Abstract
Background
Following the emergency use authorisation of the Pfizer–BioNTech mRNA COVID-19 vaccine BNT162b2 (international non-proprietary name tozinameran) in Israel, the Ministry of Health (MoH) launched a campaign to immunise the 6·5 million residents of Israel aged 16 years and older. We estimated the real-world effectiveness of two doses of BNT162b2 against a range of SARS-CoV-2 outcomes and to evaluate the nationwide public-health impact following the widespread introduction of the vaccine.
Methods
We used national surveillance data from the first 4 months of the nationwide vaccination campaign to ascertain incident cases of laboratory-confirmed SARS-CoV-2 infections and outcomes, as well as vaccine uptake in residents of Israel aged 16 years and older. Vaccine effectiveness against SARS-CoV-2 outcomes (asymptomatic infection, symptomatic infection, and COVID-19-related hospitalisation, severe or critical hospitalisation, and death) was calculated on the basis of incidence rates in fully vaccinated individuals (defined as those for whom 7 days had passed since receiving the second dose of vaccine) compared with rates in unvaccinated individuals (who had not received any doses of the vaccine), with use of a negative binomial regression model adjusted for age group (16–24, 25–34, 35–44, 45–54, 55–64, 65–74, 75–84, and ≥85 years), sex, and calendar week. The proportion of spike gene target failures on PCR test among a nationwide convenience-sample of SARS-CoV-2-positive specimens was used to estimate the prevelance of the B.1.1.7 variant.
Findings
During the analysis period (Jan 24 to April 3, 2021), there were 232 268 SARS-CoV-2 infections, 7694 COVID-19 hospitalisations, 4481 severe or critical COVID-19 hospitalisations, and 1113 COVID-19 deaths in people aged 16 years or older. By April 3, 2021, 4 714 932 (72·1%) of 6 538 911 people aged 16 years and older were fully vaccinated with two doses of BNT162b2. Adjusted estimates of vaccine effectiveness at 7 days or longer after the second dose were 95·3% (95% CI 94·9–95·7; incidence rate 91·5 per 100 000 person-days in unvaccinated vs 3·1 per 100 000 person-days in fully vaccinated individuals) against SARS-CoV-2 infection, 91·5% (90·7–92·2; 40·9 vs 1·8 per 100 000 person-days) against asymptomatic SARS-CoV-2 infection, 97·0% (96·7–97·2; 32·5 vs 0·8 per 100 000 person-days) against symptomatic COVID-19, 97·2% (96·8–97·5; 4·6 vs 0·3 per 100 000 person-days) against COVID-19-related hospitalisation, 97·5% (97·1–97·8; 2·7 vs 0·2 per 100 000 person-days) against severe or critical COVID-19-related hospitalisation, and 96·7% (96·0–97·3; 0·6 vs 0·1 per 100 000 person-days) against COVID-19-related death. In all age groups, as vaccine coverage increased, the incidence of SARS-CoV-2 outcomes declined. 8006 of 8472 samples tested showed a spike gene target failure, giving an estimated prevalence of the B.1.1.7 variant of 94·5% among SARS-CoV-2 infections.
Interpretation
Two doses of BNT162b2 are highly effective across all age groups (≥16 years, including older adults aged ≥85 years) in preventing symptomatic and asymptomatic SARS-CoV-2 infections and COVID-19-related hospitalisations, severe disease, and death, including those caused by the B.1.1.7 SARS-CoV-2 variant. There were marked and sustained declines in SARS-CoV-2 incidence corresponding to increasing vaccine coverage. These findings suggest that COVID-19 vaccination can help to control the pandemic.
Funding
None.
Introduction
As of April 3, 2021, the SARS-CoV-2 pandemic has resulted in more than 131 million cases and more than 2·8 million deaths worldwide,1 including 821 748 cases and 6236 deaths in Israel2 (population 9·1 million). Among the SARS-CoV-2 strains characterised globally in 2020, the D614G variant was dominant.3 More recently, the SARS-CoV-2 variant B.1.1.7, first identified in the UK and associated with increased transmissibility, has emerged in several countries.3 B.1.1.7 was first reported in Israel on Dec 23, 2020.4
Research in context.
Evidence before this study
The Pfizer–BioNTech mRNA COVID-19 vaccine BNT162b2, administered as two doses 21 days apart, was authorised for emergency use in Israel in December, 2020, after it was shown to have high efficacy against symptomatic laboratory-confirmed COVID-19 in a randomised controlled trial of individuals aged 16 years and older. Since the initiation of vaccine rollout, we have been closely monitoring the scientific literature (including preprint servers) and press coverage to identify reports of BNT162b2 vaccine effectiveness. Although observational studies have estimated the effectiveness of BNT162b2, precise nationwide effectiveness estimates of two doses of BNT162b2 against SARS-CoV-2 outcomes are lacking. More data are particularly needed regarding the vaccine's effectiveness against severe disease and deaths, and effectiveness in older adults. Finally, no country has yet described the nationwide public health impact of a national COVID-19 vaccination campaign.
Added value of this study
This analysis of nationwide surveillance data, done in a period when SARS-CoV-2 variant B.1.1.7 was the dominant strain, provides precise real-world estimates of the high effectiveness of two doses of BNT162b2 against a range of SARS-CoV-2 outcomes, including symptomatic and asymptomatic infection and hospitalisation or death due to COVID-19. The median follow-up time of 7 weeks after the second dose for vaccinated individuals was longer than that in previous reports. Marked and sustained declines in the incidence of SARS-CoV-2 infections were observed in all age groups as the percentage of individuals vaccinated with two BNT162b2 doses began to rise, thereby showing, at a national level, the beneficial public health impact of a nationwide vaccination campaign.
Implications of all the available evidence
Vaccination with two doses of BNT162b2 has high efficacy and effectiveness against a range of SARS-CoV-2 outcomes, including among older adults (aged ≥85 years), offering hope that COVID-19 vaccination will eventually control the pandemic. These findings are of international importance as vaccination programmes ramp up across the rest of the world, suggesting that other countries can similarly achieve marked and sustained declines in SARS-CoV-2 incidence if they can achieve high vaccine uptake.
In a randomised controlled trial (RCT), two doses of the Pfizer–BioNTech mRNA COVID-19 vaccine BNT162b2 (international non-proprietary name tozinameran) had 95% efficacy against symptomatic laboratory-confirmed COVID-19 at least 7 days after the second dose in people aged 16 years or older with no evidence of existing or previous SARS-CoV-2 infection.5 After emergency use authorisation of BNT162b2 in Israel on Dec 6, 2020, the Ministry of Health (MoH) launched a nationwide vaccination campaign to administer two doses of BNT162b2 to the 6·5 million people aged 16 years and older (71% of the population). On April 3, 2021, 61% of the population of Israel had received at least one dose of a COVID-19 vaccine, a proportion higher than that of any other country in the world.6
Preliminary estimates of the effectiveness of one dose of BNT162b2 have been reported from Denmark,7 Israel,8, 9 the UK,10, 11 and the USA,12 and estimates for two doses of BNT162b2 have been described for a subset of the Israeli population enrolled in a health maintenance organisation.13 However, no estimates of the effectiveness of two doses of BNT162b2 against a range of SARS-CoV-2 outcomes, including among older adults, have been reported. Furthermore, population-level estimates of the impact of a COVID-19 vaccine on the incidence of SARS-CoV-2 infections have not been reported.
In this study, we provide nationwide estimates of the effectiveness of two doses of BNT162b2 against a range of SARS-CoV-2 outcomes and to evaluate the nationwide public-health impact following the widespread introduction of the vaccine.
Methods
Study design and population
In this observational study, we analysed nationwide surveillance data from Jan 24 to April 3, 2021, to assess the effectiveness of the BNT162b2 vaccine against various SARS-CoV-2 outcomes. The study population consisted of residents of Israel (ie, the census population) aged 16 years and older. The start of the study period corresponded to 14 days after the first individuals received their second BNT162b2 dose.
Health care in Israel is universal, with government-funded participation in one of four nationwide medical insurance programmes that operate as health maintenance organisations:14 Clalit (in which 54% of the population are enrolled), Maccabi (26%), Meuhedet (12%), and Leumit (8%).15 All Israeli residents are assigned a unique identification number that enables data linkage in the national medical records database.
The Israel MoH planned, organised, and continues to lead the nationwide vaccination campaign, which began on Dec 20, 2020, and was initially targeted at people aged 65 years and older, health-care workers, and residents of long-term care facilities. Vaccine availability was subsequently expanded, approximately weekly, to younger age cohorts in 5-year intervals. Until Feb 28, 2021, because of an insufficient vaccine supply, individuals with a previous diagnosis of laboratory-confirmed SARS-CoV-2 infection were instructed to not seek vaccination, unless they were a resident of a long-term care facility. However, an unknown number of previously diagnosed people received vaccine. Immunisations were given at around 400 vaccination sites. At these sites, information about the administered vaccine was entered into the patient's electronic health record and reported to the national database.
Surveillance of COVID-19 and vaccine uptake are part of the national pandemic response and are collected under Public Health Ordinance number 40. Only aggregate data, with no personal identifiers, were used in this analysis.
The analysis plan for this study was internally reviewed by senior management in the MoH Public Health Services and found to be compliant with all regulatory requirements including the MoH guidelines for human subject research. As no regulatory issues were identified, it was decided that a full ethical review was not necessary. The study followed the Strengthening the Reporting of Observational studies in Epidemiology guidelines.16
Testing for SARS-CoV-2, including variant B.1.1.7
SARS-CoV-2 testing is free-of-charge and widely available in Israel. Testing is required for people returning from travel abroad, in close contact with an infected person, or with suggestive symptoms such as fever or acute respiratory illness. When seeking testing, individuals provide their identification number and a specimen is collected via nasal or nasopharyngeal swab. Specimens are tested, using national testing standards, at one of 48 clinical diagnostic laboratories with use of real-time PCR tests. B.1.1.7 prevalence was estimated on the basis of swabs tested at Leumit with the TaqPath COVID-19 test (Thermo Fisher Scientific, Pleasanton, CA, USA), which identifies spike gene target failure (SGTF) associated with gene mutations that cause deletions of amino acids 69 and 70 in the spike protein. Because these mutations are found in B.1.1.7, SGTF is used to estimate the prevalence of this variant.17, 18
Public health surveillance
MoH conducts surveillance for laboratory-confirmed SARS-CoV-2 infections, with mandatory daily reporting of PCR results by all diagnostic laboratories. An epidemiological investigation, including an interview about COVID-19 symptoms, is done for each SARS-CoV-2 infection, usually within 2 days of diagnosis. MoH also conducts surveillance of COVID-19-associated hospitalisations. Daily updates are received from all hospitals and linked to the national database using patients' identification numbers. Hospitalisations are classified as severe (if a patient has a resting respiratory rate of >30 breaths per minute, oxygen saturation on room air of <94%, or a ratio of PaO2 to FiO2 of <300) or critical (in the event of mechanical ventilation, shock, or cardiac, hepatic, or renal failure). In accordance with national guidelines, health-care providers attributed any hospitalisations and deaths among individuals with laboratory-confirmed SARS-CoV-2 infection to COVID-19.2, 19
Outcomes
Vaccine effectiveness estimates were assessed against six SARS-CoV-2 outcomes, comprising asymptomatic infections and five other hierarchical laboratory-confirmed outcomes: all SARS-CoV-2 infections (symptomatic and asymptomatic), symptomatic COVID-19 cases, and COVID-19-related hospitalisations, severe or critical hospitalisations (including those who died), and deaths. Asymptomatic infection was defined as a person with laboratory-confirmed SARS-CoV-2 infection who reported no fever and no respiratory symptoms during the symptom interview portion of the epidemiological investigation, and who was not subsequently hospitalised for or did not die from COVID-19.
Statistical analysis
Individuals were defined as unvaccinated if they had not received any doses of BNT162b2, and as fully vaccinated if at least 7 days had passed since receiving the second dose of BNT162b2. Incidence rates were calculated for unvaccinated and fully vaccinated individuals aged 16 years and older for each SARS-CoV-2 outcome after excluding people with previous laboratory-confirmed SARS-CoV-2 infection. Data were stratified by age group (16–24, 25–34, 35–44, 45–54, 55–64, 65–74, 75–84, and ≥85 years, based on 2020 census data), sex, and calendar week. In the primary analysis, cases were categorised as vaccinated if the date of laboratory confirmation of infection occurred at least 7 days after the second dose of BNT162b2. Cases were excluded from the analysis if they had received only one dose, or had received two doses of BNT162b2 and fewer than 7 days had passed since the second dose. Person-days for the fully vaccinated group were ascertained each day by multiplying the proportion of people who were fully vaccinated with two doses of BNT162b2 by the census estimates for each age stratum. Person-days for the unvaccinated group were determined each day by subtracting the number of person-days contributed by those who were vaccinated from the total census population for each age stratum; this process was repeated, summed, and aggregated for each day of the study period. Individuals with previous SARS-CoV-2 infection were excluded from person-day estimates. Using STATA (version 15), a negative binomial regression model (nbreg command), which is better suited for over-dispersion of variance than the traditional Poisson regression method,20 was used to derive incidence rate ratios (IRRs) with 95% CIs for each outcome adjusted for age group, sex, and calendar week. The exposure command was used to account for varying patient-days across strata.21 Vaccine effectiveness estimates were calculated as (1 – IRR) × 100. In sensitivity analyses, vaccine effectiveness estimates were also calculated with the same method for people who had received two BNT162b2 doses and for whom at least 14 days had passed after the second dose, as well as for those who had received one dose and for whom 14–21 days had passed after the first dose.
Role of the funding source
The Israel MoH and Pfizer separately provided in-kind support to this study. No funding was exchanged between the Israel MoH and Pfizer. MoH and Pfizer were involved in the study design and writing of the report, and approved the decision to submit for publication.
Results
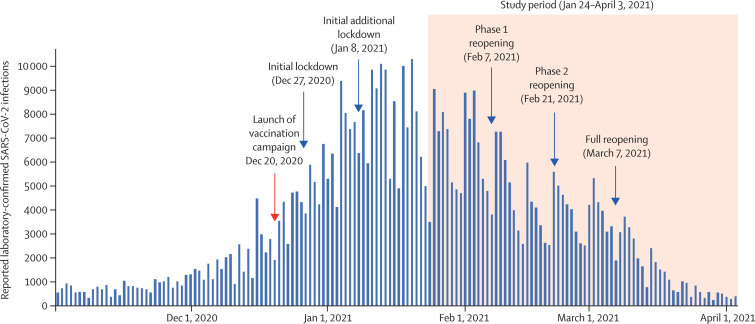
The vaccination campaign was launched on Dec 20, 2020, around the time of a surge in SARS-CoV-2 infections in Israel that resulted in a nationwide lockdown on Dec 27, 2020 (figure 1 ). Additional lockdown restrictions were implemented on Jan 8, 2021. Daily SARS-CoV-2 infections increased in December, 2020, peaking at 10 213 on Jan 20, 2021. Phased reopening occurred on Feb 7 and Feb 21, 2021, and the lockdown was lifted on March 7, 2021.
Figure 1.
Daily laboratory-confirmed SARS-CoV-2 infections in Israel (Nov 1, 2020, to April 3, 2021)
From Jan 24 to April 3, 2021 (the study period), there were 232 268 SARS-CoV-2 infections. 213 919 (92·1%) of infected individuals were interviewed and 186 109 (87·1%) answered the questions about the presence or absence of symptoms. People aged 16 years or older accounted for 154 648 (66·6%) infections, among which 31 548 (20·4%) were in the Arab sector, 24 280 (15·7%) in the ultra-Orthodox sector, and 98 220 (63·9%) in the general Jewish (non-ultra-Orthodox) sector. During the study period, 7694 COVID-19 hospitalisations, 4481 severe or critical COVID-19 hospitalisations, and 1113 COVID-19 deaths occurred in people aged 16 years or older. 8472 (60%) of Leumit PCR tests used TaqPath during the study period, of which 8006 had an SGTF, giving an estimated prevalence of 94·5% for the B.1.1.7 variant.
As of April 3, 2021, BNT162b2 was the only COVID-19 vaccine available in Israel, and more than 10·0 million doses were administered to more than 5·2 million people. Overall, 4 714 932 (72·1%) of 6 538 911 people aged 16 years or older and 1 015 620 (90·0%) of 1 127 965 people aged 65 years or older were fully vaccinated with two doses (table 1 ). By sector, among those aged 16 years or older, 669 542 (54·6%) of 1 226 788 in the Arab population, 228 479 (42·8%) of 534 146 in the ultra-Orthodox population, and 3 816 911 (79·9%) of 4 777 977 in the general Jewish population were fully vaccinated with two doses. Median follow-up for people who received two doses was 48 days (IQR 30–60).
Table 1.
Numbers and proportions of people fully vaccinated with BNT162b2 in the Israel population aged 16 years and older (Jan 24 to April 3, 2021)
| Population | Fully vaccinated* | ||
|---|---|---|---|
| Age-groups, years | |||
| 16–44 | 3 646 848 | 2 290 820 (62·8%) | |
| 45–64 | 1 764 098 | 1 408 492 (79·8%) | |
| ≥65 | 1 127 965 | 1 015 620 (90·0%) | |
| Sex† | |||
| Female | 3 337 693 | 2 398 547 (71·9%) | |
| Male | 3 201 218 | 2 310 788 (72·2%) | |
| Sector | |||
| Arab | 1 226 788 | 669 542 (54·6%) | |
| Ultra-Orthodox | 534 146 | 228 479 (42·8%) | |
| General Jewish (non-ultra-Orthodox) | 4 777 977 | 3 816 911 (79·9%) | |
| Calendar week in 2021 | |||
| Jan 17 to 23 | .. | 248 196 | |
| Jan 24 to 30 | .. | 773 331 | |
| Jan 31 to Feb 6 | .. | 767 416 | |
| Feb 7 to 13 | .. | 285 272 | |
| Feb 14 to 20 | .. | 412 001 | |
| Feb 21 to 27 | .. | 443 542 | |
| Feb 28 to March 6 | .. | 408 882 | |
| March 7 to 13 | .. | 391 983 | |
| March 14 to 20 | .. | 415 510 | |
| March 21 to 27 | .. | 382 569 | |
| March 28 to April 3 | .. | 186 230 | |
| Overall | 6 538 911 | 4 714 932 (72·1%) | |
Data are n or n (%).
Defined as people for whom at least 7 days had passed after the second dose of BNT162b2 vaccine.
Sex was not recorded for 5597 fully vaccinated individuals.
Among 154 648 SARS-CoV-2 infections in those aged 16 years and older, 109 876 (71·0%) were unvaccinated and 6266 (4·1%) were fully vaccinated (with ≥7 days after the second dose). Among 54 677 people aged 16 years and older who had symptomatic COVID-19, 39 065 (71·4%) were unvaccinated and 1692 (3·1%) received two doses (with ≥7 days after the second dose). Among 7694 people aged 16 years and older who were hospitalised with COVID-19, 5526 (71·8%) were unvaccinated and 596 (7·7%) received two doses with ≥7 days after the second dose. 4481 COVID-19-related severe or critical hospitalisations occurred in people aged 16 years and older, among which 3201 (71·4%) people were unvaccinated and 364 (8·1%) were fully vaccinated. Of the 1113 people aged 16 years and older who died from COVID-19, 715 (64·2%) were unvaccinated and 138 (12·4%) were fully vaccinated.
The incidence rate of SARS-CoV-2 infections among adults aged 16 years and older was 91·5 per 100 000 person-days in the unvaccinated group and 3·1 per 100 000 person-days in the fully vaccinated group, with an estimated vaccine effectiveness (adjusted for age group, sex, and calendar week) against SARS-CoV-2 infection of 95·3% (95% CI 94·9–95·7%; table 2 , appendix p 3). The adjusted estimates of vaccine effectiveness were 91·5% (90·7–92·2%) against asymptomatic SARS-CoV-2 infection, 97·0% (96·7–97·2%) against symptomatic COVID-19, 97·2% (95% CI 96·8–97·5%) against COVID-19 hospitalisation, 97·5% (97·1–97·8%) against severe or critical hospitalisation, and 96·7% (95% CI 96·0–97·3%) against death (table 2). Adjusted vaccine effectiveness estimates against all COVID-19 outcomes were higher than 96% among people aged 75 years and older and people aged 85 years and older (table 3 ). Vaccine effectiveness estimates adjusted for each day of the study period (rather than calendar week) yielded similar results (data not shown).
Table 2.
Estimated effectiveness of two doses of BNT162b2 (≥7 days after the second dose) against laboratory-confirmed SARS-CoV-2 outcomes by age group (Jan 24 to April 3, 2021)
|
Unvaccinated |
Fully vaccinated* |
Vaccine effectiveness |
||||
|---|---|---|---|---|---|---|
| Cases | Incidence rate per 100 000 person-days† | Cases | Incidence rate per 100 000 person-days‡ | Unadjusted | Adjusted§ | |
| SARS-CoV-2 infection¶ | ||||||
| Age 16–44 years | 84 611 | 95·1 | 1801 | 2·3 | 95·4% (94·0–96·5) | 96·1% (95·7–96·5) |
| Age 45–64 years | 19 579 | 86·1 | 2264 | 3·4 | 93·6% (91·4–95·3) | 94·9% (94·2–95·5) |
| Age ≥65 years | 5686 | 67·7 | 2201 | 3·8 | 93·4% (91·3–95·0) | 94·8% (93·9–95·5) |
| All ages | 109 876 | 91·5 | 6266 | 3·1 | 94·2% (93·2–95·1) | 95·3% (94·9–95·7) |
| Asymptomatic SARS-CoV-2 infection | ||||||
| Age 16–44 years | 40 088 | 45·1 | 1174 | 1·5 | 92·8% (90·3–94·7) | 93·6% (92·8–94·4) |
| Age 45–64 years | 7414 | 32·6 | 1343 | 2·0 | 89·1% (84·7–92·3) | 90·8% (89·6–91·9) |
| Age ≥65 years | 1636 | 19·5 | 1115 | 1·9 | 85·9% (80·2–89·9) | 88·5% (86·4–90·3) |
| All ages | 49 138 | 40·9 | 3632 | 1·8 | 90·1% (88·0–91·8) | 91·5% (90·7–92·2) |
| Symptomatic COVID-19 | ||||||
| Age 16–44 years | 28 196 | 31·7 | 352 | 0·5 | 97·8% (97·0–98·3) | 97·6% (97·3–97·8) |
| Age 45–64 years | 7790 | 34·3 | 560 | 0·8 | 96·3% (95·0–97·3) | 96·7% (96·3–97·0) |
| Age ≥65 years | 3079 | 36·6 | 780 | 1·4 | 96·1% (94·8–97·1) | 96·4% (95·9–97·0) |
| All ages | 39 065 | 32·5 | 1692 | 0·8 | 96·6% (95·8–97·2) | 97·0% (96·7–97·2) |
| COVID-19-related hospitalisation | ||||||
| Age 16–44 years | 2043 | 2·3 | 33 | <0·1 | 98·1% (97·1–98·8) | 98·1% (97·3–98·7) |
| Age 45–64 years | 1687 | 7·4 | 112 | 0·2 | 97·6% (96·9–98·2) | 97·6% (97·1–98·1) |
| Age ≥65 years | 1826 | 21·7 | 451 | 0·8 | 96·6% (95·3–97·6) | 96·8% (96·2–97·3) |
| All ages | 5526 | 4·6 | 596 | 0·3 | 96·7% (95·5–97·6) | 97·2% (96·8–97·5) |
| Severe or critical COVID-19-related hospitalisation | ||||||
| Age 16–44 years | 644 | 0·7 | 7 | 0·01 | 98·8% (97·3–99·5) | 98·9% (97·6–99·5) |
| Age 45–64 years | 1132 | 5·0 | 62 | 0·1 | 98·1% (97·2–98·6) | 98·1% (97·5–98·5) |
| Age ≥65 years | 1425 | 17·0 | 295 | 0·5 | 97·2% (95·9–98·1) | 97·3% (96·8–97·8) |
| All ages | 3201 | 2·7 | 364 | 0·2 | 97·2% (95·9–98·1) | 97·5% (97·1–97·8) |
| COVID-19-related death | ||||||
| Age 16–44 years | 36 | 0·04 | 0 | 0·0 | 100 | 100 |
| Age 45–64 years | 125 | 0·5 | 14 | <0·1 | 96·2% (92·6–98·0) | 95·8% (92·6–97·6) |
| Age ≥65 years | 554 | 6·6 | 124 | 0·2 | 96·8% (94·6–98·1) | 96·9% (96·0–97·6) |
| All ages | 715 | 0·6 | 138 | 0·1 | 96·6% (93·9–98·1) | 96·7% (96·0–97·3) |
Numbers and incidence rates of outcomes are shown for unvaccinated and fully vaccinated individuals. Vaccine effectiveness estimates are % (95% CI).
Defined as people for whom at least 7 days had passed after the second dose of BNT162b2 vaccine.
Total person-days for all outcomes were 88 938 310 for age 16–44 years, 22 734 104 for age 45–64 years, 8 403 722 for age ≥65 years, and 120 076 136 for all ages.
Total person-days for all outcomes were 77 280 829 for age 16–44 years, 67 027 668 for age 45–64 years, 57 573 686 for age ≥65 years, and 201 882 183 for all ages.
Model is adjusted for age group (16–24, 25–34, 35–44, 45–54, 55–64, 65–74, 75–84, and ≥85 years), sex, and calendar week.
Includes asymptomatic and symptomatic infections, as well as cases with positive SARS-CoV-2 tests for which the symptom interview portion of the epidemiological investigation was not completed.
Table 3.
Estimated effectiveness of two doses of BNT162b2 (≥7 days after the second dose) against laboratory-confirmed SARS-CoV-2 outcomes in the oldest age groups (Jan 24 to April 3, 2021)
|
Vaccine effectiveness* |
|||
|---|---|---|---|
| Age ≥65 years | Age ≥75 years | Age ≥85 years | |
| SARS-CoV-2 infection† | 94·8% (93·9–95·5) | 95·1% (93·9–96·0) | 94·1% (91·9–95·7) |
| Asymptomatic SARS-CoV-2 infection | 88·5% (86·4–90·3) | 87·5% (84·2–90·1) | 83·2% (76·3–88·1) |
| Symptomatic COVID-19 | 96·4% (95·9–97·0) | 96·7% (95·9–97·4) | 96·6% (95·2–97·6) |
| COVID-19-related hospitalisation | 96·8% (96·2–97·3) | 97·0% (96·2–97·7) | 96·9% (95·5–97·9) |
| Severe or critical COVID-19-related hospitalisation | 97·3% (96·8–97·8) | 97·6% (96·8–98·1) | 97·4% (95·9–98·3) |
| COVID-19-related death | 96·9% (96·0–97·6) | 97·1% (96·0–97·9) | 97·0% (94·9–98·3) |
Estimates are % (95% CI).
Model is adjusted for age group (16–24, 25–34, 35–44, 45–54, 55–64, 65–74, 75–84, and ≥85 years), sex, and calendar week.
Includes asymptomatic and symptomatic infections, as well as cases with positive SARS-CoV-2 tests for which the symptom interview portion of the epidemiological investigation was not completed.
Adjusted vaccine effectiveness estimates against all SARS-CoV-2 outcomes were slightly higher at 14 days or longer after the second dose (table 4 ; appendix p 4) and somewhat lower at 14–21 days after the first dose (appendix p 5) compared with those at 7 days or longer after the second dose. Vaccine effectiveness against deaths was estimated to be 98·1% at 14 days or longer after the second dose and 77·0·% at 14–21 days after the first dose (in contrast to 96·7% at 7 days or longer after the second dose).
Table 4.
Estimated effectiveness of two doses of BNT162b2 (≥14 days after the second dose) against laboratory-confirmed SARS-CoV-2 outcomes by age group (Jan 24 to April 3, 2021)
|
Unvaccinated |
Fully vaccinated* |
Vaccine effectiveness |
||||
|---|---|---|---|---|---|---|
| Number of cases | Incidence rate per 100 000 person-days† | Number of cases | Incidence rate per 100 000 person-days‡ | Unadjusted | Adjusted§ | |
| SARS-CoV-2 infection¶ | ||||||
| Age 16–44 years | 84 611 | 95·1 | 1066 | 1·7 | 97·2% (96·3–97·8) | 97·1% (96·7–97·3) |
| Age 45–64 years | 19 579 | 86·1 | 1292 | 2·2 | 96·5% (95·6–97·2) | 96·5% (96·3–96·7) |
| Age ≥65 years | 5686 | 67·7 | 1284 | 2·5 | 96·1% (95·1–96·9) | 95·9% (95·5–96·3) |
| All ages | 109 876 | 91·5 | 3642 | 2·1 | 96·6% (96·1–97·0) | 96·5% (96·3–96·8) |
| Asymptomatic SARS-CoV-2 infection | ||||||
| Age 16–44 years | 40 088 | 45·1 | 666 | 1·1 | 95·8% (94·4–96·9) | 95·2% (94·6–95·8) |
| Age 45–64 years | 7414 | 32·6 | 729 | 1·3 | 94·3% (92·4–95·7) | 94·0% (93·4–94·4) |
| Age ≥65 years | 1636 | 19·5 | 633 | 1·2 | 91·7% (88·8–93·8) | 91·5% (90·4–92·5) |
| All ages | 49 138 | 40·9 | 2028 | 1·2 | 94·4% (93·3–95·3) | 93·8% (93·3–94·2) |
| Symptomatic COVID-19 | ||||||
| Age 16–44 years | 28 196 | 31·7 | 230 | 0·4 | 98·4% (97·7–98·8) | 97·8% (97·5–98·1) |
| Age 45–64 years | 7790 | 34·3 | 333 | 0·6 | 97·9% (97·2–98·4) | 97·7% (97·4–97·9) |
| Age ≥65 years | 3079 | 36·6 | 437 | 0·9 | 97·9% (97·3–98·4) | 97·5% (97·2–97·8) |
| All ages | 39 065 | 32·5 | 1000 | 0·6 | 98·0% (97·6–98·3) | 97·7% (97·5–97·9) |
| COVID-19-related hospitalisation | ||||||
| Age 16–44 years | 2043 | 2·3 | 26 | 0·0 | 98·1% (97·0–98·8) | 98·1% (97·1–98·7) |
| Age 45–64 years | 1687 | 7·4 | 74 | 0·1 | 98·3% (97·6–98·7) | 98·2% (97·7–98·6) |
| Age ≥65 years | 1826 | 21·7 | 259 | 0·5 | 98·2% (97·6–98·7) | 97·9% (97·6–98·1) |
| All ages | 5556 | 4·6 | 359 | 0·2 | 98·2% (97·5–98·7) | 98·0% (97·7–98·3) |
| Severe or critical COVID-19-related hospitalisation | ||||||
| Age 16–44 years | 644 | 0·7 | 5 | 0·01 | 98·9% (97·3–99·6) | 99·0% (97·5–99·6) |
| Age 45–64 years | 1132 | 5·0 | 41 | 0·1 | 98·6% (97·9–99·0) | 98·5% (97·9–98·9) |
| Age ≥65 years | 1425 | 17·0 | 160 | 0·3 | 98·7% (98·1–99·1) | 98·3% (98·0–98·6) |
| All ages | 3201 | 2·7 | 206 | 0·1 | 98·6% (98·0–99·0) | 98·4% (98·1–98·6) |
| COVID-19-related death | ||||||
| Age 16–44 years | 36 | 0·04 | 0 | 0 | 100 | 100 |
| Age 45–64 years | 125 | 0·5 | 10 | <0·1 | 96·9% (93·5–98·5) | 96·5% (93·2–98·2) |
| Age ≥65 years | 554 | 6·6 | 61 | 0·1 | 98·7% (97·8–99·2) | 98·2% (97·7–98·7) |
| All ages | 715 | 0·6 | 71 | <0·1 | 98·5% (97·4–99·2) | 98·1% (97·6–98·5) |
Numbers and incidence rates of outcomes are shown for unvaccinated and fully vaccinated individuals. Vaccine effectiveness estimates are % (95% CI).
Defined as people for whom at least 14 days had passed after the second dose of BNT162b2 vaccine.
Total person-days for all outcomes were 88 938 310 for age 16–44 years, 22 734 104 for age 45–64 years, 8 403 722 for age ≥65 years, and 120 076 136 for all ages.
Total person-days for all outcomes were 61 397 072 for age 16–44 years, 57 734 915 for age 45–64 years, 51 302 672 for age ≥65 years, and 170 434 659 for all ages.
Model is adjusted for age group (16–24, 25–34, 35–44, 45–54, 55–64, 65–74, 75–84, and ≥85 years), sex, and calendar week.
Includes asymptomatic and symptomatic infections, as well as cases with positive SARS-CoV-2 tests for which the symptom interview portion of the epidemiological investigation was not completed.
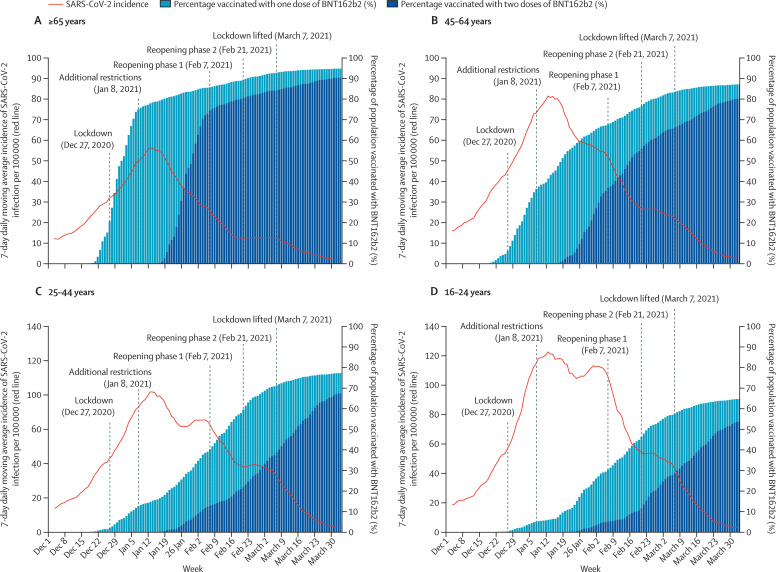
Overall, as cumulative vaccination coverage increased, the 7-day daily moving average of incident cases of SARS-CoV-2 infection (per 100 000 people) markedly declined across all age groups (figure 2 ). Notably, steeper and earlier declines were observed in older age groups, which had higher and earlier vaccine coverage. Specifically, although some declines in incident infections were evident around 2 weeks following the implementation of lockdown, sharper declines followed increased vaccine uptake. For example, steep reductions in incident cases of SARS-CoV-2 infections were observed for people aged 65 years and older starting in mid-January, 2021, but were not observed until 3–4 weeks later among people aged 16–24 years, when vaccine coverage for this age group began to increase. Similar marked declines in all age groups, corresponding to increasing vaccine coverage, were seen in the incidence of COVID-19 hospitalisations, severe or critical hospitalisations, and deaths (appendix pp 6–11). It is also noteworthy that the declines continued even after the two phases of reopening and the final lifting of the lockdown.
Figure 2.
Incident cases of SARS-CoV-2 infection and prevalence of BNT162b2 vaccination by age group in Israel (Dec 1, 2020, to April 3, 2021)
Graphs show the 7-day daily moving average of laboratory-confirmed SARS-CoV-2 infections and the percentage of the population who had received one and two doses of the vaccine at each time point in people aged 65 years and older (A), 45–64 years (B), 25–44 years (C), and 16–24 years (D). Note that y-axis scales showing infection incidence differ between age groups.
Discussion
This nationwide observational study, with a median follow-up period of almost 7 weeks after receipt of the second vaccine dose, showed high effectiveness of two doses of BNT162b2, including among older adults, against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, severe disease, and deaths. Corroborating the high effectiveness observed, marked declines in incident cases of SARS-CoV-2 infection were observed as vaccine coverage increased. Although population-level vaccine effectiveness data are ecological, and teasing apart the impact of a vaccination programme from the impact of non-pharmaceutical interventions (including a nationwide lockdown) is complex, it is noteworthy that declines in incident cases of SARS-CoV-2 for each age group corresponded with achieving high vaccine coverage in that age group rather than initiation of the nationwide lockdown. These findings suggest that the primary driver of reductions in the incidence of SARS-CoV-2 infections was high vaccine coverage, not implementation of the lockdown. Furthermore, even after reopenings occurred, SARS-CoV-2 incidence remained low, suggesting that high vaccine coverage might provide a sustainable path towards resuming normal activity. These data provide nationwide evidence of the beneficial public health impact of a COVID-19 vaccination campaign.
During the study period, 95% of 8472 tested specimens showed an SGTF, associated with SARS-CoV-2 variant B.1.1.7, thereby providing evidence that BNT162b2 is effective against B.1.1.7. These findings are consistent with laboratory studies that indicated that BNT162b2 was likely to be effective against B.1.1.7.22 Notably, another clinically important variant, B.1.351, which was initially identified in South Africa, has recently been identified in Israel.23 Vaccine effectiveness against B.1.351, however, could not be estimated in our study because of the small number of B.1.351 infections identified in Israel during the study period.
This study also suggests that two doses of BNT162b2 are effective against asymptomatic SARS-CoV-2 infections (with vaccine effectiveness estimates of 92% at ≥7 days after the second dose and 94% at ≥14 after the second dose). Estimates of effectiveness against asymptomatic infections were slightly lower than those against COVID-19, which could suggest a higher threshold of protection for asymptomatic infection compared with symptomatic illness. We conservatively defined asymptomatic infections as SARS-CoV-2 infections in interviewees who reported no fever and no respiratory symptoms at the time of the interview. Additionally, it is possible that some SARS-CoV-2-infected individuals who reported being asymptomatic at the time of interview might have instead been presymptomatic (ie, developed symptoms later). Although we made efforts to avoid this type of misclassification by excluding the small number of people who were initially reported to be asymptomatic but were later hospitalised for or died from COVID-19, some presymptomatic individuals who later developed symptoms without being hospitalised or dying might still have been included. This type of misclassification, however, was probably uncommon and would be unlikely to substantially influence the vaccine effectiveness estimate against asymptomatic infection.
Notably, Israel's SARS-CoV-2 testing policy was different for unvaccinated and vaccinated individuals during the study period. At 7 days after the second dose, vaccinated individuals were exempt from the SARS-CoV-2 testing required of individuals who either had contact with a laboratory-confirmed case or returned from travel abroad. This testing policy might have resulted in a differential bias that would cause overestimation of vaccine effectiveness against asymptomatic infection (ie, asymptomatic people who received two doses were less likely to be tested than unvaccinated asymptomatic people). However, 19% of the 4·4 million PCR tests conducted during the study period were done on exempted individuals (MoH, unpublished data). Additionally, symptomatic individuals might have been reluctant to report symptoms for fear of being blamed for infecting other individuals, in which case asymptomatic vaccine effectiveness would also be overestimated. Conversely, individuals who were hesitant to receive a COVID-19 vaccine might also have been reluctant to seek SARS-CoV-2 testing, which would lead to underestimation of vaccine effectiveness against asymptomatic infection. Further studies are needed to confirm the magnitude of BNT162b2 protection against asymptomatic infection that we observed. Specifically, studies are needed to evaluate testing behaviour of vaccinated and unvaccinated people and to determine the extent to which prevention of asymptomatic infection leads to interruption of transmission.
Two-dose BNT162b2 vaccine effectiveness estimates from this observational study align with the 95% efficacy against symptomatic SARS-CoV-2 infections shown in the pivotal RCT.5 Our study adds important new data about the effectiveness of BNT162b2 derived from outside of the RCT setting. We found high vaccine effectiveness against a wider range of SARS-CoV-2 outcomes (including severe COVID-19 and deaths) than were evaluated in the RCT, as well as high effectiveness in older adults with a level of precision not available in the RCT. In addition, although pregnant women and immunocompromised individuals were excluded from the RCT, these groups were recommended to receive BNT162b2 in Israel and an unknown number would, therefore, have been vaccinated. Finally, unlike the clinical trial, our study provides evidence that achieving high population-level coverage with BNT162b2 can lead to marked declines in the incidence of SARS-CoV-2 infections and COVID-19 outcomes.
In the primary analysis of the Israel MoH surveillance data, we evaluated the effectiveness of two doses of BNT162b2, given Israel's adherence to the authorised two-dose 21-day vaccine schedule. In the sensitivity analysis, we showed moderate effectiveness of the vaccine against all SARS-CoV-2 outcomes at 14–21 days after the first dose. This finding is similar to those of studies from Israel8, 9 early in the vaccine campaign and in the UK.10, 11 Estimated effectiveness against all outcomes at 14–21 days after the first dose was lower than that of two doses at 7 days or longer or at 14 days or longer after the second dose, demonstrating the importance of fully vaccinating adults. Furthermore, despite indications of at least partial effectiveness after one dose of BNT162b2, relying on protection against COVID-19 from a single dose might not be prudent; BNT162b2 was developed and evaluated in the RCT as a two-dose schedule,5 and substantially lower levels of neutralising antibodies were observed after one dose compared with after two doses.24 Additionally, little is known about the duration of protection of one dose and how it compares with the durability after two doses. It is possible that one dose will provide a shorter duration of protection than two doses, particularly in an environment where new SARS-CoV-2 variants continue to emerge.
Our study has some limitations. In the absence of randomisation, there could have been unmeasured differences between vaccinated and unvaccinated persons (eg, different test-seeking behaviours or levels of adherence to non-pharmaceutical interventions) which might have confounded our vaccine effectiveness estimates.25 Although we adjusted our estimates for age, sex, and calendar week, the effect of additional covariates such as location, comorbidities, race or ethnicity, socioeconomic status, and likelihood of seeking SARS-CoV-2 testing should be evaluated in future studies. Preliminary findings from a study in Israel, for example, indicate that neighbourhood might be an important confounder.9 Misclassification of exposures and outcomes in our study are potentially more common than in the RCT, although misclassification was probably limited by Israel's readily available SARS-CoV-2 testing and comprehensive surveillance system. Misclassification of vaccine history in our study was also unlikely because of comprehensive recording of vaccine administration in Israel. With nearly 7 weeks of follow-up after the second dose, our study has the longest follow-up reported so far, although longer-term data on effectiveness are needed. Another limitation is that the time from symptom onset to hospitalisation and death might have prevented identification of all hospitalisations and deaths during the study period. Such unidentified hospitalisations and deaths are unlikely, however, to be differential between the vaccinated and unvaccinated groups. Finally, given differences between countries in how vaccines are rolled out and in how the pandemic evolves, caution should be used in extrapolating our findings to other populations. Further real-world effectiveness studies of BNT162b2, and other COVID-19 vaccines, in other populations and settings are needed.
Israel provides a unique opportunity to observe the nationwide impact on SARS-CoV-2 transmission of a rapidly increasing percentage of the population with vaccine-derived immunity. SARS-CoV-2 transmission is likely to continue until the proportion of the population with immunity exceeds a herd immunity threshold,26 which has been estimated to be at least 60%,27 although the emergence of more transmissible SARS-CoV-2 variants could result in higher herd immunity thresholds. Achieving the SARS-CoV-2 herd immunity threshold might not be reached, however, without vaccinating some individuals younger than 16 years. In addition, the duration of immunity to SARS-CoV-2, either from infection or immunisation, is not known, and progress towards herd immunity in Israel could be disrupted by the emergence of new SARS-CoV-2 variants if those variants are less susceptible to the current vaccine-induced immune response and if they were to become broadly disseminated. Further studies are needed to monitor the population level of immunity, identify disruption of viral transmission, and detect and evaluate the effects of emerging SARS-CoV-2 variants.
This study showed that two doses of BNT162b2 were highly effective, including in older adults, against laboratory-confirmed SARS-CoV-2 infections and COVID-19 hospitalisations, severe disease, and deaths in a nationwide observational study where variant B.1.1.7 was the dominant strain. Marked nationwide declines in the incidence of SARS-CoV-2 infections and COVID-19 outcomes corresponded with increasing vaccine coverage, and these declines were sustained even after societal reopening. Finally, the high effectiveness against all SARS-CoV-2 infections and apparent effectiveness against infections that were asymptomatic at the time of epidemiological investigation suggest that BNT162b2 might reduce SARS-CoV-2 transmission. Taken together, these findings suggest that high vaccine uptake can meaningfully stem the pandemic and offers hope for eventual control of the SARS-CoV-2 outbreak as vaccination programmes ramp up across the rest of the world.
This online publication has been corrected. The corrected version first appeared at thelancet.com on July 15, 2021
Data sharing
The individual-level data used in this study are sensitive and cannot be publicly shared. Requests for data should be made to the Ministry of Health of Israel. Aggregated surveillance data are freely available online at https://data.gov.il/dataset/covid-19.
Declaration of interests
FJA, JMM, FK, GM, KP, JS, DLS, and LJ hold stock and stock options in Pfizer. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
We thank Natalia Bilenko, Tal Brosh, Dani Cohen, Ron Dagan, Aharona Glatman-Freedman, Michael Gdalevich, Manfred Green, Yoram Hamu, Amit Huppert, Udi Kaliner, Boaz Lev, Ella Mendelson, Ami Mizrachi, Walid Salliba, Avigdor Shafferman, Chen Stein-Zamir, Michal Stein, Dana Wolf, and Gidon Zuriely of the Israel Advisory Council for COVID-19 Vaccine Effectiveness for their guidance and feedback on data management and analysis. We also thank Rona Kaiser, Hanna Levi, Gilad Saar, Osnath Dreyfuss, and Natalia Pertsovsky from the Israel MoH for data management and programming assistance; Yotam Shenhar from Leumit Health Services and Ron Milo and Yinon Bar On from the Weizmann Institute of Science for assistance with data on SARS-CoV-2 variant B.1.1.7 in Israel; and Marc Lipsitch and Miguel Hernan from Harvard University for epidemiological guidance. We acknowledge Ugur Sahin and Özlem Türeci from BioNTech, the holder of the emergency use authorisation for BNT162b2 in Israel; BNT162b2 is produced using BioNTech proprietary mRNA technology and was developed by BioNTech and Pfizer.
Contributors
EJH and SA-P conceived the study, conducted the analysis, and edited the final manuscript. EJH, FJA, JMM, and DLS wrote the first draft of the protocol. EJH, JMM, FK, and KP cleaned and analysed the data. All authors contributed to study design, drafting the protocol, and revising the manuscript for important intellectual content, were responsible for the decision to submit for publication, and approved the final submitted version of the manuscript. All authors had full access to the deidentified and aggregated data in the study. EJH, JMM, and FK accessed and verified the data underlying the study and take responsiblity for the data.
Supplementary Material
References
- 1.Worldometer COVID-19 coronavirus pandemic. https://www.worldometers.info/coronavirus
- 2.Israel Ministry of Health COVID-19 database (in Hebrew) https://data.gov.il/dataset/covid-19
- 3.Moore JP, Offit PA. SARS-CoV-2 vaccines and the growing threat of viral variants. JAMA. 2021;325:821–822. doi: 10.1001/jama.2021.1114. [DOI] [PubMed] [Google Scholar]
- 4.Ayyub R. Reuters; Dec 23, 2020. UK COVID-19 variant detected in Israel, health ministry says.https://www.reuters.com/article/uk-health-coronavirus-israel/uk-covid-19-variant-detected-in-israel-health-ministry-says-idUSKBN28X28C [Google Scholar]
- 5.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Our World in Data Coronavirus (COVID-19) vaccinations. http://www.ourworldindata.org/covid-vaccinations
- 7.Moustsen-Helms IR, Emborg HD, Nielsen J, et al. Vaccine effectiveness after 1st and 2nd dose of the BNT162b2 mRNA COVID-19 vaccine in long-term care facility residents and healthcare workers—a Danish cohort study. medRxiv. 2021 doi: 10.1101/2021.03.08.21252200. published online March 9. (preprint). [DOI] [Google Scholar]
- 8.Amit S, Regev-Yochay G, Afek A, Kreiss Y, Leshem E. Early rate reductions of SARS-CoV-2 infection and COVID-19 in BNT162b2 vaccine recipients. Lancet. 2021;397:875–877. doi: 10.1016/S0140-6736(21)00448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chodick G, Tene L, Tene L, et al. The effectiveness of the first dose of BNT162b2 vaccine in reducing SARS-CoV-2 infection 13–24 days after immunization: real-world evidence. medRxiv. 2021 doi: 10.1101/2021.01.27.21250612. published online Jan 29. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vasileiou E, Simpson CR, Shi T, et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet. 2021;397:1646–1657. doi: 10.1016/S0140-6736(21)00677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall V, Foulkes S, Saei A, et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021 doi: 10.1016/S0140-6736(21)00790-X. published online April 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Britton A, Jacobs Slifka KM, Edens C, et al. Effectiveness of the Pfizer-BioNTech COVID-19 vaccine among residents of two skilled nursing facilities experiencing COVID-19 outbreaks—Connecticut, December 2020–February 2021. MMWR Morb Mortal Wkly Rep. 2021;70:396–401. doi: 10.15585/mmwr.mm7011e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dagan N, Barda M, Kepten E, et al. BNT162b2 mRNA COVID-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.State of Israel Rights of the insured under the national health insurance law. https://www.health.gov.il/English/Topics/RightsInsured/RightsUnderLaw/Pages/default.aspx
- 15.National Insurance Institute of Israel Calculation of the key to the distribution of health insurance funds between the health funds as of February 1, 2021. https://www.btl.gov.il/Mediniyut/Situation/haveruth1/2021/Pages/capitatia_022021.aspx (in Hebrew).
- 16.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Public Health England Investigation of novel SARS-CoV-2 variant: variant of concern 202012/01. Dec 28, 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/959360/Variant_of_Concern_VOC_202012_01_Technical_Briefing_3.pdf
- 18.Zuckerman NS, Pando R, Bucris E, et al. Comprehensive analyses of SARS-CoV-2 transmission in a public health virology laboratory. Viruses. 2020;12:854. doi: 10.3390/v12080854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Institutes of Health COVID-19 treatment guidelines: clinical spectrum of SARS-CoV-2 infection. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
- 20.Zhang X, Mallick H, Tang Z, et al. Negative binomial mixed models for analyzing microbiome count data. BMC Bioinformatics. 2017;18:4. doi: 10.1186/s12859-016-1441-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.UCLA Institute for Digital Research and Education Statistical Consulting Regression models with count data. https://stats.idre.ucla.edu/stata/seminars/regression-models-with-count-data/
- 22.Muik A, Wallisch AK, Sänger B, et al. Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine-elicited human sera. Science. 2021;371:1152–1153. doi: 10.1126/science.abg6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jewish News Syndicate Israeli Health Ministry reports four cases of South African COVID-19 variant. https://www.jns.org/israeli-health-ministry-reports-four-cases-of-south-african-covid-19-variant/
- 24.Mulligan MJ, Lyke KE, Kitchin N, et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- 25.Jackson ML, Yu O, Nelson JC, et al. Further evidence for bias in observational studies of influenza vaccine effectiveness: the 2009 influenza A(H1N1) pandemic. Am J Epidemiol. 2013;178:1327–1336. doi: 10.1093/aje/kwt124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fine P, Eames K, Heymann DL. “Herd immunity”: a rough guide. Clin Infect Dis. 2011;52:911–916. doi: 10.1093/cid/cir007. [DOI] [PubMed] [Google Scholar]
- 27.Angulo FJ, Finelli L, Swerdlow DL. Reopening society and the need for real-time assessment of COVID-19 at the community level. JAMA. 2020;323:2247–2248. doi: 10.1001/jama.2020.7872. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The individual-level data used in this study are sensitive and cannot be publicly shared. Requests for data should be made to the Ministry of Health of Israel. Aggregated surveillance data are freely available online at https://data.gov.il/dataset/covid-19.