical practice. Plast Reconstr Surg. 2009;123:1408–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallner LP, Abrahamse P, Uppal JK, et al. Involvement of primary care physicians in the decision making and care of patients with breast cancer. J Clin Oncol. 2016;34:3969–3975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jagsi R, Momoh AO, Qi J, et al. Impact of radiotherapy on complications and patient reported outcomes after breast reconstruction. J Natl Cancer Inst. 2018;110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchanan PJ, Abdulghani M, Waljee JF et al. An analysis of the decisions made for contralateral prophylactic mastectomy and breast reconstruction. Plast Reconstr Surg. 2016;138:29–40. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal S, Kidwell KM, Farberg A, Kozlow JH, Chung KC, Momoh AO. Immediate Reconstruction of the Radiated Breast: Recent Trends Contrary to Traditional Standards. Ann Surg Oncol. 2015;22:2551–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kronowitz SJ, Hunt KK, Kuerer HM, et al. Delayed-immediate breast reconstruction. Plast Reconstr Surg. 2004;113:1617–28 [DOI] [PubMed] [Google Scholar]
- 12.Kronowitz SJ, Lam C, Terefe W, et al. A multidisciplinary protocol for planned skin-preserving delayed breast reconstruction for patients with locally advanced breast cancer requiring postmastectomy radiation therapy: 3-year follow-up. Plast Reconstr Surg. 2011;127:2154–66. [DOI] [PubMed] [Google Scholar]
- 13.Cordeiro PG, Albornoz CR, McCormick B, et al. What is the Optimum Timing of Postmastectomy Radiotherapy in Two-Stage Prosthetic Reconstruction: Radiation to the Tissue Expander or Permanent Implant? Plast Reconstr Surg. 2015;135:1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chetta MD, Aliu O, Zhong L, et al. Reconstruction of the Irradiated Breast: A National Claims-Based Assessment of Postoperative Morbidity. Plast Reconstr Surg. 2017;139:783–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spear SL, Boehmler JH, Taylor NS, Prada C. The role of the latissimus dorsi flap in reconstruction of the irradiated breast. Plast Reconstr Surg. 2007;119:1–9. [DOI] [PubMed] [Google Scholar]
- 16.Ramaswami R, Morrow M, Jagsi R. Contralateral Prophylactic Mastectomy. N Engl J Med. 2017;377:1288–1291. [DOI] [PubMed] [Google Scholar]
- 17.Boughey JC, Attai DJ, Chen SL, Cody HS, et al. Contralateral Prophylactic Mastectomy (CPM) Consensus Statement from the American Society of Breast Surgeons: Data of CPM Outcomes and Risks. Ann Surg Oncol. 2016; 23:3100–3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunt KK, Euhus DM, Boughey JC, et al. Society of Surgical Oncology Breast Disease Working Group Statement on Prophylactic (Risk-Reducing) Mastectomy. Ann Surg Oncol. 2017;24:375–397. [DOI] [PubMed] [Google Scholar]
- 19.Jagsi R, Hawley ST, Griffith KA, et al. Contralateral prophylactic mastectomy decisions in a population-based sample of patients with early-stage breast cancer. JAMA Surg. 2017;152:274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Momoh AO, Cohen WA, Kidwell KM, et al. Tradeoffs Associated With Contralateral Prophylactic Mastectomy in Women Choosing Breast Reconstruction: Results of a Prospective Multicenter Cohort. Ann Surg. 2017;266:158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sando IC, Chung KC, Kidwell KM, Kozlow JH, Malay S, Momoh AO. Comprehensive breast reconstruction in an academic surgical practice: an evaluation of the financial impact. Plast Reconstr Surg. 2014;134:1131–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischer JP, Sieber B, Nelson JA, et al. Comprehensive outcome and cost analysis of free tissue transfer for breast reconstruction: An experience with 1303 flaps. Plast Reconstr Surg. 2013;131:195–203. [DOI] [PubMed] [Google Scholar]
- 23.Sheckter CC, Panchal HJ, Razdan SN, et al. The influence of physician payments on the method of breast reconstruction: a national claims analysis. Plast Reconstr Surg. 2018:142:434–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atisha D, Alderman AK, Lowery JC, Kuhn LE, Davis J, Wilkins EG. Prospective analysis of long-term psychosocial outcomes in breast reconstruction: two-year prospective results from the Michigan Breast Reconstruction Outcomes Study. Ann Surg. 2008;247:1019–28. [DOI] [PubMed] [Google Scholar]
- 25.Sando IC, Momoh AO, Chung KC, Kozlow JH. The early years of practice: an assessment of operative efficiency and cost of free flap and implant breast reconstruction at an academic institution. [DOI] [PubMed] [Google Scholar]
- 26.Birkmeyer JD, Lucas FL, Wennberg DE. Potential benefits of regionalizing major surgery in Medicare patients. Eff Clin Pract. 1999;2:277–283. [PubMed] [Google Scholar]
- 27.Albornoz CR, Cordeiro PG, Hishon L et al. A nationwide analysis of the relationship between hospital volume and outcome for autologous breast reconstruction. [DOI] [PubMed] [Google Scholar]
- 28.Weichman KE, Lam G, Wilson SC, et al. The impact of two operating surgeons on microsurgical breast reconstruction. Plast Reconstr Surg. 2017;139:277–284. [DOI] [PubMed] [Google Scholar]
- 29.Razdan SN, Panchal HJ, Hespe GE, et al. The impact of the cosurgeon model on bilateral autologous breast reconstruction. J Reconstr Microsurg. 2017;33:624–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheckter CC, Yi D, Panchal HJ, et al. Trends in physician payments for breast reconstruction. Plast Reconstr Surg. 2018;141:493–499. [DOI] [PMC free article] [PubMed] [Google Scholar]