Abstract
Orthostatic hypotension is seen in various medical conditions. It can be secondary to medications or volume depletion. It can also be due to autonomic neuropathy secondary to other diseases, such as diabetes mellitus, or to primary degenerative processes of the autonomic nervous system. Orthostatic hypotension dominates the clinical picture of patients suffering from autonomic failure. Paradoxically, about one half of these patients also suffer from supine hypertension, which induces pressure natriuresis, worsening orthostatic hypotension. It also complicates the treatment of orthostatic hypotension. Supine hypertension is mediated by an increase in peripheral vascular resistance. This is due to residual sympathetic tone in patients with multiple system atrophy (Shy‐Drager syndrome), but the cause is not known in patients with pure autonomic failure, who have increased vascular resistance despite very low levels or plasma norepinephrine and renin activity. The recent observation that patients with supine hypertension develop left ventricular hypertrophy suggests they should be treated. During the day, avoiding the supine position is often all that is required. Short‐acting vasodilators (e.g., transdermal nitroglycerin) can be used during the night.
The autonomic nervous system plays a crucial role in the regulation of acute changes of blood pressure. Its importance is clinically evident in patients with autonomic impairment. Severely affected patients are unable to stand but for a few seconds because of orthostatic hypotension. Any disorder associated with peripheral neuropathy (e.g., diabetes mellitus) can potentially affect autonomic function. There are also degenerative disorders of the autonomic nervous system that are characterized by severe autonomic failure. These are seen most often in patients in their sixth decade of life or older. There are two main neurodegenerative disorders of the autonomic nervous system. Patients with pure autonomic failure suffer from an isolated involvement of the autonomic nervous system, and symptoms include orthostatic hypotension, impotence, bladder dysfunction, constipation, and decreased sweating. In patients with multiple‐system atrophy, other neurologic systems are involved, in addition to the autonomic nervous system. They commonly have atypical parkinsonian features (Shy‐Drager syndrome), but may have ataxia or other neurologic symptoms.
The clinical picture of patients with severe failure of the autonomic nervous system is dominated by orthostatic hypotension (defined as a fall in systolic blood pressure of at least 20 mm Hg or diastolic blood pressure of at least 10 mm Hg within 3 minutes of standing). This is often disabling and patients often cannot stand for more than a few minutes before the occurrence of orthostatic symptoms or frank syncope (Figure 1). 1 , 2 The fact that patients with autonomic failure often suffer from hypertension while lying down is often overlooked, even though the association was described almost 80 years ago. 3 Indeed, supine hypertension, defined as a systolic blood pressure ≥150 mm Hg or diastolic blood pressure ≥90 mm Hg, is present in one half of patients with severe autonomic failure (Figure 2), despite normal seated and low upright blood pressures. Supine blood pressures as high as 230/140 mm Hg have been described. 4 Elevated blood pressure during recumbency complicates the treatment of orthostatic hypotension. It limits the use of pressor agents for the treatment of orthostatic hypotension and increases nocturnal sodium excretion. This latter effect worsens orthostatic hypotension the following morning. 5 , 6 Preliminary studies suggest that supine hypertension may promote left ventricular hypertrophy in these patients. 7 The negative effect of this syndrome on nocturnal sodium excretion and the possible association with chronic cardiovascular damage suggests that the condition should be treated. However, treatment of supine hypertension may make orthostatic hypotension worse and may increase the risk of falling. Recent studies suggest that in some patients, the hypertension is driven by residual sympathetic tone. In this group, manipulation of residual sympathetic function may be useful to treat supine hypertension. In others, supine hypertension is independent of the autonomic nervous system and different treatment approaches are indicated. 8
Figure 1.
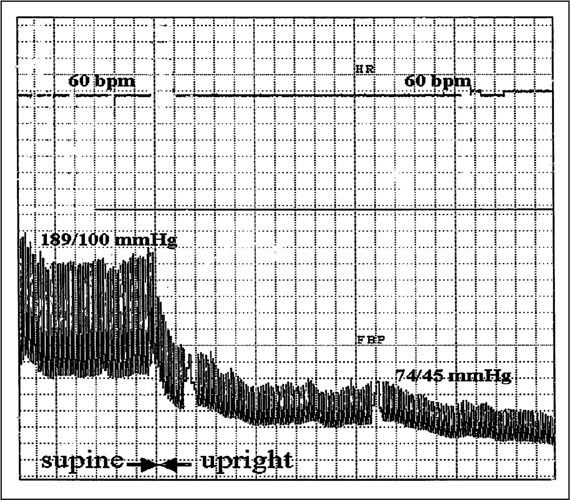
Beat‐to‐beat finger blood pressure (FBP) and heart rate (HR) recording in a patient with pure autonomic failure. The blood pressure (BP) values were determined with a brachial BP cuff. One small box indicates a 7‐second interval. In the supine position, BP readings were in the hypertensive range. Respiratory sinus arrhythmia was almost completely abolished. When the patient stood up, BP decreased profoundly. There was no adequate compensatory increase in HR. In the first few minutes, the patient did not experience orthostatic symptoms despite the profound hypotension. After several minutes of standing, a further slight decrease in BP occurred. This decrease in BP exceeded the autoregulatory capacity of the brain, and the patient became severely symptomatic and had to sit. bpm=beats per minute
Figure 2.
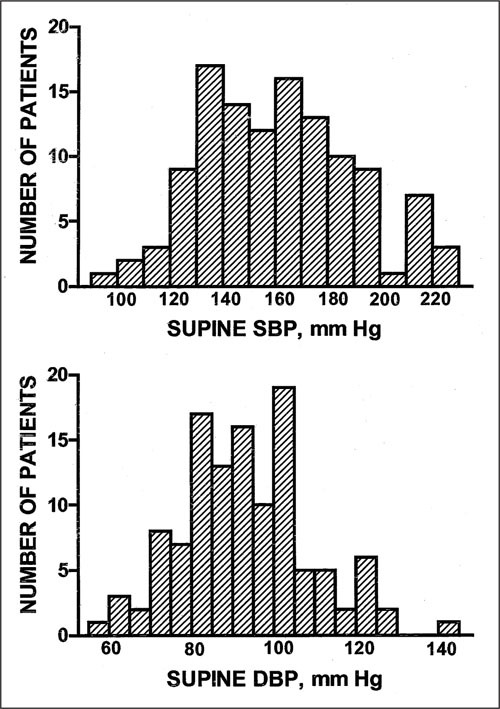
Frequency distribution of supine systolic (SBP, upper panel) and diastolic (DBP, lower panel) blood pressure in 117 primary autonomic failure patients. Patients were studied off medications and in sodium balance on a controlled diet containing 150 mEq/d sodium.
SUPINE HYPERTENSION COMPLICATES THE TREATMENT OF ORTHOSTATIC HYPOTENSION
Most patients with severe orthostatic hypotension are dependent on pressor agents in order to lead normal lives. The ideal pressor agent for the treatment of orthostatic hypotension would increase upright blood pressure without an effect on supine blood pressure. Unfortunately, current pharmacologic treatments do not selectively abolish the orthostatic decrease in blood pressure in patients with severe autonomic failure. 9 Instead, they increase both supine and upright blood pressure. Any of the drugs currently used for the treatment of orthostatic hypotension has the potential of exacerbating supine hypertension. Longer‐acting pressor agents, such as fludrocortisone 10 , 11 or erythropoietin, are particularly likely to worsen supine hypertension.
The large diurnal swings in blood pressure observed in autonomic failure patients are associated with fluctuations in volume status. During the day, while patients spend most of their time in a seated or upright position and blood pressure is low, sodium excretion is decreased. During the night, while patients are resting in the supine position and blood pressure is high, sodium excretion is increased (Figure 3). The phenomenon is probably related to pressure natriuresis. An increase in atrial natriuretic factor 12 and mineralocorticoid deficiency 13 seem to be less important. The nocturnal sodium loss is maladaptive and leads to aggravation of orthostatic hypotension the following morning, a phenomenon that coincides with worsening of symptoms early in the morning, with gradual improvement throughout the day. This phenomenon is increased in patients with supine hypertension. Conversely, sodium retention during the day would exacerbate supine hypertension.
Figure 3.
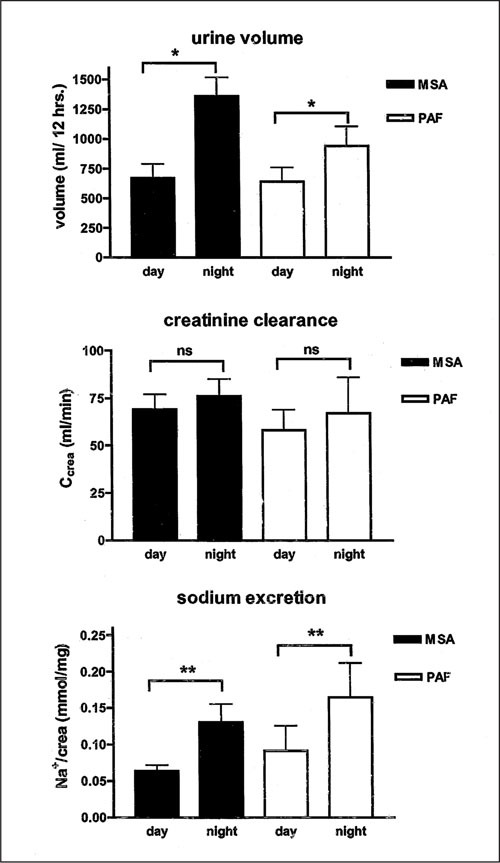
Diurnal variation in urine volume, creatinine clearance (Ccrea), and sodium excretion (Na+/crea) in patients with severe autonomic failure due to multiple‐system atrophy (MSA) and pure autonomic failure (PAF) (day: 8 a.m. to 8 p.m.; night: 8 p.m. to 8 a.m.). Ccrea was similar during the day and during the night. The urine volume and urinary sodium excretion were approximately two‐fold greater during the night than during the day. *p<0.05; **p<0.01
SHOULD WE TREAT SUPINE HYPERTENSION IN PATIENTS WITH AUTONOMIC FAILURE?
Even mild degrees of arterial hypertension increase cardiovascular morbidity and mortality in the long term. Complications, such as cerebral edema or congestive heart failure, can occur with extreme blood pressure elevations. However, there are no epidemiologic data on the cardiovascular consequences of isolated supine hypertension. To balance the risks of the hypertension against the potential side effects of antihypertensive treatment is challenging. Prolonged follow‐up in a substantial number of patients with this relatively rare disorder will be required to answer this question.
Even though epidemiologic data on the prognosis of supine hypertension are not available, a decision must be made regarding the treatment of these patients. Consideration of the differences in pathophysiology between isolated supine hypertension and essential hypertension may be useful. Obviously, patients with supine hypertension are hypertensive during the night, but during the day they may have blood pressure values in the normotensive or hypotensive range. Thus, the average blood pressure over a 24‐hour period may be only moderately elevated even in patients with severe supine hypertension. Furthermore, catecholamine concentrations 14 and plasma renin activity 15 tend to be decreased in patients with autonomic failure. End‐organ damage appears to correlate with plasma renin activity in essential hypertension. 16 , 17 We speculate that the low plasma renin activity in autonomic failure patients may provide some protection against hypertensive complications. However, this hypothesis has not been formally tested.
End‐organ damage occurs with long‐standing, untreated essential hypertension. A recent study suggested that supine hypertension may be associated with left ventricular hypertrophy. 7 However, whether or not the presence of left ventricular hypertrophy in patients with autonomic failure worsens the already poor prognosis is unknown. Patients with multiple‐system atrophy have a median survival of less than 10 years from the onset of symptoms. 18 Progression of their underlying disease rather than complications of hypertension will dominate their clinical picture. Patients with pure autonomic failure are believed to have a better prognosis and some may have a normal life expectancy. These patients have a higher likelihood of experiencing hypertensive end‐organ damage and are more likely to benefit from antihypertensive therapy than multiple‐system atrophy patients.
Supine hypertension can lead to acute cardiovascular complications. For example, we encountered a pure autonomic failure patient with severe supine hypertension who developed a hemorrhagic stroke while supine. We decided to treat this patient with a short‐acting calcium channel blocker taken at bedtime. Obviously, the decision to treat supine hypertension is much easier in patients who experience complications of supine hypertension.
Pharmacologic treatment of supine hypertension is associated with significant risks. Antihypertensive medications will decrease both supine and upright blood pressure. This effect worsens orthostatic hypotension, particularly during the night. Because most patients suffer from nocturia, treatment with antihypertensive medications during the night may increase the risk of falling when patients stand up to go to the bathroom. As a precaution, we instruct patients to use a bedside commode.
In summary, at present there is insufficient evidence to assess the risks associated with supine hypertension in patients with autonomic failure and the balance between potential benefits of antihypertensive medications and the risks associated with their side effects. The presence of left ventricular hypertrophy suggests that end‐organ damage can occur in these patients and that treatment is indicated.
AUTONOMIC FAILURE–IT'S NOT ALL THE SAME
It should be noted that orthostatic hypotension can be induced or worsened by reversible factors, such as volume loss or medication. This possibility should always be considered and managed appropriately. It is important, also, to distinguish the syndromes associated with autonomic failure (Table), because the mechanisms leading to supine hypertension are different. 8 Furthermore, the prognosis differs between the various syndromes, a consideration when deciding whether or not to treat supine hypertension. Symptoms of autonomic failure result from dysfunction of the efferent part of the autonomic nervous system. 19 The lesion can be located in the central nervous system 20 or may be limited to peripheral nerves. 21 The most common syndrome associated with central autonomic failure is multiple‐system atrophy (MSA). In these patients, peripheral autonomic neurons are, at least in part, functionally intact. 8 , 22 Most patients with MSA have additional neurologic symptoms, such as parkinsonism or signs of cerebellar dysfunction. When autonomic dysfunction dominates the clinical presentation, the syndrome is also referred to as Shy‐Drager syndrome. Patients with Parkinson's disease can also present with autonomic failure. In these patients, the lesion to the autonomic nervous system appears to be peripheral. Distinguishing MSA from Parkinson's disease with autonomic failure can be challenging. One important difference between the syndromes is the sensitivity to L‐dopa. Most patients with Parkinson's disease show a marked and rapid improvement in their motor symptoms after L‐dopa administration. In contrast, MSA patients have a poor response or may not respond at all.
Table.
Characteristics of Autonomic Failure Syndromes
| PAF | Park+ | MSA | |
| Level of the lesion | Peripheral | Peripheral | Central |
| Histopathology | Lewy bodies | Lewy bodies | Alpha‐synculein inclusions |
| CNS symptoms* | No | Yes | Yes |
| Response to L‐dopa | N/A | Good | Poor or none |
| Progression | Relatively good | Relatively good | Poor |
| PAF=pure autonomic failure; Park+=Parkinson's disease with autonomic failure; MSA= multiple system atrophy; CNS=central nervous system; *may include movement disorders or cerebral ataxia | |||
Peripheral autonomic failure may be secondary (e.g., diabetic autonomic neuropathy, amyloidosis) or it may be primary (pure autonomic failure [PAF] or Bradbury‐Eggleston syndrome). Patients with PAF typically experience symptoms at an older age. PAF is a clinical diagnosis and requires careful exclusion of secondary causes for peripheral autonomic dysfunction. A diagnosis of severe diabetic autonomic neuropathy is suggested by a long‐standing history of diabetes mellitus. In rare patients, severe orthostatic hypotension results from a genetic disorder associated with impaired function of dopamine‐β‐hydroxylase. Patients with dopamine‐β‐hydroxylase‐deficiency are not able to convert dopamine to norepinephrine. 23 Both central and peripheral forms of autonomic failure are associated with supine hypertension. In our experience, supine hypertension is uncommon in amyloidosis patients.
MECHANISMS OF SUPINE HYPERTENSION
The mechanisms that cause orthostatic hypotension have been extensively studied. In contrast, there are few studies on the mechanisms of supine hypertension. Theoretically, supine hypertension could result from excessive cardiac output or from inappropriately high systemic vascular resistance. An increase in cardiac output could result from increased cardiac preload or increased cardiac contractility. Hemodynamic studies in limited numbers of patients using radionuclide ventriculography suggest that supine hypertension is mainly due to an increase in systemic vascular resistance. 24 Cardiac output in the supine position is not excessive. However, cardiac contractility may be increased. 24 Patients with and without supine hypertension seem to have similar blood volumes. 4 Thus, supine hypertension mainly results from excessive vascular tone.
We reasoned that the increase in systemic vascular tone is probably independent of the autonomic nervous system, given the severity of autonomic dysfunction. 4 This hypothesis turned out to be wrong. We found that patients with MSA and supine hypertension exhibit an extreme decrease in blood pressure during infusion of the ganglionic blocker trimethaphan (Figure 4), 8 strongly suggesting that the autonomic nervous system is playing an important role in the elevated blood pressure. Similarly, the α‐adrenoreceptor antagonist phentolamine elicits a profound depressor response. 8 In this group of patients with central autonomic dysfunction, supine hypertension can be explained by excessive sympathetic tone in the supine position. In contrast, blood pressure responses to trimethaphan are variable in patients with PAF. Some patients have a moderate depressor response, whereas others do not respond at all. 8 In these patients with damage to peripheral autonomic neurons, supine hypertension is not mediated by the sympathetic nervous system. The factors that raise blood pressure independently of sympathetic tone are unknown.
Figure 4.
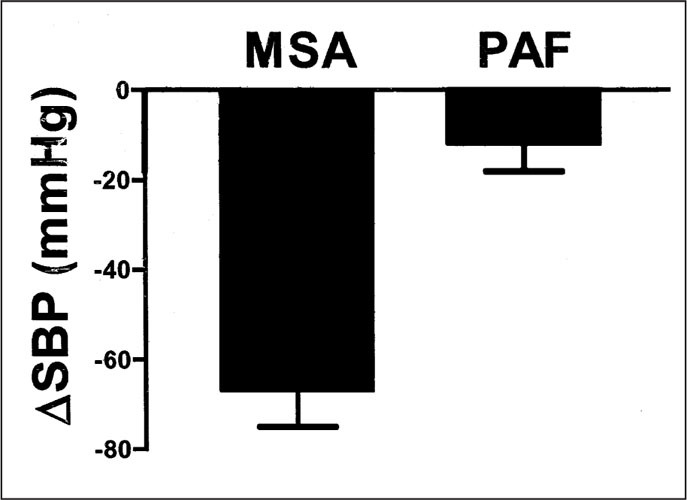
Average decrease in systolic blood pressure (δSBP) produced by 1 mg/min of the ganglionic blocker trimethaphan in patients with multiple‐system atrophy (MSA) and pure autonomic failure (PAF)
A STEPWISE APPROACH IN TREATING SUPINE HYPERTENSION
Education and Avoidance
The first and most important step in the treatment of supine hypertension is patient education. Patients need to know that simply standing up will abolish supine hypertension. They and their family members should learn to measure supine and upright blood pressure.
Interestingly, merely drinking 16 oz of plain water acutely elicits a profound increase in blood pressure in a large subgroup of patients with severe orthostatic hypotension due to autonomic failure. The effect reaches a maximum after approximately 35 minutes, and is sustained for more than 60 minutes. 25 , 26 Water drinking can be a surprisingly useful treatment of orthostatic hypotension. However, water drinking should be avoided within the hour before bedtime in patients with supine hypertension.
It is important that patients are aware that any medication that is used to treat orthostatic hypotension will worsen supine hypertension. When short‐acting pressor agents are used, patients should avoid the supine position for several hours until the effect of the medication abates. If patients notice an increase in supine blood pressure with longer‐acting pressor agents, they should discuss this issue with their physicians. Patients should know that many over‐the‐counter medications, such as nasal decongestants, ibuprofen, and other medication, can substantially increase supine blood pressure. 9 , 27 Even phenylephrine eye drops can increase blood pressure in these patients. 28 Many patients wear support stockings or abdominal binders to attenuate venous pooling. 29 They should be reminded not to use them while supine.
Nonpharmacologic Treatment
Nonpharmacologic treatments should be tried before pharmacologic therapy is initiated. It has been shown that sleeping in the head‐up tilt position reduces nocturnal sodium loss, which will improve orthostatic hypotension in the morning. 30 We encourage patients to sleep with the head of the bed elevated 6–9 inches to benefit from this phenomenon. Sleeping in the head‐up tilt position decreases blood pressure moderately. The effect may not be sufficient in patients with severe supine hypertension. Most patients with orthostatic hypotension also feature postprandial hypotension. A snack taken at bedtime can be used to reduce supine hypertension, but the effect is not sustained throughout the night. Alcohol elicits a depressor response. 31 Patients who wish to do so may have a drink before they retire, to transiently improve supine hypertension. However, we would not advocate increasing alcohol consumption above moderate levels in these patients.
Pharmacologic Treatment
Patients who do not experience sufficient improvement in supine blood pressure with nonpharmacologic treatment may be candidates for pharmacologic interventions. Nitroglycerin patches and short‐acting nifedipine used at bedtime decrease supine blood pressure substantially in patients with autonomic failure 4 , 6 (Figure 5). It is important to point out that short‐acting calcium channel blockers are avoided in the treatment of patients with essential hypertension, in whom longer‐acting preparations or other medications are preferable. In contrast, in autonomic failure patients with supine hypertension, longer‐acting calcium channel blockers would exacerbate orthostatic hypotension during the day. Minoxidil and hydralazine are less potent in reducing supine hypertension 4 (Figure 6) but may be useful in individual patients. Pharmacologic reduction in nocturnal pressure natriuresis could theoretically improve daytime orthostatic hypotension. However, neither nifedipine nor nitroglycerin decrease nocturnal natriuresis. In fact, nifedipine increases nocturnal natriuresis in autonomic failure patients. This finding is presumably explained by a direct effect of both drugs on sodium excretion that opposes the reduction in pressure‐mediated natriuresis. Furthermore, these medications do not improve orthostatic hypotension the following morning. 6
Figure 5.
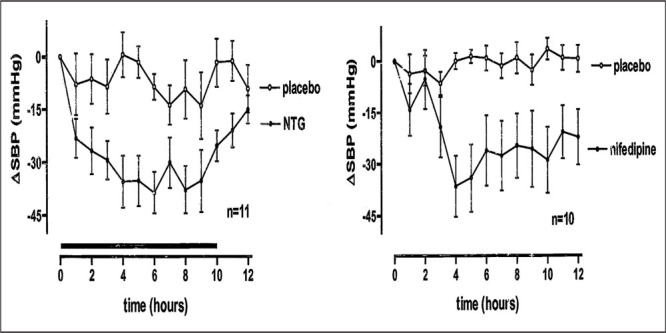
Change in systolic blood pressure (δSBP) with placebo and nitroglycerin patch (NTG) (left panel) or nifedipine (right panel) in autonomic failure patients. The medication was given at 8 p.m. (0 hours on the graph). The horizontal bar on the left panel indicates the time the patch remained on the skin. Compared to placebo, the maximal decreases in SBP were 36±10 mm Hg and 37 ±9 mm Hg 4 hours after the patch was applied or nifedipine was given, respectively. Supine SBP at 8 a.m., 2 hours after the nitroglycerin patch was removed, was similar with nitroglycerin and placebo. In contrast, the depressor effect of nifedipine was sustained throughout the observation period.
Figure 6.
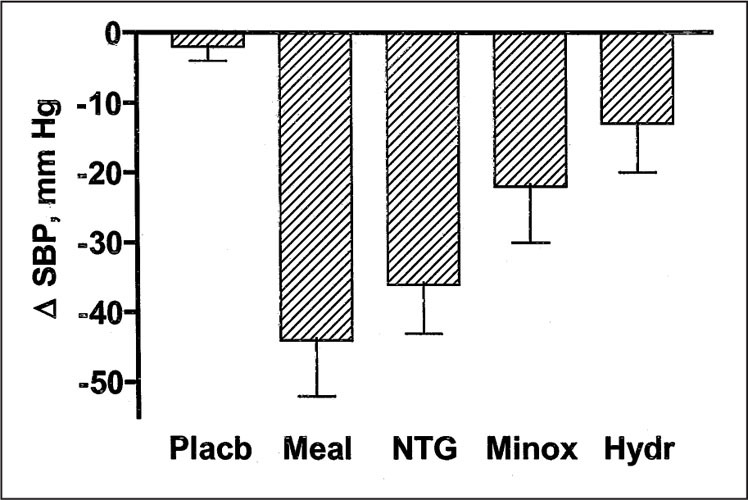
Comparison of the depressor effects produced by a standardized meal (Meal, 414 calories; n=16), transdermal nitroglycerin (NTG, 0.025–0.1 mg/hr; n=7), minoxidil (Minox, 2.5 mg p.o.; n=5), and hydralazine (Hydr, 50 mg p.o.; n=7). The maximal decrease in systolic blood pressure (?SBP) is presented in the y axis. Placb=placebo
The doses must be titrated very carefully to prevent excessive hypotension. We use a nitroglycerin preparation (Nitro‐Dur patch) that is formulated into a matrix rather than a pouch. The patch can be divided to titrate the dose without loss of active medication. Patients are instructed to remove the patch before rising in the morning. With any antihypertensive medication taken at bedtime, patients must be very careful in getting up during the night. Preliminary observations suggest that clonidine is effective in lowering blood pressure in patients with MSA.
CONCLUDING REMARKS
Paradoxically, supine hypertension is a common occurrence in patients suffering from orthostatic hypotension. Supine hypertension can be severe and may occur in patients without a previous history of hypertension. The mechanism is increased vascular resistance, but the underlying pathophysiology depends on the type of autonomic failure. Residual sympathetic tone accounts for the increased blood pressure in patients with MSA. Acute removal of sympathetic tone with ganglionic blockade produces dramatic reductions in supine blood pressure in these patients. They remain incapacitated, with profound orthostatic hypotension, because of their inability to modulate their sympathetic tone.
On the other hand, the cause of increased vascular resistance and hypertension in patients with PAF remains unknown. Supine hypertension can be treated during the day by simply avoiding the supine position. In the occasional patient, blood pressure may remain elevated even while seated. These patients remain a therapeutic challenge. During the night, blood pressure can be usually controlled with judicious use of vasodilators. Patients with supine hypertension provide unique human models of sympathetically dependent and independent hypertension. Understanding their pathophysiology, particularly that of PAF, will contribute to our knowledge of blood pressure regulation in essential hypertension.
Acknowledgments: This work was supported in part by National Institutes of Health grants RR00095 and 1PO1 HL56693 and by Deutsche Forschungsgemeinschaft grants Jo 284/1–1 and Jo 284/3–1. Dr. Jordan is the recipient of a Helmholtz fellowship from the Max Delbr?ck Center of Molecular Medicine.
References
- 1. Robertson D. Treatment of cardiovascular disorders: orthostatic hypotension. In: Melmon KL, Morrelli HF, eds. Clinical Pharmacology Basic Principles in Therapeutics. New York, NY: McGraw‐Hill; 1992:84–103. [Google Scholar]
- 2. Smit AA, Halliwill JR, Low PA, et al. Pathophysiological basis of orthostatic hypotension in autonomic failure. J Physiol. 1999;519(pt 1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bradbury S, Eggleston C. Postural hypotension: a report of three cases. Am Heart J. 1925;1:73–86. [Google Scholar]
- 4. Shannon JR, Jordan J, Costa F, et al. The hypertension of autonomic failure and its treatment. Hypertension. 1997;30:1062–1067. [DOI] [PubMed] [Google Scholar]
- 5. Wilcox CS, Aminoff MJ, Penn W. Basis of nocturnal polyuria in patients with autonomic failure. J Neurol Neurosurg Psychiatry. 1974;37:677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jordan J, Shannon JR, Pohar B, et al. Contrasting effects of vasodilators on blood pressure and sodium balance in the hypertension of autonomic failure. J Am Soc Nephrol. 1999;10:35–42. [DOI] [PubMed] [Google Scholar]
- 7. Vagaonescu TD, Saadia D, Tuhrim S, et al. Hypertensive cardiovascular damage in patients with primary autonomic failure. Lancet. 2000;355:725–726. [DOI] [PubMed] [Google Scholar]
- 8. Shannon JR, Jordan J, Diedrich A, et al. Sympathetically mediated hypertension in autonomic failure. Circulation. 2000;101:2710–2715. [DOI] [PubMed] [Google Scholar]
- 9. Jordan J, Shannon JR, Biaggioni I, et al. Contrasting actions of pressor agents in severe autonomic failure. Am J Med. 1998;105:116–124. [DOI] [PubMed] [Google Scholar]
- 10. Chobanian AV, Volicer L, Tifft CP, et al. Mineralocorticoid‐induced hypertension in patients with orthostatic hypotension. N Engl J Med. 1979;301:68–73. [DOI] [PubMed] [Google Scholar]
- 11. Shear L. Orthostatic hypotension. Treatment with sodium chloride and sodium retaining steroid hormones. Arch Intern Med. 1968;122:467–471. [DOI] [PubMed] [Google Scholar]
- 12. Kaufmann H, Oribe E, Pierotti AR, et al. Atrial natriuretic factor in human autonomic failure. Neurology. 1990;40:1115–1119. [DOI] [PubMed] [Google Scholar]
- 13. Wilcox CS, Aminoff MJ, Slater JD. Sodium homeostasis in patients with autonomic failure. Clin Sci Mol Med. 1977;53:321–328. [DOI] [PubMed] [Google Scholar]
- 14. Goldstein DS, Polinsky RJ, Garty M, et al. Patterns of plasma levels of catechols in neurogenic orthostatic hypotension. Ann Neurol. 1989;26:558–563. [DOI] [PubMed] [Google Scholar]
- 15. Biaggioni I, Garcia F, Inagami T, et al. Hyporeninemic normoaldosteronism in severe autonomic failure. J Clin Endocrinol Metab. 1993;76:580–586. [DOI] [PubMed] [Google Scholar]
- 16. Alderman MH, Sealey JE, Laragh JH. Plasma renin activity and ischemic heart disease. N Engl J Med. 1994;330:506–507. [DOI] [PubMed] [Google Scholar]
- 17. Brunner HR, Laragh JH, Baer L, et al. Essential hypertension: renin and aldosterone, heart attack and stroke. N Engl J Med. 1972;286:441–449. [DOI] [PubMed] [Google Scholar]
- 18. Wenning GK, Ben Shlomo Y, Magalhaes M, et al/ Clinical features and natural history of multiple system atrophy. An analysis of 100 cases. Brain. 1994;117:835–845. [DOI] [PubMed] [Google Scholar]
- 19. Jordan J, Shannon JR, Black B, et al. Malignant vagotonia due to selective baroreflex failure. Hypertension. 1997;30:1072–1077. [DOI] [PubMed] [Google Scholar]
- 20. Wenning GK, Tison F, Ben SY, et al. Multiple system atrophy: a review of 203 pathologically proven cases. Mov Disord. 1997;12:133–147. [DOI] [PubMed] [Google Scholar]
- 21. Kanda T, Tomimitsu H, Yokota T, et al. Unmyelinated nerve fibers in sural nerve in pure autonomic failure. Ann Neurol. 1998;43:267–271. [DOI] [PubMed] [Google Scholar]
- 22. Biaggioni I, Robertson RM, Robertson D. Manipulation of norepinephrine metabolism with yohimbine in the treatment of autonomic failure. J Clin Pharmacol. 1994;34:418–423. [DOI] [PubMed] [Google Scholar]
- 23. Robertson D, Goldberg MR, Onrot J, et al. Isolated failure of autonomic noradrenergic neurotransmission: evidence of impaired β‐hydroxylation of dopamine. N Engl J Med. 1986;314:1494–1497. [DOI] [PubMed] [Google Scholar]
- 24. Kronenberg MW, Forman MB, Onrot J, et al. Enhanced left ventricular contractility in autonomic failure: assessment using pressure‐volume relations. J Am Coll Cardiol. 1990;15:1334–1342. [DOI] [PubMed] [Google Scholar]
- 25. Jordan J, Shannon JR, Grogan E, et al. A potent pressor response elicited by drinking water. Lancet. 1999;353:723.refere [DOI] [PubMed] [Google Scholar]
- 26. Jordan J, Shannon JR, Black BK, et al. The pressor response to water drinking in humans: a sympathetic reflex? Circulation. 2000;101:504–509. [DOI] [PubMed] [Google Scholar]
- 27. Biaggioni I, Onrot J, Stewart CK, et al. The potent pressor effect of phenylpropanolamine in patients with autonomic impairment. JAMA. 1987;258:236–239. [PubMed] [Google Scholar]
- 28. Robertson D. Contraindication to the use of ocular phenylephrine in idiopathic orthostatic hypotension. Am J Ophthalmol. 1979;87:819–822. [DOI] [PubMed] [Google Scholar]
- 29. Smit AA, Wieling W, Opfer GT, et al. Patients' choice of portable folding chairs to reduce symptoms of orthostatic hypotension. Clin Auton Res. 1999;9:341–344. [DOI] [PubMed] [Google Scholar]
- 30. Bannister R, Ardill L, Fentem P. An assessment of various methods of treatment of idiopathic orthostatic hypotension. Q J Med. 1969;38:377–395. [PubMed] [Google Scholar]
- 31. Narkiewicz K, Cooley RL, Somers VK. Alcohol potentiates orthostatic hypotension: implications for alcohol–related syncope. Circulation. 2000;101:398–402. [DOI] [PubMed] [Google Scholar]


