Abstract
Renal artery stenosis is considered to be one of the more frequent causes of secondary arterial hypertension. Through its progression renal artery stenosis can cause renal insufficiency, uncontrolled hypertension, and increased cardiovascular morbidity. A thorough clinical examination and the presence of a typical abdominal bruit may provide helpful hints to identify hypertensive patients with possible renal artery stenosis. Testing for renovascular hypertension includes renal artery imaging, assessment of its functional significance, and evaluation for possible revascularization. Renal artery stenosis secondary to fibromuscular dysplasia should be mechanically corrected. For atherosclerotic renal artery stenosis, medical management can be attempted so long as it does not cause a decline of kidney function. In patients who are candidates for renovascular revascularization, surgical intervention can be helpful in improving blood pressure control and possibly halting the progression of renal failure. Randomized controlled trials comparing direct stenting with other surgical methods are necessary to define the best revascularization strategy in patients with renovascular hypertension. A careful follow‐up study after renal artery revascularization should evaluate possible benefits in halting the deterioration of chronic renal insufficiency.
Since the experiment of Goldblatt et al. 1 the renal mechanisms generating and maintaining arterial hypertension have been studied extensively. Renovascular hypertension represents one of the more frequent forms of secondary hypertension and if properly treated can theoretically be cured.
Several paradigm shifts occurred during the last decades in the diagnosis and treatment of renovascular hypertension. For the practicing physician the following questions are of particular importance: Do all renal artery stenoses cause hypertension? Who should undergo testing for renovascular hypertension? What is the “ideal” test to diagnose renovascular hypertension? When should a patient with documented renovascular hypertension be referred for revascularization of the renal artery? What type of revascularization (surgery or percutaneous intervention) is better? What are the criteria of successful revascularization?
RENAL ARTERY STENOSIS AND RENOVASCULAR HYPERTENSION
Renal artery stenosis has been described by angiographic 2 and autopsy 3 methods in both normotensive and hypertensive subjects. Generally at least a 60 mm Hg decrease in mean renal arterial pressure is necessary to generate significant changes in renal physiology to produce an elevated blood pressure. 4 Hence not all patients with renal artery stenosis have concomitant hypertension or impairment of kidney function.
The most frequent causes of renal artery stenosis are atherosclerosis and fibromuscular dysplasia. It has been estimated that atherosclerotic renal artery stenosis will progress if hypertension is not corrected in 44% of subjects during the next 5 years after diagnosis, and in 16% of the cases it may produce total arterial occlusion. 5 The progression of atherosclerosis in renal artery stenosis is significantly associated with systolic hypertension, diabetes mellitus, more than 60% stenosis (or occlusion) in the ipsilateral or contralateral renal artery, and a low ankle‐brachial pressure index. 6 Renovascular disease is estimated to account for close to 20% of all patients with end‐stage renal disease entering a dialysis program; these patients have been reported to have a 10‐year survival rate of 5%. 7 This may have improved in recent years with better therapy of hypertension. The involvement of the contralateral renal artery has been observed in up to 14% of the patients with documented unilateral atherosclerotic renal artery stenosis during 2 years of close angiographic follow‐up. 8 In patients with fibrous dysplasia the rate of renal artery obstruction was 33% over 4 years of follow‐up; however there was no total occlusion noted in this group of patients. 5
The prevalence of renovascular hypertension varies widely according to the studied population: from 1% in the general population 9 , 10 to 10% in patients referred for diagnostic testing at specific centers. 11 Thirty percent of patients with angiographically documented coronary artery disease have been found to have some degree of renal artery stenosis, and in 15% of these patients the stenosis was found to be hemodynamically significant. 12 Predictors for the presence of renal artery stenosis in subjects referred for cardiac catheterization included age above 60, female gender, presence of peripheral vascular disease, and congestive heart failure, but not arterial hypertension. 12
A hemodynamically significant renal artery stenosis will trigger the activation of the renin‐angiotensin system in the ischemic kidney, with subsequent sodium and fluid retention and elevation of peripheral resistance. The resulting elevated blood pressure, if not reduced, will in time affect the opposite kidney. The damage of the contralateral kidney can explain cases in which the treatment of renal artery stenosis is not followed by cure of hypertension. 13 It also suggests the necessity for a timely diagnosis and treatment.
THE PATIENT WITH RENOVASCULAR HYPERTENSION
Who Should Be Tested and What Test To Use
A number of clinical features in the hypertensive patient may raise the suspicion of renovascular hypertension and its probable etiology (Table I). If the patient with probable renovascular hypertension is a potential candidate for revascularization then further testing is appropriate. Due to low sensitivity and specificity, the measurement of plasma renin activity (which, if high, may confirm activation of the renin‐angiotensin system) plays no role in the further diagnosis of renovascular hypertension. Several tests are available, and each has strengths and limitations (Table II). Certain tests (intravenous urography, digital subtraction angiography) are used infrequently, while others (Duplex ultrasonography, magnetic resonance angiography, spiral computed tomographic angiography) are used more often and will gain more importance in the future with equipment and software refinement and operator experience (Figure 1). Regardless of the method used to image the renal arteries it is of paramount importance to assess both the degree of stenosis and its significance.
Table I.
| Clinical Characteristics | Pathologic Basis of Renovascular Hypertension | |
|---|---|---|
| Atherosclerosis | Medial Fibromuscular Dysplasia | |
| Morphology | Intimal plaques | Collagenous rings involving media |
| Age at onset | >50 years | <20 years |
| Duration >1 year | Yes | Yes |
| Gender risk | None | Women>Men |
| Race | Caucasians>African Americans | |
| Smoker status | Yes | No |
| Coexistence of essential hypertension | Yes | No |
| Family history of hypertension | Possible | No |
| Manifest atherosclerotic involvement in other arterial beds (e.g., carotids, coronaries) | Yes | No |
| Association with aortic dissection | Yes | Yes |
| Abdominal/flank bruits; long, high‐pitched systolic with diastolic component, and localized to the region of the renal artery. This murmur differs from other murmurs secondary to atherosclerotic changes in abdominal blood vessels. These are usually short low‐pitched systolic bruits. | Yes | Yes |
| Advanced fundus changes | Yes | Possible |
| “Refractory” hypertension | Yes | Yes |
| Recurrent pulmonary edema | Suggestive of bilateral renal artery stenosis | |
| Rapidly progressive oliguric renal failure in the absence of obstructive uropathy of bilateral renal artery stenosis | Suggestive of bilateral renal artery stenosis | |
| Deterioration of renal function after ACE inhibitor therapy | Suggestive of bilateral renal artery stenosis | |
| Congestive heart failure | Suggestive of bilateral renal artery stenosis | |
| High plasma renin activity | Yes | Yes |
| Low serum potassium | Yes | Yes |
| Proteinuria | Yes | Yes |
| ACE=angiotensin‐converting enzyme | ||
Table II.
Diagnostic Tests in Renovascular Hypertension 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36
| Diagnostic Test | Findings Suggestive of Renal Artery Stenosis | Sensitivity | Specificity | Advantage | Limitations | Predicts Response to Intervention |
|---|---|---|---|---|---|---|
| Intravenous pyelogram | Delayed calyceal appearance time | 75% | 85% | Screening for both parenchymal and vascular causes for hypertension | Unsatisfactory screening test | N/R |
| Captopril renography | Reduction of isotope uptake or slowing of isotope excretion | 90% | 86% | Most cost‐effective screening test | Less reliable in the presence of renal dysfunction | Yes |
| Captopril augmented peripheral PRA | Stimulated PRA >12 ng/mL/h and absolute increase in PRA of >10 ng/mL/h and increase in PRA >150%, or >400% if baseline PRA<3 ng/mL/h | 100% | 95% | Augmentation of the renin secretion beyond the stenosed kidney artery | Less reliable in the presence of renal dysfunction | N/R |
| 3D phase‐contrast magnetic resonance angiography | Stenosis visualization; gradient assessment | 100% | 93% | Avoids nephrotoxic contrast material | Limited availability; imaging artifacts by stents; proximal renal artery tortuosity limits accurate visualization | Yes |
| Duplex ultrasonography | Peak systolic velocity >2 m/s and a renal‐aortic ratio >3.5 Difference of renal resistance index >5% between both kidneys | 93% | 100% | Avoids nephrotoxic contrast material, widely available | Operator dependent; limited value in fibromuscular dysplasia, obesity, presence of accessory renal arteries, proximal renal artery tortuosity | Yes |
| Digital subtraction angiography | Stenosis visualization | 83% | 79% | Outpatient definition of renal anatomy | Requires nephrotoxic contrast media, poor resolution for ostial and for bifurcation stenosis | N/R |
| Renal arteriography | Stenosis visualization | 100% | 100% | “Gold standard”; can be followed by intervention in the same setting | Invasive | Yes |
| Intravascular ultrasound | Stenosis visualization | 100% | 100% | May minimize the amount of contrast media during procedure; may guide optimal stenting procedure | Invasive; requires cannulation of the ostium, and passing of the guidewired and of the imaging catheter across the stenosis | Yes |
| N/R=not reported; PRA=plasma renin activity | ||||||
Figure 1.
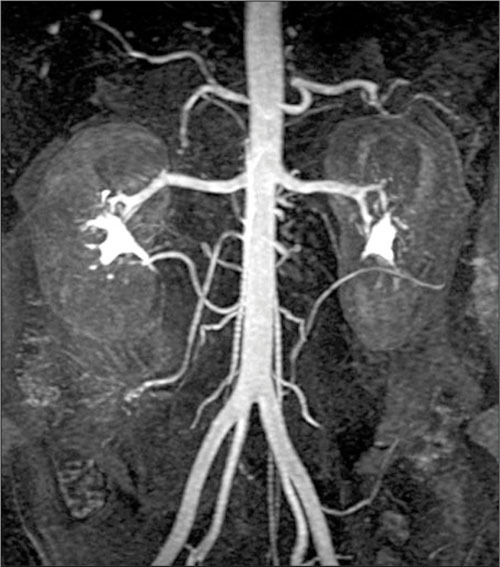
A normal magnetic resonance image of the abdominal aorta and renal arteries
The choice of the imaging test(s) to diagnose renovascular hypertension is mostly dependent on local facilities and local expertise. It has been recommended 37 that testing should be “staged” according to the pretest probability for renovascular hypertension: low probability (<5%), no further testing; intermediate probability (5%–15%), noninvasive testing first, followed by renal arteriography if positive; and high probability (>15%), renal arteriography. In most cases, percutaneous revascularization can be done at the same time as renal arteriography. Recent data 32 show that surgical or percutaneous revascularization may not be successful in all cases of renal artery stenosis, and that a noninvasively determined index from the segmental renal arteries (renal resistance index calculated as [1 − {end‐diastolic velocity divided by the peak systolic velocity} × 100]) could reliably identify patients with renal artery stenosis in whom revascularization will not improve renal function, blood pressure, or kidney survival. It seems reasonable to perform an initial noninvasive test (e.g., Doppler ultrasonography) that can predict the potential for therapeutic success before attempting revascularization.
TREATMENT OF RENOVASCULAR HYPERTENSION
Which Method for Which Patient
The goal of treatment in patients with renovascular hypertension is prevention and/or regression of target organ damage, where preservation of kidney function plays a major role. It is important to remember that in patients with known renovascular disease progressive deterioration of kidney function may occur in some patients despite adequate blood pressure control. 38 In patients with atherosclerotic renal artery disease aggressive treatment of associated risk factors (e.g., dyslipidemia, smoking, diabetes mellitus) is especially important.
Medical, interventional, and surgical methods are available for the treatment of renovascular hypertension (Table III). Only a small number of randomized controlled trials comparing the efficacy of the above mentioned methods, all with few patients enrolled, diverse patient populations (widely varying risk profiles), short follow‐up periods, and different approaches to assess their end points are available (Table IV). Therapeutic recommendations should be individualized according to the particular patient and to available expertise.
Table III.
Treatment Options in Renovascular Hypertension 12 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54
| Medical Treatment | Percutaneous Intervention | Surgical Treatment | |
|---|---|---|---|
| Preferential indications | Patients before/after revascularization | Fibromuscular dysplasia> atherosclerotic disease | Atherosclerotic disease> fibromuscular dysplasia |
| Patients not candidates for revascularization | Unilateral>bilateral disease | Failed medical treatment and/or failed balloon angioplasty | |
| Patients who failed revascularization | Ostial renal artery disease (stent>balloon angioplasty) | Bilateral high‐grade stenotic disease | |
| Pediatric renovascular hypertension | Stenosis in a solitary kidney | ||
| Transplant renal artery stenosis | Total renal artery occlusion | ||
| Renovascular disease in pregnancy | Preservation of kidney function | ||
| Concurrent aortic aneurysm/dissection | |||
| Methods | Angiotensin‐converting enzyme inhibitors, calcium channel blockers | Stenting superior to balloon angioplasty due to lower long‐term restenosis | Endarterectomy |
| Aorto‐renal artery bypass | |||
| Nephrectomy | |||
| Perioperative mortality | 0.5% | 1.7% (Isolated renal procedures); 9.2% (combined procedures) | |
| Complications | Progressive chronic renal failure | Cholesterol emboli, occlusion of renal artery side branches, renal surgery, contrast nephropathy | Cholesterol emboli |
| Hemorrhage | |||
| Restenosis | 24%–40% (balloon angioplasty alone) | Not reported | |
| 12% (stenting) | |||
| Patency rate | 84%–95% | 88.6% (19 Months follow‐up) | |
| 86.7% (50 Months follow‐up) | |||
| Blood pressure control | 74% | 47%–56% | 82% |
| Renal function stabilization or improvement | 69% | 86% |
Table IV.
| Study | No. Pts. | Inclusion Criteria | Exclusion Criteria | Treatment tested | Follow‐Up | Endpoints | BP Control | Kidney Function | Conclusions |
|---|---|---|---|---|---|---|---|---|---|
| Weibull et al. 55 1993 | 58 | Age <70 Untreated BP > 160/100 mm Hg Significant unilateral RAS (within 1 cm from the aorta) S creat <3.3 mg/dL | Diabetes mellitus | PTA vs. surgical | 24 Mo | Technical success and patency Need for reintervention | NS | NS | PTA is recommended as first choice of therapy for atherosclerotic unilateral RAS if combined with intensive follow‐up and aggressive reintervention |
| Plouin et al. 56 1998 | 49 | Age <75 CrCl >50 mL/L Ambulatory DBP >95 mm Hg Atherosclerotic RAS >75% or >60% with positive lateralization test | Malignant HTN, Hx of stroke, MI, pulmonary edema within last 6 mo | Medical (nifedipine, clonidine, prazosin ± atenolol, furosemide, or enalapril) vs. PTA (with/without stenting) | 6 Mo | Ambulatory BP Number of anti‐HTN drugs used PTA complications | NS | NS | Pts post‐PTA needed significantly less drugs to control their BP |
| Webster et al. 57 1998 | 55 | DBP >95 mm Hg on at least 2 anti‐HTN drugs RAS >50% unilateral and bilateral RAS | Age<40 Hx of stroke, MI within last 3 mo | PTA vs. medical | 3–54 Mo | BP control Kidney function evaluation | Significant drop in pts with bilateral RAS in PTA vs. medical group | NS | PTA results in a modest improvement in SBP (vs. medical treatment) in pts with bilateral RAS |
| Van de Ven et al. 58 1999 | 85 | Ostial RAS(>50%) BP> 160/95 mm Hg Positive captopril renography | Hx of cholesterol embolism; renal tumor; affected kidney size <8cm plus <25% renal function in renography | PTA vs. stenting | 6 Mo | Patency rates Renal function (improved/unchanged/deteriorated) Hypertension (cured/improved/failing) | NS | NS | Direct stenting is better than PTA to achieve vessel patency in ostial atherosclerotic RAS |
| Van Jaarsveld et al. 59 2000 | 106 | Ostial or nonostial RAS in patients with difficult to treat HTN and normal or mildy impaired (S creat <2.3 mg/dL) | Single functioning kidney and S creat >1.7 mg/dL; affected kidney <8 cm long; total occlusion of the renal artery; aortic aneurysm; RAS due to FMD | Medical (amlodipine and atenolol or enalapril and HCTZ) vs. PTA (without stenting) | 12 Mo | BP at 3 and 12 mo after randomization Number of anti‐HTN drugs used S creat and CrCl Presence of renal artery patency | NS | Better in the PTA group at 3 mo, but no difference at 12 mo | PTA has little advantage over anti‐HTN drug treatment |
| BP=blood pressure; Pts=patients; RAS=renal artery stenosis; S creat=serum creatinine; PTA=percutaneous transluminal angioplasty; NS=nonsignificant; CrCl=creatinine clearance; DBP=diastolic blood pressure; HTN=hypertension; Hx=history; MI=myocardial infarction; SBP=systolic blood pressure; HCTZ=hydrochlorothiazide; FMD=fibromuscular dysplasia | |||||||||
Criteria of successful treatment address both blood pressure control (“cure” or improvement) as well as kidney function. 60 , 61 Medical treatment may use angiotensin‐converting enzyme inhibitors (in cases of unilateral renal artery stenosis with preserved kidney function and normal contralateral kidney function) with or without added calcium channel blockers (which induce afferent arteriolar dilatation 40 ). In most cases, a diuretic is necessary for blood pressure control. If renal function does not deteriorate and if blood pressure control is achieved, medical treatment can be continued indefinitely. 62 In patients undergoing any form of revascularization, medical treatment should be continued, if needed, for blood pressure control. Usually fewer antihypertensive agents are needed for adequate hypertension control after revascularization. In many of the cases that have had surgical correction of a renal stenotic lesion the “high rates” of improvement have included many cases of patients whose doses of antihypertensive medications have been reduced but whose blood pressure was not controlled by the procedure. There are many reasons why this approach to defining success could be questioned.
Surgical revascularization is an option for patients with atherosclerotic renovascular disease. Careful evaluation of atherosclerotic disease in other vascular beds (e.g., coronaries, carotid arteries, aorta) with adequate treatment, if needed, before renal revascularization (bypass), reduces the perioperative morbidity associated with surgical intervention on the renal arteries. 43
Since its initial description, 63 percutaneous transluminal angioplasty of renal artery stenosis established itself as an efficient method of revascularization in patients with renovascular disease. Certain forms of renal artery stenosis (fibromuscular dysplasia, etc.), as shown in Table III, were considered “elective” indications for percutaneous transluminal angioplasty from its beginning. 64 The introduction of stenting dramatically reduced the rates of postprocedural restenosis and allowed successful intervention of the ostial 46 , 54 and of bilateral disease. 48 Primary stenting of renal artery stenosis had a significant beneficial effect on blood pressure control in patients with normal or mildly impaired renal function at baseline over a 4‐year follow‐up. 45 Further refinement of the technique (e.g., intravascular ultrasound‐guided interventions, 35 distal embolization protection devices, use of platelet inhibitors) may widen the spectrum of the types of renovascular disease which might benefit from percutaneous techniques. Future studies will have to address the long‐term effects of primary stenting on preservation of renal function in addition to optimal blood pressure control.
References
- 1. Goldblatt H, Lynch J, Hanzal RF, et al. Studies on experimental hypertension. I. The production of persistent elevation of systolic blood pressure by means of renal ischemia. J Exp Med. 1934;59:347–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eyler WR, Clark MD, Garman JE, et al. Angiography of the renal areas including a comparative study of renal arterial stenosis in patients with and without hypertension. Radiology. 1962;78:879–892. [DOI] [PubMed] [Google Scholar]
- 3. Holley KE, Hunt JC, Brown AL. Renal artery stenosis: a clinical‐pathologic study in normotensive patients. Am J Med. 1964;37:14–22. [DOI] [PubMed] [Google Scholar]
- 4. Selkurt EE. The effect of pulse pressure and mean arterial pressure modification of renal hemodynamics and electrolyte and water excretion. Circulation. 1951;4:541–551. [DOI] [PubMed] [Google Scholar]
- 5. Schreiber MJ, Pohl MA, Novick AC. The natural history of atherosclerotic and fibrous renal artery disease. Urol Clin North Am. 1984;11:383–392. [PubMed] [Google Scholar]
- 6. Caps MT, Perissinotto C, Zierler RE, et al. Prospective study of atherosclerotic disease progression in the renal artery. Circulation. 1998;98:2866–2872. [DOI] [PubMed] [Google Scholar]
- 7. Mailloux LU, Napolitano B, Belucci AG, et al. Renal vascular disease causing end‐stage renal disease, incidence, clinical correlates and outcomes: a 20‐year experience. Am J Kidney Dis. 1994;24:622–629. [DOI] [PubMed] [Google Scholar]
- 8. Weibull H, Bergqvist D, Bergetz SE, et al. Percutaneous transluminal renal angioplasty versus surgical reconstruction of atherosclerotic renal artery stenosis: a prospective randomized study. J Vasc Surg. 1993;18:841–852. [DOI] [PubMed] [Google Scholar]
- 9. Berglund G, Anderson O, Wilhelmsen L. Prevalence of primary and secondary hypertension. Br Med J. 1976;2:554–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Danielson M, Dammstrom BG. The prevalence of secondary and curable hypertension. Acta Med Scand. 1981;209:451–455. [DOI] [PubMed] [Google Scholar]
- 11. Bijlstra PJ, Postma CT, De Boo T, et al. Clinical and biochemical criteria in the detection of the renal artery stenosis. J Hypertens. 1996;14:1033–1040. [PubMed] [Google Scholar]
- 12. Harding MB, Smith LR, Himmelstein SI, et al. Renal artery stenosis: prevalence and associated risk factors in patients undergoing routine cardiac catheterization. J Am Soc Nephrol. 1992;2:1608–1616. [DOI] [PubMed] [Google Scholar]
- 13. Kimura G, London GM, Safar ME, et al. Glomerular hypertension in renovascular hypertensive patients. Kidney Int.1991;39:966–972. [DOI] [PubMed] [Google Scholar]
- 14. Hunt JC. Diagnosis of renovascular hypertension. Bull N Y Acad Med. 1969;45(9):877–895. [PMC free article] [PubMed] [Google Scholar]
- 15. Simon N, Franklin SS, Bleifer KH, et al. for the Cooperative Study of Renovascular Hypertension. Clinical characteristics of renovascular hypertension. JAMA. 1972,220:1209–1218. [PubMed] [Google Scholar]
- 16. Hunt JC, Strong CG. Renovascular hypertension. Mechanisms, natural history and treatment. Am J Cardiol. 1973;32:562–574. [DOI] [PubMed] [Google Scholar]
- 17. Pickering TG, Herman L, Devereux RB, et al. Recurrent pulmonary oedema in hypertension due to bilateral artery stenosis: treatment by angioplasty or surgical revascularization. Lancet. 1988;2:551–552. [DOI] [PubMed] [Google Scholar]
- 18. O'Donohoe MK, Donohoe J, Corrigan TP. Acute renal failure of renovascular origin: cure by aortorenal reconstruction after 24 days of anuria. Nephron. 1990;56:92–93. [DOI] [PubMed] [Google Scholar]
- 19. Rackson ME, Lossef SV, Sos TA. Renal artery stenosis in patients with aortic dissection: increased prevalence. Radiology. 1990;177:555–558. [DOI] [PubMed] [Google Scholar]
- 20. Rimmer JM, Gennari FJ. Atherosclerotic renovascular disease and progressive renal failure. Ann Intern Med. 1993;118:712–719. [DOI] [PubMed] [Google Scholar]
- 21. Ducloux D, Jamali M, Chalopin JM. Chronic congestive heart failure associated with bilateral renal artery stenosis. Clin Nephrol. 1997;48:54–55. [PubMed] [Google Scholar]
- 22. Mushlin AI, Thornbury JR. Intravenous pyelography: the case against its routine use. Ann Intern Med. 1989;111:58–70. [DOI] [PubMed] [Google Scholar]
- 23. Cameron HA, Close CF, Yeo WW, et al. Investigation of selected patients with hypertension by the rapid‐sequence intravenous urogram. Lancet. 1992;339:658–661. [DOI] [PubMed] [Google Scholar]
- 24. Blaufox MD, Middleton ML, Bongiovanni J, et al. Cost efficacy of the diagnosis and therapy of renovascular hypertension. J Nucl Med. 1996;37:171–177. [PubMed] [Google Scholar]
- 25. Fommei E, Ghione S, Hilson AJW, et al. Captopril radionuclide test in renovascular hypertension: a European multicentre study. Eur J Nucl Med. 1993;20:617–623. [DOI] [PubMed] [Google Scholar]
- 26. Pickering TG, Sos TA, Vaughan ED Jr, et al. Predictive value and changes of renin secretion in hypertensive patients with unilateral renovascular disease undergoing successful renal angioplasty. Am J Med. 1984;76:398–404. [DOI] [PubMed] [Google Scholar]
- 27. Muller FB, Sealey JE, Case DB, et al. Captopril test for identifying renovascular disease in hypertensive patients. Am J Med. 1986;80:633–644. [DOI] [PubMed] [Google Scholar]
- 28. Rudnick MR, Maxwell MH. Limitations of renin assays. In: Narins RG, ed. Controversies in Nephrology and Hypertension. New York, NY: Churchill Livingstone; 1984:123–160. [Google Scholar]
- 29. Olbricht CJ, Paul K, Prokop M, et al. Minimally invasive diagnosis of renal artery stenosis by spiral computed tomography angiography. Kidney Int. 1995;48:1332–1337. [DOI] [PubMed] [Google Scholar]
- 30. Beregi JP, Elkohen M, Deklunder G, et al. Helical CT angiography compared with arteriography in the detection of renal artery stenosis. AJR. 1996;167:495–501. [DOI] [PubMed] [Google Scholar]
- 31. Schoenberg S, Knopp M, Bock M, et al. Renal artery stenosis: grading of hemodynamic changes with cine phase‐contrast MR blood flow measurements. Radiology. 1997;203:45–53. [DOI] [PubMed] [Google Scholar]
- 32. Radermacher J, Chavan A, Bleck J, et al. Use of Doppler ultrasonography to predict the outcome of therapy for renal artery stenosis. N Engl J Med. 2001;344:410–417. [DOI] [PubMed] [Google Scholar]
- 33. Prince M, Schoenberg S, Ward J, et al. Hemodynamically significant atherosclerotic renal artery stenosis: MR angiographic features. Radiology. 1997;205:128–136. [DOI] [PubMed] [Google Scholar]
- 34. Hansen PB, Tribble RW, Reavis SW, et al. Renal duplex sonography: evaluation of clinical utility. J Vasc Surg. 1990;12:227–236. [DOI] [PubMed] [Google Scholar]
- 35. Dangas G, Laird JR, Mehran R, et al. Intravascular ultrasound‐guided renal artery stenting. J Endovasc Ther. 2001;8:238–247. [DOI] [PubMed] [Google Scholar]
- 36. Smith CW, Winfield AC, Price RR. Evaluation of digital venous angiography for the diagnosis of renovascular hypertension. Radiology. 1982;144:51–54. [DOI] [PubMed] [Google Scholar]
- 37. Mann SJ, Pickering TG. Detection of renovascular hypertension. State of the art. Ann Intern Med. 1992;117:845–853. [DOI] [PubMed] [Google Scholar]
- 38. National High Blood Pressure Education Program Working Group . 1995 Update of the working group reports on chronic renal failure and renovascular hypertension. Arch Intern Med. 1996;156:1938–1947. [PubMed] [Google Scholar]
- 39. Hollenberg NK. Medical treatment for renovascular hypertension: a review. Am J Hypertens. 1988;1:338S–343S. [DOI] [PubMed] [Google Scholar]
- 40. Zanchi A, Brunner HR, Waeber B, et al. Renal hemodynamic and protective effects of calcium antagonists in hypertension. J Hypertens. 1995;13:1363–1375. [PubMed] [Google Scholar]
- 41. Libertino JA, Flam TA, Zinman LN, et al. Changing concepts in surgical management of renovascular hypertension. Arch Intern Med. 1988;148:357–359. [PubMed] [Google Scholar]
- 42. Lawrie GM, Morris GC, Glaeser DH, et al. Renovascular reconstruction: factors affecting long‐term prognosis in 919 patients followed up to 31 years. Am J Cardiol. 1989;63:1085–1092. [DOI] [PubMed] [Google Scholar]
- 43. Novick AC, Ziegelbaum M, Vidt DG, et al. Trends in surgical revascularization for renal artery disease. Ten years' experience. JAMA. 1987;257:498–501. [PubMed] [Google Scholar]
- 44. Kidney D, Deutsch LS. The indications and results of percutaneous transluminal angioplasty and stenting in renal artery stenosis. Semin Vasc Surg. 1996;9:188–197. [PubMed] [Google Scholar]
- 45. Dorros G, Jaff M, Mathiak L, et al. Four‐year follow‐up of Palmaz‐Schatz stent revascularization as treatment for atherosclerotic renal artery stenosis. Circulation. 1998;98:642–647. [DOI] [PubMed] [Google Scholar]
- 46. Blum U, Krumme B, Flugel P, et al. Treatment of ostial renal artery stenoses with vascular endoprostheses after unsuccessful balloon angioplasty. N Engl J Med. 1997;336:459–465. [DOI] [PubMed] [Google Scholar]
- 47. Harden PN, MacLeod MJ, Rodger RSC, et al. Effect of renal artery stenting on progression of renovascular renal failure. Lancet. 1997;349:1133–1136. [DOI] [PubMed] [Google Scholar]
- 48. Rocha‐Singh KJ, Mishkel GJ, Katholi RE, et al. Clinical predictors of improved long‐term blood pressure control after successful stenting of hypertensive patients with obstructive renal artery atherosclerosis. Cathet Cardiovasc Diagn. 1999;47:167–172. [DOI] [PubMed] [Google Scholar]
- 49. Stanley P, Hieshima G, Mehringer M. Percutaneous transluminal angioplasty for pediatric renovascular hypertension. Radiology. 1984;153(1):101–104. [DOI] [PubMed] [Google Scholar]
- 50. Raynaud A, Bedrossian J, Remy P, et al. Percutaneous transluminal angioplasty of renal transplant arterial stenoses. AJR Am J Roentgenol. 1986;146(4):853–857. [DOI] [PubMed] [Google Scholar]
- 51. Fervenza FC, Lafayette RA, Alfrey EJ, et al. Renal artery stenosis in kidney transplants. Am J Kidney Dis. 1998;31(1):142–148. [DOI] [PubMed] [Google Scholar]
- 52. Heyborne KD, Schultz MF, Goodlin RC, et al. Renal artery stenosis during pregnancy: a review. Obstet Gynecol Surv. 1991;46(8):509–514. [DOI] [PubMed] [Google Scholar]
- 53. Rimmer JM, Gennari FJ. Atherosclerotic renovascular disease and progressive renal failure. Ann Intern Med. 1993;118:712–719. [DOI] [PubMed] [Google Scholar]
- 54. Gross CM, Kramer J, Waigand J, et al. Ostial renal artery stent placement for atherosclerotic renal artery stenosis in patients with coronary artery disease. Cathet Cardiovasc Diagn. 1998;45:1–8. [DOI] [PubMed] [Google Scholar]
- 55. Weibull H, Bergqvist D, Bergetz SE, et al. Percutaneous transluminal renal angioplasty versus surgical reconstruction of atherosclerotic renal artery stenosis: a prospective randomized study. J Vasc Surg. 1993;18:841–852. [DOI] [PubMed] [Google Scholar]
- 56. Plouin PF, Chatellier G, Darne B, et al. Blood pressure outcome of angioplasty in atherosclerotic renal artery stenosis: a randomized trial. Hypertension. 1998;31(3):823–829. [DOI] [PubMed] [Google Scholar]
- 57. Webster J, Marshall F, Abdalla M, et al. Randomized comparison of percutaneous angioplasty versus continued medical therapy for hypertensive patients with atheromatous renal artery stenosis. Scottish and Newcastle Renal Artery Stenosis Collaborative Group. J Hum Hypertens. 1998;12:329–335. [DOI] [PubMed] [Google Scholar]
- 58. Van De Ven, Peter JG, Kaate R, et al. Arterial stenting and balloon angioplasty in ostial atherosclerotic renovascular disease: a randomized trial. Lancet. 1999;353:282–286. [DOI] [PubMed] [Google Scholar]
- 59. Van Jaarsveld BC, Krijnen P, Pieterman H, et al. The effect of balloon angioplasty on hypertension in atherosclerotic renal‐artery stenosis. N Engl J Med. 2000;342:1007–1014. [DOI] [PubMed] [Google Scholar]
- 60. Maxwell MH, Bleifer KH, Franklin SS, et al. Cooperative Study of Renovascular Hypertension. Demographic analysis of the study. JAMA. 1972;220:1195–1204. [PubMed] [Google Scholar]
- 61. Standards Of Practice Committee of the Society of Cardiovascular and Interventional Radiology . Guidelines for percutaneous transluminal angioplasty. Radiology. 1990;177:619–626. [DOI] [PubMed] [Google Scholar]
- 62. Rosenthal T. Drug therapy of renovascular hypertension. Drugs. 1993;45:895–909. [DOI] [PubMed] [Google Scholar]
- 63. Gruntzig A, Kuhlmann U, Vetter W, et al. Treatment of renovascular hypertension with percutaneous transluminal dilatation of a renal‐artery stenosis. Lancet. 1978;1(8068):801–802. [DOI] [PubMed] [Google Scholar]
- 64. Tegtmeyer CT, Selby JB, Hartwell GD, et al. Results and complications of angioplasty in fibromuscular disease. Circulation. 1991;83(suppl I):I‐155–I‐161. [PubMed] [Google Scholar]


