Abstract
Background
Small airway function parameters (SAFPs) combined with fractional exhaled nitric oxide (FeNO) can predict a positive methacholine challenge test (MCT) for asthma diagnosis. However, their predictive utility in patients with forced expiratory volume in one second (FEV1) ≥80% predicted within different age ranges remains unclear. This study aimed to assess the utility of SAFPs, alone or combined with FeNO, to predict a positive MCT in patients in two age groups (<55 and ≥55 years) with asthma‐suggestive symptoms and FEV1 ≥80% predicted.
Methods
We enrolled 846 Chinese patients with suspected asthma and standard spirometry, FeNO, and MCT findings. Using the area under the curves (AUCs), the utility of SAFPs, alone or combined with FeNO, for predicting a positive MCT was analyzed in a discovery (n = 534) and validation cohort (n = 312) in both age groups with FEV1 ≥80% predicted.
Results
In the discovery cohort, the optimal cut‐off values for predicting a positive MCT in patients aged <55 years (74.2% and 74.9% for forced expiratory flow (FEF)50% and FEF25%–75%, respectively) were higher than those in patients aged ≥55 years (65.0% and 62.9% for FEF50%, FEF25%–75%, respectively). However, the optimal FeNO value in patients aged <55 years (43 ppb) was lower than that in patients aged ≥55 years (48 ppb). FeNO combined with SAFPs (FEF50%, FEF25%–75%) significantly increased the AUCs in both groups (≥55 years [0.851 for FEF50% and 0.844 for FEF25%–75%]; <55 years [0.865 for FEF50% and 0.883 for FEF25%–75%]) compared with a single parameter (p < 0.05). These findings were confirmed in the validation cohort. Compared with patients ≥55 years, those aged <55 years had higher and lower optimal cut‐off values for SAFPs and FeNO, respectively. The AUCs of FeNO combined with SAFPs for predicting a positive MCT for asthma diagnosis were significantly higher than those of the individual parameters (p < 0.05) in both age groups.
Conclusions
There were age‐group differences in the utility of SAFPs combined with FeNO for predicting a positive MCT. Patients with an asthma‐suggestive history and a normal FEV1 should be stratified by age when using SAFPs combined with FeNO to predict a positive MCT.
Keywords: age, asthma, fractional exhaled nitric oxide, methacholine challenge test, small airway
Keywords: 关键词: 年龄, 哮喘, 呼出气一氧化氮, 乙酰胆碱激发试验, 小气道
1. BACKGROUND
Asthma is a common disorder caused by chronic inflammation of the lower respiratory tract. Its diagnosis is based on typical symptoms of wheeze, recurrent breath shortness, chest tightness, and cough, as well as evidence of variable expiratory airflow limitation based on objective pulmonary function tests. 1 Both a bronchodilating test with salbutamol and a methacholine challenge test (MCT) are used for asthma diagnosis in clinical practice. 2 , 3 A positive bronchodilating test, which is indicated by significant FEV1 reversibility (a threshold of 12% and 200 ml reversibility to 400 μg salbutamol) combined with typical respiratory symptoms, is recommended by the Global Initiative for Asthma as proof of asthma. Airway hyper‐responsiveness (AHR) to MCT is reflective of another aspect of airway lability. A positive MCT with a provocative concentration of inhaled methacholine <16 mg/ml or ≤0.48 mg is highly sensitive for identifying AHR presence to allow asthma diagnosis in patients with typical symptoms suggestive of asthma and preserved baseline pulmonary function (FEV1 >70% pred). 3 , 4 , 5 However, in most hospitals in China, performing an MCT costs >$73 and at least half an hour, which is expensive and time‐consuming. Moreover, it involves 11 steps, 6 which is inconvenient for patients, technicians, and clinicians. Notably, MCT can cause unpleasant feelings in patients and can potentially induce severe bronchospasm. 6 Therefore, the use of a pulmonary function test or a test combination to predict AHR presence or a positive MCT could provide great value to clinicians for asthma diagnosis.
Small airways, which are defined by an inner diameter <2 mm, represent a "quiet zone" and provide minimal resistance to airflow in normal adult lungs 7 ; however, they are vulnerable to obstruction in asthma. 8 In the diseased state, pulmonary function changes are not detected by standard spirometry until approximately 75% of the small airways are obstructed. 9 , 10 Therefore, it is crucial for predicting AHR presence in patients with a normal FEV1 for early stage asthma diagnosis based on small airway function parameters (SAFPs). 11 Additionally, eosinophilic inflammation identified through fractional exhaled nitric oxide (FeNO) is also associated with the pathophysiological process of asthma. 12 A previous study reported that the area under the curve (AUC) of the combination of forced expiratory flow (FEF) between 25% and 75% (FEF25%–75%) and FeNO for predicting AHR presence in patients with cough‐variant asthma was significantly higher than that of the parameters being applied separately (all p < 0.05); furthermore, FeNO >43 ppb and FEF25%–75% <78.5% were the optimal cutoff values. 11 Therefore, SAFPs combined with FeNO can improve the predictive value for AHR presence in asthma diagnosis. 11 Further, compared with MCT, SAFP, and FeNO measurement are more accessible, safer, cheaper ($5–10 lower), and time‐saving (15–20 min).
Both SAFPs and FeNO are age dependent 13 , 14 with older age being an important small‐airway dysfunction (SAD) predictor in patients with asthma 9 , 15 and being associated with a greater daily dose of inhaled corticosteroid 9 and a worse clinical asthma expression. 16 Moreover, FeNO has been reported to decrease with aging in patients with asthma. 17 Under‐ or overdiagnosis using these objective tests may occur when age is not considered. 18 Therefore, age should be considered when using these parameters to predict a positive MCT for asthma diagnosis. A previous study defined older age as age ≥50 years. 9 In our recent unpublished study on a Chinese population, 55 years was used as the optimal cut‐off value for determining the impact of age on small airway function (Lili Hou, unpublished paper). This study aimed to assess the impact of age on the predictive value of SAFPs, alone or combined with FeNO, for a positive MCT in patients with asthma‐suggestive symptoms and FEV1 ≥80% predicted. This could contribute to reduced over‐ or underprediction of a positive MCT for asthma diagnosis, as well as the elucidation of the treatment effect assessed by SAFPs and FeNO in patients with asthma with different age ranges.
2. METHODS
2.1. Patients and study design
This retrospective, cross‐sectional, multicentered, observational study recruited 846 adult patients with suspected asthma involving recurrent dyspnea, cough, chest tightness, and wheeze for ≥2 months. The recruited patients were referred to the Pulmonary Outpatient Clinic from January 2016 to September 2020. Among the recruited patients, a discovery cohort of 534 patients was enrolled from the electronic medical databases (EMD) of Shanghai General Hospital affiliated to Shanghai Jiao Tong University and Tongji Hospital Affiliated to Tongji University School of Medicine. Furthermore, a validation cohort of 312 patients was enrolled from the EMD of the Second Affiliated Hospital of Zhejiang University School of Medicine. These patients underwent standard spirometry, FeNO, and MCT. The participants in both cohorts were stratified by the previously mentioned cutoff age of 55 years to clarify the effect of age on the predictive value of SAFPs, alone or combined with FeNO, in patients with asthma‐suggestive symptoms and FEV1 ≥80% predicted. This study was approved by the ethics committee of the Institutional Review Board at Shanghai General Hospital (no. 2020 [30]). All the participants provided informed written consent for study participation.
2.2. Inclusion criteria
The inclusion criteria were as follows: age 18–80 years; history of chronic dyspnea, cough, and wheeze for ≥2 months; normal chest X‐ray or computed tomography results; predicted FEV1 of ≥80% with spirometric measurement, and having undergone MCT.
2.3. Exclusion criteria
The exclusion criteria were as follows: having fever or acute respiratory tract infections within the previous 8 weeks; having taken montelukast, long‐acting β2‐agonists, theophylline, anticholinergic agents, and inhaled or oral corticosteroids within the previous 4 weeks; or having comorbid severe systemic diseases, including chronic obstructive pulmonary diseases.
2.4. FeNO measurements
FeNO was measured using NIOX MINO (Aerocrine AB) at a standard flow rate of 50 ml/s following the American Thoracic Society (ATS)/European Respiratory Society (ERS) recommendations. 11 , 19 FeNO measurements were performed before the spirometric assessments and MCT since the involved breathing maneuvers could distort FeNO results. 11
2.5. Spirometric measurements
Spirometry tests were performed between 8 and 11 a.m. using an MS‐PFT spirometer (Jaeger or SensorMedics). Spirometry tests followed the standards and recommendations of the ATS/ERS. 6 , 20 Expected values for lung function parameters were based on the prediction equation for patients in East China.
The following eight pulmonary function parameters were reviewed and analyzed: forced vital capacity (FVC), FEV1, FEV1/FVC (FEV1%), peak expiratory flow (PEF), FEF at 25% of FVC exhaled (FEF25%), FEF at 50% of FVC exhaled (FEF50%), FEF at 75% of FVC exhaled (FEF75%), and FEF between 25% and 75% (FEF25%–75%). Most of these parameters were presented as percentages of predicted values with FEV1/FVC being presented as the absolute value.
2.6. Methacholine challenge test
MCT was performed using the Jaeger APS Pro system using a Medic‐Aid sidestream nebulizer with doubling methacholine doses (0.0725–0.48 mg) following the ATS/ERS recommendations. 6 FEV1 was measured at 3 min after each provocation step. Provocative doses that caused a 20% fall in FEV1 (PD20) was recorded; moreover, AHR was defined as positive if PD20 ≤ 0.48 mg.
Well‐trained technicians in each center performed FeNO, spirometry, and MCT measurements. An experienced clinician and technician discussed whether the MEFV curves met the ATS/ERS quality criteria for spirometry. 6 , 20
2.7. Outcomes
The primary outcome was the predictive value of SAFPs, alone or combined with FeNO, for the presence of a positive MCT in asthma diagnosis of the discovery cohort enrolled from two centers located in Shanghai, China. The secondary outcome was the confirmation of the primary outcome in a validation cohort enrolled from a third center located in Hangzhou, China.
2.8. Statistical analysis
Data were analyzed for normality of distribution using Kolmogorov–Smirov test. Normally distributed data were presented as mean ± SD. Nonnormally distributed data were expressed as median and interquartile range. Independent samples were compared using Student's t test (two‐tailed) or Mann–Whitney U test. Count data were presented as the percentage and between‐group comparisons were performed using the χ 2. Between‐parameter correlations were analyzed using Spearman's analysis.
For patients with suspected asthma who presented small airway dysfunction, MCT was considered the gold standard for defining AHR. Logistic regression was applied to determine the impact of continuous test variables with dichotomous state variables. Univariate logistic regression was used to determine the impact of the variables of interest; subsequently, multiple logistic regression was used to check whether the model could be improved. Predictive values of single or combined measurements were calculated by constructing receiver‐operating characteristic (ROC) curves and measuring AUCs. 11 In the ROC plot, sensitivity was plotted against 100‐specificity. We determined the cut‐off value based on Youden's Index.
Analyses were performed using SPSS software package version 22.0 (IBM Corp.). ROC curve construction and AUCs comparisons were performed using MediCalc 19.0.4 software. AUCs were compared using the χ 2 test based on the method of Hanley and McNeil. Statistical significance was set at p < 0.05.
3. RESULTS
3.1. Baseline characteristics in the discovery cohort
The discovery cohort included 534 adults with symptoms of cough, chest tightness, dyspnea, and wheeze for ≥2 months. Among them, 276 (51.69%) patients exhibited a positive MCT. After stratification according to age, 291 and 243 participants were aged <55 and ≥55 years; among them, 148 (50.86%) and 128 (52.67%) participants had a positive MCT, respectively. Table 1 presents the baseline demographic and clinical characteristics of the patients. Based on the MCT results, there were no significant between‐group differences in age, sex, past smoking history, and BMI. Participants with positive MCT had lower PEF (all p < 0.05); however, the average value was within the normal range. Compared with the corresponding values in the negative MCT group, the FEF25%, FEF50%, FEF75%, FEF25%–75% FEV1, and FEV1/FVC, values were significantly lower while the FeNO value was significantly higher (all p < 0.001, Table 1) in the positive MCT group. Moreover, the Spearman analysis revealed a weak correlation of PD20 with FEF50% (r = 0.224, p < 0.001), FEF25%–75% (r = 0.256, p < 0.001), and FeNO (r = −0.252, p < 0.001).
TABLE 1.
Demographic data, spirometric parameters, and values for FeNO in patients with negative and positive methacholine challenge test in the discovery cohort
| Characteristic variables | Negative MCT | Positive MCT | p |
|---|---|---|---|
| <55 years | n 1 = 143 | n 2 = 148 | |
| Male (n/%) | 65 (45.45%) | 60 (40.54%) | 0.397 |
| Age, yearsa | 35.00 (30.00, 47.00) | 40.00 (30.00, 48.00) | 0.248 |
| BMI, kg/m2 a | 22.52 (19.95, 24.92) | 22.31 (20.35, 24.64) | 0.861 |
| Past smoking history (n/%) | 28 (19.58%) | 31 (20.95%) | 0.772 |
| FEF50%, % predicteda | 86.10 (68.60, 103.30) | 65.50 (60.30, 73.65) | <0.001 |
| FEF25%, % predicteda | 101.10 (86.10, 115.60) | 90.40 (75.45, 98.45) | <0.001 |
| FEF75%, % predicteda | 81.70 (61.20, 104.50) | 69.80 (50.20, 86.28) | <0.001 |
| FEF25%–75%, % predicteda | 87.70 (73.80, 99.70) | 68.35 (60.18, 73.35) | <0.001 |
| PEF, % predicteda | 103.60 (93.10, 114.60) | 99.80 (90.30, 111.63) | 0.024 |
| FEV1, % predicteda | 99.90 (94.60, 107.30) | 96.60 (89.65, 103.15) | <0.001 |
| FVC, % predicteda | 101.60 (94.70, 107.10) | 100.70 (93.20, 108.28) | 0.966 |
| FEV1/FVC, %a | 85.23 (80.30, 91.65) | 81.15 (77.61, 85.06) | <0.001 |
| FeNO, ppba | 21.00 (13.00, 41.00) | 53.00 (39.25, 93.00) | <0.001 |
| ≥55 years | n 1 = 115 | n 2 = 128 | |
| Male (n/%) | 30 (26.09%) | 34 (26.56%) | 0.993 |
| Age, yearsa | 61.00 (56.00, 65.00) | 61.00 (56.25, 64.00) | 0.696 |
| BMI, kg/m2 a | 23.31 (21.67, 25.30) | 23.62 (22.06, 25.74) | 0.326 |
| Past smoking history (n/%) | 17 (14.78%) | 14 (10.94%) | 0.370 |
| FEF50%, % predicteda | 77.50 (65.30, 102.70) | 60.90 (56.08, 65.48) | <0.001 |
| FEF25%, % predicteda | 101.00 (88.70, 117.60) | 89.00 (76.30, 103.08) | <0.001 |
| FEF75%, % predicteda | 65.60 (49.10, 82.30) | 53.45 (42.48, 70.78) | <0.001 |
| FEF25%–75%, % predicteda | 77.40 (63.70, 88.90) | 59.55 (50.68, 69.83) | <0.001 |
| PEF, % predictedb | 109.20 ± 18.76 | 103.34 ± 18.87 | 0.008 |
| FEV1, % predicteda | 108.40 (98.50, 118.70) | 97.85 (91.20, 107.98) | <0.001 |
| FVC, % predicteda | 110.90 (99.60, 122.30) | 109.05 (100.85, 116.50) | 0.130 |
| FEV1/FVC, %a | 81.23 (76.91, 85.84) | 76.98 (73.97, 79.72) | <0.001 |
| FeNO, ppba | 24.00 (16.00, 38.00) | 49.00 (22.00, 56.75) | <0.001 |
Note: Bold font indicates statistical significance.
Abbreviations: BMI, body mass index; FEF25%, forced expiratory flow at 25% of forced vital capacity; FEF50%, forced expiratory flow at 50% of forced vital capacity; FEF75%, forced expiratory flow at 75% of forced vital capacity; FEF25%–75%, Forced expiratory flow between 25% and 75%; FeNO, fractional exhaled nitric oxide; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s; PEF, peak expiratory flow; MCT, methacholine challenge test.
median (IQR) values.
mean ± SD values.
3.2. Predictive values of single and combined variables for positive MCT
The predictive value of FEV1, FEV1/FVC, FEF25%, FEF75%, FEF50%, and FEF25%–75%, alone or combined with FeNO, was evaluated using ROC curves. Tables 2 and 3 show the sensitivity, specificity, PPV, NPV, and accuracy of each variable.
TABLE 2.
Predictive values for predicting positive MCT in the discovery cohort
| Characteristic Variables | AUC | Cut off values* | Sensitivity% | Specificity % | PPV % | NPV % | Accuracy% | +LR | −LR | Variable coef. | Constant coef. | p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All (n = 534) | ||||||||||||
| FEF25%, %predicted | 0.681 | 99.7 | 75.36 | 53.10 | 63.2 | 66.8 | 59.74 | 1.61 | 0.46 | −0.033 | 3.157 | <0.001 |
| FEF50%, %predicted | 0.771 | 73.7 | 82.97 | 60.85 | 69.4 | 77.0 | 72.28 | 2.12 | 0.28 | −0.059 | 4.426 | <0.001 |
| FEF75%, %predicted | 0.635 | 68.1 | 60.14 | 60.08 | 61.7 | 58.5 | 60.11 | 1.51 | 0.66 | −0.021 | 1.561 | <0.001 |
| FEF25%–75%, %predicted | 0.774 | 75.2 | 85.51 | 65.12 | 72.4 | 80.8 | 75.66 | 2.45 | 0.22 | −0.063 | 4.649 | <0.001 |
| FEV1, %predicted | 0.652 | 97.9 | 53.62 | 68.60 | 64.6 | 58.0 | 60.86 | 1.71 | 0.68 | −0.052 | 5.311 | <0.001 |
| FEV1/FVC, % | 0.671 | 80.22 | 59.78 | 67.44 | 66.3 | 61.1 | 63.48 | 1.84 | 0.60 | −0.066 | 5.551 | <0.001 |
| FeNO | 0.754 | 43.0 | 61.23 | 84.11 | 80.5 | 67.0 | 77.28 | 3.85 | 0.46 | 0.039 | −1.437 | <0.001 |
| ≥55 years (n = 243) | ||||||||||||
| FEF50%, %predicted | 0.794 | 65.0 | 73.44 | 77.39 | 78.3 | 72.4 | 75.31 | 3.25 | 0.34 | −0.067 | 4.836 | <0.001 |
| FEF25%–75%, %predicted | 0.769 | 62.9 | 65.62 | 77.39 | 76.4 | 66.9 | 71.19 | 2.90 | 0.44 | −0.063 | 4.391 | <0.001 |
| FeNO | 0.702 | 48.0 | 50.78 | 88.70 | 83.3 | 61.8 | 68.72 | 4.49 | 0.55 | 0.036 | −1.151 | <0.001 |
| <55 years (n = 291) | ||||||||||||
| FEF50%, %predicted | 0.760 | 74.2 | 76.35 | 67.83 | 71.1 | 73.5 | 72.16 | 2.37 | 0.35 | −0.057 | 4.406 | <0.001 |
| FEF25%–75%, %predicted | 0.792 | 74.9 | 83.78 | 74.13 | 77.0 | 81.5 | 79.04 | 3.24 | 0.22 | −0.070 | 5.410 | <0.001 |
| FeNO | 0.798 | 43.0 | 68.92 | 85.31 | 82.9 | 72.6 | 76.98 | 4.69 | 0.36 | 0.042 | ‐1.709 | <0.001 |
Note: The cutoff values were selected by Youden Index.
Abbreviations: AUC, area under the curve; constant coef., constant coefficient of logistic regression; FEF25%, forced expiratory flow at 25% of forced vital capacity; FEF50%, forced expiratory flow at 50% of forced vital capacity; FEF75%, forced expiratory flow at 75% of forced vital capacity; FEF25%–75%: Forced expiratory flow between 25% and 75%; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; FeNO, fractional exhaled nitric oxide; +LR, positive likelihood ratios; −LR, negative likelihood ratios; MCT, methacholine challenge test; NPV, negative predictive values; variable p, the t test of the characteristic variables coefficient for the p‐value; PPV, positive predictive values; variable coef., characteristic variables coefficient of logistic regression.
TABLE 3.
Predictive values of small airway function parameters (FEF50%, FEF25%–75%) combined with FeNO in predicting positive MCT in the discovery cohort
| Characteristic Variables | AUC | 95% CI | Sensitivity % | Specificity % | PPV % | NPV % | Accuracy % | +LR | −LR | p a | Variable coef.(p) | FeNO coef.(p) | Constant coef. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All (n = 534) | |||||||||||||
| FEF50% + FeNO | 0.858 | 0.826–0.887 | 78.26 | 79.46 | 80.3 | 77.4 | 78.65 | 3.81 | 0.27 | <0.001 | −0.063 (<0.001) | 0.042 (<0.001) | 3.076 |
| FEF25%–75% + FeNO | 0.865 | 0.833–0.893 | 82.61 | 76.74 | 79.2 | 80.5 | 79.59 | 3.55 | 0.23 | <0.001 | −0.071 (<0.001) | 0.046 (<0.001) | 3.435 |
| ≥55 years (n = 243) | |||||||||||||
| FEF50% + FeNO | 0.851 | 0.800–0.893 | 80.47 | 75.65 | 78.6 | 77.7 | 78.19 | 3.30 | 0.26 | 0.002 | −0.079 (<0.001) | 0.047 (<0.001) | 3.996 |
| FEF25%–75% + FeNO | 0.844 | 0.792–0.887 | 85.94 | 71.30 | 76.9 | 82.0 | 78.60 | 2.99 | 0.20 | 0.001 | −0.076 (<0.001) | 0.048 (<0.001) | 3.587 |
| <55 years (n = 291) | |||||||||||||
| FEF50% + FeNO | 0.865 | 0.820–0.902 | 79.05 | 83.22 | 83.0 | 79.3 | 80.76 | 4.71 | 0.25 | 0.001 | −0.052 (<0.001) | 0.040 (<0.001) | 2.374 |
| FEF25%–75% + FeNO | 0.883 | 0.841–0.918 | 81.08 | 81.12 | 81.6 | 80.6 | 80.76 | 4.29 | 0.23 | <0.001 | −0.071 (<0.001) | 0.045 (<0.001) | 3.592 |
Note: The cutoff values were selected by the Youden Index. Bold font indicates statistical significance.
Abbreviations: AUC, area under the curve; constant coef., constant coefficient of logistic regression; FEF50%, forced expiratory flow at 50% of forced vital capacity; FEF25%–75%, forced expiratory flow between 25% and 75%; FeNO, fractional exhaled nitric oxide; FeNO coef. (p), FeNO coefficient of logistic regression and it's t‐test for the p value; +LR, positive likelihood ratios; −LR, negative likelihood ratios; MCT, methacholine challenge test; NPV, negative predictive values; PPV, positive predictive values; variable coef. (p), another characteristic variables coefficient of logistic regression and it's t test for the p value.
Compared with FEF50% or FEF25%–75% alone.
Before stratifying according to age, the AUCs of FEF50% and FEF25%–75% were 0.771 (95% confidence interval [CI]: 0.733–0.806) and 0.774 (95% CI: 0.736–0.809), respectively, which represented the two largest AUCs for a positive MCT diagnosis in the spirometry measurement (Table 2). The AUC of FeNO for a positive MCT diagnosis was 0.754 (95% CI: 0.716–0.790; Table 2).
To determine whether SAFPs combined with FeNO could improve prediction of a positive MCT, ROC analysis was performed using different combinations of SAFPs (FEF50% and FEF25%–75%) with FeNO. The AUC for FEF50% combined with FeNO was 0.858 (95% CI: 0.826–0.887), which was significantly higher than that of either FEF50% (p < 0.0001) or FeNO alone (p < 0.0001). The AUC for FEF25%–75% combined with FeNO was 0.865 (95% CI: 0.833–0.893), which was significantly higher than that of either FEF25%–75% or FeNO alone (p < 0.001 for all; Table 3, Figure 1A).
FIGURE 1.
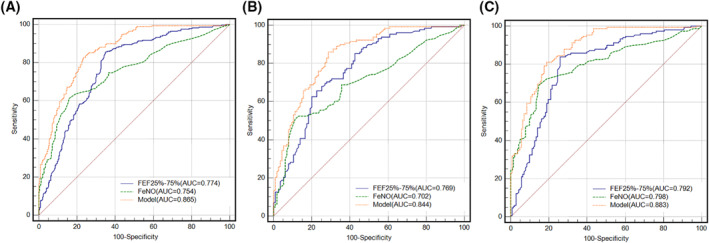
ROC curves for the model of FEF25%–75% combined with FeNO in predicting positive bronchial provocation in patients of the discovery cohort prior to being stratified by age (A), patients aged ≥55 years (B), and patients aged <55 years (C). (A) n = 534, AUCmodel = 0.865 (95% CI, 0.833–0.893); AUCFEF25%–75% = 0.774 (95% CI, 0.736–0.809; p < 0.0001, compared with the model); AUCFeNO = 0.754 (95% CI, 0.716–0.790; p = 0.5176 and <0.0001, compared with FEF25%–75% alone and the model, respectively). (B) n = 243, AUCmodel = 0.844 (95% CI, 0.792–0.887); AUCFEF25%–75% = 0.769 (95% CI, 0.711–0.820; p = 0.0005, compared with the model); AUCFeNO = 0.702 (95% CI, 0.640–0.759; p = 0.1700 and <0.0001, compared with FEF 25%–75% alone and the mode, respectively). (C) n = 291, AUCmodel = 0.883 (95% CI, 0.841–0.918); AUCFEF25%–75% = 0.792 (95% CI, 0.740–0.837; p < 0.0001, compared with the model); AUCFeNO = 0.798 (95% CI, 0.747–0.842; p = 0.8724 and 0.0001, compared with FEF25%–75% alone and the model, respectively)
In patients aged ≥55 years, the AUCs for FEF50%, FEF25%–75%, and FeNO were 0.794 (95% CI: 0.738–0.843), 0.769 (95% CI: 0.711–0.820), and 0.702 (95% CI: 0.640–0.759), respectively. The AUC for FEF50% combined with FeNO was 0.851 (95% CI: 0.800–0.893), which was higher than that for either FEF50% (p = 0.0119) or FeNO (p < 0.001) alone. The AUC for FEF25%–75% combined with FeNO was 0.844 (95% CI: 0.792–0.887), which was significantly higher than that for either FEF25%–75% or FeNO alone (all p < 0.001, Table 3, Figure 1B).
In patients aged <55 years, the AUC for FEF50%, FEF25%–75%, and FeNO was 0.760 (95% CI: 0.707–0.808), 0.792 (95% CI: 0.740–0.837), and 0.798 (95% CI: 0.747–0.842), respectively. The AUC for the combination of FEF50% and FeNO was 0.865 (95% CI: 0.820–0.902), which was significantly higher than that for FEF50% (p < 0.0001) or FeNO (p = 0.0005) alone. The AUC for FEF25%–75% combined with FeNO was 0.883 (95% CI: 0.841–0.918), which was significantly higher than that for each of them (p < 0.0001 for FEF25%–75% and p = 0.0001 for FeNO; Tables 2 and 3, Figure 1C).
3.3. Optimal cut‐off values for positive MCT prediction
The optimal cut‐off values were calculated based on Youden's Index. Prior to stratification according to age, the cut‐off values for FEF50%, FEF25%–75%, and FeNO were 73.7%, 75.2%, and 43 ppb, respectively. In patients aged ≥55 years, the cut‐off values for FEF50%, FEF25%–75%, and FeNO were 65.0%, 62.9%, and 48 ppb, respectively. On the other hand, in patients aged <55 years, the cut‐off values for FEF50%, FEF25%–75%, and FeNO were 74.2%, 74.9%, and 43 ppb, respectively (Table 2).
3.4. Predictive values of single and combined variables for positive AHR in the validation cohort
Table 4 shows the baseline demographic and pulmonary function characteristics of the validation cohort stratified according to age. In patients aged <55 years and ≥55 years, 63 (37.50%) and 57 out of 144 patients showed positive MCT, respectively.
TABLE 4.
Demographic data, spirometric parameters, and values for FeNO in patients with negative and positive bronchial provocation tests in the validation cohort
| Characteristic variables | Negative MCT | Positive MCT | p Value |
|---|---|---|---|
| <55 years | n 1 = 105 | n 2 = 63 | |
| Male (n/%) | 53 (50.48%) | 31 (49.21%) | 0.873 |
| Age, years a | 34.00 (31.00, 41.00) | 35.00 (29.00, 45.00) | 0.743 |
| BMI, kg/m 2 a | 24.45 (21.41, 28.44) | 23.11 (21.30, 25.39) | 0.156 |
| Past smoking history (n/%) | 19 (18.10%) | 19 (30.16%) | 0.070 |
| FEF 50% , % predicted a | 97.00 (79.90, 115.35) | 74.70 (64.80, 92.70) | <0.001 |
| FEF 25% , % predicted b | 101.30 ± 21.74 | 92.19 ± 15.37 | 0.002 |
| FEF 75% , % predicted b | 97.01 ± 27.71 | 75.24 ± 21.54 | <0.001 |
| FEF 25‐75% , % predicted a | 94.10 (77.45, 111.50) | 73.60 (61.04, 93.20) | <0.001 |
| PEF, % predicted a | 97.20 (86.85, 109.55) | 90.40 (84.40, 100.20) | 0.015 |
| FEV 1 , % predicted b | 105.04 ± 11.48 | 98.73 ± 9.27 | <0.001 |
| FVC, % predicted b | 102.58 ± 11.06 | 101.04 ± 8.53 | 0.312 |
| FEV 1 /FVC, % b | 86.37 ± 5.48 | 82.36 ± 5.56 | <0.001 |
| FeNO, ppb a | 22.00 (14.00, 38.50) | 80.00 (28.00, 113.00) | <0.001 |
| ≥55 years | n 1 = 87 | n 2 = 57 | |
| Male (n/%) | 30 (34.48%) | 14 (24.56%) | 0.206 |
| Age, years a | 62.00 (58.00, 67.00) | 61.00 (59.00, 64.00) | 0.352 |
| BMI, kg/m 2 a | 24.20 (22.27, 28.08) | 24.03 (21.75, 25.85) | 0.393 |
| Past smoking history (n/%) | 16 (18.39%) | 8 (14.04%) | 0.493 |
| FEF 50% , % predicted a | 78.00 (64.30, 98.8) | 62.00 (57.90, 66.70) | <0.001 |
| FEF 25% , % predicted b | 98.38 ± 27.15 | 86.13 ± 14.34 | 0.001 |
| FEF 75% , % predicted a | 94.30 (73.40, 113.90) | 62.00 (57.90, 66.70) | <0.001 |
| FEF 25‐75% , % predicted a | 79.40 (64.10, 101.30) | 62.80 (56.70, 66.00) | <0.001 |
| PEF, % predicted a | 96.80 (84.30, 109.20) | 87.00 (79.45, 100.20) | 0.011 |
| FEV 1 , % predicted a | 106.60 (97.40, 115.10) | 94.70 (89.80, 106.40) | <0.001 |
| FVC, % predicted a | 100.80 (90.50, 111.90) | 99.30 (90.20, 107.50) | 0.491 |
| FEV 1 /FVC, % b | 85.44 ± 4.88 | 81.46 ± 5.70 | 0.001 |
| FeNO, ppb a | 24.00 (15.00, 44.00) | 53.00 (29.50, 82.00) | <0.001 |
Note: Bold font indicates statistical significance.
Abbreviations: BMI, body mass index; FEF25%, forced expiratory flow at 25% of forced vital capacity; FEF50%, forced expiratory flow at 50% of forced vital capacity; FEF75%, forced expiratory flow at 75% of forced vital capacity; FEF25%–75%, Forced expiratory flow between 25% and 75%; FeNO, fractional exhaled nitric oxide; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s; PEF, peak expiratory flow.
median (IQR) values.
mean ± SD values.
Prior to stratification according to age, the AUCs for FEF50%, FEF25%–75%, and FeNO were 0.737 (95% CI: 0.684–0.785), 0.738 (95% CI: 0.686–0.786), and 0.761 (95% CI: 0.710–0.807) respectively. The AUC for FEF50% combined with FeNO was 0.842 (95% CI: 0.797–0.881), which was significantly higher than that for FEF50% (p < 0.0001) or FeNO alone (p = 0.0003). The AUC for FEF25%–75% combined with FeNO was 0.840 (95% CI: 0.795–0.879), which was significantly higher than that for either FEF25%–75% (p < 0.0001) or FeNO used alone (p = 0.0006; Tables 5 and 6, Figure 2A).
TABLE 5.
Predictive values for predicting positive MCT in the validation cohort
| Characteristic Variables | AUC | Cut off values | Sensitivity% | Specificity% | PPV% | NPV% | Accuracy% | +LR | −LR | Variable coef. | Constant coef. | p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All (n = 312) | ||||||||||||
| FEF50%, %predicted | 0.737 | 75.8 | 68.33 | 69.27 | 58.2 | 77.8 | 68.91 | 2.22 | 0.46 | −0.041 | 2.878 | <0.001 |
| FEF25%–75%, %predicted | 0.738 | 74.0 | 65.83 | 72.40 | 59.8 | 77.2 | 69.87 | 2.38 | 0.47 | −0.043 | 2.971 | <0.001 |
| FeNO | 0.761 | 40.0 | 71.67 | 77.60 | 66.7 | 81.4 | 75.32 | 3.20 | 0.37 | 0.028 | −1.795 | <0.001 |
| ≥55 years (n = 144) | ||||||||||||
| FEF50%, %predicted | 0.775 | 67.7 | 80.70 | 66.67 | 61.3 | 84.1 | 72.22 | 2.42 | 0.29 | −0.056 | 3.657 | <0.001 |
| FEF25%–75%, %predicted | 0.760 | 66.7 | 80.70 | 65.52 | 60.5 | 83.8 | 71.53 | 2.34 | 0.29 | −0.054 | 3.517 | <0.001 |
| FeNO | 0.752 | 47.0 | 70.18 | 77.01 | 66.7 | 79.8 | 74.31 | 3.05 | 0.39 | 0.028 | −1.654 | <0.001 |
| <55 years (n = 168) | ||||||||||||
| FEF50%, %predicted | 0.727 | 75.8 | 55.56 | 80.95 | 63.6 | 75.2 | 71.43 | 2.92 | 0.55 | −0.041 | 3.080 | <0.001 |
| FEF25%–75%, %predicted | 0.728 | 75.4 | 55.56 | 80.00 | 62.5 | 75.0 | 70.83 | 2.78 | 0.56 | −0.042 | 3.075 | <0.001 |
| FeNO | 0.768 | 40.0 | 73.02 | 80.00 | 68.7 | 83.2 | 77.38 | 3.65 | 0.34 | 0.028 | −1.958 | <0.001 |
Note: The cutoff values were selected by the Youden Index.
Abbreviations: AUC, area under the curve; constant coef., constant coefficient of logistic regression; FEF50%, forced expiratory flow at 50% of forced vital capacity; FEF25%–75%, Forced expiratory flow between 25% and 75%; FeNO, fractional exhaled nitric oxide; +LR, positive likelihood ratios; −LR, negative likelihood ratios; MCT, methacholine challenge test; NPV, negative predictive values; p, the t test of the characteristic variables coefficient for the p value; PPV, positive predictive values; variable coef., characteristic variables coefficient of logistic regression.
TABLE 6.
Predictive values of small airway function parameters (FEF50%, FEF25%–75%) combined with FeNO in predicting positive MCT in the validation cohort
| Characteristic variables | AUC | 95% CI | Sensitivity % | Specificity% | PPV % | NPV % | Accuracy% | +LR | −LR | p a | Variable coef.(p) | FeNO coef.(p) | Constant coef. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All (n = 312) | |||||||||||||
| FEF50% + FeNO | 0.842 | 0.797–0.881 | 64.17 | 90.62 | 81.1 | 80.2 | 80.45 | 6.84 | 0.40 | <0.001 | −0.046 (<0.001) | 0.029 (<0.001) | 1.879 |
| FEF25%–75% + FeNO | 0.840 | 0.795–0.879 | 68.33 | 85.94 | 75.2 | 81.3 | 79.17 | 4.86 | 0.37 | 0.001 | −0.046 (<0.001) | 0.029 (<0.001) | 1.822 |
| ≥55 years (n = 144) | |||||||||||||
| FEF50% + FeNO | 0.851 | 0.782–0.905 | 73.68 | 89.66 | 82.4 | 83.9 | 83.33 | 7.12 | 0.29 | 0.021 | −0.068 (<0.001) | 0.033 (<0.001) | 3.145 |
| FEF25%–75% + FeNO | 0.842 | 0.772–0.898 | 75.44 | 87.36 | 79.6 | 84.4 | 81.94 | 5.97 | 0.28 | 0.017 | −0.059 (<0.001) | 0.030 (<0.001) | 2.618 |
| <55 years (n = 168) | |||||||||||||
| FEF50% + FeNO | 0.842 | 0.777–0.893 | 84.13 | 71.43 | 63.9 | 88.2 | 76.19 | 2.94 | 0.22 | 0.011 | −0.039 (<0.001) | 0.027 (<0.001) | 1.525 (<0.001) |
| FEF25%–75% + FeNO | 0.842 | 0.778–0.894 | 76.19 | 78.10 | 67.6 | 84.5 | 77.38 | 3.48 | 0.30 | 0.015 | −0.040 (<0.001) | 0.027 (<0.001) | 1.490 |
Note: The cutoff values were selected by the Youden Index. Bold font indicates statistical significance.
Abbreviations: AUC, area under the curve; constant coef., constant coefficient of logistic regression; FEF50%, forced expiratory flow at 50% of forced vital capacity; FEF25%–75%, Forced expiratory flow between 25% and 75%; FeNO, fractional exhaled nitric oxide; FeNO coef. (p), FeNO coefficient of logistic regression and it's t test for the p value; +LR, positive likelihood ratios; −LR, negative likelihood ratios; MCT, methacholine challenge test; NPV, negative predictive values; PPV, positive predictive values; variable coef. (p), another characteristic variables coefficient of logistic regression and it's t test for the p value.
Compared with FEF50% or FEF25%–75% alone.
FIGURE 2.
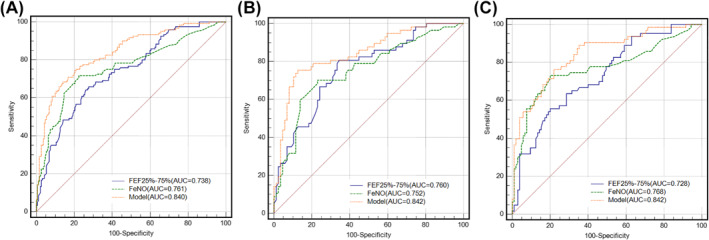
ROC curves for the model of FEF25%–75% combined with FeNO in predicting positive bronchial provocation in the patients of validation cohort prior to being stratified by age (A), patients aged ≥ 55 years (B) and patients aged < 55 years (C). (A) n = 312, AUCmodel = 0.840 (95% CI, 0.795–0.879); AUCFEF25%–75% = 0.738 (95% CI, 0.686–0.786; p < 0.0001, compared with the model); AUCFeNO = 0.761 (95% CI, 0.710–0.807; p = 0.5812 and 0.0006, compared with FEF 25%–75% alone and the model, respectively). (B) n = 144, AUCmodel = 0.842 (95% CI, 0.772–0.898); AUCFEF25%–75% = 0.760 (95% CI, 0.682–0.827; p = 0.0084, compared with the model); AUCFeNO = 0.752 (95% CI, 0.674–0.821; p = 0.9018 and 0.0171, compared with FEF25%–75% alone and the model, respectively). (C) n = 168, AUCmodel = 0.842 (95% CI, 0.778–0.894); AUCFEF25%–75% = 0.728 (95% CI, 0.655–0.794; p = 0.0007, compared with the model); AUCFeNO = 0.768 (95% CI, 0.697–0.830; p = 0.4900, and 0.0149, compared with FEF25%–75% alone and the model, respectively)
In patients aged ≥55 years, the AUCs for FEF50%, FEF25%–75%, and FeNO were 0.775 (95% CI: 0.689–0.840), 0.760 (95% CI: 0.682–0.827), and 0.752 (95% CI: 0.674–0.830), respectively. The AUC for FEF50% combined with FeNO was 0.851 (95% CI: 0.782–0.905), which was significantly higher than each of them used individually (both p < 0.05). The AUC for FEF25%–75% combined with FeNO was 0.842 (95% CI: 0.772–0.898), which was significantly higher than that for either FEF25%–75% (p = 0.0084) or FeNO (p = 0.0171) used alone (Tables 5 and 6, Figure 2B).
In patients aged <55 years, the AUCs for FEF50%, FEF25%–75%, and FeNO were 0.727 (95% CI: 0.653–0.793), 0.728 (95% CI: 0.655–0.794), and 0.768 (95% CI: 0.697–0.830), respectively. The AUC for FEF50% combined with FeNO was 0.842 (95% CI: 0.777–0.893), which was significantly higher than that for FEF50% (p = 0.0007) or FeNO (p = 0.01137) used alone. The AUC for FEF25%–75% combined with FeNO was 0.842 (95% CI: 0.778–0.894), which was higher than each of them used singly (p = 0.0007 for FEF25%–75% and p = 0.0149 for FeNO; Tables 5 and 6, Figure 2C).
Before stratifying according to age, the cut‐off values for FEF50%, FEF25%–75%, and FeNO were 75.8%, 74.0%, and 40 ppb, respectively. In patients aged ≥55 years, the cut‐off values for FEF50%, FEF25%–75%, and FeNO were 67.7%, 66.7%, and 47 ppb, respectively. In patients aged <55 years, the cut‐off values for FEF50%, FEF25%–75%, and FeNO were 75.8%, 75.4%, and 40 ppb, respectively (Tables 5 and 6).
4. DISCUSSION
This study aimed to determine the age effect on the predictive utility of SAFPs, alone or combined with FeNO, for a positive MCT in patients in two age groups (54 years and under, 55 years and over) with asthma‐suggestive symptoms and FEV1 ≥ 80% predicted. The main findings of this study are as follows: compared with the corresponding values in the negative MCT group, in the positive MCT group, the FEF50% and FEF25%–75% values were significantly lower while the FeNO value was higher; compared with participants aged ≥55 years, patients aged <55 years had a higher optimal cutoff value of SAFPs (FEF25%–75% and FEF50%), a lower optimal cutoff value of FeNO and a higher AUCs for the combination of SAFPs and FeNO (>0.86); and the predictive value of SAFPs combined with FeNO in both age groups for a positive MCT diagnosis was significantly improved in patients with asthma‐suggestive symptoms and FEV1 ≥ 80% predicted.
In patients with asthma‐suggestive symptoms and normal or near‐normal pulmonary functions values, MCT is appropriate for confirming or excluding an asthma diagnosis. 6 However, MCT is expensive, time‐consuming, and inconvenient. Therefore, there is a need for cheaper, safer, and simpler tests for predicting a positive MCT, especially in hospitals lacking access to MCT. Previous studies have reported that SAFPs combined with FeNO can predict AHR presence in patients with cough‐variant asthma, 11 as well as patients with asthma‐suggestive symptoms and a normal FEV1 (Min Zhang et al., unpublished paper). This combination was confirmed to be easier, safer, cheaper, and time‐saving than MCT. However, these studies did not address the age effect on the predictive value of these parameters.
In our study, the AUCs of FEF50% and FEF25%–75% for predicting AHR in the discovery cohort did not significantly differ in both age groups (Table 2). This is consistent with previous findings that these two SAFPs had similar predictive values and good correlation for predicting a positive MCT for asthma diagnosis. 11 , 21
The current study showed that FEF25%–75% and FEF50% values in patients with a positive MCT were <80% predicted were much lower than those in patients with a negative MCT independent of age stratification. This indicated that SAD was present in early stage asthma. The FEF50% and FEF25%–75% values were lower in patients aged ≥55 years with a positive MCT than in those aged <55 years (all p < 0.01, data not shown). This is consistent with previous results that normal aging contributed to SAD in different subgroups of patients with asthma 9 , 22 , 23 ; moreover, it indicates that SAD might be more severe and common in older patients than in younger patients. Consistent with this finding, in our study, the cut‐off value of FEF25%–75% and FEF50% for a positive MCT prediction for asthma diagnosis in patients aged ≥55 years (65.0% for FEF25%–75% and 62.9% for FEF50%) was much lower than that in patients aged <55 years (74.2% for FEF25%–75% and 74.9% for FEF50%), which is consistent with previous findings that the optimal cut‐off value of FEF25%–75% was lower in Chinese adults with CVA (78.5%) 11 than in Chinese children with CVA (80.5%). 24 Moreover, the cut‐off values of FEF25%–75% and FEF50% in older patients with a positive MCT (65.0% for FEF25%–75% and 62.9% for FEF50%) decreased significantly compared with those in patients not stratified by age (75.2% for FEF25%–75% and 73.7% for FEF50%). This is indicative of an overdiagnosis in older patients with asthma if not stratified by age when using FEF25%–75% and FEF50% to predict a positive MCT in patients with suspected asthma, which supports the previous finding that age‐specific reference values should be considered for asthma diagnosis. 18 Compared with patients aged <55 years with a positive MCT, patients aged ≥55 years had lower FVC, FEV1, and FEV1/FVC, although it was within the normal range (all p < 0.05). There were no significant between‐age‐group differences in the FEF25% and PEF (all p > 0.05) in patients with a positive MCT. Furthermore, we found that the predictive value of FEF25%–75% was lower in elder patients (Table 2), which was consistent with previous findings (0.800 for children and 0.702 for adults with CVA, respectively). 11 , 24 Notably, regardless of age stratification, the FEF25%–75% and FEF50% alone could not predict AHR in patients with suspected asthma since the AUC was <0.8. Interestingly, in our study, the predictive value of SAFPs (FEF25%–75% and FEF50%) combined with FeNO for AHR diagnosis was significantly improved in both age groups.
FeNO is widely used as a noninvasive biomarker for monitoring airway eosinophilic inflammation and predicting corticosteroid sensitivity in allergic diseases such as asthma. 25 Specifically, FeNO is currently helpful for ruling out asthma. With 43 ppb as the optimal cut‐off value, Bao et al. 11 reported that the sensitivity, specificity, PPV, and NPV were 71.59%, 82.02%, 66.30%, and 85.40% respectively, in clinical AHR prediction for Chinese patients with CVA, which indicated that FeNO was valuable as a negative predictive parameter for discriminating patients with AHR. Schleich et al. 26 reported that for patients with suspected asthma, FeNO >34 ppb had a relatively low predictive value (AUC = 0.62) for AHR diagnosis. Before age stratification, we found that FeNO >43 ppb has a sensitivity, specificity, PPV, and NPV of 61.23%, 84.11%, 80.50%, and 67.0%, respectively, for predicting a positive MCT in patients with suspected asthma. The FeNO value has been reported to be affected by age. 13 , 14 Specifically, FeNO >25.5 ppb had a high predictive value (AUC = 0.905) with a sensitivity and specificity of 82.2% and 90.0%, respectively, for CVA diagnosis in Chinese children with an average age of 8 years, 24 which differed from that in the aforementioned Chinese adults with CVA. 11 Consistent with this trend, our study showed that the FeNO value was higher in patients with positive AHR aged <55 years than in those aged ≥55 years (p < 0.05), which indicated that airway eosinophilic inflammation might be more severe in younger patients with asthma who may be more sensitive to corticosteroids. This is consistent with previous findings that older patients with asthma had lower FeNO level, which indirectly indicates predominant neutrophilic bronchial inflammation in elderly patients that contributes to greater airflow limitation. 17 However, our findings are inconsistent with a previous report that FeNO level increased with aging. 27 However, this previous study was conducted in a healthy population without a smoking history. 27 Compared with aging, bronchial inflammation might be more crucially involved in affecting the FeNO level in the present study. Before age stratification, the cut‐off value of FeNO for predicting AHR was 43 ppb; however, for patients aged ≥55 years, this value was 48 ppb, which indicated an overdiagnosis in older participants. In patients aged ≥55 years, FeNO >48 ppb had a high specificity (88.70%), but a relative low sensitivity (50.78%), for identifying patients with asthma, which indicated that FeNO is limited as a sole diagnostic test for these patients. In patients aged <55 years, FeNO >43 ppb had a relative low sensitivity 68.92% and NPV 72.60%, but a higher specificity 85.31% and PPV 82.90%, for predicting AHR in patients with typical asthma‐like symptoms and an asthma‐suggestive history.
The AUC for FEF50% (FEF25%–75%) combined with FeNO was 0.858 (0.865), 0.865 (0.883), and 0.851 (0.844) in patients without age stratification, patients aged <55 years, and patients aged ≥55 years, respectively, which was significantly higher than that for either FEF50% (FEF25%–75%) or FeNO used alone. These findings suggest that FEF50% (FEF25%–75%) combined with FeNO could improve the predictive value for AHR diagnosis, which is consistent with a previous study on patients with CVA. 11 In patients aged ≥55 years, compared with the corresponding values calculated from SAFPs used alone, SAFPs combined with FeNO had a higher specificity (80.47% for FEF50%, 85.94% for FEF25%–75%) and negative predictive value (77.70% for FEF50%, 82.00% for FEF25%–75%); in patients aged <55 years, the corresponding values of specificity (83.22% for FEF50%, 81.12% for FEF25%–75%) and positive predictive value (83.00% for FEF50%, 81.60% for FEF25%–75%) also improved when SAFPs were combined with FeNO. This indicated that the combined use of optimal cut‐off values of SAFPs and FeNO contributed to improved prediction for a positive MCT detection in patients of both age groups with suspected asthma with FEV1 ≥80% predicted.
Finally, we validate the findings from the discovery cohort using a validation cohort. This indicates that our findings could be generalized in patients with asthma in other care centers in China.
This study has several limitations. First, SAD included airway wall thickening, airway narrowing, and air trapping, and so forth. 28 However, we did not further confirm these changes using computed tomography or tissues. Further studies should elucidate the associations among changes of pulmonary function and imaging and histological structures of peripheral airways. Second, FeNO was specifically correlated with airway eosinophilic, but not neutrophilic, inflammation. In previous studies, the diagnostic accuracy of FeNO increased upon exclusion of patients with neutrophilic inflammation. 29 , 30 There is a need for sputum eosinophil and neutrophil measurements to confirm asthma phenotypes. Third, comorbid diseases, including rhinitis, gastroesophageal reflux disease, and so forth, may affect result interpretation, which should have been considered in this study. However, given the retrospective and cross‐sectional design of this study, there was limited information regarding these comorbid diseases. Furthermore, the predictive value of SAFPs, alone or combined with FeNO, for the presence of a positive MCT was only assessed in two age groups (≤54 years and ≥55 years). However, asthma can occur at any age 31 , 32 and exhibits a similar rate among adults. 33 Moreover, small airway function has been shown to decline with aging 22 , 23 , 34 and contribute to asthma emergence and exacerbation. 23 , 35 Unfortunately, there is limited information regarding the starting age of decline for small airway function in Chinese healthy individuals and patients with asthma. Therefore, the trend of the value for predicting a positive MCT in these patients should be evaluated in more different age groups. Unfortunately, some groups stratified at 10‐year intervals in this study had few patients with asthma; therefore, future studies should recruit a larger study group to better represent the population.
5. CONCLUSIONS
Patients with asthma with SAD, which was affected by aging, were more prone to have a positive MCT. Therefore, Chinese patients with FEV1 ≥ 80% and an asthma‐suggestive history should be stratified by age (55 years) when SAFPs (FEF50%, FEF25%–75%), alone or combined with FeNO, are used to predict the presence of a positive MCT in asthma diagnosis.
CONFLICT OF INTERESTS
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
Lili Hou, Lei Zhu, and Min Zhang were responsible for conceptualization, design of the study, and drafting the manuscript. Min Zhang and Lei Zhu was responsible for funding acquisition. Lili Hou, Gang Huang, Huahao Shen, and Li Yu were responsible for data collection and interpretation. Lili Hou, Huijuan Hao, and Huahao Shen were responsible for data analysis and interpretation. All authors reviewed the article critically for important intellectual content and approved the final version to be submitted.
ACKNOWLEDGMENTS
This study is conducted by the Shanghai General Hospital affiliated to Shanghai Jiao Tong University in close collaboration with Second Affiliated Hospital of Zhejiang University School of Medicine and Tongji Hospital Affiliated to Tongji University School of Medicine. The authors would like to acknowledge the contribution of hospitals, technicians, and patients in every research center. This study is made possible by financial support from Shanghai Municipal Health System, Science and Technology Commission of Shanghai municipality, and Shanghai Jiao Tong University. The study was financially supported by Appropriate technique application Program of Shanghai Municipal Health System (Grant no. 2019SY042), Scientific and Technological Innovation program funded by Science and Technology Commission of Shanghai municipality (Grant no. 20Y11902400), and Shanghai Jiao Tong University scientific research fund for COVID‐19 prevention and control (Grant no. YG2020YQ22) to Min Zhang, and Science and Technology Support Project of Medical Guidance in Shanghai to Lei Zhu (19411969700).
Contributor Information
Lei Zhu, Email: zhuleizhongshan@163.com.
Huahao Shen, Email: huahaoshen@163.com.
Min Zhang, Email: smzhlcf@163.com.
References
- 1. Brigham EP, West NE. Diagnosis of asthma: diagnostic testing. Int Forum Allergy Rhinol. 2015;5(suppl 1):S27‐S30. [DOI] [PubMed] [Google Scholar]
- 2. Popa V. ATS guidelines for methacholine and exercise challenge testing. Am J Respir Crit Care Med. 2001;163(1):292‐293. [DOI] [PubMed] [Google Scholar]
- 3. Louis R, Bougard N, Guissard F, Paulus V, Henket M, Schleich F. Bronchodilation test with inhaled salbutamol versus bronchial methacholine challenge to make an asthma diagnosis: do they provide the same information? J Allergy Clin Immunol Pract. 2020;8(2):618‐625. [DOI] [PubMed] [Google Scholar]
- 4. Bougard N, Nekoee H, Schleich F, et al Assessment of diagnostic accuracy of lung function indices and FeNO for a positive methacholine challenge. Biochem Pharmacol. 2020:179:113981. [DOI] [PubMed] [Google Scholar]
- 5. Nair P, Martin JG, Cockcroft DC, et al. Airway hyperresponsiveness in asthma: measurement and clinical relevance. J Allergy Clin Immunol Pract. 2017;5(3):649‐659. [DOI] [PubMed] [Google Scholar]
- 6. Coates AL, Wanger J, Cockcroft DW, et al. ERS technical standard on bronchial challenge testing: general considerations and performance of methacholine challenge tests. Eur Respir J. 2017;49(5): 1601526 [DOI] [PubMed] [Google Scholar]
- 7. Hogg JC, Macklem PT, Thurlbeck WM. Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med. 1968;278(25):1355‐1360. [DOI] [PubMed] [Google Scholar]
- 8. Hogg JC, Hackett TL. Structure and function relationships in diseases of the small airways. Ann Am Thorac Soc. 2018;15(suppl 1):S18‐S25. [DOI] [PubMed] [Google Scholar]
- 9. Cottini M, Licini A, Lombardi C, Berti A. Clinical characterization and predictors of IOS‐defined small‐airway dysfunction in asthma. J Allergy Clin Immunol Pract. 2020;8(3):997‐1004. [DOI] [PubMed] [Google Scholar]
- 10. Cosio M, Ghezzo H, Hogg JC, et al. The relations between structural changes in small airways and pulmonary‐function tests. N Engl J Med. 1978;298(23):1277‐1281. [DOI] [PubMed] [Google Scholar]
- 11. Bao W, Zhang X, Lv C, et al. The value of fractional exhaled nitric oxide and forced mid‐expiratory flow as predictive markers of bronchial hyperresponsiveness in adults with chronic cough. J Allergy Clin Immunol Pract. 2018;6(4):1313‐1320. [DOI] [PubMed] [Google Scholar]
- 12. Jatakanon A, Lim S, Kharitonov SA, Chung KF, Barnes PJ. Correlation between exhaled nitric oxide, sputum eosinophils, and methacholine responsiveness in patients with mild asthma. Thorax. 1998;53(2):91‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Olin AC, Rosengren A, Thelle DS, Lissner L, Bake B, Toren K. Height, age, and atopy are associated with fraction of exhaled nitric oxide in a large adult general population sample. Chest. 2006;130(5):1319‐1325. [DOI] [PubMed] [Google Scholar]
- 14. Kovesi T, Kulka R, Dales R. Exhaled nitric oxide concentration is affected by age, height, and race in healthy 9‐ to 12‐year‐old children. Chest. 2008;133(1):169‐175. [DOI] [PubMed] [Google Scholar]
- 15. Niewoehner DE, Kleinerman J, Liotta L. Elastic behavior of postmortem human lungs: effects of aging and mild emphysema. J Appl Physiol. 1975;39(6):943‐949. [DOI] [PubMed] [Google Scholar]
- 16. van der Wiel E, ten Hacken NH, Postma DS, van den Berge M. Small‐airways dysfunction associates with respiratory symptoms and clinical features of asthma: a systematic review. J Allergy Clin Immunol. 2013;131(3):646‐657. [DOI] [PubMed] [Google Scholar]
- 17. Ciprandi G, Schiavetti I, Ricciardolo FLM. The impact of aging on outpatients with asthma in a real‐world setting. Respir Med. 2018;136:58‐64. [DOI] [PubMed] [Google Scholar]
- 18. Kavanagh J, Jackson DJ, Kent BD. Over‐ and under‐diagnosis in asthma. Breathe. 2019;15(1):e20 e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602‐615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miller A, Enright PL. PFT interpretive strategies: American Thoracic Society/European Respiratory Society 2005 guideline gaps. Respir Care. 2012;57(1):127‐133. [DOI] [PubMed] [Google Scholar]
- 21. Yuan H, Liu X, Li L, et al. Clinical and pulmonary function changes in cough variant asthma with small airway disease. Allergy Asthma Clin Immunol. 2019;15:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Skloot GS. The effects of aging on lung structure and function. Clin Geriatr Med. 2017;33(4):447‐457. [DOI] [PubMed] [Google Scholar]
- 23. Martinez CH, Diaz AA, Meldrum C, et al. Age and small airway imaging abnormalities in subjects with and without airflow obstruction in SPIROMICS. Am J Respir Crit Care Med. 2017;195(4):464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhu H, Zhang R, Hao C, Yu X, Tian Z, Yuan Y. Fractional exhaled nitric oxide (FeNO) combined with pulmonary function parameters shows increased sensitivity and specificity for the diagnosis of cough variant asthma in children. Med Sci Monit. 2019;25:3832‐3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Price D, Ryan D, Burden A, et al. Using fractional exhaled nitric oxide (FeNO) to diagnose steroid‐responsive disease and guide asthma management in routine care. Clin Transl Allergy. 2013;3(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schleich FN, Asandei R, Manise M, Sele J, Seidel L, Louis R. Is FENO50 useful diagnostic tool in suspected asthma? Int J Clin Pract. 2012;66(2):158‐165. [DOI] [PubMed] [Google Scholar]
- 27. Gelb AF, George SC, Camacho F, Fraser C, Flynn Taylor C, Shakkottai S. Increased nitric oxide concentrations in the small airway of older normal subjects. Chest. 2011;139(2):368‐375. [DOI] [PubMed] [Google Scholar]
- 28. Inoue H, Niimi A, Takeda T, et al. Pathophysiological characteristics of asthma in the elderly: a comprehensive study. Ann Allergy Asthma Immunol. 2014;113(5):527‐533. [DOI] [PubMed] [Google Scholar]
- 29. Schneider A, Schwarzbach J, Faderl B, Welker L, Karsch‐Volk M, Jorres RA. FENO measurement and sputum analysis for diagnosing asthma in clinical practice. Respir Med. 2013;107(2):209‐216. [DOI] [PubMed] [Google Scholar]
- 30. Skloot GS, Busse PJ, Braman SS, et al. An official American Thoracic Society Workshop report: evaluation and management of asthma in the elderly. Ann Am Thorac Soc. 2016;13(11):2064‐2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hedman L, Bjerg A, Lundback B, Ronmark E. Conventional epidemiology underestimates the incidence of asthma and wheeze‐a longitudinal population‐based study among teenagers. Clin Transl Allergy. 2012;2(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dunn RM, Busse PJ, Wechsler ME. Asthma in the elderly and late‐onset adult asthma. Allergy. 2018;73(2):284‐294. [DOI] [PubMed] [Google Scholar]
- 33. Reed CE. Asthma in the elderly: diagnosis and management. J Allergy Clin Immunol. 2010;126(4):681‐687. [DOI] [PubMed] [Google Scholar]
- 34. Boudewijn IM, Postma DS, Telenga ED, et al. Effects of ageing and smoking on pulmonary computed tomography scans using parametric response mapping. Eur Respir J. 2015;46(4):1193‐1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nagasaki T, Matsumoto H. Influences of smoking and aging on allergic airway inflammation in asthma. Allergol Int. 2013;62(2):171‐179. [DOI] [PubMed] [Google Scholar]


