A significant challenge to clinicians is the patient with both diabetes and hypertension. The magnitude of this problem is considerable. 1 Diabetes is epidemic throughout the world; at present, there are approximately 124 million patients with diabetes in the world and this number will probably double by the year 2010. Virtually all of these patients are type 2 diabetics. The morbidity from diabetes includes: coronary disease, peripheral vascular disease, blindness, nephropathy, stroke, neuropathy, amputations, and a decrease in both the quality and quantity of life.
The burden of diabetes is unevenly distributed throughout the world and among various ethnic groups. Particularly, the Asian population and the African American, Hispanic, and American Indian groups in the United States suffer from the combination of diabetes and hypertension more frequently than other populations. Hypertension is common among those with diabetes mellitus. The combination of these two diseases adds greatly to the prevalence of cardiovascular (CV) complications. Figure 1 represents an estimate of the prevalence of diabetes in various parts of the world over the next 20 years. It is of interest to note the large numbers of diabetics in the Western Pacific and Southeast Asian areas, both at present and in projections for the future.
Figure 1.

Current and projected prevalence rates for diabetes, worldwide from World Health Organization Statistics, 2000
As with most diseases, there is a genetic‐environmental interaction that sets the pathophysiologic process of diabetes in motion. Some of the environmental factors are modifiable and include nutritional habits and physical exercise.
As the genetic and environmental interactions occur, one of the early phenotypes of diabetes is the cardiovascular dysmetabolic syndrome (CDS). The features of this syndrome are shown in Table I. Insulin resistance appears to be the basis for much of the pathophysiology of the CDS (Figure 2). Patients with insulin resistance do not metabolize glucose in the periphery or store glucose in the liver with the same levels of insulin as other individuals. Along with this, the metabolic changes listed in Figure 2 occur. 2 The process of atherosclerosis precedes the clinical recognition of diabetes. One study has reported that an individual with a diagnosis of diabetes has the same risk for a myocardial infarction as someone without diabetes who has already suffered an event (Figure 3). 3 Microvascular complications, e.g., retinopathy, nephropathy and neuropathy, usually occur after the diagnosis of diabetes is made. The advanced phenotypes of diabetes include end‐stage renal disease, which requires dialysis or transplantation, coronary heart disease, stroke, and amputation.
Table I.
The Cardiovascular Dysmetabolic Syndrome—Diagnostic Criteria
| Dyslipidemia | Over weight |
| Fasting TG >140 mg/dL | Body mass index >25 kg/m2 |
| HDL‐C <40 mg/dL | Waist/hip >0.85 |
| LDL‐C particle size <260Ä | Waist (females) >75 cm (>34 in) |
| Waist (males) >100 cm (39 in) | |
| Insulin Resistance | High Blood Pressure |
| Fasting plasma glucose ≥110 mg/dL | SBP ≥140 mm Hg |
| Type 2 diabetes mellitus | DBP ≥90 mm Hg |
| TG=triglycerides; HDL‐C/LDL‐C=high/low‐density lipoprotein cholesterol; SBP=systolic blood pressure; DBP=diastolic blood pressure Data are derived from Fagan T, Deedwania PC. The cardiovascular dysmetabolic syndrome. Am J Med. 1998;105:77S–82S. | |
Figure 2.

Role of insulin resistance in the metabolic syndrome Data derived from Deedwania PC. The deadly quartet revisited. Am J Med. 1998;105(1A):1S–3S.
Figure 3.

Cardiovascular (CV) risk similar in patients with type 2 diabetes and no prior myocardial infarction (MI) as in nondiabetics with prior MI; *p<0.001 for diabetes vs. no diabetes Data derived from Haffner et al. 3
INSULIN RESISTANCE
Insulin resistance is central to much of the pathophysiology of the CDS (Figure 2). As noted, in this situation, an excess of insulin is required to metabolize glucose—eventually, the amount necessary is inadequate—diabetes results. A concomitant decrease in pancreatic β‐cell function is a major factor in the appearance of clinical diabetes. An increase in systemic arterial blood pressure (BP) is contributed to by insulin resistance in a variety of ways including sodium metabolism (retention) and autonomic activity (increased sympathetic nerve activity). Diabetes contributes to the development of CV disease in large measure due to hyperglycemia and its effect on vascular endothelium. Small, dense low‐density‐lipoprotein (LDL) is more prevalent in the metabolic syndrome. Therefore, for any concentration or level of LDL, the individual with diabetes has a higher risk perhaps because small, dense LDL is more atherogenic. Low high‐density lipoprotein, a common finding in this syndrome, is a marker for inability to clear cholesterol from peripheral tissues. The presence of hyper‐coagulability underlies many of the problems, including the complications of a prothrombotic state in diabetics. An elevated triglyceride level completes the findings of the metabolic syndrome.
THE IMPORTANCE OF SYSTOLIC BP (SBP)
SBP is now recognized as being the most important component of BP for determination of CV risk. For years, the focus had been on diastolic BP (DBP). In fact, the first two JNC reports in 1977 4 and 1980 5 presented no recommendations for the treatment of SBP. Even to this day, antihypertensive drug approval is based on the lowering of DBP. Figure 4 shows the importance of elevation of SBP in diabetic patients. For example, risk in a diabetic with a SBP of 140–159 mm Hg (stage or grade 1 hypertension) is more than twice as great as in a nondiabetic.
Figure 4.
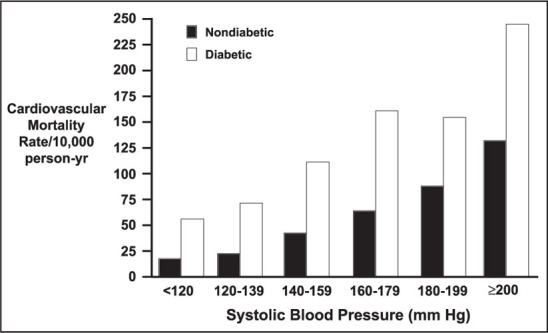
Association of systolic blood pressure and cardiovascular death in type 2 diabetes Data derived from Stamler et al. 1
DIABETES AND THE KIDNEY
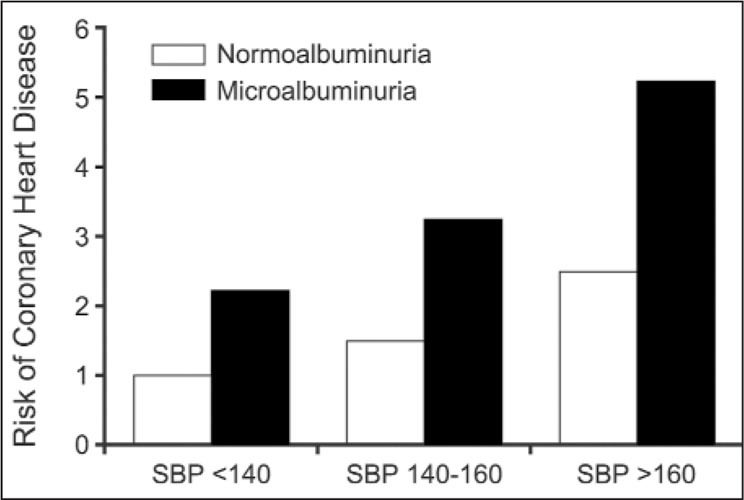
Microalbuminuria (30–300 mg/day) is an indicator of CV disease and correlates with the presence of left ventricular hypertrophy (LVH) and other evidence of coronary heart disease 6 , 7 , 8 , 9 (Figure 5, Table II). The presence of even smaller amounts of protein in the urine is associated with an increased risk of coronary heart disease at all levels of SBP (Figure 6). Thus, this marker, which is often not tested for, is an important predictor of future CV events. (Most physicians use a standard dipstick for urinary protein testing. This will not detect quantities of below 300–400 mg/day.) There are, however, dipsticks that are available to detect microproteinuria.
Figure 5.
Association of microalbuminuria and cardiovascular morbidity and mortality in type 2 diabetes. Microalbuminuria is a strong predictor of all‐cause mortality and cardiovascular (CV) morbidity and mortality in type 2 diabetes. A meta‐analysis of prospective trials of patients with type 2 diabetes found an overall odds ratio of 3.1 for total mortality and 1.8 for CV morbidity and mortality in patients with microproteinuria. The presence of microalbuminuria may reflect a generalized defect in vascular permeability leading to atherogenesis. Hypertension is a major risk factor for the development of microalbuminuria. Data derived from Dinneen and Gerstein. 16
Table II.
Microproteinuria
| What Is It? |
| Excretion of small amounts (30–300 mg/d) of protein in the urine. May be present in patients with insulin resistance prior to development of clinical diabetes. |
| How Is It Determined? |
| Spot morning urine sample for protein (creatinine levels in the urine to determine the percentage of 24‐hour urine volume excreted). Use albumin‐to‐creatinine ratio; <0.03 indicates proteinuria |
| What Testing Methods are Available? |
| Micral II dipstick ($4–$7/strip) and spot urine for albumin‐to‐creatinine ratio ($12–$14) |
| What Does It Mean? |
| Is suggestive of vascular injury not just in the kidney but in blood vessels elsewhere (correlates with cardiovascular risk) |
| What Should Be Done About It? |
| Glycemic control; control of blood pressure to levels <130–135/80–85 mm Hg |
| Is Specific Therapy Needed? |
| The use of an angiotensin‐converting enzyme inhibitor or an angiotensin receptor blocker usually with a diuretic; probably represents the most appropriate therapy to lower blood pressure and reduce proteinuria. |
| Adapted from Moser and Sowers. 11 |
Figure 6.

Microalbuminuria and ischemic heart disease risk. The presence of microalbuminuria (24‐hour excretion of between 30 and 300 mg/d of urinary protein) is associated with an increased risk of coronary heart disease at all levels of systolic blood pressure (SBP). (n=2085; 10‐year follow‐up) Data derived from Borch‐Johnsen K, Feldt‐Rasmussen B, Strandgaard S, et al. Urinary albumin excretion. An independent predictor of ischemic heart disease. Arterioscler Thromb Vasc Biol. 1999;19:1992–1997.
Microproteinuria often progresses to macroproteinuria as renal function deteriorates. This progression may be prevented in hypertensive diabetes by lowering BP (Figure 6).
Diabetes is the most common cause of end‐stage renal disease; and accounts for approximately 40% of all cases on dialysis. In many instances, patients with end‐stage renal disease have both diabetes and hypertension. Keeping BP as close to an optimal level of <120/80 mm Hg should be a priority in diabetics, but it is difficult to define an “optimal” BP for a diabetic. Current guidelines for treatment of hypertension in patients with diabetes suggest a goal for reduction of BP to levels of 130/80–85 mm Hg, but attempts can also be made to achieve pressures as close to 120/80 mm Hg as possible. 10 The prevalence of hypertension in type 2 diabetes is defined as a BP >140/90 mm Hg on several occasions.
STRATEGIES FOR THE TREATMENT OF DIABETIC HYPERTENSION
Until recent years, most efforts in managing diabetes have concentrated on glycemic control with diet, insulin, or antidiabetic medication. These efforts have proved to be only marginally successful in reducing CV events in type 2 diabetic patients. With extremely tight glycemic control, some success has been noted in preventing the microvascular complications of diabetes (e.g., retinopathy, neuropathy, renal disease), but effects on CV end points have not been consistently demonstrated. Too many diabetic patients are unable or unwilling to alter their lifestyle enough or take the appropriate medication to bring about the degree of blood glucose control required to prevent complications. In recent years, more attention has been focused on interventions to control the other common risk factors that occur in the diabetic; controlling hyperglycemia should be only one part of the effort in diabetics to reduce vascular complications. More emphasis should be placed on controlling elevated BP levels. Recent data clearly indicate the benefits of lowering BP in diabetic subjects, benefits that may exceed those achieved by glucose control. Not only has BP control been shown to reduce macrovascular events such as heart failure, stroke, and myocardial infarctions, but also microvascular events such as retinopathy and proteinuria.
Specific Interventions—Hyperglycemia
Hyperglycemia constitutes an increased risk for CV disease. Data from the United Kingdom Prospective Diabetes Study (UKPDS) indicated an increased risk for the development of heart failure associated with increasing levels of glycosylated hemoglobin levels (Hb) A1c (normal=6.5). 12 , 13 Hyperglycemia not only increases the risk of coronary atherosclerosis, but also produces changes in the myocardium, i.e., diabetic cardiomyopathy. Lowering HbA1C reduces the risk of CV complications. This should clearly be one goal in the management of the hypertensive diabetic.
ELEVATED BP
Evidence for the importance of reducing BP in patients with diabetes mellitus is provided by an analysis of The Hypertension Optimal Treatment (HOT) trial. 14 Impressive reductions in CV risk were obtained with relatively small decreases in BP in the diabetic cohort compared to patients who were not diabetic (Figure 7). For example, there was a decrease of DBP to 81 mm Hg in the tighter BP control group compared to a decrease to 85 mm Hg in the less tight control group (difference of only −4 mm Hg). There was a statistically significant decrease in CV end points in the diabetic subjects. Other examples illustrating the benefit of lowering BP are the Systolic Hypertension in the Elderly (SHEP) study 15 and the Systolic Hypertension in Europe (Syst‐Eur). 16 In both of these trials, risk was reduced more in diabetic subjects with BP lowering than in nondiabetics.
Figure 7.

Blood pressure (BP) control reduces cardiovascular events: Hypertension Optimal Treatment (HOT) Trial MI=myocardial infarction; CV=cardiovascular Data derived from Hansson et al. 14
Heart failure, as well as stroke risk, was also reduced with BP control in patients who have diabetes. In the UKPDS, a comparison of groups of type 2 diabetic patients with hypertension assigned to less tight BP control with those assigned to tight control achieved BP 154/87 mm Hg compared to 144/82 mm Hg (a difference of 10/5 mm Hg) resulted in a substantial reduction in stroke and heart failure, as well as in microvascular complications (retinopathy and proteinuria) (Figure 8). 13 In this trial, BP control resulted in a greater reduction in CV end points than the degree of glycemic control that was achieved (Figure 9). 12
Figure 8.

Reduction in congestive heart failure risk as a result of tight blood pressure control in patients with type 2 diabetes: UK Prospective Diabetes Study 38 MI=myocardial infarction; HF=heart failure NS=not significant. Data derived from UKPDS Group. 13
Figure 9.

Both tight glucose and blood pressure (BP) control reduce cardiovascular outcomes: UK Prospective Diabetes Study (UKPDS) *p<0.05 Tight BP control = 144/82 mm Hg; Tight glucose control = HbA1c = 7.0%. Data derived from UKPDS Group 12, 13
Thus, while glycemic control is of importance for prevention of complications associated with diseases of the microcirculation, reductions of macrovascular complications may be greater with reduction in BP. (Glycemic control [HbA1c <6] is of obvious importance in diabetic subjects, but control of elevated BP is of equal and perhaps more importance.)
“Numbers needed to treat” calculations reveal that to prevent one event secondary to microvascular disease with diabetes and elevated BP, 20 people need to be treated for 3 years; but, for any diabetes‐related end point, only approximately nine or 10 people need to be treated. These are impressive numbers, since with many diseases more than 100 people will have to be treated for benefit to be noted.
ANTIHYPERTENSIVE MEDICATIONS IN PATIENTS WITH DIABETES
Angiotensin I Converting Enzyme Inhibitors (ACEIs)
ACEIs have been particularly effective in the diabetic hypertensive patient. In part this may be due to the dysregulation of the ACE and the renin‐angiotensin‐aldosterone system that occurs in this disease. Upregulation of the ACE not only increases the production of angiotensin II but also increases the degradation of bradykinin and other substrates and contributes to an increase in oxidative stress and a reduction in vasodilator prostaglandins and nitric oxide. Blocking the effects of this enzyme with an ACEI not only decreases the generation of angiotensin II, but increases the amount of bradykinin by blocking its degradation.
The benefits of ACEIs in diabetes are shown by data from the Microalbuminuria, Cardiovascular, and Renal Outcomes—Heart Outcomes Prevention Evaluation (MICRO‐HOPE); MICRO‐HOPE was a study of the diabetic subpopulation of the Heart Outcomes Prevention Evaluation (HOPE) trial. 17 , 18 An ACEI, ramipril, at a dose of 10 mg, in addition to other medications in this high‐risk population, reduced the incidence of myocardial infarction, stroke, and CV death (Table III) more than in a group of subjects treated with medications that did not include an ACEI. Nephropathy and heart failure were also reduced. This, as in all of the other studies of high‐risk patients, involved patients who were treated with several other drugs in addition to an ACEI. It should be remembered that a large majority of diabetics will not respond until two or three medications are used.
Table III.
Results of the Heart Outcome Prevention Evaluation (HOPE) Study in Diabetes*
| Event | Reduction in Risk † |
|---|---|
| Combined primary outcome | ↓25% |
| Myocardial infarction | ↓22% |
| Stroke | ↓33% |
| Cardiovascular death | ↓37% |
| Total mortality | ↓27% |
| Overt nephropathy | ↓24% |
| *Number of patients studied=3577, age >55 years; †angiotensin‐converting enzyme (ACE) inhibitor group compared with subjects not receiving an ACE inhibitor. The use of this agent in addition to other medications reduced cardiovascular events. | |
In addition, the Fosinopril vs. Amlodipine Cardiovascular Events Randomized Trial (FACET) 19 and Appropriate Blood Pressure Control in Diabetes (ABCD) trial 20 also demonstrate that an ACEI‐based program was more effective than regimens that did not include an ACEI‐based program. In these studies a calcium channel blocker (CCB)‐based treatment program was the comparator.
Antiotensin Receptor Blockers (ARBs)
Several studies have evaluated the use of an ARB in patients with diabetes and varying degrees of nephropathy. In the Irbesartan Type 2 Diabetes With Microalbuminuria (IRMA) trial 21 in diabetic patients with some degree of proteinuria the use of irbesartan (with other medications) reduced progression to more severe nephropathy compared to a group of subjects who were on medications other than an ARB or ACEI. In another trial, (the Irbesartan Type II Diabetic Nephropathy Trial [IDNT]), 22 patients with hypertension and a more advanced degree of diabetic nephropathy experienced a decrease in progression of renal disease when they were treated with an ARB (irbesartan) compared to a cohort of patients on a CCB (amlodipine)‐based program or a control group not receiving the study medication.
In the Reduction of Endpoints in Non‐Insulin Dependent Diabetes Mellitus With the Angiotensin II Antagonist Losartan (RENAAL) trial, 23 an ARB (losartan)‐based treatment program reduced the incidence of hospitalizations for heart failure by 32% and significantly reduced the occurrence of end‐stage renal disease when compared to a treatment regimen that did not include an ARB or an ACEI (Table IV). These data indicate the benefits of an ARB‐based regimen compared to therapies that do not include an ARB or an ACEI in patients with hypertension and diabetic nephropathy. The majority of patients in these trials also received a diuretic to reduce BP to goal levels.
Table IV.
Effects of Losartan Plus Conventional Therapy Compared With Conventional Therapy Without an ACEI or an ARB in Type 2 Diabetics With Nephropathy (RENAAL Study)
| Significantly reduced incidence of end‐stage renal disease |
| Reduced proteinuria and the rate of decline in renal function |
| Reduced incidence of hospitalization for heart failure |
| ACE=angiotensin‐converting enzyme inhibitor; ARB=angiotensin receptor blocker; RENAAL=Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan |
The Losartan Intervention for Endpoint Reduction (LIFE) trial, 24 which included a subgroup of 1195 patients with diabetes, hypertension, and LVH, compared an ARB (losartan)‐based regimen to a β blocker (atenolol)‐based program. Achieved Bps were similar in both groups. A reduction of 24% occurred in the composite end point (Table V) in the losartan group. CV death was significantly reduced by 37% and heart failure by 40% in the diabetics treated with ARB‐based therapy compared to those on β blocker‐based therapy. An analysis of the overall population of the LIFE study noted a reduction in overall end points, largely due to a 25% reduction in strokes.
Table V.
Results of an ARB‐Based (Losartan) Compared With a β Blocker‐based (Atenolol) Treatment Program in 1195 Diabetic Hypertensive Patients With Left Ventricular Hypertrophy*
| Event | Difference ARB/β Blocker |
|---|---|
| Achieved blood pressure (mm Hg) | −2/0† |
| Primary end point (CV mortality stroke, myocardial infarction) | −24 |
| Death from CV disease | −37 |
| Heart failure | −41 |
| All‐cause mortality | −39 |
| ARB=angiotensin receptor blocker; CV=cardiovascular; *No significant differences between medications in stroke and myocardial infarction; †ARB, 146/79 mm Hg; β blocker, 148/79 mm Hg Data derived from Lindholm et al. 24 | |
Beta Blockers
Beta blockers are among the most indicated, and underused drugs in patients with diabetes. In the recent UKPDS group report, BP lowering with a β blocker (atenolol)‐based regimen was equal to that achieved by an ACEI (captopril)‐based regimen. There was no difference in the incidence of both microvascular and macrovascular complications (Table VI). Most of these patients required one of the study drugs plus a diuretic for “tight control of 144/82 mm Hg.” In those patients assigned to less tight control (154/87 mm Hg), there was less use of multiple antihypertensive agents. As noted, in addition to a reduction in heart failure, reductions in risk in the group assigned to tight BP control were 24% in diabetes related end points, 32% in deaths related to diabetes, 44% in strokes, and 37% in microvascular end points, especially diabetic retinopathy. These results suggest that combination therapy with either an ACEI or a β blocker is effective in reducing macrovascular as well as microvascular events as long as BP is adequately lowered. Although there was a 2.2 kg greater weight gain and a higher prevalence of cold extremities in the β blocker group, no other inordinate side effects were noted in this treatment group. This study appears to dispel the notion that β blockers are contraindicated in diabetic hypertensives because of concerns such as hypoglycemia.
Table VI.
United Kingdom Prospective Diabetes Study: Effect of Blood Pressure Control on Diabetic Events
| Tight blood pressure control (144/82 mm Hg) compared with less effective control (154/87 mm Hg) |
| Reduction of: |
| 24% in diabetic‐related end points |
| 32% reduction in deaths related to diabetes |
| 37% in microvascular events, renal failure, and severe retinopathy |
| From the United Kingdom Prospective Studies Group. BMJ. 1998;317:703–713. 13 |
A further example of benefit of β blockers in diabetics is seen in the MERIT trial 25 The reduction in all‐cause mortality and hospitalization for heart failure was the same for the diabetic population as for the population as a whole. Difficulties with glycemic control or discontinuance of drug use because of adverse events was no greater for the β blocker metoprolol than for patients who did not receive a β blocker.
Of interest is that in the whole UKPDS, a β blocker‐based treatment program produced benefits equivalent to the ACEI‐based program in type 2 diabetic subjects; another trial (Captopril Prevention Project [CAPPP]) 26 comparing a β blocker‐based regimen to an ACEI group in diabetics, reported that benefit was greater in the ACEI group. In other trials, progression to diabetes has also been less frequent with medications such the ARBs and ACEIs when compared to β blockers. Thus, although it appears to be safe and beneficial to use β blockers in a diabetic patient, there may be some advantages to using a medication such as an ACEI or ARB that has a more lasting and greater effect on reducing the activity of angiotensin II. Again, it should be noted that in all of the studies reviewed, a diuretic was used as part of the regimen in more than 70% of patients. This is an important fact to keep in mind when planning a treatment program in a diabetic hypertensive patient, especially if there is any evidence of LVH or nephropathy.
Calcium Channel Blockers
The CCBs have not been regarded as first‐line antihypertensive therapy drugs for patients with diabetes. They are often used as comparator drugs when evaluating renal effects with other drug classes. Recently, many of these studies have involved a comparison of a CCB and an ACEI or angiotensin AT1 receptor blocker. Results indicate that a CCB‐based regimen is not as effective in preventing CV events as a treatment program that involves an agent that effects the renin‐angiotensin‐aldosterone system.
Nevertheless, the CCBs are often required as part of a multidrug regimen to control BP in patients with diabetes. In this regard, data from FACET are instructive. The FACET trial was a comparison of a CCB, amlodipine, and an ACEI, fosinopril, in patients with diabetes and hypertension. As noted, the study demonstrated that the ACEI was superior to the CCB, in reducing CV events. The numbers of patients were small but the results are consistent with those of other trials. Importantly, the combination of the ACEI and the CCB produced a beneficial effect that exceeded the benefits of the two drugs assessed independently. Thus, in the diabetic patient, CCBs may be useful as part of a multidrug regimen but should be given with drugs from classes that perturb the renin angiotensin system.
Thiazide Diuretics
Thiazide diuretics have been used successfully to treat hypertension in diabetic patients. 27
Low‐dose diuretic‐based (chlorthalidone) treatment prevented major CV disease events in both non‐insulin‐treated diabetic and nondiabetic older patients with isolated systolic hypertension. 15 These agents lower BP to a degree equal to other agents and cause regression of LVH (if present); their use results in a reduction of morbidity and mortality. Diuretics are also useful when given with other antihypertensive drugs, particularly ACEIs, ARBs, or β blockers. The thiazides are underused; many cases of so‐called resistant hypertension can be controlled by the appropriate use a diuretic. 28
GUIDELINES
BP in the patient with diabetes should be reduced to at least 130/80 mm Hg. 29 The optimal BP for an individual patient is not known but is probably lower, e.g., < 120/80 mm Hg. A reasonable guideline is to reduce BP to as close to 120/80 as possible without producing side effects or interfering with the enjoyment of life.
Data from many clinical trials suggest that the number of drugs required to achieve control in the patient with diabetes and hypertension is more than two. Treating a patient with diabetic nephropathy may even require more than three different agents. Fixed‐combination therapy may provide an effective way to achieve control with fewer pills per day. And, cost to the patient may be reduced. If a diuretic is not used initially it should be the second medication of choice. A suggested treatment algorithm is noted in Table VII. The American Diabetes Association and the National Kidney Foundation 10 have issued specific recommendations (Table VIII).
Table VII.
Suggested Regimen for the Management of Hypertension in a Diabetic Patient
| Confirm presence of hypertension (BP >140/90 mm Hg) |
| Institute lifestyle changes if appropriate (weight loss, exercise, sodium restriction) |
| Simultaneously begin drug therapy |
| Diuretics, ACE inhibitor, or ARB, or in some cases, a β blocker are appropriate first‐step drugs |
| It is also appropriate to begin therapy with a diuretic/ACE inhibitor, diuretic/ARB, or diuretic/β blocker combination |
| If BP is not controlled at BP <130–135/80–85 mm Hg, a CCB or in some cases an a blocker can be added |
| BP=blood pressure; ACE=angiotensin‐converting enzyme; ARB=angiotensin receptor blocker; CCB=calcium channel blocker |
Table VIII.
ADA and NKF Recommendations on Treatment of Hypertension in Diabetes
| Blood pressure goal: 130/80 mm Hg |
| Blood pressure lowering medications should reduce both blood pressure and proteinuria |
| Both these goals reduce: |
| Renal disease progression |
| Incidence of ischemic heart disease |
| ADA=American Diabetes Association; NKF=National Kidney Foundations Executive Working Group. Data derived from ADA. Diabetes Care. 2001;24(suppl 1):S33–S43 and Bakris et al. 10 |
SUMMARY AND CONCLUSION
Strategies for the treatment of the patient with hypertension and diabetes include both glycemic and BP control. In addition, any dyslipidemia should be corrected. ACEIs, ARBs, and β blockers, usually with a diuretic, are probably the drugs of choice. The addition of a CCB may be necessary to achieve goal Bps. Achieving a goal BP of >130–135/80–85 mm Hg should be the objective of treatment. The reductions in BP produce impressive reductions in overall CV diseases, myocardial infarction, stroke, heart failure, and death.
References
- 1. Stamler J, Vaccaro O, Neaton JD, et al. Diabetes, other risk factors, and 12‐yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993; 16:434–444. [DOI] [PubMed] [Google Scholar]
- 2. McFarlane SI, Banerji M, Sowers JR. Insulin resistance and cardiovascular disease. J Clin Endocrinol Metab. 2001;86(2): 713–718. [DOI] [PubMed] [Google Scholar]
- 3. Haffner SM, Lehto S, Ronnemaa T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. [DOI] [PubMed] [Google Scholar]
- 4. Joint National Committee. Report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure. A cooperative study. JAMA. 1977;237(3):255–261. [PubMed] [Google Scholar]
- 5. Joint National Committee . The 1980 report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure. Arch Intern Med. 1980;140(10):1280–1285. [PubMed] [Google Scholar]
- 6. Dinneen SF, Gerstein HC. The association of microalbuminuria and mortality in non‐insulin‐dependent diabetes mellitus. A systematic overview of the literature. Arch Intern Med. 1997;157:1413–1418. [PubMed] [Google Scholar]
- 7. Valmadrid CT, Klein R, Moss SE, et al. The risk of cardiovascular disease mortality associated with microalbuminuria and gross proteinuria in persons with older‐onset diabetes mellitus. Arch Intern Med. 2000;160:1093–1100. [DOI] [PubMed] [Google Scholar]
- 8. Miettinen H, Haffner SM, Lehto S, et al. Proteinuria predicts stroke and other atherosclerotic vascular disease events in nondiabetic and non‐insulin‐dependent diabetic subjects. Stroke. 1996;27:2033–2039. [DOI] [PubMed] [Google Scholar]
- 9. Gerstein HC, Mann JF, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and non‐diabetic individuals. JAMA. 2001;286:421–426. [DOI] [PubMed] [Google Scholar]
- 10. Bakris GL, Williams M, Dworkin L, et al. Preserving renal function in adults with hypertension and diabetes: a consensus approach. National Kidney Foundation Hypertension and Diabetes Executive Committees Working Group. Am J Kidney Dis. 2000;36:646–661. [DOI] [PubMed] [Google Scholar]
- 11. Moser M, Sowers JR. Clinical Management of Cardiovascular risk Factors in Diabetes. Caddo, OK: Professional Communications; 2002. [Google Scholar]
- 12. UK Prospective Diabetes Study Group . Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. BMJ. 1998;317:713–20. [PMC free article] [PubMed] [Google Scholar]
- 13. UK Prospective Diabetes Study Group . Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 14. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood‐pressure lowering and low‐dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755–1762. [DOI] [PubMed] [Google Scholar]
- 15. Curb JD, Pressel SL, Cutler JA, et al. Effect of diuretic‐based antihypertensive treatment on cardiovascular disease risk in older diabetic patients with isolated systolic hypertension. Systolic Hypertension in the Elderly Program Cooperative Research Group. JAMA. 1996;276:1886–1892. [PubMed] [Google Scholar]
- 16. Tuomilehto J, Rastenyte D, Birkenhager WH, et al. Effects of calcium‐channel blockade in older patients with diabetes and systolic hypertension. Systolic Hypertension in Europe Trial Investigators. N Engl J Med. 1999;340:677–684. [DOI] [PubMed] [Google Scholar]
- 17. Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin‐converting‐enzyme inhibitor, ramipril, on cardiovascular events in high‐risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–153. [DOI] [PubMed] [Google Scholar]
- 18. Heart Outcomes Prevention Evaluation Study Investigators . Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO‐HOPE substudy. Lancet. 2000;355(9200):253–259. [PubMed] [Google Scholar]
- 19. Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21(4):597–603. [DOI] [PubMed] [Google Scholar]
- 20. Estacio RO, Jeffers BW, Hiatt WR, et al. The effect of nisoldipine as compared with enalapril on cardiovascular outcomes in patients with non‐insulin‐dependent diabetes and hypertension. N Engl J Med. 1998;338(10):645–652. [DOI] [PubMed] [Google Scholar]
- 21. Parving HH, Lehnert H, Brochner‐Mortensen J, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345:870–878. [DOI] [PubMed] [Google Scholar]
- 22. Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin‐receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001; 345:851–860. [DOI] [PubMed] [Google Scholar]
- 23. Brenner BM, Cooper ME, De Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001; 345:861–869. [DOI] [PubMed] [Google Scholar]
- 24. Lindholm LH, Ibsen H, Dahlof B, et al. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359(9311): 1004–1010. [DOI] [PubMed] [Google Scholar]
- 25. Waagstein F, Bristow MR, Swedberg K, et al. For the Metoprolol in Dilated Cardiomyopathy (MDC) Trial Study Group. Beneficial effects of metoprolol in idiopathic dilated cardiomyopathy. Lancet. 1993;342:1441–1446. [DOI] [PubMed] [Google Scholar]
- 26. Hansson L, Lindholm LH, Niskanen L, et al. Effect of angiotensin‐converting‐enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: the Captopril Prevention Project (CAPPP) randomised trial. Lancet. 1999;353(9153):611–616. [DOI] [PubMed] [Google Scholar]
- 27. Moser M, Ross H. The treatment of hypertension in diabetic patients. Diabetes Care. 1993;16(2):542–547. [DOI] [PubMed] [Google Scholar]
- 28. Moser M. Why are physicians not prescribing diuretics more frequently in the management of hypertension? JAMA. 1998; 279(22):1813–1816. [DOI] [PubMed] [Google Scholar]
- 29. White WB, Prisant LM, Wright JT Jr. Management of patients with hypertension and diabetes mellitus: advances in the evidence for intensive treatment. Am J Med. 2000;108:238–245. [DOI] [PubMed] [Google Scholar]


