PURPOSE
TP53-mutated (TP53m) myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML) have very poor outcome irrespective of the treatment received, including 40% responses (20% complete remission [CR]) with azacitidine (AZA) alone, short response duration, and a median overall survival (OS) of approximately 6 months. Eprenetapopt (APR-246), a novel first-in-class drug, leads to p53 protein reconformation and reactivates its proapoptotic and cell-cycle arrest functions.
PATIENTS AND METHODS
This phase II study assessed the safety and efficacy of eprenetapopt in combination with AZA in untreated high or very high International Prognostic Scoring System-R TP53m MDS and AML patients.
RESULTS
Fifty-two TP53m patients (34 MDS, 18 AML [including seven with more than 30% blasts]) were enrolled. In MDS, we observed an overall response rate (ORR) of 62%, including 47% CR, with a median duration of response at 10.4 months. In AML, the ORR was 33% including 17% CR (27% and 0% CR in AML with less than and more than 30% marrow blasts, respectively). Seventy-three percent of responders achieved TP53 next-generation sequencing negativity (ie, variant allele frequency < 5%). The main treatment-related adverse events were febrile neutropenia (36%) and neurologic adverse events (40%), the latter correlating with a lower glomerular filtration rate at treatment onset (P < .01) and higher age (P = .05), and resolving with temporary drug interruption without recurrence after adequate eprenetapopt dose reduction. With a median follow-up of 9.7 months, median OS was 12.1 months in MDS, and 13.9 and 3.0 months in AML with less than and more than 30% marrow blasts, respectively.
CONCLUSION
In this very high-risk population of TP53m MDS and AML patients, eprenetapopt combined with AZA was safe and showed potentially higher ORR and CR rate, and longer OS than reported with AZA alone.
INTRODUCTION
TP53 mutations are seen in 5%-10% tof de novo myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML) and in 25%-40% of therapy-related MDS and AML.1,2 They are also observed in 50% of patients with complex karyotype and 20% of lower-risk MDS with isolated deletion 5q, with the former generally having biallelic mutation, whereas the latter generally having monoallelic mutation.3 Treatment outcome with currently available therapies is poor, especially in patients with complex karyotype or when TP53 mutation is biallelic.4,5 The hypomethylating agents azacitidine (AZA) and decitabine (DAC), and intensive chemotherapy, yield poor and very short responses in these patients, with complete remission (CR) rates of 15%-20% and a median overall survival (OS) around 6 months.3,6-8 A high risk of relapse is also observed after allogeneic stem cell transplantation (allo-SCT), particularly in patients with TP53 mutation with complex karyotype, thus limiting improvements in OS.9
CONTEXT
Key Objective
TP53-mutated myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) have very poor outcome irrespective of the treatment received, including 40% responses (20% complete remission [CR] with azacitidine [AZA] alone), short response duration, and a median overall survival (OS) of approximately 6 months. This phase II study assessed the safety and efficacy of eprenetapopt in combination with AZA in untreated patients with high or very high International Prognostic Scoring System-R TP53-mutated MDS and AML.
Knowledge Generated
Fifty-two patients (34 MDS and 18 AML) were enrolled. In MDS, we observed an overall response rate (ORR) of 62% (including 47% CR), and in AML, an ORR of 33% (including 17% CR). Median OS was 12.1 months in MDS and 10.4 months in AML.
Relevance
Eprenetapopt combined with AZA was safe and showed potentially higher ORR and CR rate, and longer OS than reported with AZA alone.
Eprenetapopt (APR-246) is a novel, first-in-class, small molecule that targets TP53-mutant cancer cells. Eprenetapopt is a prodrug that is spontaneously converted to methylene quinuclidinone, a Michael acceptor that covalently binds to Cys residues in mutant p53, leading to thermodynamic stabilization of p53 protein by shifting the equilibrium toward the wild-type conformation and restoring protein function.10 The excretion mechanism is renal, and a relationship of APR-246 clearance to creatinine clearance was previously reported.11 Plasma protein binding is low, and albumin binding is not expected to be relevant to estimate effective drug levels. In addition, the metabolizing enzymes for APR-246 have not yet been identified, but oxidation seems to play a minor role in APR-246 elimination, and clinically relevant drug-drug interactions with CYP inhibitors are unlikely (unpublished data).
Eprenetapopt monotherapy showed efficacy in a phase I study including patients with AML. In vitro synergy was demonstrated with AZA in SKM1 TP53-mutated (TP53m) cell line. In vivo synergy was also observed in mice transplanted with the SKM1 TP53m cell line, and ex vivo synergistic effect was confirmed in bone marrow samples of TP53m MDS and AML patients.12
Here, we report the safety and efficacy of eprenetapopt combined with AZA in TP53m MDS and AML patients in a phase II clinical trial.
PATIENTS AND METHODS
Study Design
This was a phase II open-label multicenter study, whose design was similar to that of a US phase II study (ClinicalTrials.gov identifier: NCT03072043),13 except that our study also included AML with more than 30% blasts, and allowed maintenance therapy with eprenetapopt and AZA for up to 1 year after allo-SCT. Eprenetapopt was administered by 6-hour intravenous infusion daily at a fixed dose of 4,500 mg on days 1-4 of each 28-day cycle. AZA was administered at the standard dose of 75 mg/m2 subcutaneous injection daily on days 4-10 of each 28-day cycle. In patients consolidated with allo-SCT, maintenance treatment was administered with reduced doses of AZA (36 mg/m2 daily on days 1-5) and eprenetapopt at a fixed dose of 3,700 mg on days 1-4 of each 28-day cycle. The protocol provided for dose adjustments depending on adverse events (AEs) and management guidelines for neurologic AEs, including use of chlorpromazine and temporary interruption and/or dose reduction of eprenetapopt. The initial fixed starting dose of eprenetapopt was 4,500 mg, and the dose was reduced in 500-mg increments (500 mg down for DL-1 and 1,000 mg for DL-2) for neurologic AEs, if needed.
The clinical trial was approved by a French research ethics committee (Comité de Protection des Personnes Ile de France IV) and by the French National competent authority (Agence National de Securité du Médicament). Written informed consent was provided by all patients before screening and enrollment. The study was conducted in accordance with the Declaration of Helsinki and registered in ClinicalTrials.gov (identifier: NCT03588078).
Patients
Patients ≥18 years of age with an Eastern Cooperative Oncology Group performance status of 0-2 and adequate renal and hepatic function, who had de novo MDS or chronic myelomonocytic leukemia classified as intermediate, high, or very high using International Prognostic Scoring System (IPSS)-R, or AML including low blast count AML (ie, with less than 30% marrow blasts) and AML with more than 30% of blasts were included. All patients were hypomethylating agent-naive and were not previously allografted. All patients had at least one TP53 mutation identified based on local next-generation sequencing testing with central review. Therapy-related MDS and AML were eligible if they had at least 1-year disease-free survival from prior malignancy. Prior treatment with growth factors, lenalidomide, and/or hydroxyurea was permitted. All inclusion and exclusion criteria are listed in the Appendix (online only).
Study Assessments
The primary end point of the study was response based on intention to treat and in evaluable patients, using IWG 2006 criteria for MDS and IWG 2003 criteria for AML.14,15 Secondary end points were safety using National Cancer Institute-Common Terminology Criteria for Adverse Events 4.03 criteria, OS, response duration, AML progression rate, and correlation between TP53 variant allele frequency (VAF) and response. Responses, in MDS, included CR, marrow CR (mCR), partial remission (PR), and stable disease (SD) with hematologic improvement (SD with HI). Responses, in AML, included CR and CR with incomplete count recovery (CRi). Progression, in AML, was defined by a 50% increase of baseline blast percentage, as defined by ELN 2017.16 Minimal residual disease (MRD) was evaluated by detection of TP53 mutation (Appendix).
Statistical Analysis
It is described in detail in the supplemental data.
The intention-to-treat (ITT) population included all patients enrolled in this study. The evaluable population per protocol included all patients receiving at least three cycles of treatment and who had a bone marrow assessment after the third cycle. Treatment-emergent AEs were evaluated according to National Cancer Institute-Common Terminology Criteria for Adverse Events 4.03 criteria. Statistical analyses were performed on both the ITT and the evaluable population.
RESULTS
Baseline Patient Characteristics
Patients were enrolled between September 2018 and July 2019, and the data cutoff was April 1, 2020, with all patients enrolled for ≥ 9 months. As mentioned in the protocol, more than six patients achieved CR in the first 24 patients and allowed to continue until a total of 52 patients treated with eprenetapopt combined with AZA. As described below, although this patient number led to accrual of only 39 evaluable patients, ie, less than the planned 45 evaluable patients, the study eventually did meet the threshold of observing more than 14 CR in the evaluable population.
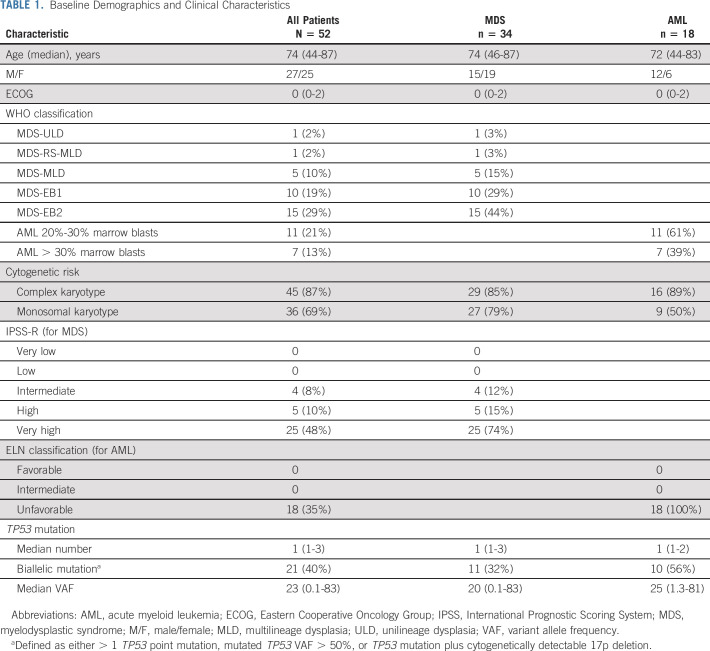
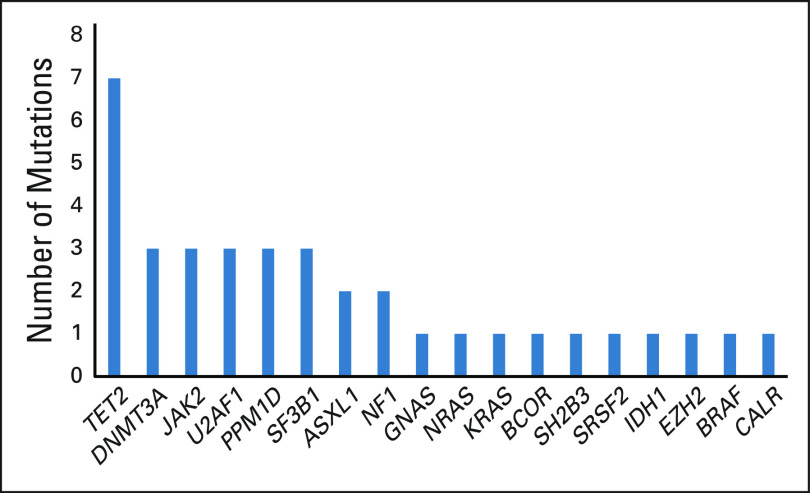
At the cutoff date, 12 (23%) patients were continuing on study treatment and four (8%) had received allogeneic SCT, two of whom started post-transplant maintenance treatment. Early discontinuation, before cycle 3 bone marrow evaluation, was observed in 13 (25%) patients and was related to severe infection (n = 6), progression (n = 4, who all had AML at inclusion, including two with more than 30% blasts at baseline), multiorgan dysfunction (n = 2), and consent withdrawal (n = 1). The median treatment duration was 8.6 months (range, 0.3-17.3 months). Baseline demographic and clinical characteristics are shown in Table 1. Thirty-four patients had MDS and 18 had AML. Using conventional cytogenetics, 80% of the patients had complex karyotype. 17p deletion was cytogenetically detectable in only in 25% of the patients but was probably often overlooked in those patients with complex karyotypes, as fluorescent in situ hybridization analysis of 17p was not assessed in most cases. The median number of TP53 point mutations was 1 (range, 1-3), with 25% of patients having two or more TP53 point mutations. The TP53m clone was dominant (ie, with VAF greater than that of the other mutated clones) in 83% of the patients. Median baseline TP53m VAF was 20% (range, 0.1-83%), and 10 (19%) had a baseline VAF > 50%. Forty-nine (94%) patients had at least one mutation in the DNA-binding domain (including 44 [90%] with a missense mutation) (Fig 1). Based on having either > 1 TP53 point mutation, TP53m VAF > 50%, or TP53 mutation plus cytogenetically detectable 17p deletion, including potential loss of heterozygosity, 21 (40%) patients were estimated to have biallelic mutation. Co-occurring somatic mutations were found in 48% of patients and are shown in Figure 3.
TABLE 1.
Baseline Demographics and Clinical Characteristics
FIG 1.
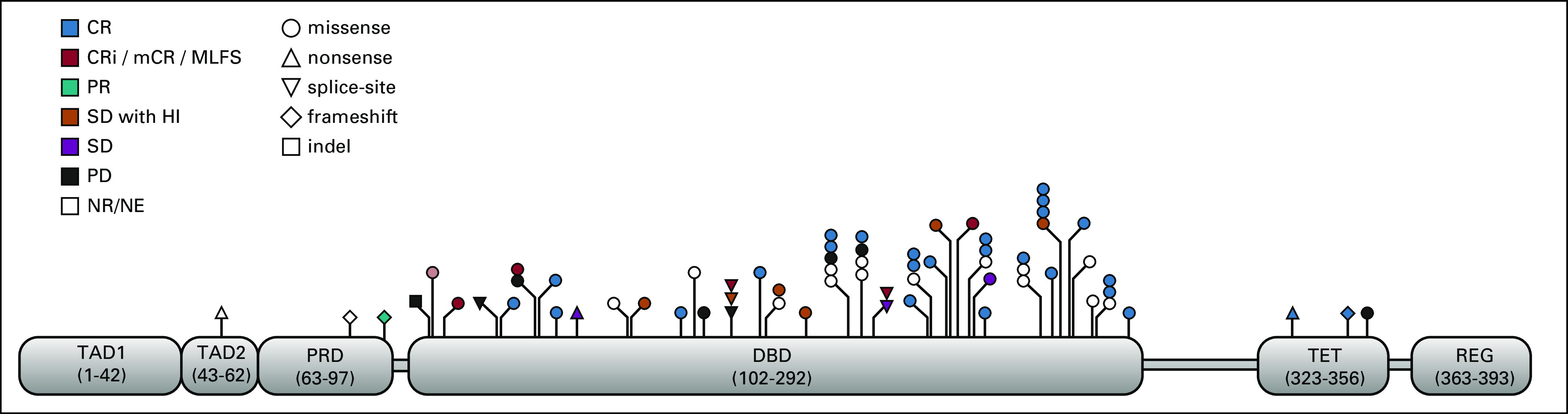
Spectrum of TP53 mutations in all patients (N = 52) at baseline. CR, complete remission; CRi, CR with incomplete count recovery; DBD, DNA binding domain; HI, hematologic improvement; mCR, marrow CR; NE, not evaluable; PR, partial remission; PRD, proline-rich domain; REG, C-terminal regulatory domain; SD, stable disease; TAD, transactivation domain; TET, tetramerization domain.
FIG 3.
Co-occurring somatic mutations.
Efficacy
Intention-to-treat population.
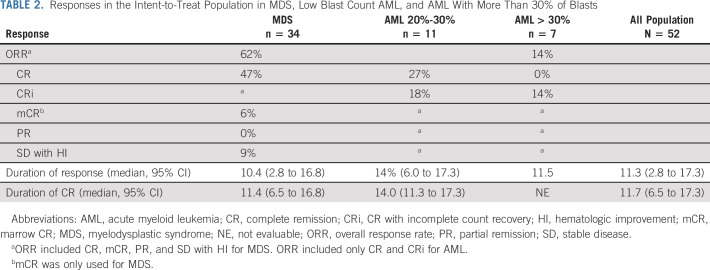
Fifty-two patients received combination therapy (ITT cohort). In MDS, the overall response rate (ORR) was 62%, including 47% CR, 6% mCR, and 9% SD with HI. In AML, the ORR was 33%, including 17% CR and 10% CRi. The ORR was 45% for low blast count AML (including 27% CR) and 14% for AML with more than 30% blasts (with no CR) (Table 2). When combining AML and MDS, the ORR was 52%, including 37% CR.
TABLE 2.
Responses in the Intent-to-Treat Population in MDS, Low Blast Count AML, and AML With More Than 30% of Blasts
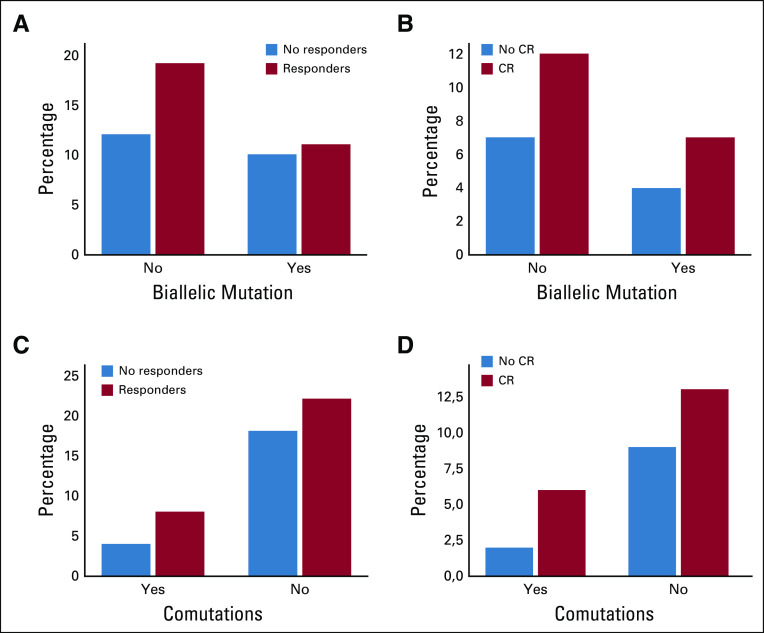
In MDS, median duration of overall duration response and CR was 10.4 (2.8-16.8+) and 11.4 (6.5-16.8+) months, respectively. In AML, median duration of overall duration response and CR was 12.7 (6.0-17.3+) and 14.0 (11.3-17.3+) months, respectively. When combining AML and MDS, median duration of overall response and CR was 11.3 (95% CI, 2.8 to 17.3+) and 11.7 (95% CI, 6.5 to 17.3+) months, respectively, with no significant difference between MDS, low blast AML, and AML with more 30% of blasts. When combining MDS and AML to have sufficient patient numbers, presence of biallelic TP53 mutation and presence of comutations had no impact on the ORR and CR rate (Appendix Fig A1, online only).
Evaluable population per protocol.
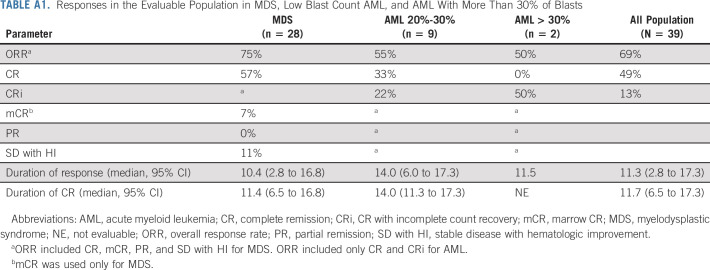
Only 39 patients received at least three cycles of combination treatment and had a bone marrow evaluation after 3 cycles, and were considered evaluable. Although accruing fewer than 45 evaluable patients, the study did meet the threshold of observing 19 CR, ie, more than 14 CR in the evaluable population. In MDS, the ORR was 75%, including 57% CR, 7% mCR, and 11% SD with HI. In AML, the ORR was 55%, including 36% CR and 18% CRi. The ORR was 55% for low blast count AML (including 50% CR) and 50% for AML with more than 30% blasts (with no CR) (Appendix). When combining AML and MDS, the ORR was 69%, including 19% CR.
In MDS, median duration of overall duration response and CR was 10.4 (2.8-16.8+) and 11.4 (6.5-16.8+) months, respectively. In AML, median duration of overall duration response and CR was 12.7 (6.0-17.3+) and 14.0 (11.3-17.3+) months, respectively. When combining AML and MDS, median duration of overall responses and CR was 11.3 (95% CI, 2.8 to 17.3+) and 11.7 (95% CI, 6.5 to 17.3+) months, respectively, with no significant difference between MDS, low blast AML, and AML with more 30% of blasts (ORR, 95% CI, 10.4 v 14.0 v11.5 months and CR, 95% CI, 11.4 v 14.0 months v not evaluable, respectively).
Safety
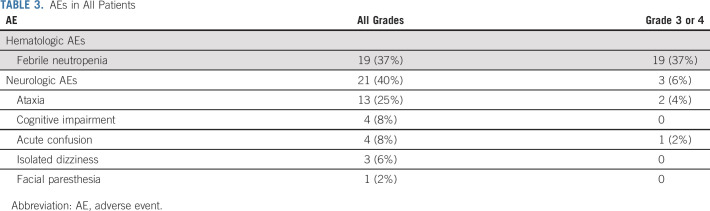
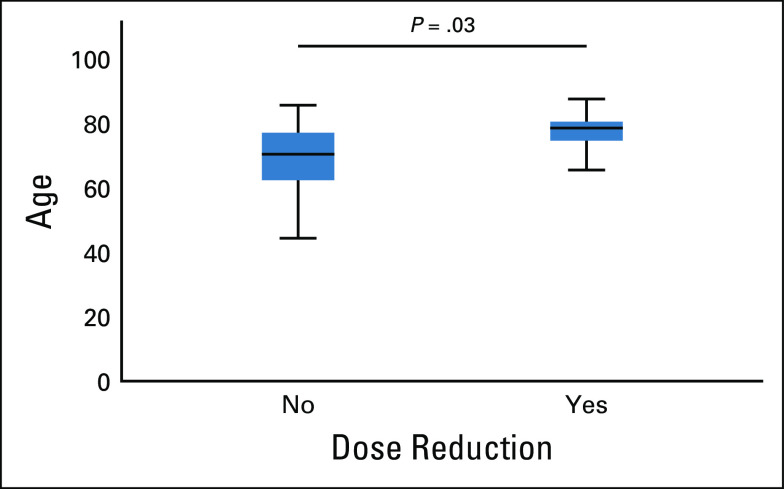
All AEs are reported in Table 3. AEs observed in ≥ 20% were febrile neutropenia (37%) and neurologic (40%). Hematologic toxicities appeared similar to those reported for AZA monotherapy. Neurologic AEs reached grade 3 in only three patients (one acute confusion and two ataxia). Occurrence of neurologic AEs was correlated with lower glomerular filtration rate at treatment onset (P < .01) and higher age (P = .003, Appendix Fig A2, online only). Neurologic toxicity was fully reversible within 5 days of drug discontinuation and showed no recurrence after dose reduction (one dose reduction [n = 13] and two dose reductions [n = 4]). Nine (69%) of the 13 patients who required one dose reduction, and two (50%) of the four patients who required two dose reductions responded, including six and one CR, respectively. Only one early discontinuation was because of eprenetapopt-related neurologic AE, and occurred in a patient with antibiotic-related renal failure. The 30-day and 60-day mortality was 0% and 8%, respectively.
TABLE 3.
AEs in All Patients
Survival
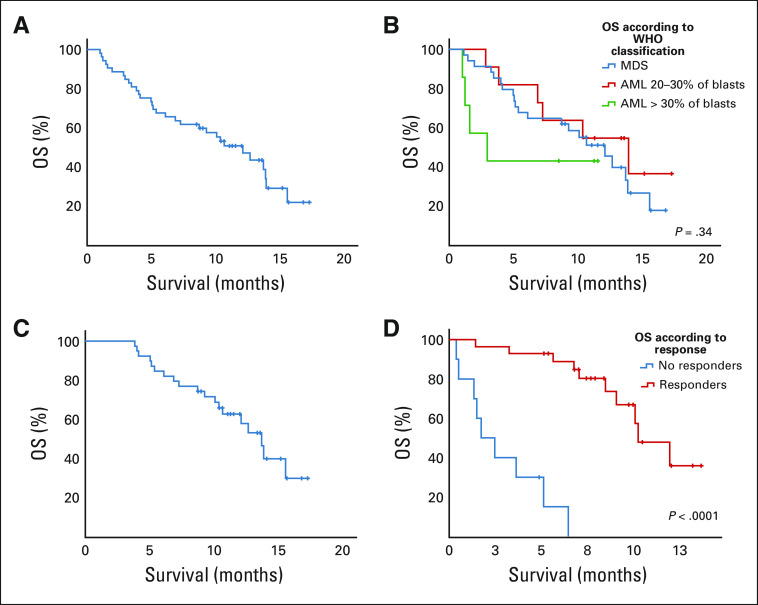
At a median follow-up time of 9.7 months, median OS was 12.1 months for MDS and 10.4 months for AML without significant difference between the two populations (P = .92). Nevertheless, we observed a trend for shorter OS in AML with more than 30% blasts versus MDS and low blast count AML: median 3.0 versus 12.1 versus 13.9 months, respectively (P = .34, Fig 2B). Median OS in the ITT population was 12.1 months (95% CI, 8.1 to 13.4 months; Fig 2A). Median OS was 13.7 months (95% CI, 11.7 to 15.7 months) in patients who received at least three treatment cycles (Fig 2C).
FIG 2.
OS (A) in the overall population, (B) in MDS and AML, (C) in patients who received at least three cycles, and (D) in responders versus nonresponders. AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; OS, overall survival.
When combining MDS and AML to have sufficient patient numbers, using a landmark analysis analyzing survival according to response, OS was significantly longer in responders (median 15.6 months, 95% CI, 13.0 to 18.1 months) than in nonresponders (median, 3.8 months, 95% CI, 2.8 to 4.9 months, P < .0001) (Fig 2D). In responders, there was no significant difference in OS between CR and non-CR patients (median 13.9 months v not reached, P = .37).
When combining MDS and AML to have sufficient patient numbers, presence of biallelic TP53 mutation or presence of comutations had no impact on OS. Four patients received allogeneic SCT, two of whom started maintenance treatment, precluding analysis of the impact of transplant. No relapse has so far been observed after allogeneic SCT. Sixty-eight percent of the patients who died had progressed before death. Forty one percent of patients with MDS progressed in AML during treatment. Analysis of the molecular profile at relapse is underway by pooling data from the US and French trials, to determine whether the frequency of TP53 mutant disease at relapse is negatively selected or not, akin to the lower frequency of FLT3-mutant AML often observed at relapse among patients exposed to an FLT3 inhibitor in combination with chemotherapy.
Correlation Between TP53 VAF and Response
When combining MDS and AML to have sufficient patient numbers, 22 (73%) of the responders achieved TP53 VAF levels below the 5% threshold, and nine (30%) TP53 VAF levels below the 0.1% threshold. Eighteen of the 22 patients with no detectable mutant TP53 at the 5% threshold were already negative at the first response assessment after cycle 3, and the remaining four patients after cycle 6. Eight of the nine patients with no detectable mutant TP53 at the 0.1% threshold were already negative after cycle 3, and the remaining patient after cycle 6. TP53 VAF decrease was strongly associated with response (P < .0001), CR achievement (P = .002), and duration of response (P < .0001). Achieving TP53 VAF level below the 5% threshold was associated with significantly longer response duration (P < .001) and significantly longer OS (13.9 months v 5.0 months, P < .0001). Achieving TP53 VAF level below the 0.1% threshold was also associated with better outcome (median OS not reached v 10.7 months, P = .05).
DISCUSSION
In this phase II clinical trial evaluating eprenetapopt and AZA in a very high-risk population, we report in MDS an ORR of 62% including 47% CR with a median duration of response at 10.4 months; in AML, the ORR of 33% including 17% CR in the ITT population with a median duration of response of 12.7 months. The ORR was 75% in patients with evaluable MDS and 55% in patients with evaluable AML (ie, who received at least 3 cycles). ORR was 55% in patients with low blast count AML. Outcome was poorer in AML with > 30% marrow blasts, but similar in MDS and low blast count AML.
Patients with TP53m MDS and AML have a poor prognosis, with poor and very short responses to all available treatments, and a median OS of about 6 months when treated with AZA alone in previous studies (median 7, 9, 8, and 4 months, respectively, in four large series where patient characteristics, including median Eastern Cooperative Oncology Group, were similar to those of this study).3,8,10,17 Recently, the combination of AZA and venetoclax demonstrated improved OS over AZA alone in patients with AML not eligible for intensive chemotherapy.18,19 In TP53m patients, however, although the combination was significantly associated with an increased CR/CRi rate (55.3% v 0% for AZA alone), it failed to improve OS. DiNardo et al20 reported, in AML, three patterns of resistance to venetoclax including TP53 mutations. In another study, a 10-day schedule of DAC showed a CR rate of 100% in 21 TP53m MDS or AML patients, with a median OS of 12.7 months, similar to that seen in TP53 wild-type patients.21 However, those results were not reproduced in a randomized phase II clinical trial comparing 5-day and 10-day schedules of DAC in patients with AML. In that study, with the 10-day schedule, the CR rate and median OS in the TP53m patients were 47% and 5.5 months, respectively.22
Responding patients had a deep molecular response with 73% achieving TP53 VAF levels below the 5% threshold, and 30% achieving TP53 VAF levels below the 0.1% threshold. TP53 VAF levels, using both sensitivity thresholds of 5% and 0.1%, were associated with a significant longer duration of response (P < .001) and a significant better OS.
Finally, the eprenetapopt plus AZA combination was generally well tolerated, with relatively frequent but reversible neurologic side effects that appeared more prevalent in elderly patients and/or those with renal failure, as already reported.11 Neurologic AEs were managed using supportive measures, including rare use of chlorpromazine, and with temporary interruption or dose reductions of eprenetapopt, if needed, and their precise mechanism is being explored. Given the potential relationship with impaired renal function, eprenetapopt should potentially be contraindicated or used with extreme precautions in patients with serum creatinine > 2 upper limit normal. Close monitoring of the renal function should be performed, especially in elderly patients, in patients with serum creatinine between normal and ≤2 upper limit normal, and those receiving concomitant potentially nephrotoxic agents.
In conclusion, the addition of eprenetapopt to AZA appeared to compare favorably with AZA alone in TP53m MDS or AML, and our results are consistent with results from a US phase I or II of eprenetapopt and AZA in TP53m MDS and low blast count AML showing an ORR of 71% with 44% achieving CR in the ITT population.23 Those data support the ongoing international phase III, multicenter, randomized study of eprenetapopt in combination with AZA versus AZA alone in patients with TP53-mutant MDS (ClinicalTrials.gov identifier: NCT03745716). The combination of AZA and eprenetapopt will have to be compared, in patients with AML, with the AZA and venetoclax combination. A phase I, multicenter, study of eprenetapopt in combination with AZA and venetoclax in patients with AML with TP53-mutant AML is also ongoing (ClinicalTrials.gov identifier: NCT04214860).
In January 2020, based on our current results and results from the US phase I or II trial of eprenetapopt and AZA, the Food and Drug Administration granted breakthrough therapy designation for the treatment of patients with TP53-mutant MDS with the combination of eprenetapopt and AZA.
APPENDIX. SUPPLEMENTAL PATIENTS AND METHODS
Study Design
Bone marrow aspiration was performed at baseline and every three cycles until progression or relapse. Adverse events were declared using the National Cancer Institute Common Terminology Criteria for AEs version 4.03 criteria and managed by standard of care.
Study Assessments
Minimal residual disease was evaluated by detection of TP53 mutation using (1) a classical next-generation sequencing (NGS) method with a 5% sensitivity (Genoptix, Carlsbad, CA) and (2) a high-sensitivity NGS method (human serum albumin panel from Genoptix, Carlsbad, CA) with a sensitivity of 0.1%.
Statistical Analysis
To determine the sample size, we used a Simon mini-max two-stage design, considering a proportion of patients achieving complete remission [CR] ≤ 20% with azacitidine alone versus the alternative hypothesis ≥ 40% with the combination treatment. Using a type I error 0.05 and a type II error rate of 0.10, it was computed that 24 patients should be enrolled at the first stage, with at least six patients achieving CR to continue to stage II. Then, 21 additional patients were required for a total of 45, with at least 14 CR to conclude there was sufficient evidence to support further study of APR-246 in combination with azacitidine in phase III. We decided to enroll 52 patients to take into account possible nonevaluable patients. Continuous variables were described using medians [interquartile ranges] (minimum; maximum) and qualitative variables were described using counts and percentages. Mann-Spearman correlation and Mann-Whitney test were used for continuous variables. The median follow-up time was calculated as the median time from initiation of treatment to last follow-up. Duration of response was estimated as the time between response and either loss of response, progression, or death. Kaplan-Meier estimates of the median duration of response were computed with 95% CI. Overall survival was calculated from the date of treatment initiation to date of death or last follow-up and was not censored at date of allogeneic stem cell transplantation. Survival curves were estimated using the Kaplan-Meier method and were compared by the logrank test. To compare survival curves according to response, and TP53 NGS variant allele frequency levels, a landmark analysis was performed at 109 days, corresponding to the maximum time of response evaluation.
FIG A1.
Correlation between response and presence or absence of comutation or biallelic mutation. Correlation between CR and presence or absence of comutation or biallelic mutation. CR, complete remission.
FIG A2.
Correlation between dose reduction and age.
TABLE A1.
Responses in the Evaluable Population in MDS, Low Blast Count AML, and AML With More Than 30% of Blasts
Thomas Cluzeau
Consulting or Advisory Role: Abbvie, Agios, Bristol-Myers Squibb, Jazz Pharmaceuticals, Novartis, Roche, Takeda, Syros Pharmaceuticals
Speakers' Bureau: Novartis, Amgen, Sanofi, Astellas Pharma
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Novartis, Pfizer, Sanofi
Jacqueline Lehmann-Che
Consulting or Advisory Role: AstraZeneca, Roche
Pierre Peterlin
Consulting or Advisory Role: Jazz Pharmaceuticals, Abbvie, Astellas Pharma, Daiichi Sankyo/Lilly
Odile Beyne Rauzy
Travel, Accommodations, Expenses: Novartis
Christian Recher
Consulting or Advisory Role: Roche, Pfizer, Incyte, Novartis, Otsuka, Astellas Pharma, Daiichi Sankyo, Macrogenics, Janssen, Abbvie, Jazz Pharmaceuticals, Amgen, Celgene
Research Funding: Abbvie, Roche, MaaT Pharma, Daiichi Sankyo, Agios, Chugai Pharma, Astellas Pharma, Jazz Pharmaceuticals, Novartis, Amgen, Celgene
Travel, Accommodations, Expenses: Gilead Sciences, Daiichi Sankyo, Novartis, Amgen, Celgene, Sanofi, Incyte
Aspasia Stamatoullas
Honoraria: Celgene
Consulting or Advisory Role: Takeda
Travel, Accommodations, Expenses: Pfizer
Lise Willems
Honoraria: Abbvie, Janssen, Novartis
Emmanuel Raffoux
Travel, Accommodations, Expenses: Abbvie, Roche
Bruno Quesnel
Research Funding: Daiichi Sankyo Europe GmbH
Michael Loschi
Consulting or Advisory Role: Astellas Pharma, Iqone, Novartis
Travel, Accommodations, Expenses: Novartis/Pfizer, MSD
Antoine F. Carpentier
Stock and Other Ownership Interests: Altevax
Honoraria: Gilead Sciences
Consulting or Advisory Role: Bristol-Myers Squibb
David A. Sallman
Consulting or Advisory Role: Syndax, Novartis, Magenta Therapeutics, Kite Pharma, Intellia Therapeutics, Gilead Sciences, Bristol-Myers Squibb, Aprea AB, Abbvie, Celyad, Agios
Speakers' Bureau: Bristol-Myers Squibb, Incyte, Agios
Research Funding: Jazz Pharmaceuticals, Celgene
Patents, Royalties, Other Intellectual Property: Intellectual Property Patent for LB-100 in MDS
Rami Komrokji
Stock and Other Ownership Interests: Abbvie
Consulting or Advisory Role: Acceleron Pharma, Abbvie, Geron, Jazz Pharmaceuticals, Bristol-Myers Squibb, Incyte, Novartis
Speakers' Bureau: Bristol-Myers Squibb, Jazz Pharmaceuticals
Travel, Accommodations, Expenses: Agios, Bristol-Myers Squibb, Jazz Pharmaceuticals, Incyte
Lionel Ades
Research Funding: Celgene
Honoraria: Novartis, Silence Therapeutics, BerGenBio, Jazz Pharmaceuticals, Abbvie, Celgene
Pierre Fenaux
Honoraria: Celgene
Research Funding: Celgene
No other potential conflicts of interest were reported.
See accompanying article on page 1595
PRIOR PRESENTATION
Presented in part in abstract form at the 2019 American Society of Hematology Annual Meeting, Orlando, FL, December 9, 2019; and the 2020 European Hematology Virtual Meeting, June 19, 2020.
SUPPORT
Supported by Groupe Francophone des Myelodysplasies (GFM).
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Thomas Cluzeau, Habiba Attalah, David A. Sallman, Rami Komrokji, Sylvie Chevret, Lionel Ades, Pierre Fenaux
Administrative support: Habiba Attalah, Fatiha Chermat, Sylvie Chevret
Provision of study materials or patients: Thomas Cluzeau, Jacqueline Lehmann-Che, Habiba Attalah, Elsa Miekoutima, Christian Recher, Aspasia Stamatoullas, Lise Willems, Emmanuel Raffoux, Bruno Quesnel, Lionel Ades
Collection and assembly of data: Thomas Cluzeau, Marie Sebert, Ramy Rahmé, Jacqueline Lehmann-Che, Pierre Peterlin, Blandine Bève, Fatiha Chermat, Elsa Miekoutima, Odile Beyne Rauzy, Christian Recher, Aspasia Stamatoullas, Lise Willems, Emmanuel Raffoux, Céline Berthon, Bruno Quesnel, Lionel Ades, Pierre Fenaux
Data analysis and interpretation: Thomas Cluzeau, Marie Sebert, Stefania Cuzzubbo, Blandine Bève, Habiba Attalah, Céline Berthon, Bruno Quesnel, Michael Loschi, Antoine F. Carpentier, David A. Sallman, Rami Komrokji, Anouk Walter-Petrich, Sylvie Chevret, Lionel Ades, Pierre Fenaux
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Eprenetapopt Plus Azacitidine in TP53-Mutated Myelodysplastic Syndromes and Acute Myeloid Leukemia: A Phase II Study by the Groupe Francophone des Myélodysplasies (GFM)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Thomas Cluzeau
Consulting or Advisory Role: Abbvie, Agios, Bristol-Myers Squibb, Jazz Pharmaceuticals, Novartis, Roche, Takeda, Syros Pharmaceuticals
Speakers' Bureau: Novartis, Amgen, Sanofi, Astellas Pharma
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Novartis, Pfizer, Sanofi
Jacqueline Lehmann-Che
Consulting or Advisory Role: AstraZeneca, Roche
Pierre Peterlin
Consulting or Advisory Role: Jazz Pharmaceuticals, Abbvie, Astellas Pharma, Daiichi Sankyo/Lilly
Odile Beyne Rauzy
Travel, Accommodations, Expenses: Novartis
Christian Recher
Consulting or Advisory Role: Roche, Pfizer, Incyte, Novartis, Otsuka, Astellas Pharma, Daiichi Sankyo, Macrogenics, Janssen, Abbvie, Jazz Pharmaceuticals, Amgen, Celgene
Research Funding: Abbvie, Roche, MaaT Pharma, Daiichi Sankyo, Agios, Chugai Pharma, Astellas Pharma, Jazz Pharmaceuticals, Novartis, Amgen, Celgene
Travel, Accommodations, Expenses: Gilead Sciences, Daiichi Sankyo, Novartis, Amgen, Celgene, Sanofi, Incyte
Aspasia Stamatoullas
Honoraria: Celgene
Consulting or Advisory Role: Takeda
Travel, Accommodations, Expenses: Pfizer
Lise Willems
Honoraria: Abbvie, Janssen, Novartis
Emmanuel Raffoux
Travel, Accommodations, Expenses: Abbvie, Roche
Bruno Quesnel
Research Funding: Daiichi Sankyo Europe GmbH
Michael Loschi
Consulting or Advisory Role: Astellas Pharma, Iqone, Novartis
Travel, Accommodations, Expenses: Novartis/Pfizer, MSD
Antoine F. Carpentier
Stock and Other Ownership Interests: Altevax
Honoraria: Gilead Sciences
Consulting or Advisory Role: Bristol-Myers Squibb
David A. Sallman
Consulting or Advisory Role: Syndax, Novartis, Magenta Therapeutics, Kite Pharma, Intellia Therapeutics, Gilead Sciences, Bristol-Myers Squibb, Aprea AB, Abbvie, Celyad, Agios
Speakers' Bureau: Bristol-Myers Squibb, Incyte, Agios
Research Funding: Jazz Pharmaceuticals, Celgene
Patents, Royalties, Other Intellectual Property: Intellectual Property Patent for LB-100 in MDS
Rami Komrokji
Stock and Other Ownership Interests: Abbvie
Consulting or Advisory Role: Acceleron Pharma, Abbvie, Geron, Jazz Pharmaceuticals, Bristol-Myers Squibb, Incyte, Novartis
Speakers' Bureau: Bristol-Myers Squibb, Jazz Pharmaceuticals
Travel, Accommodations, Expenses: Agios, Bristol-Myers Squibb, Jazz Pharmaceuticals, Incyte
Lionel Ades
Research Funding: Celgene
Honoraria: Novartis, Silence Therapeutics, BerGenBio, Jazz Pharmaceuticals, Abbvie, Celgene
Pierre Fenaux
Honoraria: Celgene
Research Funding: Celgene
No other potential conflicts of interest were reported.
REFERENCES
- 1.Papaemmanuil E Gerstung M Malcovati L, et al. : Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood 122:3616-3627, 2013; quiz 3699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bejar R Stevenson K Abdel-Wahab O, et al. : Clinical effect of point mutations in myelodysplastic syndromes. N Engl J Med 364:2496-2506, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haase D Stevenson KE Neuberg D, et al. : TP53 mutation status divides myelodysplastic syndromes with complex karyotypes into distinct prognostic subgroups. Leukemia 33:1747-1758, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montalban-Bravo G Kanagal-Shamanna R Benton CB, et al. : Genomic context and TP53 allele frequency define clinical outcomes in TP53-mutated myelodysplastic syndromes. Blood Adv 4:482-495, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sallman DA Komrokji R Vaupel C, et al. : Impact of TP53 mutation variant allele frequency on phenotype and outcomes in myelodysplastic syndromes. Leukemia 30:666-673, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bally C Ades L Renneville A, et al. : Prognostic value of TP53 gene mutations in myelodysplastic syndromes and acute myeloid leukemia treated with azacitidine. Leuk Res 38:751-755, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Fenaux P Mufti GJ Hellstrom-Lindberg E, et al. : Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: A randomised, open-label, phase III study. Lancet Oncol 10:223-232, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kulasekararaj AG Smith AE Mian SA, et al. : TP53 mutations in myelodysplastic syndrome are strongly correlated with aberrations of chromosome 5, and correlate with adverse prognosis. Br J Haematol 160:660-672, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Ciurea SO Chilkulwar A Saliba RM, et al. : Prognostic factors influencing survival after allogeneic transplantation for AML/MDS patients with TP53 mutations. Blood 131:2989-2992, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambert JM Gorzov P Veprintsev DB, et al. : PRIMA-1 reactivates mutant p53 by covalent binding to the core domain. Cancer Cell 15:376-388, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Lehmann S Bykov VJ Ali D, et al. : Targeting p53 in vivo: A first-in-human study with p53-targeting compound APR-246 in refractory hematologic malignancies and prostate cancer. J Clin Oncol 30:3633-3639, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Maslah N Salomao N Drevon L, et al. : Synergistic effects of PRIMA-1(Met) (APR-246) and 5-azacitidine in TP53-mutated myelodysplastic syndromes and acute myeloid leukemia. Haematologica 105:1539-1551, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas Cluzeau MP Marie Sebert M Ramy Rahmé M, et al. : APR-246 combined with azacitidine (AZA) in TP53 mutated myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML). A phase 2 study by the Groupe Francophone des Myélodysplasies (GFM). Blood 134 (suppl 1):677, 2019 [Google Scholar]
- 14.Cheson BD Greenberg PL Bennett JM, et al. : Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood 108:419-425, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Cheson BD Bennett JM Kopecky KJ, et al. : Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol 21:4642-4649, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Dohner H Estey E Grimwade D, et al. : Diagnosis and management of AML in adults: 2017 ELN recommendations from an International Expert Panel. Blood 129:424-447, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung J, Sallman DA, Padron E: TP53 and therapy-related myeloid neoplasms. Best Pract Res Clin Haematol 32:98-103, 2019 [DOI] [PubMed] [Google Scholar]
- 18.DiNardo CD Jonas BA Pullarkat VP, et al. : A randomized, double-blind, placebo-controlled study of venetoclax with azacitidine vs azacitidine in treatment-naive patients with acute myeloid leukemia ineligible for intensive therapy-viale_A. Clin Lymphoma Myeloma 20:S179, 2020. (suppl 1) [Google Scholar]
- 19.DiNardo CD Jonas BA Pullarkat V, et al. : Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med 383:617-629, 2020 [DOI] [PubMed] [Google Scholar]
- 20.DiNardo CD Tiong IS Quaglieri A, et al. : Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood 135:791-803, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welch JS, Petti AA, Ley TJ: Decitabine in TP53-mutated AML. N Engl J Med 376:797-798, 2017 [DOI] [PubMed] [Google Scholar]
- 22.Short NJ Kantarjian HM Loghavi S, et al. : Treatment with a 5-day versus a 10-day schedule of decitabine in older patients with newly diagnosed acute myeloid leukaemia: A randomised phase 2 trial. Lancet Haematol 6:e29-e37, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sallman DA DeZern AE. Sweet KL, et al. : Phase 2 results of APR-246 and azacitidine (AZA) in patients with TP53 mutant myelodysplastic syndromes (MDS) and oligoblastic acute myeloid leukemia (AML). Blood 134 (suppl 1):676, 2019 [Google Scholar]