Abstract
Background
The consequences of COVID-19 in those who recover from acute infection requiring hospitalisation have yet to be clearly defined. We aimed to describe the temporal trends in respiratory outcomes over 12 months in patients hospitalised for severe COVID-19 and to investigate the associated risk factors.
Methods
In this prospective, longitudinal, cohort study, patients admitted to hospital for severe COVID-19 who did not require mechanical ventilation were prospectively followed up at 3 months, 6 months, 9 months, and 12 months after discharge from Renmin Hospital of Wuhan University, Wuhan, China. Patients with a history of hypertension; diabetes; cardiovascular disease; cancer; and chronic lung disease, including asthma or chronic obstructive pulmonary disease; or a history of smoking documented at time of hospital admission were excluded at time of electronic case-note review. Patients who required intubation and mechanical ventilation were excluded given the potential for the consequences of mechanical ventilation itself to influence the factors under investigation. During the follow-up visits, patients were interviewed and underwent physical examination, routine blood test, pulmonary function tests (ie, diffusing capacity of the lungs for carbon monoxide [DLCO]; forced expiratory flow between 25% and 75% of forced vital capacity [FVC]; functional residual capacity; FVC; FEV1; residual volume; total lung capacity; and vital capacity), chest high-resolution CT (HRCT), and 6-min walk distance test, as well as assessment using a modified Medical Research Council dyspnoea scale (mMRC).
Findings
Between Feb 1, and March 31, 2020, of 135 eligible patients, 83 (61%) patients participated in this study. The median age of participants was 60 years (IQR 52–66). Temporal improvement in pulmonary physiology and exercise capacity was observed in most patients; however, persistent physiological and radiographic abnormalities remained in some patients with COVID-19 at 12 months after discharge. We found a significant reduction in DLCO over the study period, with a median of 77% of predicted (IQR 67–87) at 3 months, 76% of predicted (68–90) at 6 months, and 88% of predicted (78–101) at 12 months after discharge. At 12 months after discharge, radiological changes persisted in 20 (24%) patients. Multivariate logistic regression showed increasing odds of impaired DLCO associated with female sex (odds ratio 8·61 [95% CI 2·83–26·2; p=0·0002) and radiological abnormalities were associated with peak HRCT pneumonia scores during hospitalisation (1·36 [1·13–1·62]; p=0·0009).
Interpretation
In most patients who recovered from severe COVID-19, dyspnoea scores and exercise capacity improved over time; however, in a subgroup of patients at 12 months we found evidence of persistent physiological and radiographic change. A unified pathway for the respiratory follow-up of patients with COVID-19 is required.
Funding
National Natural Science Foundation of China, UK Medical Research Council, and National Institute for Health Research Southampton Biomedical Research Centre.
Translation
For the Chinese translation of the abstract see Supplementary Materials section.
Introduction
The spread of the COVID-19 pandemic worldwide has placed an enormous burden on health authorities across the world. The symptoms associated with COVID-19 are diverse, ranging from mild upper respiratory tract symptoms to severe acute respiratory distress syndrome. Additionally, several non-respiratory presentations have been reported in the literature, including haematological, gastroenterological, renal, dermatological, neurological, and psychiatric manifestations.1 To date, over 100 million people worldwide have recovered from COVID-19, but concern remains that some organs, including the lungs, might have long-term impairment following infection.
Although data to accurately estimate the extent of post-COVID-19 sequelae are missing, post-viral syndromes are well documented following other viral infections, including previous coronavirus outbreaks such as severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). SARS resulted in significant effects on pulmonary function, chronic musculoskeletal pain, and long-term mental disorders in survivors.2, 3, 4, 5 In MERS survivors, at a median follow-up point of 6 weeks, 13 (36%) of 36 patients had residual chest radiographic changes, almost all of which were due to pulmonary fibrosis.6 Moreover, data collected from the COVID Symptom Study suggest that although most people recover from COVID-19 within 2 weeks, approximately 10% of patients might still have symptoms after 3 weeks and some for months. Post-COVID-19 sequelae have been reported to include pulmonary fibrosis; pulmonary and systemic vascular disease; bronchiectasis; chronic fatigue; and mental disorders, including post-traumatic stress disorder, depression, and anxiety.7 Therefore, these patients should be followed up to detect and manage pulmonary sequelae and functional impairment.
Research in context.
Evidence before this study
We searched PubMed without language restriction for studies published from database inception until March 30, 2021, using keywords “2019 novel coronavirus”, “2019-nCoV”, “SARS-CoV-2”, “COVID-19” AND “follow-up” OR “pulmonary function” OR “sequelae”. We included all reports on outcomes up to 6 months in patients admitted to hospital with COVID-19. Although there are reports on outcomes up to 6 months following discharge for patients admitted to hospital with COVID-19 pneumonia, the temporal changes as well as 12-month outcomes have not previously been reported.
Added value of this study
We present the 3-month, 6-month, 9-month, and 12-month outcomes of a prospective cohort of 83 patients with severe COVID-19 who did not require mechanical ventilation. Serial pulmonary function, exercise capacity, and chest high-resolution CT were examined at 3 months, 6 months, 9 months, and 12 months after discharge. In most patients who recovered from severe COVID-19, exercise capacity improved over this time period; however, we found evidence of persistent physiological and radiographic change in a subgroup of patients, with women having a higher risk than men of persistent lung diffusion impairment.
Implications of all the available evidence
Routine respiratory follow-up of patients hospitalised with COVID-19 pneumonia is warranted. Investigation into potential sex-specific differences in longitudinal recovery and whether standardised pulmonary rehabilitation interventions improve the short-term, medium-term, and long-term outcomes of patients hospitalised with COVID-19 pneumonia should be considered.
Here, we aimed to present the temporal trends in respiratory outcomes over 12 months in a prospective cohort of patients hospitalised with severe COVID-19 pneumonia without intubation.
Methods
Study design and participants
In this prospective, longitudinal, follow-up study, patients aged at least 18 years with severe COVID-19 discharged from Renmin Hospital of Wuhan University, Wuhan, China, were identified through electronic case-note review and approached for study participation.
A diagnosis of severe COVID-19 pneumonia was based on the WHO interim guidance and all patients had subsequent laboratory confirmation of SARS-CoV-2 using real-time RT-PCR with a standard protocol recommended by the China Center for Disease Control and Prevention.8, 9 Patients with any of the following features were categorised as having severe disease: respiratory rate of at least 30 breaths per min; oxygen saturation of 93% or less at a rest state; arterial partial pressure of oxygen to fractional inspired oxygen ratio of 300 mm Hg or less; and more than 50% progression of lesions on lung imaging within 24–48 h. Patients with a history of hypertension, diabetes, cardiovascular disease, cancer, and chronic lung disease, including asthma or chronic obstructive pulmonary disease (COPD), or a history of smoking documented at time of hospital admission were excluded at time of electronic case-note review. Patients who required intubation and mechanical ventilation were excluded given the potential for the consequences of mechanical ventilation itself to influence the factors under investigation.
Written informed consent was obtained from all study participants. This study was approved by the Ethics Commission of Renmin Hospital of Wuhan University (WDRY2020-K143).
Procedures
Patients were assessed at 3 months, 6 months, 9 months, and 12 months after discharge. During the visit, patients were interviewed and underwent a physical examination, routine blood test, pulmonary function tests, chest high-resolution CT (HRCT) scan, and a standardised 6-min walk distance (6MWD) test.10 Additionally, all patients were assessed with a modified Medical Research Council dyspnoea scale (mMRC).11, 12
Pulmonary function tests were done according to American Thoracic Society (ATS)–European Respiratory Society guidelines.13 The following parameters were measured: diffusing capacity of the lungs for carbon monoxide (DLCO); forced expiratory flow between 25% and 75% of forced vital capacity (FVC); functional residual capacity; FVC; FEV1; residual volume; total lung capacity; and vital capacity. DLCO was measured using the single-breath test. The haemoglobin value was taken for correcting the DLCO. For spirometry, flow-volume curves were obtained through a dry spirometer (Vmax 229, SensorMedics, Yorba Linda, CA, USA) and the greatest volume of the three manoeuvres was expressed as the percentage of predicted normal and used for analysis. All pulmonary function test measurements were expressed as percentages of predicted normal values. Diffusion deficit was considered as DLCO less than 80% of predicted values.
Patients underwent chest non-contrast enhanced CT examinations in the supine position and with breath-holding following inspiration (Optima CT680, GE Healthcare, Beijing, China). The technical parameters included a 64-section scanner with 1 mm collimation at 5 mm intervals. Images were obtained with both mediastinal (width 350 HU; level 50 HU) and parenchymal (width 1500 HU; level −700 HU) window settings. The follow-up patients completed HRCT scan testing every 3 months.
For imaging assessments, two radiologists with 5 years and 27 years of thoracic radiology experience, reviewed the images independently, with a final finding reached by consensus when a discrepancy was found. The radiologists were masked to the clinical information or clinical progress of the patients, except for the knowledge that these images were of patients with COVID-19. The pneumonia CT scores of patients during hospitalisation were recorded with a method described previously.8 The peak pneumonia CT score was the highest pneumonia CT score for a patient during COVID-19-related hospitalisation. To analyse follow-up HRCT scans, HRCT findings were initially assessed on the basis of key features14 and then scored using a method adapted from Ichikado and colleagues,15 here named HRCT follow-up score (appendix 2 pp 1–4), which allowed us to assess interstitial changes in lungs.15
6MWD test was done according to the ATS practical guidelines.10 Each follow-up patient walked on the flat ground as fast as possible without oxygen inhalation and completed the 6MWD test independently. The results were expressed as metres and percentage of predicted values calculated using a method described by Enright and colleagues.16
The severity of dyspnoea was measured using an mMRC scale.11 The mMRC scale is a self-rating tool to measure the degree of disability that breathlessness poses on day-to-day activities on a scale from 0 to 4. Details of this scoring system are: 0, no breathlessness except on strenuous exercise; 1, shortness of breath when hurrying on the level or walking up a slight hill; 2, walks slower than people of the same age on the level because of breathlessness or has to stop to catch breath when walking at their own pace on the level; 3, stops for breath after walking approximately 100 m or after a few minutes on the level; and 4, too breathless to leave the house, or breathless when dressing or undressing.12
Statistical analysis
Continuous variables were expressed as median (IQR) and compared using two-sample t test, Welch's two-sample t test, Mann-Whitney U test, one-way ANOVA, Kruskal–Wallis test, or repeated-measure ANOVA if appropriate; categorical variables were expressed as n (%) and compared by χ2 test or Fisher's exact test if appropriate. Because of the sample size, measurable variables with significant differences between groups were considered in subsequent univariate and multivariate logistic regression analysis.17, 18 p values less than 0·05 were considered statistically significant. All data analyses and graphs were done in R (version 3.6.1) or SPSS (version 26.0).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
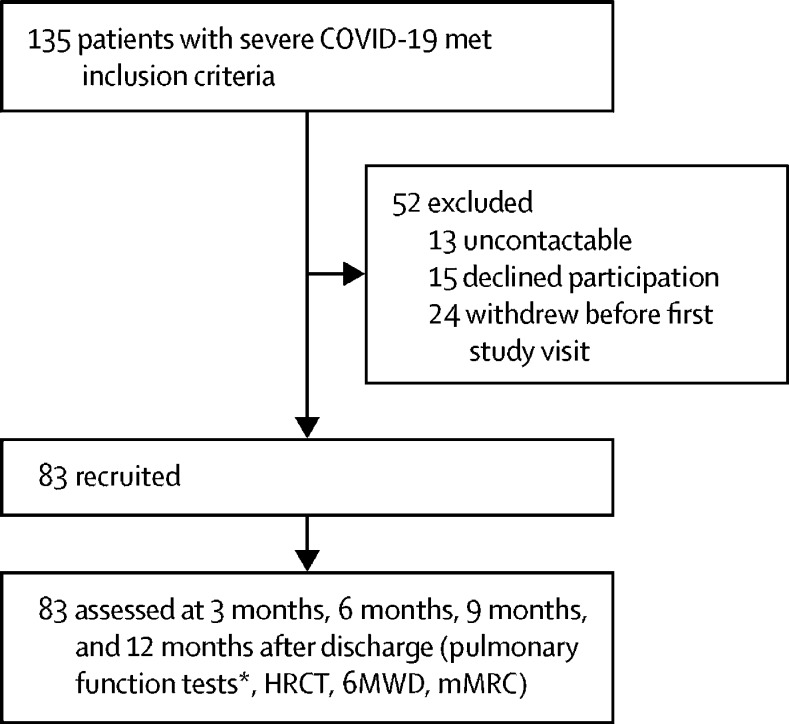
Between Feb 1, and March 31, 2020, 399 patients who were admitted to hospital with severe COVID-19 were screened for eligbility, of whom 135 (34%) patients met the inclusion criteria. 52 (39%) patients were excluded and 83 (61%) patients were prospectively enrolled after discharge for follow-up at 3 months, 6 months, 9 months, and 12 months (figure 1 ). No patients were lost to follow-up.
Figure 1.
Trial profile
HRCT=high-resolution CT. 6MWD=6-min walk distance. mMRC=modified Medical Research Council dyspnoea scale. *Because of changes in local guidance, pulmonary function tests were precluded at 9 months after discharge.
Patients were followed up for a median of 98 days (IQR 92–101) at 3 months, 189 (185–195) at 6 months, and 275 (269–280) at 9 months, and 348 (341–359) at 12 months (table 1 ). 47 (57%) patients were male and 36 (43%) were female. The median age was 60 years (52–66). The median body-mass index was 25·1 kg/m2 (23·8–26·9) in men and 24·3 kg/m2 (22·8–27·2) in women (table 1). All patients had never smoked and no patient had a history of hypertension, diabetes, cardiovascular diseases, cancer, asthma, or COPD. During hospitalisation all patients received antiviral drugs, including oseltamivir (n=53 [64%]), ribavirin (n=83 [100%]), and ganciclovir (n=42 [51%]; table 1). No patient received treatment with corticosteroids (table 1). 37 (45%) patients received supplemental oxygen only via nasal cannula or mask and 46 (55%) patients required high-flow nasal cannula (HFNC), non-invasive ventilation (NIV), or both (table 1). The median length of hospital stay was 29 days (25–35; table 1).
Table 1.
Patient characteristics
| All patients (n=83) | ||
|---|---|---|
| Age, years | 60 (52–66) | |
| Sex | ||
| Male | 47 (57%) | |
| Female | 36 (43%) | |
| BMI, kg/m2 | 25·0 (23·5–27·1) | |
| Cigarette smoking | ||
| Never smoked | 83 (100%) | |
| Comorbidities* | 0 | |
| Hospitalisation | ||
| Length of hospital stay, days | 29 (25–35) | |
| Peak CT pneumonia score during hospitalisation | 30 (24–36) | |
| Oxygen supply | ||
| Nasal cannula or mask | 37 (45%) | |
| HFNC or NIV | 46 (55%) | |
| Antivirals | ||
| Oseltamivir | 53 (64%) | |
| Ribavirin | 83 (100%) | |
| Ganciclovir | 42 (51%) | |
| Corticosteroids | 0 | |
| Follow-up visit, days | ||
| Month 3 | 98 (92–101) | |
| Month 6 | 189 (185–195) | |
| Month 9 | 275 (269–280) | |
| Month 12 | 348 (341–359) | |
Data are median (IQR) or n (%). BMI=body-mass index. HFNC=high-flow nasal cannula. NIV=non-invasive mechanical ventilation.
Patients with a history of hypertension, diabetes, cardiovascular diseases, cancer, and chronic lung disease including asthma or chronic obstructive pulmonary disease at time of hospital admission were excluded at time of screening.
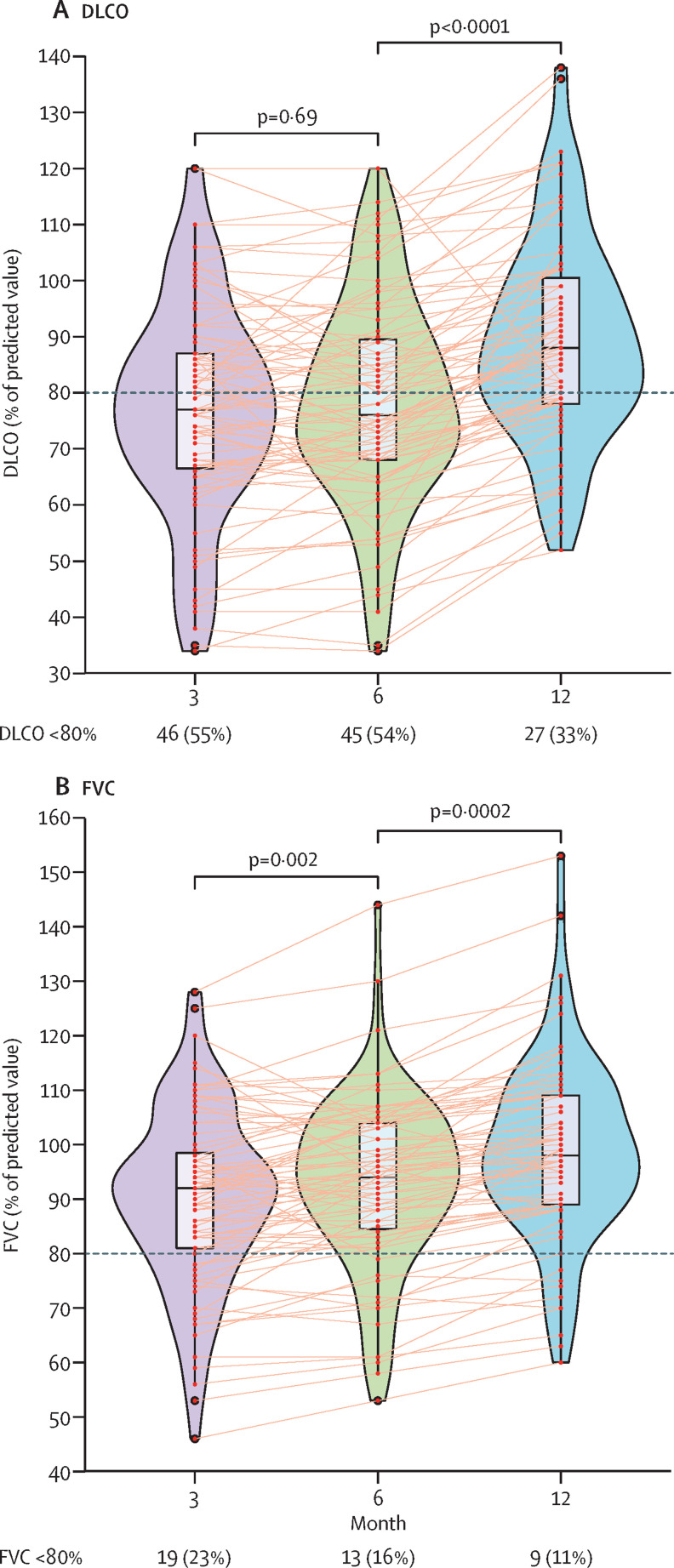
Pulmonary function tests were completed in all patients at 3 months, 6 months, and 12 months following discharge. Most patients showed an improvement in their pulmonary function at each timepoint of 3 months, 6 months, and 12 months (figure 2 ; appendix 2 p 8). Because of changes in local aerosolisation guidance, pulmonary function tests were precluded at 9 months. Median DLCO was 77% of predicted (IQR 67–87) at 3 months, 76% of predicted (68–90) at 6 months, and increased to 88% of predicted (78–101) at 12 months (figure 2). Median FVC was 92% of predicted (81–99) at 3 months, 94% of predicted (85–104) at 6 months, and increased to 98% of predicted (89–109) at 12 months (figure 2).
Figure 2.
Temporal changes in pulmonary function following severe COVID-19-related hospitalisation
Graphs show temporal changes in DLCO (A) or FVC (B) at 3 months, 6 months, and 12 months after discharge in patients with severe COVID-19. Data are median (IQR). Figure shows n (%) of patients with abnormal DLCO or FVC. Horizontal dotted lines indicate the normal cutoff of 80%. DLCO=diffusing capacity of the lungs for carbon monoxide. FVC=forced vital capacity.
The frequency of pulmonary function parameters below 80% of predicted values is shown in appendix 2 (p 9). Nine (11%) patients had reduced FVC measurements (<80% predicted value) at 12 months and 27 (33%) patients had impaired DLCO at 12 months (<80% predicted value; appendix 2 p 9). Comparisons in patients with severe COVID-19 with normal versus abnormal DLCO at 12 months after discharge were done. A higher proportion of patients with DLCO less than 80% predicted at 12 months after discharge was observed in women when compared with men (p<0·0001; table 2 ). Patients with impaired DLCO at 12 months after discharge also had increased peak HRCT pneumonia scores during hospitalisation; however, this finding was not statistically significant (table 2).
Table 2.
DLCO in patients with severe COVID-19 at 12 months after discharge
| DLCO at month 12 ≥80% predicted value (n=56) | DLCO at month 12 <80% predicted value (n=27) | p value | |||
|---|---|---|---|---|---|
| Age, years | 58 (52–66) | 62 (57–66) | 0·14 | ||
| Sex | |||||
| Male (n=47) | 40 (71%) | 7 (26%) | <0·0001 | ||
| Female (n=36) | 16 (29%) | 20 (74%) | .. | ||
| BMI, kg/m2 | 25·1 (23·6–27·1) | 24·4 (23·1–27·0) | 0·81 | ||
| Hospitalisation | |||||
| Length of hospital stay, days | 29 (25–34) | 31 (24–35) | 0·39 | ||
| Peak CT pneumonia score during hospitalisation | 28 (24–36) | 30 (26–39) | 0·11 | ||
| Oxygen supply | |||||
| Nasal cannula or mask (n=37) | 26 (46%) | 11 (41%) | 0·63 | ||
| HFNC or NIV (n=46) | 30 (54%) | 16 (59%) | .. | ||
| Antivirals | |||||
| Oseltamivir | |||||
| No (n=30) | 20 (36%) | 10 (37%) | 0·91 | ||
| Yes (n=53) | 36 (64%) | 17 (63%) | .. | ||
| Ganciclovir | |||||
| No (n=41) | 30 (54%) | 11 (41%) | 0·27 | ||
| Yes (n=42) | 26 (46%) | 16 (59%) | .. | ||
Data are median (IQR) or n (%). BMI=body-mass index. DLCO=diffusing capacity of the lungs for carbon monoxide. HFNC=high-flow nasal cannula. NIV=non-invasive mechanical ventilation.
To explore the risk factors associated with impaired DLCO at 12 months after discharge, univariate and multivariate logistic regression models were used. Multivariate logistic regression showed increasing odds of impaired DLCO associated with female sex (odds ratio [OR] 8·61 [95% CI 2·83–26·2; p=0·0002), independent of age and peak HRCT pneumonia scores (appendix 2 p 10).
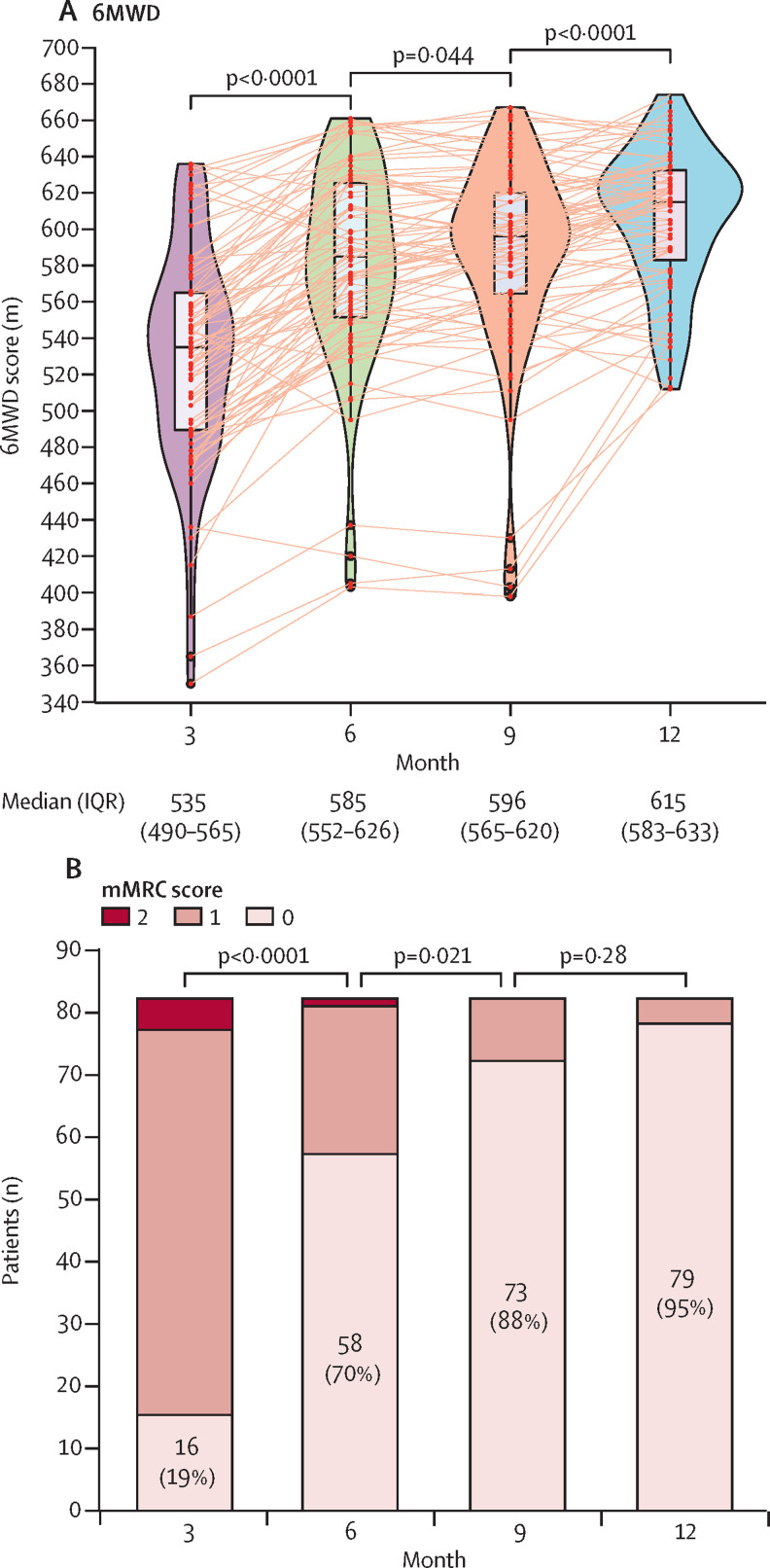
Results of the median 6MWD test increased significantly, from 535 m (IQR 490–565) at 3 months to 585 m (552–626) at 6 months (p<0·0001; figure 3 ). A further detectable increase was observed at 9 and 12 months (figure 3). Similar results were obtained if normalised to predicted values calculated according to a method described by Enright and colleagues (appendix 2 p 5).16
Figure 3.
Effect of severe COVID-19 on follow-up 6MWD test and mMRC score
Panel A shows temporal changes in 6MWD at 3 months, 6 months, 9 months, and 12 months after discharge. Data are median (IQR). Panel B shows the distributions of mMRC scores at 3 months, 6 months, 9 months, and 12 months after discharge. Figure shows n (%) of patients without dyspnoea. 6MWD=6-minute walking distance. mMRC=modified Medical Research Council dyspnoea scale.
Dyspnoea symptoms assessed using the mMRC scale were very frequent in patients at 3 months, with 67 (81%) patients with an mMRC score of at least 1 and five (6%) patients with an mMRC score of least 2 (figure 3). The number of patients with various levels of dyspnoea symptoms progressively and significantly reduced at 6 months, 9 months, and 12 months (figure 3). Four (5%) patients reported persistent symptoms of dyspnoea at 12 months.
65 (78%) patients had residual changes on CT at 3 months after discharge with ground-glass opacity (GGO; 65 [78%]), interlobular septal thickening (28 [34%]), reticular opacity (27 [33%]), and subpleural curvilinear opacity (nine [11%]) being the most common CT features found (appendix 2 p 11). At 6 months, 40 (48%) patients still had abnormal chest radiographic scores, with GGO (38 [46%]), interlobular septal thickening (11 [13%]), reticular opacity (13 [16%]), subpleural curvilinear opacity (four [5%]), mosaic attenuation (three [4%]), and bronchiectasis (one [1%]; appendix 2 pp 6, 11). Typical features such as interlobular septal thickening (four [5%]), reticular opacity (three [4%]), and subpleural curvilinear opacity (one [1%]) were almost resolved at 9 months but the radiological changes did not fully resolve in 22 (27%) patients, predominantly with GGO (20 [24%]; appendix 2 pp 7, 11). No significant improvement at 12 months was identified when compared with 9 months (appendix 2 pp 7, 11). 20 (24%) patients had abnormal HRCT at 12 months. None of the HRCT scans showed evidence of established fibrosis (appendix 2 pp 7, 11) and none showed evidence of progressive interstitial changes (appendix 2 pp 7, 11).
Comparisons in patients with severe COVID-19 with normal versus abnormal chest HRCT at 12 months after discharge were done. Patients with abnormal radiographic changes at 12 months had increased length of hospital stay (p=0·027; table 3 ) and increased peak HRCT pneumonia scores (p<0·0001; table 3; appendix 2 p 7). Patients receiving HFNC, NIV, or both were more likely to have abnormal HRCT at 12 months, compared with patients who did not have HFNC or NIV (p=0·043; table 3). We found a significant difference in pulmonary function test parameters (including DLCO, functional residual capacity, FVC, residual volume, total lung capacity, and vital capacity) between patients with normal versus abnormal HRCT follow-up scores at 12 months after discharge (table 3).
Table 3.
HRCT scores in patients with severe COVID-19 at 12 months after discharge
| HRCT normal (n=63) | HRCT abnormal (n=20) | p value | |||
|---|---|---|---|---|---|
| Age, years | 59 (51–68) | 61 (56–65) | 0·27 | ||
| Sex | |||||
| Male (n=47) | 38 (60%) | 9 (45%) | 0·23 | ||
| Female (n=36) | 25 (40%) | 11 (55%) | .. | ||
| BMI, kg/m2 | 25·3 (23·5–27·2) | 24·2 (23·3–25·7) | 0·36 | ||
| Hospitalisation | |||||
| Length of hospital stay, days | 28 (24–33) | 35 (29–37) | 0·027 | ||
| Peak CT pneumonia score during hospitalisation | 27 (24–33) | 36 (30–44) | <0·0001 | ||
| Oxygen supply | |||||
| Nasal cannula or mask (n=37) | 32 (51%) | 5 (25%) | 0·043 | ||
| HFNC or NIV (n=46) | 31 (49%) | 15 (75%) | .. | ||
| Antivirals | |||||
| Oseltamivir | |||||
| No (n=30) | 25 (40%) | 5 (25%) | 0·23 | ||
| Yes (n=53) | 38 (60%) | 15 (75%) | .. | ||
| Ganciclovir | |||||
| No (n=41) | 33 (52%) | 8 (40%) | 0·34 | ||
| Yes (n=42) | 30 (48%) | 12 (60%) | .. | ||
| Pulmonary function at month 12* | |||||
| DLCO | 90 (82–102) | 77 (66–81) | <0·0001 | ||
| FEF25–75% | 89 (76–109) | 92 (72–123) | 0·28 | ||
| FEV1:FVC | 82 (78–85) | 85 (80–86) | 0·34 | ||
| FRC | 107 (89–126) | 99 (76–106) | 0·031 | ||
| FVC | 99 (90–111) | 92 (79–100) | 0·012 | ||
| FEV1 | 97 (87–110) | 88 (75–106) | 0·054 | ||
| RV | 88 (74–102) | 75 (64–89) | 0·031 | ||
| TLC | 95 (88–104) | 88 (72–94) | 0·0074 | ||
| VC | 101 (91–112) | 92 (79–100) | 0·0037 | ||
Data are median (IQR) or n (%). BMI=body-mass index. DLCO=diffusing capacity of the lungs for carbon monoxide. FEF25–75%=forced expiratory flow between 25% and 75% of FVC. FRC=functional residual capacity. FVC=forced vital capacity. RV=residual volume. TLC=total lung capacity. VC=vital capacity. HFNC=high-flow nasal cannula. HRCT=high resolution CT. NIV=non-invasive mechanical ventilation.
Values are percentage of predicted value.
To explore the risk factors associated with abnormal radiographic changes at 12 months after discharge, univariate and multivariate logistic regression models were used. In univariate analysis, length of hospital stay, peak HRCT pneumonia scores during hospitalisation, and receiving HFNC or NIV were associated with abnormal HRCT at 12 months after discharge (appendix 2 p 12). We then identified peak HRCT pneumonia score (OR 1·36 [95% CI 1·13–1·62; p=0·0009) as an independent risk factor of abnormal HRCT at 12 months after discharge, including in the multivariate analysis with length of hospital stay and receiving HFNC or NIV (appendix 2 p 12).
Discussion
The consequences of COVID-19 in those who recover from acute infection are uncertain despite a few reports on outcomes up to 6 months after discharge,19, 20, 21, 22, 23, 24 although data from previous coronavirus outbreaks such as SARS and MERS suggests that some patients will have long-term respiratory complications. In this study, we report serial pulmonary function, exercise capacity, and chest HRCT changes in non-intubated patients hospitalised with severe COVID-19 pneumonia at 3 months, 6 months, 9 months, and 12 months following hospital discharge. Although in most patients exercise capacity improved over this time period, we found evidence of persistent physiological and radiographic changes in a subgroup of patients.
At 12 months after discharge, residual abnormalities of pulmonary function were observed in a third of patients, with the most common finding being a reduction in gas transfer as measured by DLCO. Studies have shown that gas–blood exchange is impaired in patients who have been discharged following hospital admission with COVID-19 pneumonia,19, 20, 24, 25, 26 including a 6-month cohort study of COVID-19 in patients discharged from hospital.23 Low DLCO could be the consequence of interstitial abnormalities or pulmonary vascular abnormalities caused by COVID-19.27, 28, 29 Longer-term follow-up will be required to confirm this observation. Consistent with our findings, in follow-up studies of patients recovering from SARS, impaired pulmonary function could last for months or even years.2, 3, 4, 5 Our findings are consistent with a previous COVID-19 follow-up study at 6 months, and we extend a recent report23 to identify that female sex strongly predicts impaired DLCO at 12 months after discharge. Notably, female sex was not significantly associated with persistent HRCT abnormalities, suggesting that distinct mechanisms might underlie the identified persistent radiological abnormalities and gas–blood exchange abnormalities; the underlying mechanisms merit further investigation.
At 12 months after discharge, the radiological changes did not resolve fully in 24% of patients, including findings potentially consistent with evolving fibrosis in some patients with the presence of interstitial thickening and reticular opacity. Although none of the HRCT scans showed any development of definitive fibrosis nor progressive interstitial change, plausibly the burden of pulmonary fibrosis after COVID-19 recovery could be substantial given these observations and the huge numbers of individuals affected by COVID-19.30, 31 Therefore, ongoing longitudinal follow-up is warranted to further understand the natural history of the identified radiological changes.
The prospective enrolment of patients enabled us to study the temporal pulmonary physiology, exercise capacity, and radiographic abnormalities. To better understand the consequences of COVID-19 pneumonia itself, we selected patients meeting the criteria for severe COVID-19 pneumonia while excluding patients requiring intubation, given the potential for mechanical ventilation itself to alter the natural history of the factors under investigation, in particular the potential for triggering mechanical-ventilation associated lung fibrosis in patients with acute respiratory distress syndrome.
This study has several limitations. By choosing a cohort of patients with severe COVID-19 pneumonia, we did not fully define the potential long-term consequences for varying severities of disease. Additionally, because of changes in local guidance, pulmonary function tests were precluded at 9 months. The study was not designed to collect data on any medication prescriptions following hospital discharge. Lastly, we did not have pulmonary function, exercise capacity, or CT results before COVID-19 that would enable longitudinal assessment of the effect of COVID-19. However, the studied cohort had no history of significant cardiovascular or respiratory disease and the temporal improvement observed suggests that at least some changes were related to COVID-19 or hospitalisation.32, 33
Our findings highlight the importance of respiratory follow-up of patients with COVID-19, and that studies to mitigate the long-term consequences of COVID-19 pneumonia, including pulmonary rehabilitation as well as novel therapeutic approaches, are required.34
For reported cases worldwide see https://www.worldometers.info/coronavirus/#countries
For the COVID Symptom Study see https://covid.joinzoe.com/post/covid-long-term
For the WHO guidance for the clinical management of patients with COVID-19 see https://www.who.int/publications/i/item/clinical-management-of-covid-19
Data sharing
The data that support the findings of this study are available from the corresponding author (HN) upon reasonable request and with permission of Renmin Hospital of Wuhan University, Hubei, China.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
This project was funded by the National Natural Science foundation of China (81970024). YZ was supported by an Institute for Life Sciences (University of Southampton, Southampton, UK) PhD studentship. YW was funded by the UK Medical Research Council (MR/S025480/1). MGJ and YW acknowledge the support of the UK National Institute for Health Research Southampton Biomedical Research Centre. We acknowledge all the patients involved in this study and appreciate all the front-line medical and nursing staff involved in the diagnosis, treatment, and follow-up of patients in Wuhan, China. We also thank Yan Jiang and Tao Li for their support in HRCT imaging assessments.
Contributors
YW, HN, YH, MGJ, XW, and XL had the idea for and designed the study. All authors had full access to the data and had final responsibility for the decision to submit for publication. YW, MGJ, HN, YH, and RME drafted the paper. YZ, XW, XL, HY, RL, QZ, and YW did the analysis and all authors critically revised the manuscript for important intellectual content and gave final approval for the version to be published. XW, XL, HY, RL, QZ, FN, SF, YL, XD, and HL collected the data. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Supplementary Materials
References
- 1.AlSamman M, Caggiula A, Ganguli S, Misak M, Pourmand A. Non-respiratory presentations of COVID-19, a clinical review. Am J Emerg Med. 2020;38:2444–2454. doi: 10.1016/j.ajem.2020.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moldofsky H, Patcai J. Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post-SARS syndrome; a case-controlled study. BMC Neurol. 2011;11:37. doi: 10.1186/1471-2377-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hui DS, Joynt GM, Wong KT, et al. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax. 2005;60:401–409. doi: 10.1136/thx.2004.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hui DS, Wong KT, Ko FW, et al. The 1-year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors. Chest. 2005;128:2247–2261. doi: 10.1378/chest.128.4.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ngai JC, Ko FW, Ng SS, To KW, Tong M, Hui DS. The long-term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology. 2010;15:543–550. doi: 10.1111/j.1440-1843.2010.01720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Das KM, Lee EY, Singh R, et al. Follow-up chest radiographic findings in patients with MERS-CoV after recovery. Indian J Radiol Imaging. 2017;27:342–349. doi: 10.4103/ijri.IJRI_469_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long-COVID: analysis of COVID cases and their symptoms collected by the Covid Symptoms Study App. medRxiv. 2020 doi: 10.1101/2020.10.19.20214494. published online Oct 21. (preprint). [DOI] [Google Scholar]
- 8.Liu X, Zhou H, Zhou Y, et al. Temporal radiographic changes in COVID-19 patients: relationship to disease severity and viral clearance. Sci Rep. 2020;10 doi: 10.1038/s41598-020-66895-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X, Zhou H, Zhou Y, et al. Risk factors associated with disease severity and length of hospital stay in COVID-19 patients. J Infect. 2020;81:e95–e97. doi: 10.1016/j.jinf.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 11.Rajala K, Lehto JT, Sutinen E, Kautiainen H, Myllärniemi M, Saarto T. mMRC dyspnoea scale indicates impaired quality of life and increased pain in patients with idiopathic pulmonary fibrosis. ERJ Open Res. 2017;3 doi: 10.1183/23120541.00084-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54:581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham BL, Steenbruggen I, Miller MR, et al. Standardization of spirometry 2019 update. An official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med. 2019;200:e70–e88. doi: 10.1164/rccm.201908-1590ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246:697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 15.Ichikado K, Suga M, Müller NL, et al. Acute interstitial pneumonia: comparison of high-resolution computed tomography findings between survivors and nonsurvivors. Am J Respir Crit Care Med. 2002;165:1551–1556. doi: 10.1164/rccm.2106157. [DOI] [PubMed] [Google Scholar]
- 16.Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158:1384–1387. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 17.Courvoisier DS, Combescure C, Agoritsas T, Gayet-Ageron A, Perneger TV. Performance of logistic regression modeling: beyond the number of events per variable, the role of data structure. J Clin Epidemiol. 2011;64:993–1000. doi: 10.1016/j.jclinepi.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 18.Agoritsas T, Merglen A, Shah ND, O'Donnell M, Guyatt GH. Adjusted Analyses in Studies Addressing Therapy and Harm: Users' Guides to the Medical Literature. JAMA. 2017;317:748–759. doi: 10.1001/jama.2016.20029. [DOI] [PubMed] [Google Scholar]
- 19.Zhao YM, Shang YM, Song WB, et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25 doi: 10.1016/j.eclinm.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah AS, Wong AW, Hague CJ, et al. A prospective study of 12-week respiratory outcomes in COVID-19-related hospitalisations. Thorax. 2020 doi: 10.1136/thoraxjnl-2020-216308. published online Dec 3. [DOI] [PubMed] [Google Scholar]
- 21.Liu C, Ye L, Xia R, et al. Chest computed tomography and clinical follow-up of discharged patients with COVID-19 in Wenzhou City, Zhejiang, China. Ann Am Thorac Soc. 2020;17:1231–1237. doi: 10.1513/AnnalsATS.202004-324OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang Y, Tan C, Wu J, et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir Res. 2020;21:163. doi: 10.1186/s12931-020-01429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin W, Chen S, Zhang Y, et al. Diffusion capacity abnormalities for carbon monoxide in patients with COVID-19 at three-month follow-up. Eur Respir J. 2021 doi: 10.1183/13993003.03677-2020. published online Feb 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mo X, Jian W, Su Z, et al. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J. 2020;55 doi: 10.1183/13993003.01217-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guler SA, Ebner L, Beigelman C, et al. Pulmonary function and radiological features four months after COVID-19: first results from the national prospective observational Swiss COVID-19 lung study. Eur Respir J. 2021 doi: 10.1183/13993003.03690-2020. published online Jan 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanidziar D, Robson SC. Hyperoxia and modulation of pulmonary vascular and immune responses in COVID-19. Am J Physiol Lung Cell Mol Physiol. 2021;320:L12–L16. doi: 10.1152/ajplung.00304.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel BV, Arachchillage DJ, Ridge CA, et al. Pulmonary angiopathy in severe COVID-19: physiologic, imaging, and hematologic observations. Am J Respir Crit Care Med. 2020;202:690–699. doi: 10.1164/rccm.202004-1412OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lang M, Som A, Mendoza DP, et al. Hypoxaemia related to COVID-19: vascular and perfusion abnormalities on dual-energy CT. Lancet Infect Dis. 2020;20:1365–1366. doi: 10.1016/S1473-3099(20)30367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.George PM, Wells AU, Jenkins RG. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir Med. 2020;8:807–815. doi: 10.1016/S2213-2600(20)30225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spagnolo P, Balestro E, Aliberti S, et al. Pulmonary fibrosis secondary to COVID-19: a call to arms? Lancet Respir Med. 2020;8:750–752. doi: 10.1016/S2213-2600(20)30222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7:611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pierce M, Hope H, Ford T, et al. Mental health before and during the COVID-19 pandemic: a longitudinal probability sample survey of the UK population. Lancet Psychiatry. 2020;7:883–892. doi: 10.1016/S2215-0366(20)30308-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.George PM, Barratt SL, Condliffe R, et al. Respiratory follow-up of patients with COVID-19 pneumonia. Thorax. 2020;75:1009–1016. doi: 10.1136/thoraxjnl-2020-215314. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (HN) upon reasonable request and with permission of Renmin Hospital of Wuhan University, Hubei, China.