Abstract
The biological mechanisms driving disability worsening in multiple sclerosis (MS) are only partly understood. Monitoring changes in lesion load on MRI has a limited predictive value on the progression of clinical disability, and there is an essential need for novel imaging markers specific for the main candidate mechanisms underlying neurodegeneration which include failing myelin repair, innate immune cell activation and gray matter neuronal damage. Positron Emission Tomography (PET) is an imaging technology based on the injection of radiotracers directed against specific molecular targets, which has recently allowed the selective quantification in‐vivo of the key biological mechanisms relevant to MS pathophysiology. Pilot PET studies performed in patients with all forms of MS allowed to revisit the contribution of MS lesions to disability worsening and showed that the evolution of lesions toward chronic activation, together with their remyelination profile were relevant predictors of disability worsening. PET offers the opportunity to bridge a critical gap between neuropathology and in‐vivo imaging. This technique provides an original approach to disentangle some of the most relevant pathological components driving MS progression, to follow‐up their temporal evolution, to investigate their clinical relevance and to evaluate novel therapeutics aimed to prevent disease progression.
Keywords: multiple sclerosis, clinical progression, disability worsening, positron emission tomography, remyelination, innate immune cell, microglia, white matter lesions
During the past decades, an extensive amount of research has been devoted to understand how macroscopic multiple sclerosis (MS) lesions visualized on MRI could drive neurological disability over the course of the disease 77. This has led to the general view that while an increase in brain white matter (WM) lesion number and/or load was strongly predictive of subsequent relapses, disability worsening, at least to some extent, developed independently of the change in WM lesion load, especially following the first years of disease 12, 32, 52. While the debate remains active, the disease is commonly considered as a dual entity, with an adaptative immune‐driven inflammatory component linked to WM lesions and relapses, and a diffuse neurodegenerative component responsible for longer term disability accrual. However, the interpretation of correlative studies between MRI‐visible lesions and disability course should be cautious because of potential limitations: first, the contribution of spinal cord and/or cortical lesions together with WM lesions is often unaccounted for, a point that is beyond the scope of this review; second, WM lesions are generally identified through T1 or T2‐weighted sequences, which are both very sensitive to WM damage, but not specific for the underlying biological processes; third, these studies usually take into account only the increase in either the number or volume of lesions over time, while a decrease in lesion size could also reflect a deleterious event 76. Overall, lesion heterogeneity, which has been well described in pathological investigations, has not been fully explored in previous longitudinal clinical studies. Indeed, postmortem studies have shown that individual patients are characterized by extremely variable regenerative capacities, with only a minority of subjects being able to extensively remyelinate WM lesions 63, and a small proportion of lesions being classified as shadow plaques 34. The clinical correlates of these observations are challenging to derive from post mortem data, but it has been suggested that an efficient remyelination was linked to older age at death 63. Several distinct inflammatory profiles of chronic lesions have also been described, with a proportion of WM lesions evolving toward chronic activation and/or a smoldering state, and others becoming inactive. These profiles have recently been shown to have an impact on disease severity 34, 53.
To investigate the role of this lesion heterogeneity in contributing to axonal damage and neuronal retrograde degeneration, and in turn to disability, it is essential to use imaging modalities specific for the underlying mechanisms in longitudinal studies. Several advanced MRI tools have been developed over recent years with the objective to quantify the different processes implicated in the pathophysiology of MS. However, these techniques, while very sensitive to microstructural changes, are not characterized by the pathological specificity required to quantify myelin repair or neuroinflammation, as they reflect changes in the physical characteristics of brain tissues, rather than specific pathological processes. Positron emission tomography (PET) is a nuclear medicine imaging technology that measures the distribution of specific ligands labeled with positron emitters in vivo: as ligands are specific for the targets of interest, this imaging technology provides the highest possible specificity at the cellular and/or tissular level to investigate brain pathological changes in neurological diseases. PET has a lower spatial resolution than MRI and is more challenging to be implemented as it involves the use of radiation and requires multidisciplinary teams, but it allows an absolute quantification of tracer binding that directly reflects the concentration of the biological target in the tissue of interest, with excellent sensitivity to changes. Therefore, PET opens the unique perspective to directly and specifically quantify the mechanisms underlying MS in vivo, and to explore the role of lesion heterogeneity in contributing to neurodegeneration and clinical disability over the course of the disease.
IMAGING MYELIN DYNAMICS BY PET
Selection of myelin radiotracers
Up to now, the search of myelin radiotracers has been mainly focused on the development of small radiolabeled compounds that could selectively bind to myelin, aiming to quantify myelin content changes in WM regions of interest. We first identified a stilbene derivative, the 1,4‐bis(p‐aminostyryl)‐2‐methoxy benzene), also named BMB, and discovered that it selectively binds to myelin ex‐vivo and in‐vivo 80. Following this first evidence, several compounds belonging to the stilbene chemical class were shown to have similar affinity properties for myelin such as BDB, CIC, GE3111, 20, 39, 93, 94, or more recently C‐11‐labeled N‐methyl‐4,4′‐diaminostilbene ([11C]MeDAS), which has been demonstrated to have optimal biodistribution and pharmacokinetic properties 94, 95.
An intriguing question regarding the specificity of stilbene derivatives toward myelin relates to the characterization of the molecular target responsible for the binding of these compounds. Originally developed as amyloid markers, they are thought to bind to proteins or aggregates displaying a particular molecular conformation with adjacent beta‐sheet structures 40, 46. Interestingly, this conformation is also found in some myelin‐specific proteins such as myelin basic protein (MBP) 68. While this target has not been fully characterized yet, a large body of evidence supports a selective binding to myelin of these compounds: the binding to myelin remained preserved after lipids removal, and this evidence excludes a non‐specific hydrophobic binding to the lipids contained in myelin; the level of binding correlated with myelin content in several dysmyelinating mutants such as the shiverer or the quacking, and could differentiate ex‐vivo normal WM, MS demyelinated lesions and shadow plaques on postmortem MS samples 80; a direct chemical interaction between MBP protein and several stilbene derivative has been demonstrated 5; finally, in longitudinal microPET in vivo experimental studies performed in rodents with chemically induced demyelination, [11C]MEDAS was shown to have the potential to quantify myelin loss and repair 24, 95.
Following the same hypothesis of a common molecular target between amyloid plaques and CNS myelin, other amyloid markers related to the benzothiazole chemical class were investigated for their potential as myelin markers. The thioflavin T derivative 2‐(4′‐methylaminophenyl)‐6‐hydroxybenzothiazole (Pittsburg Compound B, PIB) was shown to stain myelin ex‐vivo in rodent and human postmortem brain samples, with a drastic decrease in the WM of shiverer mice79. In rodent models79, [11C]PiB microPET could capture remyelination 25, and in non‐human primates [11C]PiB allowed to generate promising PET images of myelinated WM areas 79, therefore appearing as a natural candidate for a proof‐of‐concept clinical study in patients with MS.
Pilot PET human studies: lesion repair has a strong impact on clinical disability
A major prerequisite for the clinical application of myelin PET has been the development of accurate non‐invasive quantification methods of WM binding that do not require arterial sampling. A semi‐quantitative approach using standardized uptake value ratios (SUVR) has been applied to a cohort of late MS patients, showing a decrease in SUVR in WM lesions that correlated with visuo‐spatial performances 96. A supervised clustering algorithm has been implemented to extract reference regions from the gray matter, which were used to estimate [11C]PIB binding using the LOGAN reference region approach. This approach allowed to generate voxel‐wise maps of [11C]PIB distribution volume ratio (DVR), reflecting myelin density. Test–retest analyses in healthy controls have shown a more accurate reproducibility of DVR compared to SUV 86. Further supporting the [11C]PIB selective binding to myelin in vivo, [11C]PIB DVR maps of the WM generated from a group of healthy controls showed a remarkable correlation with the mRNA maps of the Allen Brain Atlas for those genes coding for the major proteins composing the structure of human myelin 86.
In a longitudinal pilot study, a group of 20 patients with a radiologically active relapsing‐remitting form of MS (RRMS), along with age‐ and gender‐matched healthy controls underwent a [11C]PiB PET at study entry and after 2‐4 months 10. The cross‐sectional analysis showed a progressive reduction in [11C]PIB binding from normal‐appearing WM (NAWM) to the center of MS lesions, which mirrored the postmortem evidence of a gradient in myelin density from normal‐appearing tissues to the core of WM lesions 23, 60, 75. Interestingly, when we compared the [11C]PIB binding in the NAWM of patients with that in the WM of healthy controls, we did not detect any significant change. This suggests that the subtle pathological changes classically described on advanced MRI sequences in the NAWM of patients with MS, are not characterized by a significant demyelinating component. Over the follow‐up period, negative and positive changes in [11C]PIB binding were observed in lesions, reflecting dynamic demyelination and remyelination (Figure 1), and several dynamic indices of myelin content change were calculated. The first key finding of this study was that [11C]PIB PET allowed to demonstrate a significant between‐patient variability for the index of dynamic remyelination, and to identify “good” and “bad” remyelinators. This result is in line with the notion of a patient‐specific “remyelination profile,” already supported by neuropathological evidence 63. This index of dynamic remyelination generated with PET was able to measure the extent of myelin regeneration of each individual patient in response to a demyelinating insult. The second key finding of this study was the strong correlation between dynamic remyelination and clinical disability scores. This evidence supports the hypothesis that an efficient remyelination process taking place in an appropriate time window after a demyelinating insult may be a critical factor in determining a favorable prognosis in patients with MS. As a further confirmation of this hypothesis, when myelin PET and multimodal MRI were combined, we showed that patients with a higher remyelination potential (“good remyelinators”) were characterized by less axonal damage in WM tracts as reflected by the measure of fractional anisotropy on diffusion weighted images, and by less gray matter damage as reflected by the measure of thalamic volume, when compared with patients with a lower remyelination potential (“bad remyelinators”).
Figure 1.
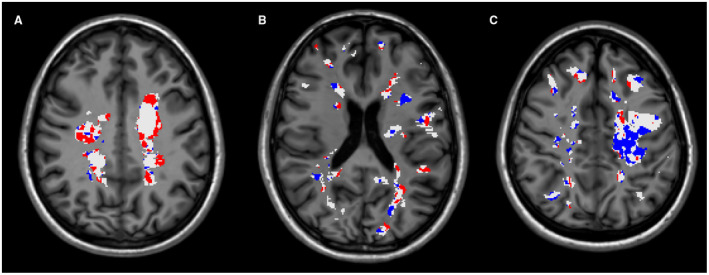
Three single‐patient maps of lesional myelin content changes showing demyelinating (in red) and remyelinating (in blue) voxels derived from longitudinal [11C]PIB PET, localized inside white matter (WM) lesions (in white), overlaid onto the corresponding MPRAGE scans. A. Map of lesional myelin content changes of a 20‐year‐old woman with a disease duration of 3 years, where a clear prevalence of demyelination over remyelination is visible. B. The map of myelin content changes of this 27‐year‐old man with a 10‐year history of MS shows active demyelination together with moderate remyelination in all visible WM lesions. C. An extensive process of remyelination characterizes the map of myelin content changes of this 32‐year‐old woman with a disease duration of 3 years. Of note, the extensive lesion visible in the left‐hemispheric WM corresponds to a recent lesional area, characterized by large gadolinium‐enhancing regions on the corresponding T1 spin‐echo scans.
Limitation of the current approach and perspectives
A wide application of PET to quantify myelin content in MS lesion is limited by several drawbacks. The spatial resolution of PET is usually limited, above 4‐5 mm for most of the systems, 2.5 mm for the high resolution research tomograph, which displays the highest resolution available to date. As demyelination and remyelination in patients are selectively detected within MS plaques, and not in the NAWM, the limited resolution of PET imposes a perfect co‐registration between MRI and PET: the synergistic acquisition of PET and MRI allowed by novel PET‐MRI systems offers the opportunity to minimize the partial volume effect inherent to PET imaging, and to obtain an accurate between‐modality co‐registration. In this respect, PET‐MRI systems may find an ideal application in MS. Moreover, the application of PET to measure myelin content in the cortex is hampered by the low thickness of the cortical ribbon, by the low concentration of myelin in cortical areas compared to WM regions, and by the use of cortical voxels as reference region for myelin quantification. This justifies to develop advanced MRI tools characterized by an acceptable sensitivity and specificity to cortical myelin content changes that could be combined with myelin PET for a comprehensive investigation of demyelination and remyelination in all brain tissues: recent results suggested that this could be achieved by magnetization transfer imaging (MTI) 19, 29. Building up on these results, our group has recently developed a postprocessing approach of MTI images that allowed to generate individual indices of cortical remyelination in patients, which in combination with PET‐derived WM remyelination profiles explained above 80% of neurological disability (unpublished results).
PET‐derived remyelination indices have the potential to provide transformative insights in future studies aiming to reconstruct the natural history of the key biological events underlying neurodegeneration, to stratify patient in cohort studies, or to evaluate candidate remyelinating drugs in early phase trials of promyelinating therapies. The necessary step to allow a wide application of myelin PET is the validation of fluorinated myelin radiotracers, a goal nearly reached by ongoing pilot studies in primates and humans which indicate that fluorinated stilbene derivatives such as [18F]florbetaben or [18F]florbetapir, present a higher signal to noise ratio in the WM compared to [11C]PiB. Another alternative may consist in developing novel chemical classes of myelin‐binding compounds targeting myelin, oligodendrocytes, or their precursors, the oligodendrocyte precursor cells (OPCs). Several compounds have been proposed, either belonging to the coumarine 90 or to the sphingosine‐1‐phosphate receptor ligands 13, 14, but no human application has been performed so far. Moreover, none of these compounds reaches the specificity required for a selective imaging of myelinating cells.
Another innovative approach consists in the development of positive markers of demyelinated axons that could be used for PET imaging. On myelinated axons, voltage‐gated potassium channel (VGKC) are localized within juxtaparanodal regions, and cannot be reached by pharmacological compounds such as 4‐aminopyridine (4‐AP) derivatives, as they are masked by myelin sheets. Following demyelination VGKC channels become accessible, and can be targeted by 4AP radiotracers, generating a positive PET signal. A candidate fluorinated radiotracer for VGKC has been produced and its specificity validated 15. Moreover, pilot animal PET imaging experiments with this radiotracer have been conducted, showing that an increased uptake of the compound was indeed associated with demyelination 15. This method has not yet been translated to humans. The interpretation of the signal derived from this compound is challenging: while it is possible to speculate that demyelinated regions are easily identified with an increased binding of the radiotracer, the interpretation of the progressive reduction of the tracer uptake with time can be tricky, as it may equally result from axonal loss or remyelination.
Finally, the development of molecular imaging probes able to visualize and quantify in vivo the content in oligodendrocyte precursor cells would be a transformative achievement that could open the perspective of personalized remyelinating strategies, promoting either OPCs recruitment or oligodendrocyte differentiation. Molecular targets specific for OPCs have been described, such as platelet derived growth factor alpha (67), but all the attempts to generate radiotracers suitable for PET imaging have not been successful to date.
IMAGING THE INFLAMED BRAIN BY PET
While the adaptative immune system has always been considered as the key player in the formation of MS plaques, only recently the innate immune system has emerged as a major contributor to the earliest step of plaque formation, described as the pre‐phagocytic stage 8, but also to lesion remyelination 31, 58 and disease progression 48. Both monocyte‐derived cells and microglial cells, the brain innate immune cells, are known to acquire different phenotypes depending on environmental stimuli and to have the capability to shift functions to maintain tissue homeostasis 71. They can be polarized into a predominant inflammatory phenotype that expresses pro‐inflammatory cytokines, or into a more regulatory phenotype that is oriented toward tissue protection and repair. The activation of innate immune cells with a pro‐inflammatory phenotype was shown to be prominent in patients with progressive MS, 34, 48, 97, and is considered one of the key mechanisms that could underlie oxidative damage and neurodegeneration in MS 49, 55. In addition, pathological studies have well identified specific lesional stages based on the innate immune system activation, namely chronic active or smoldering lesions that could drive disability worsening 34, 53.
Targeting TSPO to assess innate immune cells by PET: selection of tracers, technical issues
The most popular target to image innate immune cells is the translocator protein (18 kDa) (TSPO) that is part of the permeability transition pore, a macromolecular complex primarily localized in the outer mitochondrial membrane of steroid‐synthesizing cells. While the precise function of this complex remains elusive, it has been shown that in the context of inflammatory conditions such as MS, TSPO is strongly up‐regulated, with an expression mainly driven by innate immune cells activation 7, 85. Of note, an additional contribution also comes from astrocytic activation 51 and endothelial cells 73, limiting the specificity of this target for innate immune cells.
The first compound developed for TSPO imaging has been the [11C]PK11195, whose increased binding has been described in relapsing and progressive patients 38, 69. However, [11C]PK11195 PET imaging is limited by a modest target affinity and a suboptimal signal to noise ratio 84.
In the attempt to overcome [11C]PK11195 limitations, several second‐generation TSPO radioligands belonging to different chemical classes and with improved affinity to TSPO have been developed (17). Only a few of them have been applied in MS to date. Unexpected negative results were initially obtained with [18F]PBR111 and [18F]FEDAA1106, probably explained by a between‐patient heterogeneity in binding affinity that was not accounted for in these first studies. Further investigations clearly showed that the heterogeneity in binding affinity is related to a rs6971 TSPO gene single nucleotide polymorphism 62. At present, patients are stratified according to genetic polymorphism in clinical studies, which allows to neutralize this bias. One second‐generation compound that has recently emerged is the pyrazolopyrimidine [18F]DPA‐714 42. This is a fluorinated tracer that has been successfully employed in animal models of MS 1, 18, and showed an excellent in‐vivo biodistribution 3, as well as a reliable kinetic modeling 50 in human studies. However, additional biases may still influence the quantification process for this family of tracers. First, the affinity to platelets, monocytes and plasma proteins alters the free plasma concentration of the tracers across subjects, with a possible impact on input function calculation, when input function is used for signal modeling 2, 84. This evidence supports the application of quantification methods based on the extraction of reference regions directly from brain images in order to avoid fluctuation in the measure of input function, a strategy that was applied for PBR28 21, 22, 41 and [18F]DPA‐714 36. Second, a blood brain barrier (BBB) signal from endothelial TSPO, in particular for high affinity TSPO tracers may also interfere with the signal quantification from brain tissues 84. Novel models that take into account endothelial binding have been developed 73, but to date they still require a full modelling with input function 72, 92.
TSPO PET imaging in MS: a biomarker of disability worsening?
First human studies applying [11C]PK11195 PET to patients with MS showed an increased uptake of the tracer in some active WM lesions 7, 26, 88. However, the increase in TSPO expression was not limited to active lesions identified by gadolinium enhancement on MRI, but also extended to some chronic lesions and peri‐lesional areas, especially in progressive forms of the disease 69, and to lesions appearing as hypointense “black holes” on T1‐weighted MRI sequences 37, potentially reflecting an additional chronic neuro‐inflammatory component of these lesions. Beyond lesions, a milder uptake increase was also described in normal‐appearing tissues since the clinically isolated syndrome stage, where it was found to be associated with lesion load and could predict the conversion toward MS 38. An increase in [11C]PK11195 binding was also found in the periventricular WM and in the gray matter of secondary progressive patients 66, 69. Based on these results, [11C]PK11195 PET has recently been proposed to evaluate the therapeutic response to second line disease modifying therapies such as fingolimod 81 and natalizumab 45. Interestingly, these drugs drastically decrease relapse rate in relapsing MS but fail to reduce disability worsening in progressive MS. Moreover, these treatments were shown to decrease [11C]PK11195 binding in WM lesions only but not in normal appearing tissues. Taken together, these data suggest that both fingolimod and natalizumab might have an impact on innate immune inflammation which is limited to the acute inflammatory component within lesions.
The development of second‐generation TSPO radiotracers has provided further insight into the understanding of the innate immune cell contribution to the pathophysiology of MS. Using [11C]PBR‐28 and WM as a pseudo‐reference region, a neuroinflammatory component was identified in the cortical gray matter of patients with MS that correlated with cognitive testing 41. Using the same tracer, Datta and collaborators have followed‐up 21 patients with MS (14 relapsing MS, and 7 progressive MS) in a longitudinal study, employing a non‐invasive quantification approach based on the use of striatum as a reference region. The cross‐sectional analysis showed an increased binding both in WM lesions and in the NAWM. Of note, WM lesions were characterized by a great heterogeneity in TSPO expression, with some active lesions being found in all forms of the disease 21. In the longitudinal follow‐up, [11C]PBR‐28 binding at study entry was shown to predict WM lesion volume enlargement over one year in relapsing patients, and brain atrophy in progressive patients, supporting the complex pathogenic role played by innate immune cells over the course of the disease 22. Our group has recently developed a novel processing approach allowing to generate individual maps of active innate immune cells using [18F]DPA‐714 dynamic PET acquired with a high resolution camera. Patient‐specific maps of activated innate immune cells were generated through a voxel‐wise randomized permutation‐based analysis between patients and healthy controls which determined a threshold of significant activation. This threshold was employed to classify each voxel on patients’ PET scans in “active” or “not active,” comparing its value with the mean value of the voxels localized in the same position in the control group. This procedure was applied to a cohort of 36 MS patients, 10 with a relapsing‐remitting form, and 26 with a progressive form of the disease. We found an increased percentage of activated voxels in WM lesions, in peri‐lesional areas, in the NAWM and in the gray matter, with a large individual heterogeneity regarding the extent of activation, in particular within and around WM lesions (Figure 2). Each lesion and each perilesional area was subsequently analyzed separately: different lesion patterns were identified based on the extent of activation within and around lesions (Figure 2) that reflected the histopathologic classification of lesions as active, smoldering and inactive 34, 53. We used conservative thresholds to identify active lesions and found that while only few WM lesions were enhanced by gadolinium on MRI scans, a large proportion of them were classified as chronically active based on [18F]DPA‐714 PET, in relapsing remitting but also in progressive patients. Finally, we analyzed the relationship between [18F]DPA‐714 PET measures and disability worsening assessed with the Expanded Disability Status Scale—EDSS 47 step‐changes over the 2 years preceding the PET examination. Interestingly, the best predictive model of EDSS step‐change was obtained combining the number of active lesions identified on 18F DPA‐PET with the percentage of activated voxels in perilesional areas. Taken together, our results clearly identify the inflammatory profile of chronic WM lesions as a key player in disability worsening in MS, especially during the progressive phases of the disease. To which extent the level of activation of innate immune cells in normal appearing tissues that correlates with lesion activation, is a secondary consequence of chronic active and smoldering lesions is a fascinating question that should now be addressed in vivo in longitudinal PET studies.
Figure 2.
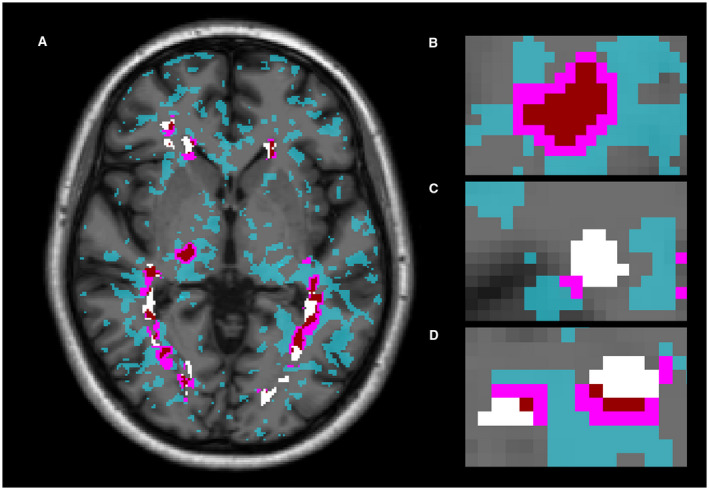
A. Map of activated innate immune cells in a single patient with secondary progressive multiple sclerosis, measured with [18F]DPA‐714 PET, overlaid onto the corresponding MPRAGE. White matter (WM) lesions are displayed in white. Voxels characterized by activated innate immune cells are displayed in dark red if localized inside lesions, in purple if localized in the perilesional area, and in aquamarine if localized in normal‐appearing tissues. B. Example of a WM lesion classified as “active,” characterized by an extensive activation of innate immune cells inside and around the lesion edge. C. Example of a lesion characterized by a lack of innate immune cell activation, defined as “inactive.” D. Single lesion with no activation of innate immune cells inside its border, but with an extensive activation in the perilesional area. Lesions characterized by this profile of innate immune cell activation are suggestive of “smoldering plaques.”
Limitations of the current approach and perspectives
While TSPO PET has successfully allowed to revisit the contribution of chronic active lesions to disability worsening in MS, the interpretation of results is still challenged by the limited cell specificity of TSPO expression. First, some expression has been described in astrocytes in an inflammatory environment 51 as well as in endothelial cells 72, 84, 92. Whether the changes in TSPO tracer binding described in MS tissues is influenced by these cellular components remains an open question. The methodology we have developed for the regional mapping of [18F]DPA‐714 binding minimizes biases related to regional variation in endothelial expression of TSPO. However, there is still a need to exclude any endothelial overexpression specifically related to MS, which requires a full modeling of [18F]DPA‐714 binding derived from input function in patients. Second, TSPO expression does not discriminate myeloid derived from microglial CNS resident innate immune cells, on the one hand, and does not distinguish between functional states of cells (pro‐inflammatory or protective) on the other hand. Therefore, a key objective would be to generate original compounds targeting polarized states of activated microglia, which would represent a promising step toward the characterization of the cascade of events leading to clinical progression 87 and the development of new therapeutic strategies effective on preventing progressive deterioration 11, 65. Promising targets potentially able to distinguish between polarized states of activated cells have been recently proposed 83. In particular, the purinergic receptors P2X7R has been associated with a pro‐inflammatory phenotype of microglial cells both in vitro and in vivo 9 and the first radiotracer specific for this receptor has been produced and should be translated soon to clinical studies 43. In contrast, the P2Y12R may identify an anti‐inflammatory or a homeostatic phenotype 44, 97, and the first specific compounds for this receptor will soon be available. Radioligands for the A2A adenosine receptor have already been applied to MS patients, and showed increased binding in the NAWM of patients with the secondary progressive form of disease, but it is still unclear whether they bind to a subtype of microglial cells or to other cell types 70, 89. Beyond purinergic receptors, specific ligands for the cannabinoid receptor‐2 (CB2) 78 may hold promises to image neuroinflammation in the CNS, with several compounds ready for pilot clinical studies 16. However, the CB2 radiotracer is not characterized by an absolute specificity for microglial cells, as it is also expressed by lymphocyte populations. Targeting the xc‐ system, which controls the extra‐cellular concentration of glutamate and has been shown to be overexpressed by pro‐inflammatory microglial cells may also represent a promising strategy 30, 56 but no human data have been produced to date. Finally, reactive astrocytes have been described to express the monocarboxylate transporters (MCT) and in consequence may preferentially absorb acetate, a property that could be applied to PET imaging using [11C]‐acetate with the goal to identify the gliotic reaction within MS lesions and in normal appearing tissues 82.
PET BEYOND MYELIN DYNAMICS AND NEUROINFLAMMATION
PET could also provide exciting clues to improve our understanding of the relationship between the neurodegenerative component of the disease and MS lesions by selectively targeting neuronal damage. A reduced [18F]Fluoro‐desoxy‐glucose [18F]FDG uptake in gray matter regions, potentially reflecting neuronal damage, has been described in MS and correlates with fatigue and cognitive function 6, 28, 64, 74. However, [18F]FDG cannot be interpreted as a pure neuronal marker. Indeed, it reflects glucose transport and metabolism within glial cells and neurons in the context of a narrow neuro‐glial coupling 35, 54, but its uptake may also increase in some inflammatory cells 4. Interestingly, we have recently observed that in WM lesions [18F]FDG signal was globally reduced, but also correlated with [18F]DPA‐714 binding. These results illustrate the complex interpretation of this PET measure that reflects both metabolic breakdown and inflammatory burden. The neuronal‐specific radiotracer [11C]flumazenil ([11C]FMZ) provides a more specific neuronal signal for PET studies: it is an antagonist of the central benzodiazepine receptor, a component of the ubiquitous GABAA receptor complex, which is localized on axo‐somatic and axo‐dendritic neuronal synapses throughout the gray matter 57, 59. Using a non‐invasive quantification methodology 27, we have evaluated neuronal damage using [11C]FMZ in a pilot study investigating both relapsing and progressive patients with MS. We showed a worsening gradient of neuronal damage from RRMS to progressive MS patients, which correlated with cognitive scores 33. In the next future, the application of novel and specific synaptic radiotracers may allow an in depth investigation of the pathophysiology of synaptic degeneration, one of the earliest neurodegenerative process occurring in the gray matter of patients with MS 61, 91.
Conclusion
We are entering into a new era in the field of MS diagnostic and care, where the identification and measure of the mechanisms underlying disease progression in‐vivo may become key tools to support the development of novel therapies aimed to prevent the accrual of clinical disability. PET provides promising imaging metrics specific and sensitive for key processes identified by pathological investigations such as myelin repair, innate immune system activation and neurodegeneration. Pilot clinical PET studies performed at different stages of the disease have recently shown that patient‐specific metrics related to MS lesion biology, reflecting individual profiles of lesion remyelination and chronic inflammation, could underlie individual trajectories of disability worsening. Future PET studies will allow to analyze more in depth the relationship between MS lesions heterogeneity and neurodegeneration over the course of the disease, and to develop innovative therapeutic trials aimed to control neuroinflammation and promote remyelination. Bridging the gap between pathology and clinical evaluation, the combination of PET with MRI holds great promises and now represents the next frontier in the field of in‐vivo imaging of the disease.
CONFLICT OF INTEREST
We have no conflict of interest related to this article.
B. Stankoff received honoraria from Biogen, Teva, Novartis, Genzyme, Roche and research support from Genzyme, Merck‐Serono and Roche.
B. Bodini receives rese arch support from ARSEP. She has received funding for traveling and/or speaker's honoraria from Novartis, Genzyme, Roche and Merck Serono.
M Tonietto and E Poirion have nothing to disclose.
ACKNOWLEDGMENTS
We thank the whole staff of the Centre d'Investigation Clinique of the ICM in Paris, and of the Service Hospitalier Frederic Joliot, CEA, Orsay. PET studies have been funded by Fondation ARSEP, ECTRIMS, ELA (European Leukodystrophy Association), ANR (Agence Nationale de la Recherche) MNP2008‐007125, INSERM‐DHOS, PHRC 2010 (Programme Hospitalier de Recherche Clinique), JNLF (Journées de Neurologie de Langue Française) and FRM (Fondation pour la Recherche Médicale). APHP (Assistance Publique des Hôpitaux de Paris) sponsored the clinical PET studies performed in Paris.
References
- 1. Abourbeh G, Theze B, Maroy R, Dubois A, Brulon V, Fontyn Y et al (2012) Imaging microglial/macrophage activation in spinal cords of experimental autoimmune encephalomyelitis rats by positron emission tomography using the mitochondrial 18 kDa translocator protein radioligand [18F]DPA‐714. J Neurosci [Internet] April 25 [cited 2018 June 20];32:5728–5736. Available at: http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.2900-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Albrecht DS, Granziera C, Hooker JM, Loggia ML (2016) In vivo imaging of human neuroinflammation. ACS Chem Neurosci [Internet] April 20 [cited 2018 June 20];7:470–483. Available at: http://pubs.acs.org/doi/10.1021/acschemneuro.6b00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arlicot N, Vercouillie J, Ribeiro M‐J, Tauber C, Venel Y, Baulieu J‐L et al (2012) Initial evaluation in healthy humans of [18F]DPA‐714, a potential PET biomarker for neuroinflammation. Nucl Med Biol [Internet] May [cited 2018 June 20];39:570–578. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22172392. [DOI] [PubMed] [Google Scholar]
- 4. Backes H, Walberer M, Ladwig A, Rueger MA, Neumaier B, Endepols H et al (2016) Glucose consumption of inflammatory cells masks metabolic deficits in the brain. Neuroimage [Internet] March [cited 2018 June 20];128:54–62. Available at: http://linkinghub.elsevier.com/retrieve/pii/S105381191501157X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bajaj A, LaPlante NE, Cotero VE, Fish KM, Bjerke RM, Siclovan T et al (2013) Identification of the protein target of myelin‐binding ligands by immunohistochemistry and biochemical analyses. J Histochem Cytochem [Internet] January 23 [cited 2018 June 19];61:19–30. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23092790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bakshi R, Miletich RS, Kinkel PR, Emmet ML, Kinkel WR (1998) High‐resolution fluorodeoxyglucose positron emission tomography shows both global and regional cerebral hypometabolism in multiple sclerosis. J Neuroimaging [Internet] October [cited 2018 June 20];8:228–234. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9780855. [DOI] [PubMed] [Google Scholar]
- 7. Banati RB, Newcombe J, Gunn RN, Cagnin A, Turkheimer F, Heppner F et al (2000) The peripheral benzodiazepine binding site in the brain in multiple sclerosis: quantitative in vivo imaging of microglia as a measure of disease activity. Brain [Internet] November [cited 2018 June 20];123(Pt 1):2321–2337. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11050032 [DOI] [PubMed] [Google Scholar]
- 8. Barnett MH, Prineas JW (2004) Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann Neurol [Internet] April [cited 2018 June 19];55:458–468. Available at: 10.1002/ana.20016. [DOI] [PubMed] [Google Scholar]
- 9. Beaino W, Janssen B, Kooij G, van der Pol SMA, van Het Hof B, van Horssen J et al (2017) Purinergic receptors P2Y12R and P2X7R: potential targets for PET imaging of microglia phenotypes in multiple sclerosis. J Neuroinflammation [Internet] December 22 [cited 2018 June 20];14:259. Available at: https://jneuroinflammation.biomedcentral.com/articles/10.1186/s12974-017-1034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bodini B, Veronese M, García‐Lorenzo D, Battaglini M, Poirion E, Chardain A et al (2016) Dynamic imaging of individual remyelination profiles in multiple sclerosis. Ann Neurol [Internet] May;79:726–738. Available at: 10.1002/ana.24620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bogie JFJ, Stinissen P, Hendriks JJA (2014) Macrophage subsets and microglia in multiple sclerosis. Acta Neuropathol [Internet] August 22 [cited 2018 June 20];128:191–213. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24952885. [DOI] [PubMed] [Google Scholar]
- 12. Brex PA, Ciccarelli O, O'Riordan JI, Sailer M, Thompson AJ, Miller DH (2002) A longitudinal study of abnormalities on MRI and disability from multiple sclerosis. N Engl J Med [Internet]January 17 [cited 2018 June 19];346:158–164.Available at: http://www.nejm.org/doi/abs/10.1056/NEJMoa011341. [DOI] [PubMed] [Google Scholar]
- 13. Briard E, Orain D, Beerli C, Billich A, Streiff M, Bigaud M et al (2011) BZM055, an iodinated radiotracer candidate for PET and SPECT imaging of myelin and FTY720 brain distribution. ChemMedChem [Internet] April 4 [cited 2018 June 19];6:667–77. Available at: 10.1002/cmdc.201000477. [DOI] [PubMed] [Google Scholar]
- 14. Briard E, Rudolph B, Desrayaud S, Krauser JA, Auberson YP (2015) MS565: a SPECT tracer for evaluating the brain penetration of BAF312 (Siponimod). ChemMedChem [Internet] June [cited 2018 June 19];10:1008–18. Available at: 10.1002/cmdc.201500115. [DOI] [PubMed] [Google Scholar]
- 15. Brugarolas P, Sánchez‐Rodríguez JE, Tsai H‐M, Basuli F, Cheng S‐H, Zhang X et al (2018) Development of a PET radioligand for potassium channels to image CNS demyelination. Sci Rep [Internet] December 12 [cited 2018 June 19];8:607. Available at: http://www.nature.com/articles/s41598-017-18747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Caillé F, Cacheux F, Peyronneau M‐A, Jego B, Jaumain E, Pottier G et al (2017)From structure‐activity relationships on thiazole derivatives to the in vivo evaluation of a new radiotracer for cannabinoid subtype 2 PET imaging. Mol Pharm [Internet] November 6 [cited 2018 June 20];14:4064–4078. Available at: http://www.ncbi.nlm.nih.gov/pubmed/28968497. [DOI] [PubMed] [Google Scholar]
- 17. Chauveau F, Boutin H, Van Camp N, Dollé F, Tavitian B (2008) Nuclear imaging of neuroinflammation: a comprehensive review of [11C]PK11195 challengers. Eur J Nucl Med Mol Imaging [Internet] December 1 [cited 2018 June 20];35:2304–2319. Available at: http://link.springer.com/10.1007/s00259-008-0908-9. [DOI] [PubMed] [Google Scholar]
- 18. Chauveau F, Van Camp N, Dollé F, Kuhnast B, Hinnen F, Damont A et al (2009) Comparative evaluation of the translocator protein radioligands 11C‐DPA‐713, 18F‐DPA‐714, and 11C‐PK11195 in a rat model of acute neuroinflammation. J Nucl Med [Internet] March 17 [cited 2018 June 20];50:468–476. Available at: http://jnm.snmjournals.org/cgi/doi/10.2967/jnumed.108.058669. [DOI] [PubMed] [Google Scholar]
- 19. Chen JT, Collins DL, Atkins HL, Freedman MS, Arnold DL (2008) Magnetization transfer ratio evolution with demyelination and remyelination in multiple sclerosis lesions. Ann Neurol [Internet] February [cited 2018 June 19];63:254–262. Available at: 10.1002/ana.21302. [DOI] [PubMed] [Google Scholar]
- 20. Cotero VE, Siclovan T, Zhang R, Carter RL, Bajaj A, LaPlante NE et al (2012) Intraoperative fluorescence imaging of peripheral and central nerves through a myelin‐selective contrast agent. Mol Imaging Biol [Internet] December 10 [cited 2018 June 19];14:708–717. Available at: http://link.springer.com/10.1007/s11307-012-0555-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Datta G, Colasanti A, Kalk N, Owen D, Scott G, Rabiner EA et al (2017) 11C‐PBR28 and 18F‐PBR111 detect white matter inflammatory heterogeneity in multiple sclerosis. J Nucl Med [Internet] September [cited 2018 June 20];58:1477–1482. Available at: http://www.ncbi.nlm.nih.gov/pubmed/28302760 . [DOI] [PubMed] [Google Scholar]
- 22. Datta G, Colasanti A, Rabiner EA, Gunn RN, Malik O, Ciccarelli O et al (2017) Neuroinflammation and its relationship to changes in brain volume and white matter lesions in multiple sclerosis. Brain [Internet] (September):1–12. Available at: http://academic.oup.com/brain/article/doi/10.1093/brain/awx228/4210384/Neuroinflammation-and-its-relationship-to-changes [DOI] [PubMed] [Google Scholar]
- 23. De Groot CJA, Bergers E, Kamphorst W, Ravid R, Polman CH, Barkhof F et al (2001) Post‐mortem MRI‐guided sampling of multiple sclerosis brain lesions: increased yield of active demyelinating and (p)reactive lesions. Brain [Internet] August 1 [cited 2018 June 19];124:1635–1645. Available at: https://academic.oup.com/brain/article-lookup/doi/10.1093/brain/124.8.1635. [DOI] [PubMed] [Google Scholar]
- 24. De Paula Faria D, de Vries EFJ, Sijbesma JWA, Dierckx RAJO, Buchpiguel CA, Copray S (2014) PET imaging of demyelination and remyelination in the cuprizone mouse model for multiple sclerosis: a comparison between [11C]CIC and [11C]MeDAS. Neuroimage [Internet] February [cited 2018 June 19];15:395–402. Available at: https://www.sciencedirect.com/science/article/pii/S105381191301080X. [DOI] [PubMed] [Google Scholar]
- 25. De Paula Faria D, Copray S, Sijbesma JWA, Willemsen ATM, Buchpiguel CA, Dierckx RAJO et al (2014) PET imaging of focal demyelination and remyelination in a rat model of multiple sclerosis: comparison of [11C]MeDAS, [11C]CIC and [11C]PIB. Eur J Nucl Med Mol Imaging 41:995–1003. [DOI] [PubMed] [Google Scholar]
- 26. Debruyne JC, Versijpt J, Van Laere KJ, De Vos F, Keppens J, Strijckmans K et al (2003) PET visualization of microglia in multiple sclerosis patients using [11C]PK11195. Eur J Neurol [Internet] May [cited 2018 June 20];10:257–264. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12752399. [DOI] [PubMed] [Google Scholar]
- 27. Delforge J, Pappata S, Millet P, Samson Y, Bendriem B, Jobert A et al (1995) Quantification of benzodiazepine receptors in human brain using PET, [11C]flumazenil, and a single‐experiment protocol. J Cereb Blood Flow Metab 15:284–300. [DOI] [PubMed] [Google Scholar]
- 28. Derache N, Marié R‐M, Constans J‐M, Defer G‐L (2006) Reduced thalamic and cerebellar rest metabolism in relapsing‐remitting multiple sclerosis, a positron emission tomography study: correlations to lesion load. J Neurol Sci [Internet] June 15 [cited 2018 June 20];245:103–109. Available at: http://linkinghub.elsevier.com/retrieve/pii/S0022510X06001110. [DOI] [PubMed] [Google Scholar]
- 29. Derakhshan M, Caramanos Z, Narayanan S, Arnold DL, Louis Collins D (2014) Surface‐based analysis reveals regions of reduced cortical magnetization transfer ratio in patients with multiple sclerosis: a proposed method for imaging subpial demyelination. Hum Brain Mapp [Internet] July [cited 2018 June 19];35:3402–3413. Available at: 10.1002/hbm.22410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Domercq M, Szczupak B, Gejo J, Gómez‐Vallejo V, Padro D, Gona KB et al (2016)PET imaging with [18F]FSPG evidences the role of system xc—on brain inflammation following cerebral ischemia in rats. Theranostics [Internet] [cited 2018 June 20];6:1753–1767. Available at: http://www.ncbi.nlm.nih.gov/pubmed/27570548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. El Behi M, Sanson C, Bachelin C, Guillot‐Noël L, Fransson J, Stankoff B et al (2017) Adaptive human immunity drives remyelination in a mouse model of demyelination. Brain [Internet] April 1 [cited 2018 June 19];140:967–980. Available at: http://www.ncbi.nlm.nih.gov/pubmed/28334918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fisniku LK, Brex PA, Altmann DR, Miszkiel KA, Benton CE, Lanyon R et al (2008) Disability and T2 MRI lesions: a 20‐year follow‐up of patients with relapse onset of multiple sclerosis. Brain [Internet] February 7 [cited 2018 June 19];131:808–817.Available at: https://academic.oup.com/brain/article-lookup/doi/10.1093/brain/awm329. [DOI] [PubMed] [Google Scholar]
- 33. Freeman L, Garcia‐Lorenzo D, Bottin L, Leroy C, Louapre C, Bodini B et al (2015) The neuronal component of gray matter damage in multiple sclerosis: a [(11) C]flumazenil positron emission tomography study. Ann Neurol 78:554–567. [DOI] [PubMed] [Google Scholar]
- 34. Frischer JM, Weigand SD, Guo Y, Kale N, Parisi JE, Pirko I et al (2015) Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Ann Neurol 78:710–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fünfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J et al (2012) Glycolytic oligodendrocytes maintain myelin and long‐term axonal integrity. Nature [Internet] May 29 [cited 2018 June 20];485:517–521. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22622581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. García‐Lorenzo D, Lavisse S, Leroy C, Wimberley C, Bodini B, Remy P et al (2017) Validation of an automatic reference region extraction for the quantification of [18F]DPA‐714 in dynamic brain PET studies. J Cereb Blood Flow Metab [Internet] January;0271678X1769259. Available at: http://journals.sagepub.com/doi/10.1177/0271678X17692599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Giannetti P, Politis M, Su P, Turkheimer F, Malik O, Keihaninejad S et al (2014) Microglia activation in multiple sclerosis black holes predicts outcome in progressive patients: an in vivo [(11)C](R)‐PK11195‐PET pilot study. Neurobiol Dis [Internet] May [cited 2018 June 20];65:203–210. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24508617. [DOI] [PubMed] [Google Scholar]
- 38. Giannetti P, Politis M, Su P, Turkheimer FE, Malik O, Keihaninejad S et al (2015) Increased PK11195‐PET binding in normal‐appearing white matter in clinically isolated syndrome. Brain [Internet] January [cited 2018 June 20];138:110–119. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25416179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gibbs‐Strauss SL, Nasr KA, Fish KM, Khullar O, Ashitate Y, Siclovan TM et al (2011) Nerve‐highlighting fluorescent contrast agents for image‐guided surgery. Mol Imaging [Internet] April [cited 2018 June 19];10:91–101. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21439254. [PMC free article] [PubMed] [Google Scholar]
- 40. Glenner GG, Page DL, Eanes ED (1972) The relation of the properties of congo red‐stained amyloid fibrils to the β‐conformation. J Histochem Cytochem [Internet] October 26 [cited 2018 June 19];20:821–826. Available at: http://journals.sagepub.com/doi/10.1177/20.10.821. [DOI] [PubMed] [Google Scholar]
- 41. Herranz E, Giannì C, Louapre C, Treaba CA, Govindarajan ST, Ouellette R et al (2016) Neuroinflammatory component of gray matter pathology in multiple sclerosis. Ann Neuro [Internet] November [cited 2018 June 20];80:776–790. Available at: 10.1002/ana.24791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. James ML, Fulton RR, Vercoullie J, Henderson DJ, Garreau L, Chalon S et al (2008)DPA‐714, a new translocator protein‐specific ligand: synthesis, radiofluorination, and pharmacologic characterization. J Nucl Med [Internet] April 15 [cited 2018 June 20];49:814–822. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18413395. [DOI] [PubMed] [Google Scholar]
- 43. Janssen B, Vugts DJ, Wilkinson SM, Ory D, Chalon S, Hoozemans JJM et al (2018) Identification of the allosteric P2X7 receptor antagonist [11C]SMW139 as a PET tracer of microglial activation. Sci Rep [Internet] December 26 [cited 2018 June 20];8:6580. Available at: http://www.ncbi.nlm.nih.gov/pubmed/29700413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Janssen B, Vugts D, Windhorst A, Mach R (2018) PET Imaging of Microglial Activation—Beyond Targeting TSPO. Molecules [Internet] March 8 [cited 2018 June 20];23:607. Available at: http://www.ncbi.nlm.nih.gov/pubmed/29518005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kaunzner UW, Kang Y, Monohan E, Kothari PJ, Nealon N, Perumal J et al (2017) Reduction of PK11195 uptake observed in multiple sclerosis lesions after natalizumab initiation. Mult Scler Relat Disord [Internet] July [cited 2018 June 20];15:27–33. Available at: http://www.ncbi.nlm.nih.gov/pubmed/28641769. [DOI] [PubMed] [Google Scholar]
- 46. Klunk WE, Pettegrew JW, Abraham DJ (1989) Quantitative evaluation of congo red binding to amyloid‐like proteins with a beta‐pleated sheet conformation. J Histochem Cytochem 37:1273–1281. [DOI] [PubMed] [Google Scholar]
- 47. Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33:1444–1452. [DOI] [PubMed] [Google Scholar]
- 48. Kutzelnigg A, Lucchinetti CF, Stadelmann C, Brück W, Rauschka H, Bergmann M et al (11) Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain [Internet] November 1 [cited 2018 June 19];128:2705–2712. Available at: http://academic.oup.com/brain/article/128/11/2705/339613/Cortical-demyelination-and-diffuse-white-matter. [DOI] [PubMed] [Google Scholar]
- 49. Lassmann H (2007) Multiple sclerosis: is there neurodegeneration independent from inflammation? J Neurol Sci [Internet] August 15 [cited 2018 June 20];259:3–6. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17367814. [DOI] [PubMed] [Google Scholar]
- 50. Lavisse S, García‐Lorenzo D, Peyronneau M‐AM‐A, Bodini B, Thiriez C, Kuhnast B et al (2015)Optimized quantification of translocator protein radioligand 18F‐DPA‐714 uptake in the brain of genotyped healthy volunteers. J Nucl Med [Internet] July 1 [cited 2018 June 20];56:1048–1054. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26025960. [DOI] [PubMed] [Google Scholar]
- 51. Lavisse S, Guillermier M, Herard A‐S, Petit F, Delahaye M, Van Camp N et al (2012) Reactive astrocytes overexpress TSPO and are detected by TSPO positron emission tomography imaging. J Neurosci [Internet] August 8 [cited 2018 June 20];32:10809–10818. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22875916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Li DKB, Held U, Petkau J, Daumer M, Barkhof F, Fazekas F et al (2006) MRI T2 lesion burden in multiple sclerosis: a plateauing relationship with clinical disability. Neurology [Internet] May 9 [cited 2018 June 19];66:1384–1389.Available at: http://www.ncbi.nlm.nih.gov/pubmed/16682671. [DOI] [PubMed] [Google Scholar]
- 53. Luchetti S, Fransen NL, van Eden CG, Ramaglia V, Mason M, Huitinga I (2018) Progressive multiple sclerosis patients show substantial lesion activity that correlates with clinical disease severity and sex: a retrospective autopsy cohort analysis. Acta Neuropathol [Internet] April 13 [cited 2018 June 19];135:511–528.Available at: http://link.springer.com/10.1007/s00401-018-1818-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Magistretti PJ (2011) Neuron‐glia metabolic coupling and plasticity. Exp Physiol [Internet] April 1 [cited 2018 June 20];96:407–410. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21123364. [DOI] [PubMed] [Google Scholar]
- 55. Mahad DH, Trapp BD, Lassmann H (2015) Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol [Internet] February 1 [cited 2018 June 20];14:183–193. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25772897. [DOI] [PubMed] [Google Scholar]
- 56. Martín A, Vázquez‐Villoldo N, Gómez‐Vallejo V, Padro D, Soria FN, Szczupak B et al (2016) In vivo imaging of system xc‐ as a novel approach to monitor multiple sclerosis. Eur J Nucl Med Mol Imaging [Internet] June 10 [cited 2018 June 20];43:1124–1138. Available at: http://link.springer.com/10.1007/s00259-015-3275-3. [DOI] [PubMed] [Google Scholar]
- 57. Maziere M, Prenant C, Sastre J, Crouzel M, Comar D, Cepeda C et al (1983) In vivo study of benzodiazepine receptors using positron emission tomography. Encephale [Internet] [cited 2018 June 20];9(4 Suppl 2):151B–160B. Available at: http://www.ncbi.nlm.nih.gov/pubmed/6327227. [PubMed] [Google Scholar]
- 58. Miron VE, Franklin RJM. Macrophages and CNS remyelination (2014) J Neurochem [Internet] July [cited 2018 June 19];130:165–171. Available at: 10.1111/jnc.12705. [DOI] [PubMed] [Google Scholar]
- 59. Möhler H (2009) Role of GABAA receptors in cognition: table 1. Biochem Soc Trans [Internet] December 1 [cited 2018 June 20];37:1328–1333. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19909270. [DOI] [PubMed] [Google Scholar]
- 60. Moore GRW, Laule C, MacKay A, Leung E, Li DKB, Zhao G et al (2008) Dirty‐appearing white matter in multiple sclerosis. J Neurol [Internet] November 24 [cited 2018 June 19];255:1802–1811. Available at: http://link.springer.com/10.1007/s00415-008-0002-z. [DOI] [PubMed] [Google Scholar]
- 61. Nabulsi NB, Mercier J, Holden D, Carré S, Najafzadeh S, Vandergeten M‐C et al (2016) Synthesis and preclinical evaluation of 11C‐UCB‐J as a PET tracer for imaging the synaptic vesicle glycoprotein 2A in the brain. J Nucl Med [Internet] May 1 [cited 2018 June 20];57:777–784. Available at: http://jnm.snmjournals.org/cgi/doi/10.2967/jnumed.115.168179. [DOI] [PubMed] [Google Scholar]
- 62. Owen DR, Yeo AJ, Gunn RN, Song K, Wadsworth G, Lewis A et al (2012) An 18‐kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J Cereb Blood Flow Metab [Internet] January 19 [cited 2018 June 20];32:1–5. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22008728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Patrikios P, Stadelmann C, Kutzelnigg A, Rauschka H, Schmidbauer M, Laursen H et al (2006) Remyelination is extensive in a subset of multiple sclerosis patients. Brain [Internet] June 9 [cited 2018 June 19];129:3165–3172.Available at: https://academic.oup.com/brain/article-lookup/doi/10.1093/brain/awl217. [DOI] [PubMed] [Google Scholar]
- 64. Paulesu E, Perani D, Fazio F, Comi G, Pozzilli C, Martinelli V et al (1996) Functional basis of memory impairment in multiple sclerosis: a[18F]FDG PET study. Neuroimage October [cited 2018 June 20];4:87–96. Available at: http://linkinghub.elsevier.com/retrieve/pii/S1053811996900324. [DOI] [PubMed] [Google Scholar]
- 65. Perry VH, Nicoll JAR, Holmes C (2010) Microglia in neurodegenerative disease. Nat Rev Neurol [Internet] April 16 [cited 2018 June 20];6:193–201. Available at: http://www.nature.com/articles/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- 66. Politis M, Giannetti P, Su P, Turkheimer F, Keihaninejad S, Wu K et al (2012) Increased PK11195 PET binding in the cortex of patients with MS correlates with disability. Neurology [Internet] August 7 [cited 2018 June 20];79:523–530. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22764258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pringle NP, Mudhar HS, Collarini EJ, Richardson WD (1992) PDGF receptors in the rat CNS: during late neurogenesis, PDGF alpha‐receptor expression appears to be restricted to glial cells of the oligodendrocyte lineage. Development 115:???–???. [DOI] [PubMed] [Google Scholar]
- 68. Ridsdale RA, Beniac DR, Tompkins TA, Moscarello MA, Harauz G (1997) Three‐dimensional structure of myelin basic protein. II. Molecular modeling and considerations of predicted structures in multiple sclerosis. J Biol Chem [Internet] February 14 [cited 2018 June 19];272:4269–4275. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9020143. [DOI] [PubMed] [Google Scholar]
- 69. Rissanen E, Tuisku J, Rokka J, Paavilainen T, Parkkola R, Rinne JO et al (2014) In vivo detection of diffuse inflammation in secondary progressive multiple sclerosis using PET imaging and the radioligand 11C‐PK11195. J Nucl Med [Internet] June 1 [cited 2018 June 20];55:939–944. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24711650. [DOI] [PubMed] [Google Scholar]
- 70. Rissanen E, Virta JR, Paavilainen T, Tuisku J, Helin S, Luoto P et al (2013) Adenosine A2A receptors in secondary progressive multiple sclerosis: a [(11)C]TMSX brain PET study. J Cereb Blood Flow Metab [Internet] September 22 [cited 2018 June 20];33:1394–1401. Available at: http://journals.sagepub.com/doi/10.1038/jcbfm.2013.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rivest S (2009) Regulation of innate immune responses in the brain. Nat Rev Immunol [Internet] June 1 [cited 2018 June 20];9:429–439. Available at: http://www.nature.com/articles/nri2565. [DOI] [PubMed] [Google Scholar]
- 72. Rizzo G, Veronese M, Tonietto M, Bodini B, Stankoff B, Wimberley C et al (2017) Generalization of endothelial modelling of TSPO PET imaging: considerations on tracer affinities. J Cereb Blood Flow Metab [Internet] November 14;0271678X1774200. Available at: http://journals.sagepub.com/doi/10.1177/0271678X17742004%0Ahttp://www.ncbi.nlm.nih.gov/pubmed/29135382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rizzo G, Veronese M, Tonietto M, Zanotti‐Fregonara P, Turkheimer FE, Bertoldo A (2014) Kinetic modeling without accounting for the vascular component impairs the quantification of [11C]PBR28 brain PET data. J Cereb Blood Flow Metab 34:???–???. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Roelcke U, Kappos L, Lechner‐Scott J, Brunnschweiler H, Huber S, Ammann W et al (1997) Reduced glucose metabolism in the frontal cortex and basal ganglia of multiple sclerosis patients with fatigue: a 18F‐fluorodeoxyglucose positron emission tomography study. Neurology [Internet] June [cited 2018 June 20];48:1566–1571. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9191767. [DOI] [PubMed] [Google Scholar]
- 75. Seewann A, Vrenken H, van der Valk P, Blezer ELA, Knol DL, Castelijns JA et al (2009) Diffusely abnormal white matter in chronic multiple sclerosis. Arch Neurol [Internet] May 1 [cited 2018 June 19];66:601–609. Available at: http://archneur.jamanetwork.com/article.aspx?doi=10.1001/archneurol.2009.57. [DOI] [PubMed] [Google Scholar]
- 76. Sethi V, Nair G, Absinta M, Sati P, Venkataraman A, Ohayon J et al (2017) Slowly eroding lesions in multiple sclerosis. Mult Scler J[Internet] March 11 [cited 2018 June 19];23:464–472.Available at: http://journals.sagepub.com/doi/10.1177/1352458516655403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Simon JH (2014) MRI outcomes in the diagnosis and disease course of multiple sclerosis. Handb Clin Neurol [Internet] January 1 [cited 2018 June 20];122:405–425.Available at: https://www.sciencedirect.com/science/article/pii/B9780444520012000170. [DOI] [PubMed] [Google Scholar]
- 78. Spinelli F, Mu L, Ametamey SM (2018) Radioligands for positron emission tomography imaging of cannabinoid type 2 receptor. J Label Compd Radiopharm [Internet] March [cited 2018 June 20];61:299–308. Available at: http://www.ncbi.nlm.nih.gov/pubmed/29110331. [DOI] [PubMed] [Google Scholar]
- 79. Stankoff B, Freeman L, Aigrot MS, Chardain A, Dollé F, Williams A et al (2011) Imaging central nervous system myelin by positron emission tomography in multiple sclerosis using [methyl‐11C]‐2‐(4‐methylaminophenyl)‐ 6‐hydroxybenzothiazole. Ann Neurol 69:673–680. [DOI] [PubMed] [Google Scholar]
- 80. Stankoff B, Wang Y, Bottlaender M, Aigrot M‐S, Dolle F, Wu C et al (2006) Imaging of CNS myelin by positron‐emission tomography. Proc Natl Acad Sci [Internet] 103:9304–9309.Available at: http://www.pnas.org/cgi/doi/10.1073/pnas.0600769103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sucksdorff M, Rissanen E, Tuisku J, Nuutinen S, Paavilainen T, Rokka J et al (2017) Evaluation of the effect of fingolimod treatment on microglial activation using serial PET imaging in multiple sclerosis. J Nucl Med [Internet] October [cited 2018 June 20];58:1646–1651. Available at: http://www.ncbi.nlm.nih.gov/pubmed/28336784. [DOI] [PubMed] [Google Scholar]
- 82. Takata K, Kato H, Shimosegawa E, Okuno T, Koda T, Sugimoto T et al (2014) 11C‐acetate PET imaging in patients with multiple sclerosis. suzumura A, editor. PLoS One [Internet] November 4 [cited 2018 June 20];9:e111598. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25369426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tronel C, Largeau B, Santiago Ribeiro M, Guilloteau D, Dupont A‐C, Arlicot N (2017) Molecular targets for PET imaging of activated microglia: the current situation and future expectations. Int J Mol Sci [Internet] April 11 [cited 2018 June 20];18:802. Available at: http://www.ncbi.nlm.nih.gov/pubmed/28398245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Turkheimer FE, Rizzo G, Bloomfield PS, Howes O, Zanotti‐Fregonara P, Bertoldo A et al (2015) The methodology of TSPO imaging with positron emission tomography. Biochem Soc Trans [Internet] August 1 [cited 2018 June 20];43:586–592. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26551697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Venneti S, Lopresti BJ, Wiley CA (2006) The peripheral benzodiazepine receptor (Translocator protein 18kDa) in microglia: from pathology to imaging. Prog Neurobiol [Internet] December [cited 2018 June 20];80:308–322. Available at: http://linkinghub.elsevier.com/retrieve/pii/S0301008206001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Veronese M, Bodini B, García‐Lorenzo D, Battaglini M, Bongarzone S, Comtat C et al (2015) Quantification of [11C]PIB PET for imaging myelin in the human brain: a test‐retest reproducibility study in high‐resolution research tomography. J Cereb Blood Flow Metab 35:1771–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Vogel DY, Vereyken EJ, Glim JE, Heijnen PD, Moeton M, van der Valk P et al (2013) Macrophages in inflammatory multiple sclerosis lesions have an intermediate activation status. J Neuroinflammation [Internet] December 4 [cited 2018 June 20];10:809. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23452918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Vowinckel E, Reutens D, Becher B, Verge G, Evans A, Owens T et al (1997) PK11195 binding to the peripheral benzodiazepine receptor as a marker of microglia activation in multiple sclerosis and experimental autoimmune encephalomyelitis. J Neurosci Res [Internet] October 15 [cited 2018 June 20];50:345–353. Available at: . [DOI] [PubMed] [Google Scholar]
- 89. Vuorimaa A, Rissanen E, Airas L (2017) In vivo PET imaging of adenosine 2A receptors in neuroinflammatory and neurodegenerative disease. Contrast Media Mol Imaging [Internet] [cited 2018 June 20];2017:6975841. Available at: https://www.hindawi.com/journals/cmmi/2017/6975841/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wang C, Wu C, Zhu J, Miller RH, Wang Y (2011) Design, synthesis, and evaluation of coumarin‐based molecular probes for imaging of myelination. J Med Chem [Internet] April 14 [cited 2018 June 19];54:2331–2340. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21391687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Warnock GI, Aerts J, Bahri MA, Bretin F, Lemaire C, Giacomelli F et al (2014) Evaluation of 18F‐UCB‐H as a novel PET tracer for synaptic vesicle protein 2A in the brain. J Nucl Med [Internet] August 1 [cited 2018 June 20];55:1336–1341. Available at: http://jnm.snmjournals.org/cgi/doi/10.2967/jnumed.113.136143. [DOI] [PubMed] [Google Scholar]
- 92. Wimberley C, Lavisse S, Brulon V, Peyronneau M‐A, Leroy C, Bodini B et al (2017) Impact of endothelial TSPO on the quantification of 18F‐DPA‐714. J Nucl Med [Internet]. jnumed.117.195396. Available at: http://jnm.snmjournals.org/lookup/doi/10.2967/jnumed.117.195396 [DOI] [PubMed] [Google Scholar]
- 93. Wu C, Tian D, Feng Y, Polak P, Wei J, Sharp A et al (2006) A novel fluorescent probe that is brain permeable and selectively binds to myelin. J Histochem Cytochem [Internet] September 1 [cited 2018 June 19];54:997–1004.Available at: http://journals.sagepub.com/doi/10.1369/jhc.5A6901.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wu C, Wang CCCC, Popescu DC, Zhu W, Somoza EA, Zhu J et al (2010) A novel PET marker for in vivo quantification of myelination. Bioorg Med Chem [Internet] December 15 [cited 2018 June 19];18:8592–8599. Available at: 10.1016/j.bmc.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wu C, Zhu J, Baeslack J, Zaremba A, Hecker J, Kraso J et al (2013) Longitudinal positron emission tomography imaging for monitoring myelin repair in the spinal cord. Ann Neurol [Internet] November [cited 2018 June 19];74:688–698. Available at: 10.1002/ana.23965. [DOI] [PubMed] [Google Scholar]
- 96. Zeydan B, Lowe VJ, Schwarz CG, Przybelski SA, Tosakulwong N, Zuk SM et al (2018) Pittsburgh compound‐B PET white matter imaging and cognitive function in late multiple sclerosis. Mult Scler J [Internet] May 5 [cited 2018 June 19];24:739–749. Available at: http://journals.sagepub.com/doi/10.1177/1352458517707346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zrzavy T, Hametner S, Wimmer I, Butovsky O, Weiner HL, Lassmann H (2017) Loss of ‘homeostatic’ microglia and patterns of their activation in active multiple sclerosis. Brain [Internet] July 1 [cited 2018 June 20];140:1900–1913. Available at: https://academic.oup.com/brain/article/140/7/1900/3852560. [DOI] [PMC free article] [PubMed] [Google Scholar]


