PURPOSE
Treatment options are limited for patients with lower-risk myelodysplastic syndromes (LR-MDS). This phase III, placebo-controlled trial evaluated CC-486 (oral azacitidine), a hypomethylating agent, in patients with International Prognostic Scoring System LR-MDS and RBC transfusion–dependent anemia and thrombocytopenia.
METHODS
Patients were randomly assigned 1:1 to CC-486 300-mg or placebo for 21 days/28-day cycle. The primary end point was RBC transfusion independence (TI).
RESULTS
Two hundred sixteen patients received CC-486 (n = 107) or placebo (n = 109). The median age was 74 years, median platelet count was 25 × 109/L, and absolute neutrophil count was 1.3 × 109/L. In the CC-486 and placebo arms, 31% and 11% of patients, respectively, achieved RBC-TI (P = .0002), with median durations of 11.1 and 5.0 months. Reductions of ≥ 4 RBC units were attained by 42.1% and 30.6% of patients, respectively, with median durations of 10.0 and 2.3 months, and more CC-486 patients had ≥ 1.5 g/dL hemoglobin increases from baseline (23.4% v 4.6%). Platelet hematologic improvement rate was higher with CC-486 (24.3% v 6.5%). Underpowered interim overall survival analysis showed no difference between CC-486 and placebo (median, 17.3 v 16.2 months; P = .96). Low-grade GI events were the most common adverse events in both arms. In the CC-486 and placebo arms, 90% and 73% of patients experienced a grade 3-4 adverse event. Overall death rate was similar between arms, but there was an imbalance in deaths during the first 56 days (CC-486, n = 16; placebo, n = 6), most related to infections; the median pretreatment absolute neutrophil count for the 16 CC-486 patients was 0.57 × 109/L.
CONCLUSION
CC-486 significantly improved RBC-TI rate and induced durable bilineage improvements in patients with LR-MDS and high-risk disease features. More early deaths occurred in the CC-486 arm, most related to infections in patients with significant pretreatment neutropenia. Further evaluation of CC-486 in MDS is needed.
INTRODUCTION
Myelodysplastic syndromes (MDS) encompass a heterogenous group of hematologic disorders characterized by bone marrow dysplasia and peripheral blood cytopenias.1 The International Prognostic Scoring System (IPSS) stratifies patients into four risk groups based on cytogenetic features, number of cytopenias, and blast percentage2; Low and Intermediate-1 risks are considered lower-risk MDS (LR-MDS), and Intermediate-2 and High risks are considered higher-risk MDS.
CONTEXT
Key Objective
Myelodysplastic syndromes (MDS) are a diverse group of hematologic disorders with a range of prognoses. Within the International Prognostic Scoring System lower-risk MDS population exists a subgroup of patients with high-risk disease features including multiple cytopenias. The AZA-MDS-003 study was a randomized, placebo-controlled phase III trial of CC-486, an oral hypomethylating agent, in patients with International Prognostic Scoring System lower-risk MDS with transfusion-dependent anemia and thrombocytopenia.
Knowledge Generated
CC-486 was associated with a significantly higher rate of RBC transfusion independence versus placebo and with improvements in hemoglobin and platelet counts over time. The most common adverse events were GI and hematologic events. Although overall death rate was similar between treatment arms, more early deaths occurred in the CC-486 arm, most related to infections in patients with significant pretreatment neutropenia.
Relevance
Further evaluation of CC-486 in patients with MDS is needed, including rational identification of patients most likely to benefit from the drug.
Anemia is the most frequent clinical finding in MDS. RBC transfusion dependence (TD) is associated with decreased overall survival (OS) and health-related quality of life (HRQoL) and increased risk of transformation to acute myeloid leukemia (AML).3,4 Thrombocytopenia has especially poor prognostic implications for patients with LR-MDS and is among the strongest predictors of decreased OS across all IPSS MDS risk categories.5,6
Supportive care measures have traditionally been the mainstay of LR-MDS therapy, including RBC and platelet transfusions, erythropoiesis stimulating agents, and hematopoietic growth factors such as granulocyte CSF.7 Thrombopoietin receptor agonists are under investigation for treatment of MDS-related thrombocytopenia, although these agents are not yet recommended for routine use.8,9 Injectable hypomethylating agents (HMAs) may address anemia and thrombocytopenia in patients with MDS,10,11 and luspatercept, an erythroid maturation agent, was recently approved for treatment of anemia for patients with LR-MDS with ring sideroblasts. Within the LR-MDS population exists a subgroup of patients with poor prognosis and high-risk disease features, including RBC–transfusion dependent (RBC-TD) anemia and thrombocytopenia, who require therapeutic agents that address both anemia and thrombocytopenia.
CC-486 is an oral HMA that has been evaluated in patients with hematologic malignancies.12-16 CC-486 (ONUREG; azacitidine tablets) was recently approved in the United States for continuous treatment of adult patients with AML in first remission following induction chemotherapy, who are not able to complete curative therapy (eg, transplant).16 CC-486 has a unique pharmacokinetic or pharmacodynamic profile from injectable azacitidine, and the two are not bioequivalent. CC-486 allows for extended dosing schedules over each treatment cycle to sustain drug exposure. Longer HMA dosing regimens have been associated with higher response rates compared with shorter schedules,17-19 and extended CC-486 dosing has shown more prolonged hypomethylation compared with a 7-day schedule.14,20
Reported here are outcomes from a randomized, placebo-controlled, phase III trial of CC-486 in patients with IPSS-defined LR-MDS, with RBC-TD anemia and thrombocytopenia at study entry.
METHODS
Trial Design
This trial (ClinicalTrials.gov identifier: NCT01566695) enrolled patients at 101 sites in 20 countries between April 2013 and February 2018. The sponsor designed the trial in collaboration with an independent steering committee and with advice from regulatory agencies. The Protocol (online only) was approved at each participating site. The study was conducted in accordance with the principles outlined in the Declaration of Helsinki. The sponsor supplied study drug and contributed to data collection and analysis. The authors are responsible for all content in this report. The corresponding author had full access to study data and had final responsibility for the decision to submit for publication.
The study comprised an initial screening phase, a double-blind treatment period, and a survival follow-up period (Data Supplement, online only). Eligible patients were age ≥ 18 years, with IPSS-defined2 LR-MDS (the Revised IPSS [IPSS-R]21 was not available when this study was designed), Eastern Cooperative Oncology Group performance status score ≤ 2, RBC-TD anemia, and thrombocytopenia. RBC-TD was defined according to International Working Group (IWG) 2006 criteria22 as an average requirement of ≥ 2 RBC units per 28 days for ≥ 56 days, with no transfusion-free period of ≥ 28 consecutive days during the 56 days before random assignment. Thrombocytopenia was defined as two platelet counts ≤ 75 × 109/L, measured ≥ 21 days apart. Full eligibility criteria are provided in the Data Supplement.
Patients were randomly assigned 1:1 to CC-486 300-mg or placebo, administered once daily for 21 days per 28-day treatment cycle. All patients could receive best supportive care as needed. Erythropoiesis stimulating agents and other growth factors were not allowed. All patients signed informed consent following institutional guidelines.
Procedures and Assessments
Clinical laboratory assessments are reported per central review. Disease status and treatment response were assessed centrally at the end of cycle 6; patients who achieved a response or showed no evidence of disease progression could continue receiving randomized treatment.
End Points
The primary end point was rate of RBC transfusion independence (TI) lasting ≥ 56 consecutive days, per IWG 2006 criteria.22 The primary end point was assessed after all patients had completed 12 months of treatment or discontinued study drug. The key secondary end point was OS, defined as the time from random assignment to death (or last follow-up). Other end points included time to and duration of RBC-TI, rate and duration of ≥ 84-day RBC-TI, rate and duration of platelet-TI (≥ 56 days), hematologic improvement (HI) in the erythroid (HI-E) and platelet (HI-P) lineages, morphologic response among patients with > 5% blasts at baseline and with ≥ 1 postbaseline assessment (IWG 2006 criteria22), and AML progression (WHO 2008 criteria23). The Functional Assessment of Cancer Therapy-Anemia (FACT-An)24 questionnaire was used to measure effects of treatment on patient-reported HRQoL related to anemia and fatigue. The primary HRQoL end points were changes from baseline in the Functional Well-Being (FWB) domain and the Trial Outcome Index (TOI).
All patients were evaluated for treatment-emergent adverse events (TEAEs) from first dose through 28 days following last dose. Study drug dosing could be interrupted, delayed, or modified to ameliorate TEAEs (Data Supplement).
Statistical Analyses
The prospectively planned sample size was 386 patients, which would provide 80% power to determine a treatment effect on OS after approximately 250 deaths. However, in January 2018, the Data Monitoring Committee recommended halting study enrollment because of safety concerns related to an excess of early mortality in the CC-486 arm. In consultation with the US Food and Drug Administration, a partial clinical hold was placed on the study. In July 2018, the clinical hold was lifted; however, given historically slow recruitment, the sponsor decided not to reopen enrollment. The number of patients randomly assigned at the time of clinical hold (N = 216) provided only approximately 72% power to detect a difference in OS between arms at a significance level of 0.05 with 205 deaths but remained sufficient to detect a difference in RBC-TI with approximately 99% power. At data cutoff (January 25, 2019), 140 deaths had occurred.
RBC-TI rates were compared between arms using a stratified Mantel-Haenszel chi-squared test with a two-sided alpha of .05. Durations of RBC-TI and platelet-TI, time to AML progression, and OS from time of randomization were estimated using Kaplan-Meier methods, and OS was compared between treatment groups by stratified log-rank test. No adjustments for multiplicity were made for other secondary end points, and the associated P values should not be used to infer treatment effects.
RESULTS
Patients
In all, 499 patients were screened for participation, but 283 (56.7%) failed screening, most commonly because of unconfirmed LR-MDS (n = 180) and not meeting protocol-defined criteria for thrombocytopenia or RBC-TD anemia (n = 76). Twenty-four patients died during the screening period, most often because of infections (n = 7) or MDS progression (n = 6).
Overall, 216 patients were randomly assigned to receive CC-486 (n = 107) or placebo (n = 109) (Fig 1). At data cutoff, all patients had completed 12 months of treatment or had discontinued treatment. Baseline characteristics were generally well-balanced between treatment groups (Table 1), except that a higher proportion of patients in the placebo arm had > 5% blasts (28.4% v 15.9% in the CC-486 arm). The median age was 74 years (range, 30-89), 70.8% of patients had refractory cytopenia with multilineage dysplasia MDS, and all but one patient had IPSS Intermediate-1 risk disease. Post hoc assessment showed that 28.3% of patients had IPSS-R–defined21 high- or very high-risk MDS. Median RBC transfusion requirement was 6.7 units/56 days (range, 2.6-20.0). The most common gene mutations were ASXL1 (33.2%) and TET2 (21.5%).
FIG 1.
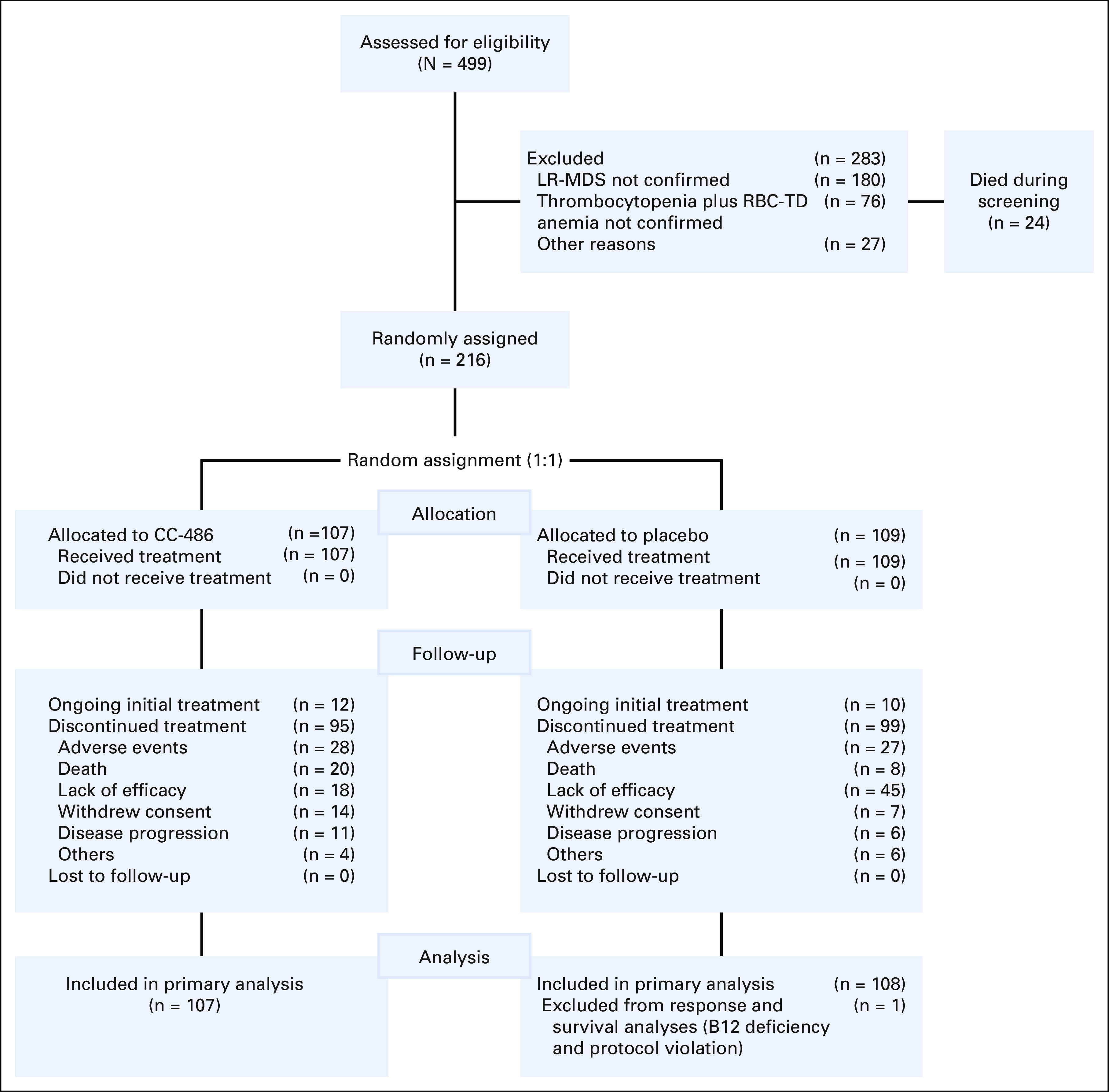
Patient disposition. Data cutoff: January 25, 2019. LR-MDS, lower-risk myelodysplastic syndromes; RBC-TD, RBC–transfusion dependent.
TABLE 1.
Baseline Demographic and Disease Characteristics
Efficacy
Significantly more patients in the CC-486 arm than in the placebo arm achieved RBC-TI for ≥ 56 consecutive days (30.8% v 11.1%, respectively; odds ratio [OR], 3.6 [95% CI, 1.7 to 7.4]; P = .0002). In univariate analysis, RBC-TI rates appeared to consistently favor CC-486 across subgroups defined by prognostic baseline characteristics (Data Supplement). Multivariate analysis confirmed the significant treatment effect of CC-486 on RBC-TI after controlling for prognostic baseline characteristics (P = .0002) (Data Supplement). No clear associations were observed between baseline gene mutations and achievement of RBC-TI (Data Supplement).
The median time to RBC-TI was 2.4 months in the CC-486 arm and 2.0 months in the placebo arm, and estimated median RBC-TI durations were 11.1 and 5.0 months, respectively (P = .42) (Fig 2A). Similar to 56-day RBC-TI rates, the rate of RBC-TI lasting ≥ 84 days was higher in the CC-486 arm (28.0%) than in the placebo arm (5.6%) (OR, 6.6 [95% CI, 2.6 to 16.7]; P < .0001).
FIG 2.
Kaplan-Meier estimated durations of (A) RBC transfusion independence and (B) RBC transfusion reductions (≥ 4 units). Data cutoff: January 25, 2019. NR, not reached; RBC-TI, RBC transfusion independence.
An HI-E response was attained by 46 patients (43.0%) in the CC-486 arm and 34 patients (31.5%) in the placebo arm (OR, 1.6 [95% CI, 0.9 to 2.9]; P = .12). Twenty-five patients (23.4%) in the CC-486 arm and 5 (4.6%) in the placebo arm had a ≥ 1.5 g/dL increase in hemoglobin concentration from baseline (OR, 6.3 [95% CI, 2.3 to 17.1]; P < .0001). Forty-five patients (42.1%) in the CC-486 arm and 33 (30.6%) in the placebo arm achieved RBC transfusion reductions of ≥ 4 units/56 days (OR, 1.7 [95% CI, 0.9 to 2.9]; P = .12). RBC transfusion reductions were sustained for the median of 10.0 months with CC-486 and 2.3 months with placebo (Fig 2B); 29 patients (27.1%) in the CC-486 arm sustained these transfusion reductions for ≥ 16 weeks, compared with only six patients (5.6%) in the placebo arm.
Among 75 patients who were platelet-TD at baseline, five of 30 (16.7%) in the CC-486 arm and five of 35 (14.3%) in the placebo arm attained platelet-TI and sustained for the median of 12.1 months (95% CI, 8.3 to not estimable) and 4.4 months (2.3 to not estimable), respectively. However, HI-P rate was higher in the CC-486 arm than in the placebo arm: 24.3% versus 6.5%, respectively (OR, 4.6 [95% CI, 1.9 to 11.2]; P = .0003).
Within the CC-486 arm, 25 patients (23.3%) achieved both HI-E and HI-P (ie, bilineage response) (Data Supplement). Patients in the CC-486 arm showed early and sustained improvement in mean hemoglobin levels and platelet counts on study (Fig 3).
FIG 3.
Mean (±SE) changes from baseline in (A) hemoglobin concentrations and (B) platelet counts. BL, baseline; SE, standard error.
Few patients (n = 37; 17%) were eligible to achieve an IWG-defined morphologic response (ie, had ≥ 5% blasts at baseline). Of 13 eligible patients in the CC-486 arm, one achieved complete remission (CR), no patient achieved partial remission, and three achieved marrow CR. Among 24 eligible patients in the placebo arm, no patient achieved CR or partial remission and one patient attained marrow CR.
Baseline FACT-An FWB and TOI scores were similar between arms and lower (worse) than reference values from a general population.25 During treatment, FACT-An FWB scores generally improved from baseline over time in both arms and TOI scores trended toward improvement only in the CC-486 arm. No significant between-group differences in changes from baseline FWB or TOI scores were reported. HRQoL outcomes will be reported in full detail elsewhere.
Overall Survival
At data cutoff, 140 patients (CC-486, n = 69; placebo, n = 71) had died (April 2013 to January 2019). Thirty-nine deaths (CC-486, n = 25; placebo, n = 14) occurred during treatment (ie, from first dose through 28 days after last dose), and 101 (CC-486, n = 44; placebo, n = 57) occurred during post-treatment follow-up (most attributed to disease progression).
In interim survival analysis (median follow-up 13.3 months), the estimated median OS was 17.3 months in the CC-486 arm and 16.2 months in the placebo arm (hazard ratio, 0.99 [95% CI, 0.70 to 1.40]; P = .96) (Data Supplement). There were no significant OS differences between treatment arms among patient subgroups defined by baseline characteristics. Rate of AML progression in the CC-486 arm (7.5% [n = 8]) was approximately one half of that in the placebo arm (16.7% [n = 18]) (HR, 0.40 [95% CI, 0.16 to 0.97]; P = .04). In the CC-486 arm, three patients progressed to AML on study and five progressed after discontinuing treatment. Estimated median time to AML progression was not reached in either arm in interim analysis; among patients who did progress, the median time to progression was 17.9 months (range, 0.8-32.9) in the CC-486 arm and 9.9 months (1.0-35.2) in the placebo arm.
Safety
Patients received a median of 5 (range, 1-70) CC-486 cycles and 6 (1-69) placebo cycles. In the CC-486 arm, the median adherence to CC-486 was 97% and the median cycle length was 28 days.
During the treatment period, all patients treated with CC-486 experienced ≥ 1 TEAE (any grade), as did all but one patient (99.1%) who received placebo. Most patients (89.7% in the CC-486 arm and 73.4% in the placebo arm) experienced a grade 3-4 TEAE. GI and hematologic events were the most common TEAEs in both arms but occurred more frequently in the CC-486 arm (Data Supplement). GI TEAEs were mostly grade 1-2 in severity; grade 3-4 events were uncommon and occurred primarily during early treatment cycles. Grade 3-4 neutropenia, thrombocytopenia, and febrile neutropenia were reported more frequently in the CC-486 arm (Table 2); incidence of these events was generally higher during early treatment cycles (Data Supplement). Grade 3-4 infections were reported in 46 CC-486 patients (43.0%) and 30 placebo patients (27.5%).
TABLE 2.
Grade 3-4 Treatment-Emergent Adverse Events Reported in ≥ 10% of Patients Randomly Assigned to CC-486
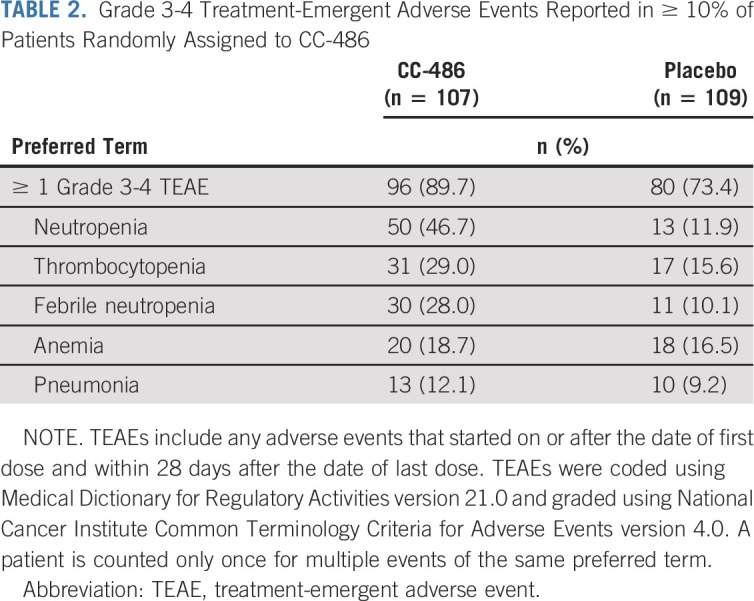
Thirty-one patients (29.0%) required ≥ 1 CC-486 dose reduction because of a TEAE, most commonly related to neutropenia (14.0%), thrombocytopenia (4.7%), diarrhea (3.7%), and febrile neutropenia (2.8%). Thirty-two patients (29.9%) in the CC-486 arm and 31 patients (28.4%) in the placebo arm permanently discontinued study drug because of a TEAE. The most common TEAEs requiring discontinuation of CC-486 were nausea (3.7%), febrile neutropenia (2.8%), and sepsis (2.8%) (Data Supplement).
Infections were the most common TEAEs leading to death (CC-486, n = 10; placebo, n = 4). There was an imbalance in early deaths between treatment arms, with 16 in the CC-486 arm and six in the placebo arm occurring between study days 1 and 56. Post hoc analyses showed that the 16 patients in the CC-486 arm who died early were severely neutropenic and thrombocytopenic at baseline, with a median absolute neutrophil count (ANC) of 0.57 × 109/L and a platelet count of 15.5 × 109/L; for the six patients in the placebo arm, the median ANC was 2.31 × 109/L and the platelet count was 22.0 × 109/L at baseline. The most common reason for early death in the CC-486 arm was infection (primarily bacterial).
DISCUSSION
The primary end point of this phase III study was met: CC-486 was associated with a significantly higher rate of RBC-TI compared with placebo. The estimated median duration of RBC-TI was 11.1 months with CC-486, compared with 5.0 months with placebo, and the median duration of RBC transfusion reduction of ≥ 4 units was substantially longer with CC-486 (10.0 v 2.3 months, respectively). The durability of these responses is consistent with the observed increases in hemoglobin levels during CC-486 treatment. RBC-TD is associated with transformation to AML4; consistent with higher rates of RBC-TI, the rate of AML progression in the CC-486 arm was one half of that in the placebo arm.
The HI-E rate in the placebo arm (31.5%) was substantially higher than what has been reported for placebo in other LR-MDS trials.26-28 The HI-E responses reported in the placebo arm were driven by 30.6% of patients who sustained RBC transfusion reductions for ≥ 8 weeks (transfusions were administered at physician discretion), and hemoglobin increases of ≥ 1.5 g/dL were infrequent; thus, transfusion reductions did not necessarily reflect meaningful improvements in hemoglobin levels, highlighting a limitation of the IWG 2006 HI-E criteria.22 Moreover, the median duration of transfusion reductions was shorter with placebo than with CC-486. The 2018 IWG MDS response criteria,29 which were not available when this study was designed, require a 16-week transfusion-reduction duration for an HI-E response; had those criteria been in place (our study did not require a 16-week screening period), the HI-E rate would have been substantially lower.
Almost one half (43%) of all patients in our study had baseline platelet counts of < 20 × 109/L. Although platelet-TI rate was not significantly different between treatment arms, patients in the CC-486 arm experienced sustained platelet count increases over time, and patients were > 4 times more likely to achieve HI-P. Notably, nearly one fourth of patients treated with CC-486 achieved both HI-E and HI-P.
Patients in this study represent a particularly unfavorable subgroup of the IPSS Intermediate-1 risk LR-MDS population. Indeed, 28% of these patients would have had high- or very high-risk MDS at study entry by IPSS-R classification. The genetic landscape of this lower-risk study population at baseline was enriched with adverse-risk mutations, also suggestive of a higher-risk phenotype.30-33 There were also a high number of deaths during screening (n = 24), mainly because of infections or disease progression. During the first 56 days on study, a higher rate of early death was observed in the CC-486 arm than in the placebo arm, most related to infections. The propensity to develop fatal infections early in the study could be partly attributed to poor prognostic baseline characteristics of these mostly older patients (median age 74 years), but this does not explain the imbalance between treatment arms (except for the notable difference in baseline ANC) and a causal relationship between CC-486 and these early deaths cannot be excluded. Given the unfavorable prognostic features of patients in this study, there might have been a subgroup of patients who were more sensitive to the myelosuppressive effects of CC-486, inducing an ANC reduction during early treatment that could have rendered already significantly neutropenic patients more prone to fatal infections. Currently, the ONUREG prescribing information does not recommend using CC-486 for MDS outside of a clinical trial.16
At data cutoff, underpowered interim OS analysis showed no survival benefit for patients receiving CC-486. The relatively poor OS seen in both arms of this trial also speaks to the severity of illness in this population.2
Hematologic and GI TEAEs were the most frequently observed toxicities with CC-486. These events occurred most often during cycles 1-2, after which they were less frequent, suggesting a lack of cumulative toxicity. The majority of GI TEAEs were mild or moderate in severity. Grade 3-4 hematological TEAEs and infections occurred more frequently in the CC-486 arm. Similarly, more patients in the CC-486 arm had TEAEs leading to a dose reduction, but similar proportions of patients in each arm discontinued treatment because of a TEAE, suggesting that CC-486 dose modifications allowed some patients to continue treatment with better tolerance.
These results demonstrate that CC-486 can provide clinically meaningful reductions in RBC transfusion burden and ameliorate thrombocytopenia in patients with LR-MDS with symptomatic disease and refractory cytopenias. A higher rate of early deaths occurred in the CC-486 arm, most related to infections in patients with significant pretreatment neutropenia. Ongoing molecular and HRQoL analyses may provide additional context for these clinical outcomes. Further evaluation of CC-486 in patients with MDS is needed and may help identify patients who may benefit from this therapy.
Guillermo Garcia-Manero
Honoraria: Celgene, Astex Pharmaceuticals, Acceleron Pharma, Helssin, Abbvie
Consulting or Advisory Role: Celgene, Astex Pharmaceuticals, Acceleron Pharma, Jazz Pharmaceuticals, Bristol-Myers Squibb, Helsinn Therapeutics
Research Funding: Celgene, Astex Pharmaceuticals, Amphivena, Helsinn Therapeutics, Novartis, Abbvie, Bristol-Myers Squibb, Onconova Therapeutics, H3 Biomedicine, Merck
Valeria Santini
Honoraria: Celgene/Bristol-Myers Squibb, Novartis, Janssen-Cilag
Consulting or Advisory Role: Celgene/Bristol-Myers Squibb, Novartis, Menarini, Takeda, Pfizer, Geron, Gilead Sciences
Research Funding: Celgene
Travel, Accommodations, Expenses: Janssen-Cilag, Celgene
Antonio Almeida
Honoraria: Novartis, Celgene
Consulting or Advisory Role: Novartis, Celgene
Speakers' Bureau: Novartis, Celgene
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Uwe Platzbecker
Honoraria: Celgene/Jazz
Consulting or Advisory Role: Celgene/Jazz
Research Funding: Amgen, Janssen, Novartis, BerGenBio, Celgene
Patents, Royalties, Other Intellectual Property: part of a patent for a TFR-2 antibody (Rauner et al. Nature Metabolics 2019)
Travel, Accommodations, Expenses: Celgene
Lewis R. Silverman
Research Funding: Celgene, MedImmune, Onconova Therapeutics, Bayer
Patents, Royalties, Other Intellectual Property: Patent for the combination of azacitidine and rigosertib
Francesco Buccisano
Consulting or Advisory Role: Novartis
Speakers' Bureau: Novartis
Pierre Fenaux
Honoraria: Celgene
Research Funding: Celgene
Rena Buckstein
Honoraria: Celgene, Taiho Pharmaceutical
Consulting or Advisory Role: Celgene
Research Funding: Celgene, Takeda, Otsuka US
Uncompensated Relationships: Celgene/Jazz
Maria Diez Campelo
Honoraria: Celgene, Novartis
Consulting or Advisory Role: Celgene, Takeda, Novartis, BerGenBio
Travel, Accommodations, Expenses: Celgene, Novartis
David Valcarcel
Consulting or Advisory Role: Celgene, Amgen, GlaxoSmithKline, Novartis, Takeda, Pfizer, Bristol-Myers Squibb, Sanofi, Jazz Pharmaceuticals, SOBI
Speakers' Bureau: Celgene, Novartis, Amgen, GlaxoSmithKline, Astellas Pharma, Pfizer, Jazz Pharmaceuticals, Sanofi/Aventis, Bristol-Myers Squibb
Travel, Accommodations, Expenses: Celgene, Amgen, Pfizer, GlaxoSmithKline, Jazz Pharmaceuticals
Paresh Vyas
Stock and Other Ownership Interests: OxStem
Honoraria: Celgene, Pfizer, Jazz Pharmaceuticals, Abbvie, Daiichi Sankyo
Research Funding: Celgene, Forty Seven
Patents, Royalties, Other Intellectual Property: Patent for flow cytometric detection of leukaemic stem cells
Esther Natalie Olíva
Honoraria: Celgene, Novartis, Amgen, Alexion Pharmaceuticals
Consulting or Advisory Role: Amgen, Celgene, Novartis
Speakers' Bureau: Celgene, Novartis
Patents, Royalties, Other Intellectual Property: Royalties for QOL-E instrument
Jake Shortt
Consulting or Advisory Role: Astellas Pharma, Novartis
Speakers' Bureau: Bristol-Myers Squibb
Research Funding: Astex Pharmaceuticals, Amgen, Celgene/Bristol-Myers Squibb
Dietger Niederwieser
Consulting or Advisory Role: Cellectis, Amgen, Novartis
Speakers' Bureau: Novartis
Research Funding: Daiichi Sankyo
Moshe Mittelman
Honoraria: Celgene
Consulting or Advisory Role: Onconova Therapeutics
Speakers' Bureau: Novartis
Research Funding: Novartis, Takeda, Janssen-Cilag, Roche, Medison, Abbvie, Gilead Sciences
Luana Fianchi
Consulting or Advisory Role: Sanofi
Travel, Accommodations, Expenses: Celgene
Ignazia La Torre
Employment: Bristol-Myers Squibb
Stock and Other Ownership Interests: Bristol-Myers Squibb
Jianhua Zhong
Employment: Bristol-Myers Squibb
Stock and Other Ownership Interests: Bristol-Myers Squibb
Eric Laille
Employment: Bristol-Myers Squibb, Celgene, Catalent,
Stock and Other Ownership Interests: Bristol-Myers Squibb, Moderna Therapeutics, Catalent
Daniel Lopes de Menezes
Employment: Celgene, Bristol-Myers Squibb
Stock and Other Ownership Interests: Celgene, Bristol-Myers Squibb, Novartis
Patents, Royalties, Other Intellectual Property: Published and Issues patents at BMS, Novartis
Barry Skikne
Employment: Celgene/Bristol-Myers Squibb
Consulting or Advisory Role: Celgene/Bristol-Myers Squibb
Research Funding: Daiichi Sankyo/UCB Japan
C.L. Beach
Employment: Bristol-Myers Squibb
Stock and Other Ownership Interests: Bristol-Myers Squibb
Aristoteles Giagounidis
Stock and Other Ownership Interests: Novartis, Roche
Honoraria: Celgene, Amgen, Novartis
Consulting or Advisory Role: Celgene
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in abstract form at the 25th EHA Annual Congress (June 11–21, 2020; Virtual).
SUPPORT
Supported by Celgene Corporation, a Bristol-Myers Squibb company. Editorial support on an early draft of the manuscript was provided by Sheila Truten and Brian Kaiser from Medical Communication Company, Inc (Wynnewood, PA), funded by Bristol-Myers Squibb Company and in accordance with Good Publication Practice guidelines.
CLINICAL TRIAL INFORMATION
G.G.-M. and V.S. contributed equally to this work.
DATA SHARING STATEMENT
See Bristol-Myers Squibb Company policy on data sharing at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
AUTHOR CONTRIBUTIONS
Conception and design: Guillermo Garcia-Manero, Valeria Santini, Lewis R. Silverman, Pierre Fenaux, Paresh Vyas, Moshe Mittelman, Ignazia La Torre, Eric Laille, Barry Skikne, C.L. Beach, Aristoteles Giagounidis
Administrative support: Uwe Platzbecker, Barry Skikne
Provision of study materials or patients: Valeria Santini, Uwe Platzbecker, Anna Jonasova, Lewis R. Silverman, Gianluigi Reda, Francesco Buccisano, Rena Buckstein, Maria Diez Campelo, David Valcarcel, Paresh Vyas, Esther Natalie Oliva, Jake Shortt, Moshe Mittelman, Luana Fianchi, Ignazia La Torre, Daniel Lopes de Menezes, Barry Skikne
Collection and assembly of data: Guillermo Garcia-Manero, Valeria Santini, Antonio Almeida, Uwe Platzbecker, Anna Jonasova, Jose Falantes, Francesco Buccisano, Pierre Fenaux, Maria Diez Campelo, Stephen Larsen, David Valcarcel, Paresh Vyas, Esther Natalie Oliva, Jake Shortt, Dietger Niederwieser, Moshe Mittelman, Luana Fianchi, Ignazia La Torre, Eric Laille, Daniel Lopes de Menezes, Barry Skikne, C.L. Beach, Aristoteles Giagounidis
Data analysis and interpretation: Guillermo Garcia-Manero, Valeria Santini, Antonio Almeida, Uwe Platzbecker, Lewis R. Silverman, Gianluigi Reda, Rena Buckstein, Maria Diez Campelo, David Valcarcel, Paresh Vyas, Valentina Giai, Esther Natalie Oliva, Jake Shortt, Moshe Mittelman, Luana Fianchi, Ignazia La Torre, Jianhua Zhong, Eric Laille, Daniel Lopes de Menezes, Barry Skikne, C.L. Beach, Aristoteles Giagounidis
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase III, Randomized, Placebo-Controlled Trial of CC-486 (Oral Azacitidine) in Patients With Lower-Risk Myelodysplastic Syndromes
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Guillermo Garcia-Manero
Honoraria: Celgene, Astex Pharmaceuticals, Acceleron Pharma, Helssin, Abbvie
Consulting or Advisory Role: Celgene, Astex Pharmaceuticals, Acceleron Pharma, Jazz Pharmaceuticals, Bristol-Myers Squibb, Helsinn Therapeutics
Research Funding: Celgene, Astex Pharmaceuticals, Amphivena, Helsinn Therapeutics, Novartis, Abbvie, Bristol-Myers Squibb, Onconova Therapeutics, H3 Biomedicine, Merck
Valeria Santini
Honoraria: Celgene/Bristol-Myers Squibb, Novartis, Janssen-Cilag
Consulting or Advisory Role: Celgene/Bristol-Myers Squibb, Novartis, Menarini, Takeda, Pfizer, Geron, Gilead Sciences
Research Funding: Celgene
Travel, Accommodations, Expenses: Janssen-Cilag, Celgene
Antonio Almeida
Honoraria: Novartis, Celgene
Consulting or Advisory Role: Novartis, Celgene
Speakers' Bureau: Novartis, Celgene
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Uwe Platzbecker
Honoraria: Celgene/Jazz
Consulting or Advisory Role: Celgene/Jazz
Research Funding: Amgen, Janssen, Novartis, BerGenBio, Celgene
Patents, Royalties, Other Intellectual Property: part of a patent for a TFR-2 antibody (Rauner et al. Nature Metabolics 2019)
Travel, Accommodations, Expenses: Celgene
Lewis R. Silverman
Research Funding: Celgene, MedImmune, Onconova Therapeutics, Bayer
Patents, Royalties, Other Intellectual Property: Patent for the combination of azacitidine and rigosertib
Francesco Buccisano
Consulting or Advisory Role: Novartis
Speakers' Bureau: Novartis
Pierre Fenaux
Honoraria: Celgene
Research Funding: Celgene
Rena Buckstein
Honoraria: Celgene, Taiho Pharmaceutical
Consulting or Advisory Role: Celgene
Research Funding: Celgene, Takeda, Otsuka US
Uncompensated Relationships: Celgene/Jazz
Maria Diez Campelo
Honoraria: Celgene, Novartis
Consulting or Advisory Role: Celgene, Takeda, Novartis, BerGenBio
Travel, Accommodations, Expenses: Celgene, Novartis
David Valcarcel
Consulting or Advisory Role: Celgene, Amgen, GlaxoSmithKline, Novartis, Takeda, Pfizer, Bristol-Myers Squibb, Sanofi, Jazz Pharmaceuticals, SOBI
Speakers' Bureau: Celgene, Novartis, Amgen, GlaxoSmithKline, Astellas Pharma, Pfizer, Jazz Pharmaceuticals, Sanofi/Aventis, Bristol-Myers Squibb
Travel, Accommodations, Expenses: Celgene, Amgen, Pfizer, GlaxoSmithKline, Jazz Pharmaceuticals
Paresh Vyas
Stock and Other Ownership Interests: OxStem
Honoraria: Celgene, Pfizer, Jazz Pharmaceuticals, Abbvie, Daiichi Sankyo
Research Funding: Celgene, Forty Seven
Patents, Royalties, Other Intellectual Property: Patent for flow cytometric detection of leukaemic stem cells
Esther Natalie Olíva
Honoraria: Celgene, Novartis, Amgen, Alexion Pharmaceuticals
Consulting or Advisory Role: Amgen, Celgene, Novartis
Speakers' Bureau: Celgene, Novartis
Patents, Royalties, Other Intellectual Property: Royalties for QOL-E instrument
Jake Shortt
Consulting or Advisory Role: Astellas Pharma, Novartis
Speakers' Bureau: Bristol-Myers Squibb
Research Funding: Astex Pharmaceuticals, Amgen, Celgene/Bristol-Myers Squibb
Dietger Niederwieser
Consulting or Advisory Role: Cellectis, Amgen, Novartis
Speakers' Bureau: Novartis
Research Funding: Daiichi Sankyo
Moshe Mittelman
Honoraria: Celgene
Consulting or Advisory Role: Onconova Therapeutics
Speakers' Bureau: Novartis
Research Funding: Novartis, Takeda, Janssen-Cilag, Roche, Medison, Abbvie, Gilead Sciences
Luana Fianchi
Consulting or Advisory Role: Sanofi
Travel, Accommodations, Expenses: Celgene
Ignazia La Torre
Employment: Bristol-Myers Squibb
Stock and Other Ownership Interests: Bristol-Myers Squibb
Jianhua Zhong
Employment: Bristol-Myers Squibb
Stock and Other Ownership Interests: Bristol-Myers Squibb
Eric Laille
Employment: Bristol-Myers Squibb, Celgene, Catalent,
Stock and Other Ownership Interests: Bristol-Myers Squibb, Moderna Therapeutics, Catalent
Daniel Lopes de Menezes
Employment: Celgene, Bristol-Myers Squibb
Stock and Other Ownership Interests: Celgene, Bristol-Myers Squibb, Novartis
Patents, Royalties, Other Intellectual Property: Published and Issues patents at BMS, Novartis
Barry Skikne
Employment: Celgene/Bristol-Myers Squibb
Consulting or Advisory Role: Celgene/Bristol-Myers Squibb
Research Funding: Daiichi Sankyo/UCB Japan
C.L. Beach
Employment: Bristol-Myers Squibb
Stock and Other Ownership Interests: Bristol-Myers Squibb
Aristoteles Giagounidis
Stock and Other Ownership Interests: Novartis, Roche
Honoraria: Celgene, Amgen, Novartis
Consulting or Advisory Role: Celgene
No other potential conflicts of interest were reported.
REFERENCES
- 1.Gangat N, Patnaik MM, Tefferi A: Myelodysplastic syndromes: Contemporary review and how we treat. Am J Hematol 91:76-89, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Greenberg P Cox C LeBeau MM, et al. : International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 89:2079-2088, 1997 [PubMed] [Google Scholar]
- 3.Jansen AJ Essink-Bot ML Beckers EA, et al. : Quality of life measurement in patients with transfusion-dependent myelodysplastic syndromes. Br J Haematol 121:270-274, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Malcovati L Germing U Kuendgen A, et al. : Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol 25:3503-3510, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Al Ameri A Jabbour E Garcia-Manero G, et al. : Significance of thrombocytopenia in myelodysplastic syndromes: Associations and prognostic implications. Clin Lymphoma Myeloma Leuk 11:237-241, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez-Porras JR Cordoba I Such E, et al. : Prognostic impact of severe thrombocytopenia in low-risk myelodysplastic syndrome. Cancer 117:5529-5537, 2011 [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network : NCCN Clinical Practice Guidelines in Oncology. Myelodysplastic Syndromes Version 2.2020. https://www.nccn.org/professionals/physician_gls/pdf/mds.pdf, 2020 [Google Scholar]
- 8.Kantarjian H Fenaux P Sekeres MA, et al. : Safety and efficacy of romiplostim in patients with lower-risk myelodysplastic syndrome and thrombocytopenia. J Clin Oncol 28:437-444, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Oliva EN Alati C Santini V, et al. : Eltrombopag versus placebo for low-risk myelodysplastic syndromes with thrombocytopenia (EQoL-MDS): Phase 1 results of a single-blind, randomised, controlled, phase 2 superiority trial. Lancet Haematol 4:e127-e136, 2017 [DOI] [PubMed] [Google Scholar]
- 10.Jung HA Maeng CH Kim M, et al. : Platelet response during the second cycle of decitabine treatment predicts response and survival for myelodysplastic syndrome patients. Oncotarget 6:16653-16662, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Helm LH Alhan C Wijermans PW, et al. : Platelet doubling after the first azacitidine cycle is a promising predictor for response in myelodysplastic syndromes (MDS), chronic myelomonocytic leukaemia (CMML) and acute myeloid leukaemia (AML) patients in the Dutch azacitidine compassionate named patient programme. Br J Haematol 155:599-606, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Manero G Gore SD Kambhampati S, et al. : Efficacy and safety of extended dosing schedules of CC-486 (oral azacitidine) in patients with lower-risk myelodysplastic syndromes. Leukemia 30:889-896, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Manero G Scott BL Cogle CR, et al. : CC-486 (oral azacitidine) in patients with myelodysplastic syndromes with pretreatment thrombocytopenia. Leuk Res 72:79-85, 2018 [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Manero G Gore SD Cogle C, et al. : Phase I study of oral azacitidine in myelodysplastic syndromes, chronic myelomonocytic leukemia, and acute myeloid leukemia. J Clin Oncol 29:2521-2527, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Manero G Savona MR Gore SD, et al. : CC-486 (oral azacitidine) in patients with hematological malignancies who had received prior treatment with injectable hypomethylating agents (HMAs): Results from phase 1/2 CC-486 studies. Blood 128:905, 2016 [Google Scholar]
- 16.ONUREG® (Azacitidine Tablets) Prescribing Information. Summit, NJ, Celgene Corporation (A Wholly Owned Subsidiary of Bristol-Myers Squibb). [Google Scholar]
- 17.Santini V, Kantarjian HM, Issa JP: Changes in DNA methylation in neoplasia: Pathophysiology and therapeutic implications. Ann Intern Med 134:573-586, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Stresemann C, Lyko F: Modes of action of the DNA methyltransferase inhibitors azacytidine and decitabine. Int J Cancer 123:8-13, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Saunthararajah Y: Key clinical observations after 5-azacytidine and decitabine treatment of myelodysplastic syndromes suggest practical solutions for better outcomes. Hematol Am Soc Hematol Educ Program 2013:511-521, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Laille E Shi T Garcia-Manero G, et al. : Pharmacokinetics and pharmacodynamics with extended dosing of CC-486 in patients with hematologic malignancies. PLoS One 10:e0135520, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenberg PL Tuechler H Schanz J, et al. : Revised international prognostic scoring system for myelodysplastic syndromes. Blood 120:2454-2465, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheson BD Greenberg PL Bennett JM, et al. : Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood 108:419-425, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Vardiman JW Thiele J Arber DA, et al. : The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: Rationale and important changes. Blood 114:937-951, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Cella D: The functional assessment of cancer therapy-anemia (FACT-An) scale: A new tool for the assessment of outcomes in cancer anemia and fatigue. Semin Hematol 34:13-19, 1997. (3 suppl 2) [PubMed] [Google Scholar]
- 25.Brucker PS Yost K Cashy J, et al. : General population and cancer patient norms for the functional assessment of cancer therapy-general (FACT-G). Eval Health Prof 28:192-211, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Fenaux P Platzbecker U Mufti GJ, et al. : Luspatercept in patients with lower-risk myelodysplastic syndromes. N Engl J Med 382:140-151, 2020 [DOI] [PubMed] [Google Scholar]
- 27.Platzbecker U Symeonidis A Oliva EN, et al. : A phase 3 randomized placebo-controlled trial of darbepoetin alfa in patients with anemia and lower-risk myelodysplastic syndromes. Leukemia 31:1944-1950, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fenaux P Santini V Spiriti MAA, et al. : A phase 3 randomized, placebo-controlled study assessing the efficacy and safety of epoetin-alpha in anemic patients with low-risk MDS. Leukemia 32:2648-2658, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Platzbecker U Fenaux P Ades L, et al. : Proposals for revised IWG 2018 hematological response criteria in patients with MDS included in clinical trials. Blood 133:1020-1030, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Papaemmanuil E Gerstung M Malcovati L, et al. : Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood 122:3616-3627, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bejar R Lord A Stevenson K, et al. : TET2 mutations predict response to hypomethylating agents in myelodysplastic syndrome patients. Blood 124:2705-2712, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haferlach T Nagata Y Grossmann V, et al. : Landscape of genetic lesions in 944 patients with myelodysplastic syndromes. Leukemia 28:241-247, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bejar R: Implications of molecular genetic diversity in myelodysplastic syndromes. Curr Opin Hematol 24:73-78, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
See Bristol-Myers Squibb Company policy on data sharing at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.





