Abstract
The main corneal infections reported worldwide are caused by bacteria and viruses but, recently, the number of Acanthamoeba keratitis (AK) cases has increased. Acanthamoeba genus is an opportunistic free living protozoa widely distributed in environmental and clinical sources, with two life-cycle stages: the trophozoite and the cyst. AK presents as primary symptoms eye redness, epithelial defects, photophobia and intense pain. An early diagnosis and an effective treatment are crucial to avoid blindness or eye removal but, so far, there is no established treatment to this corneal infection. Diverse research studies have reported the efficacy of commercialized eye drops and ophthalmic solutions against the two life cycle stages of Acanthamoeba strains, that usually present preservatives such as Propylene Glycol of Benzalkonium chloride (BAK). These compounds present toxic effects in corneal cells, favouring the inflammatory response in the so sensitive eye tissue. In the present work we have evaluated the efficacy of nine proprietary ophthalmic solutions with and without preservatives (ASDA Dry Eyes Eyedrops, Miren®, ODM5®, Ectodol®, Systane® Complete, Ocudox®, Matrix Ocular®, Alins® and Coqun®) against the two life cycle stages of three Acanthamoeba strains. Our work has demonstrated the high anti-Acanthamoeba activity of Matrix Ocular®, which induces the programmed cell death mechanisms in Acanthamoeba spp. trophozoites. The high efficacy and the absence of ocular toxic effects of Matrix Ocular®, evidences the use of the Arabinogalactan derivatives as a new source of anti-AK compounds.
Keywords: Matrix Ocular®, Ophthalmic solution, Acanthamoeba, Programmed cell death, Arabinogalactan
Graphical abstract
1. Introduction
The eye is a complex organ with highly specialized constituent tissues derived from different primordial cell lineages (Hayashi, 2021). Currently, several corneal epithelial disorders such as oedema, blepharitis, glaucoma or the dry eye syndrome, represent the most common ophthalmic epithelial disorders (Seino et al., 2021). Different corneal infections are reported worldwide, mainly caused by bacteria but also by viruses and, as the most recent pathogenic microorganisms, opportunistic free living protozoa such as Acanthamoeba spp. (Oliveira-Ferreira et al., 2019). Acanthamoeba is widely distributed in environmental and clinical sources and presents two life-cycle stages: the vegetative form named trophozoite, and a high dormant resistant form or cyst (Siddiqui and Khan, 2012; Lorenzo-Morales et al., 2015).
The most common human pathology produced by this genus is the Acanthamoeba Keratitis (AK), that primary symptoms are eye redness, epithelial defects, photophobia and intense pain. An early diagnosis and an effective treatment are crucial to avoid blindness or eye removal (Lorenzo-Morales et al., 2015). Unfortunately, so far there is no established treatment for this corneal infection, but the recommended treatment regimen for AK includes a biguanide (0.02% polyhexamethylene biguanide (PHMB)/0.02% chlorhexidine digluconate) combined with diamidine (0.1% propamidine isethionate –Brolene-/0.1% hexamidine –Desomedine-) (Martín-Navarro et al., 2008, 2013). Recently, voriconazole has been demonstrated to be effective against different clinical strains of Acanthamoeba (Cabello-Vílchez et al., 2014; Rocha-Cabrera et al., 2015) and it has been successfully used in clinical cases too (Montiel et al., 2012).
Contact lenses wearers represent the highest risk group vulnerable to suffer AK, mainly due to the ocular traumas produced by the contact lenses or the lack of a correct hygiene (Lorenzo-Morales et al., 2015). Moreover, other corneal disorders such as the dry eye syndrome, is other disease derived from the use of contact lenses. Lately, diverse research studies have reported the efficacy of commercialized eye drops and ophthalmic solutions used for these other epithelial disorders, against the two life cycle stages of Acanthamoeba strains (Sifaoui et al., 2017, 2018, 2020a, b; Reyes-Batlle et al., 2019). Several commercialized ophthalmic solutions present preservatives such as Propylene Glycol, Benzalkonium chloride (BAK) or its derivatives such as POLYQUAD®, which have demonstrated antimicrobial (Kinnunen et al., 1991; Nalawade et al., 2015) or anti-Acanthamoeba activity (Sunada et al., 2014). However, both Propylene Glycol and BAK or its derivatives, induce inflammation on the ocular surface cells as cytotoxic effect (Paimela et al., 2012).
In the present work we have evaluated the efficacy of nine proprietary ophthalmic solutions (ASDA Dry Eyes Eyedrops, Miren®, ODM5®, Ectodol®, Systane® Complete, Ocudox®, Matrix Ocular®, Alins® and Coqun®) against the two life cycle phases of three Acanthamoeba strains, as well as the cell death mechanisms produced by the most active solution against A. castellanii Neff trophozoites. To the best of our knowledge, from the total of the evaluated solutions, only two of the contained preservatives. Furthermore, the most active eye drop, Matrix Ocular®, does not contain any preserving agent.
2. Material and methods
2.1. Chemicals
Nine eye drop solutions available commercially for topical use against DED were selected for analysis. Table 2 shows the details of the composition of these solutions.
Table 2.
Detailed composition of the 9 commercialized solutions evaluated in the present study.
| Eye Drop | Company | Components | Preservative |
|---|---|---|---|
| ASDA Dry Eyes Eyedrops | ASDA | Hyaluronic Acid 0.1%, Disodium Edetate | PHMB 0,0001% |
| Miren® | Brill Pharma | Riboflavin sodium phosphate, Vitamin E TPGS, Sodium Hyaluronate, MSM (methylsulfonylmethane), L-proline, L-glicine, chlorhydrate L-lisine, L-leucine, Sodium dibasic phosphate, Sodium chloride | No |
| ODM5® | Horus Pharma | Sodium chloride 5%, Sodium hyaluronate 0,15% | No |
| Ectodol® | Brill Pharma | Pentylene glycol, Glycerin, Ectoine, Polyglyceril-4 caprate, Glycerin glucoside, xanthan gum, panthenol, 4-terpineol, Cananga ordorata flower oil, Citrus lemon peel oil, Eugenol, Benzyl salicylate, geraniol, arnesol, linalool, benzyl benzoate, citral, limonene, water | No |
| Systane® Complete | Alcon | Boric acid, dimyristoyl phosphatidylglycerol, edetate disodium, hydroxypropyl guar, mineral oil, polyoxyl 40 stearate, sorbitan tristearate, sorbitol and purified water. | POLYQUAD® (polyquaternium-1) 0.001% |
| Ocudox® | Brill Pharma | Sodium chloride, Sosium phospate, Sodium sulphate, Sodium hypochlorite, Hypochlorous acid, Electrolysed water | No |
| Matrix Ocular® | Brill Pharma | Arabinogalactan; sodium tetraborate; boric acid, sodium chloride, water | No |
| Alins® | Brill Pharma | Sodium chloride 5%, injection water | No |
| Coqun® | VISUfarma | Co Enzyme Q10, vitamin E, Buffered isotonic solution | No |
2.2. Acanthamoeba spp. strains tested
The anti-Acanthamoeba activity of selected eye drops were initially evaluated against the trophozoite stage of Acanthamoeba castellanii Neff (ATCC 30010) type strain from the American Type Culture Collection. Subsequently, the most active eye drop solution was tested against two clinical isolates: Acanthamoeba griffini, genotype T3 obtained in a previous study (González-Robles et al., 2014) and Acanthamoeba polyphaga, genotype T4 (ATCC 30461). Those three strains were grown axenically in PYG medium (0.75% (w/v) proteose peptone, 0.75% (w/v) yeast extract and 1.5% (w/v) glucose) containing 40 μg gentamicin ml−1 (Biochrom AG, Cultek, Granollers, Barcelona, Spain).
2.3. In vitro effect against the trophozoite stage of Acanthamoeba spp.
The anti-Acanthamoeba activities of eye drop solutions were determined by the alamarBlue™ assay as previously described (McBride et al., 2005). Briefly, Acanthamoeba strains were seeded in duplicate on a 96-well microtiter plate with 50 μl from a stock solution of 2 × 104 cells ml−1. Amoebae were allowed to adhere for 15 min and 50 μl of serial dilution series of the eye drop solution were added. Finally, the alamarBlue™ Reagent (Life Technologies, Madrid, Spain) was added into each well at an amount equal to 10% of the medium volume. The plates were then incubated for 96 h at 26 °C with soft agitation. Finally, the plates were measured with the Enspire® microplate reader (PerkinElmer, Massachusetts, USA) using the emitted fluorescence (570/585 nm). Percentages of growth inhibition, 50% and 90% inhibitory concentrations (IC50 and IC90) were calculated by non-linear regression analysis with 95% confidence limits. All experiments were performed three times, and the mean values were calculated. Differences between the values were assessed using one-way analysis of variance (ANOVA). Data are presented as means ± SD (N = 3); same letters indicate no significant differences when comparing different mean values of each eye drop.
2.4. In vitro effect against the cyst stage of A. castellanii Neff (ATCC300010)
The cysticidal activity was determined by the alamarBlue™ assay at 168 h and confirmed visually by inverted microscopy. A. castellanii Neff cysts were prepared as described by Lorenzo-Morales et al. (2008). Briefly, trophozoite were transferred from PYG medium based cultures (trophozoite medium) to Neff's encystment medium (NEM; 0.1 M KCl, 8 mM MgSO4·7H2O, 0.4 mM CaCl2·2H2O, 1 mM NaHCO3, 20 mM ammediol [2-amino-2-methyl-1,3-propanediol; Sigma Aldrich Chemistry Ltd., Madrid, Spain], pH 8.8, at 25 °C) and were cultured in this medium with gently shaking for a week in order to obtain mature cysts. After that, mature cysts were harvested and washed twice using PYG medium.
A serial dilution of the most active eye drop was prepared in PYG medium in sterile 96-well microtiter plates (NUNC, Thermo Scientific™). After this step, 5 × 104 Acanthamoeba spp. mature cysts ml−1 were added, obtained a final volume of 100 μL in each well. Plates were then incubated for 168 h at 26 °C with slight agitation as it has been described before (Sifaoui et al., 2018). After this initial incubation period, the 100 μL of each well were removed and added 100 μL of fresh PYG medium, in order to evaluate the excystation capacity of the treated cysts. Finally, 10 μL of the alamarBlue™ Reagent (Life Technologies, Madrid, Spain) was placed into each well, and the plates were then incubated for another 168 h at 26 °C with slight agitation. Subsequently, the plates were analysed, with an Enspire® microplate reader (PerkinElmer, Massachusetts, USA) using the emitted fluorescence (570/585 nm). Percentages of growth inhibition, 50% inhibitory concentration (IC50) was calculated by linear regression analysis with 95% confidence limits. All experiments were performed three times each in duplicate, and the mean values were calculated.
2.5. Double-stain assay for programmed cell death determination
A double-stain apoptosis detection kit (Hoechst 33342/PI) (ThermoFisher™) and an inverted microscope EVOS FL Cell Imaging System (AMF4300, Life Technologies, USA) were used. The experiment was carried out by following the manufacturer's recommendations, and 105 cells ml−1 were incubated for 24 h with the previously calculated IC90. The double-staining pattern allows the identification of three groups in a cellular population: dead cells with low-blue and high-red fluorescence (as the propidium Iodide stain enters the nucleus), live cells with low level or absence of fluorescence and cells developing PCD with a higher level of blue fluorescence.
2.6. Plasma membrane permeability
The SYTOX Green assay was performed to detect parasite's membrane permeability alterations. Initially, 105 ml−1 trophozoites were treated with the eye drop IC90 for 24 h. After this incubation, cells were washed and incubated in saline solution with SYTOX Green reagent (ThermoFischer™) at a final concentration of 1 μM for 15 min in darkness and following the manufacturer's instructions. Cells were observed and pictures were taken on the EVOS FL inverted microscope.
2.7. Analysis of mitochondrial membrane potential
The collapse of an electrochemical gradient across the mitochondrial membrane during apoptosis was measured using a JC-1 mitochondrial membrane potential detection kit (Cayman Chemical) as described by the manufacturer. Trophozoites treated with the IC90 of the evaluated eye drop were incubated for 24 h and then washed and resuspended in JC-1 buffer. 10 μL of JC-1 was added and incubated at 26 °C for 30 min in the darkness. Images were taken on the EVOS FL inverted microscope. The staining pattern allows to identify two different groups in the cellular population: live cells will show only red fluorescence, while cells with low mitochondrial potential (undergoing PCD) will show also red fluorescence and a higher level of green fluorescence.
2.8. Measurement of ATP
ATP level was measured using a CellTiter-Glo Luminescent Cell Viability Assay (ThermoFisher™) and following the manufacturer's recommendations. The effect of the evaluated eye drop on the ATP production was evaluated by incubating 105 of cells/ml with the previously calculated IC90 of the selected solution, adding the CellTiter-Glo reagent and measuring the luminescence in the Enspire® microplate reader.
2.9. Detection of reactive oxygen species (ROS)
During the apoptosis induction under both physiologic and pathologic conditions, reactive oxygen species play an important role. These species include a number of molecules that damage DNA and RNA and oxidize proteins and lipids. In order to detect ROS, we have used the CellROX® Deep Red Flow Cytometry Assay Kit. Treated cells were incubated with CellROX® at a final concentration of 5 μM for 30 min in darkness. Finally, those cells where the ROS have been produced, will show an intense red fluorescence. Images were taken on the EVOS FL inverted microscope.
3. Results
3.1. In vitro biological activity against Acanthamoeba spp.
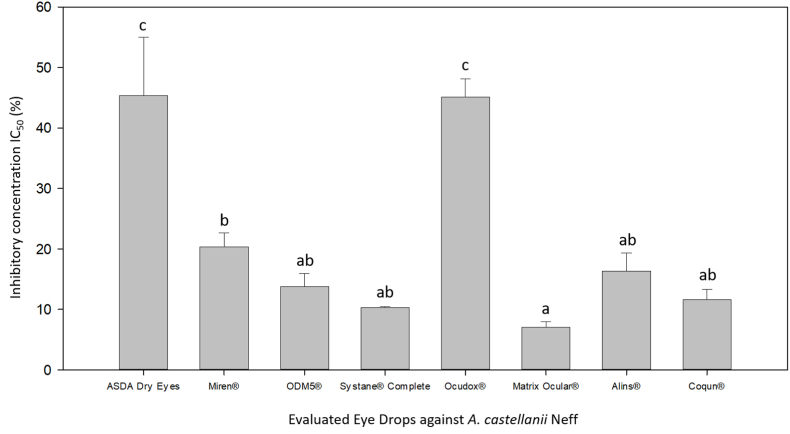
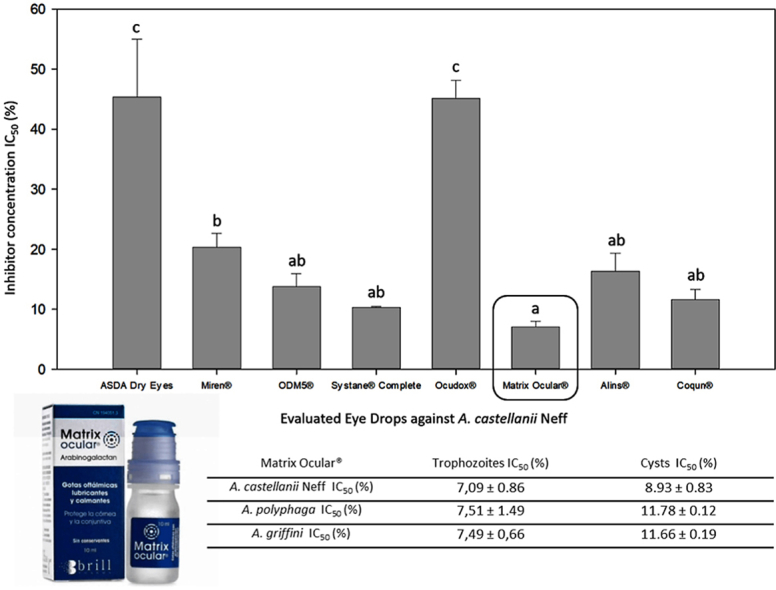
From the 9 evaluated already commercialized eye drops, we would like to remark the in vitro efficacy of Matrix Ocular®, with the lowest IC50 value (7,09 ± 0.86%). Form the rest of the evaluated solutions, all of them have presented anti-Acanthamoeba in vitro activity with the exception of Ectodol® (Fig. 1). Among this other active eye drops, ASDA Dry Eyes and Ocudox® showed the highest inhibitory concentration (IC50) values: 45,35 ± 9,66% and 45,14 ± 2.98%, respectively. However, the other six active compounds were able to inhibit the 50% of the Acanthamoeba population growth at concentrations lower than 20% (Fig. 1). As it could be observed in Fig. 1, there was no significant difference between Matrix Ocular® and ODM5®, Systane® Complete, Alins® and Coqun®. However, these last four compounds did not present a statistically significant difference with Miren either, which is significantly different to Matrix Ocular. Therefore, accordingly with these results, Matrix Ocular® was evaluated against the two different life cycle phases (trophozoites and cysts) of A. castellanii Neff and two clinical strains A. polyphaga and A. griffini (Table 1).
Fig. 1.
Comparison of the amoebicidal effects of the 8 active commercialized eye drops against Acanthamoeba castellanii Neff trophozoites. Bars represent mean concentrations with each standard deviation. Differences between the values were assessed using one-way analysis of variance (ANOVA). Same letters indicate no significant differences when comparing different mean values of each eye drop.
Table 1.
Inhibitory concentration 50 (IC50) (%) of Matrix Ocular against the two different life cycle phases (trophozoites and cysts) of A. castellanii Neff, A. polyphaga and A. griffini.
| Matrix Ocular® | Trophozoites IC50 (%) | Cysts IC50 (%) |
|---|---|---|
| A. castellanii Neff IC50 (%) | 7,09 ± 0.86 | 8.93 ± 0.83 |
| A. polyphaga IC50 (%) | 7,51 ± 1.49 | 11.78 ± 0.12 |
| A. griffini IC50 (%) | 7,49 ± 0,66 | 11.66 ± 0.19 |
3.2. Evaluation of the mechanism of action of Matrix Ocular in A. castellanii Neff trophozoites
Regarding to the preliminary results, Matrix Ocular was chosen to continue the studies on the mechanism of action on the trophozoites of the three evaluated Acanthamoeba strains. Morphological events part of the PCD such as chromatin condensation, plasmatic membrane integrity, assessment of mitochondrial function disruption and the production of reactive oxygen species (ROS) (Kaczanowski et al., 2011) were evaluated as follow.
3.2.1. Double-stain assay for programmed cell death determination
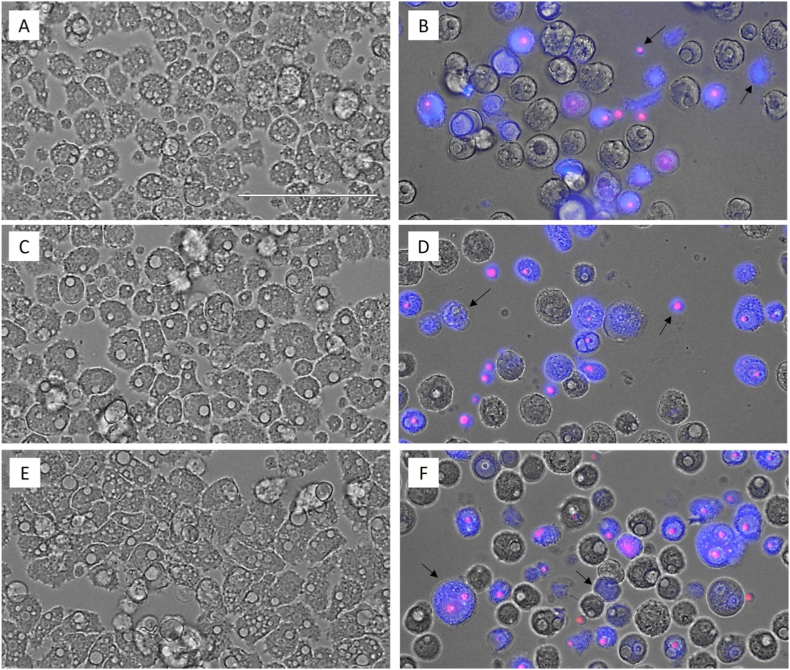
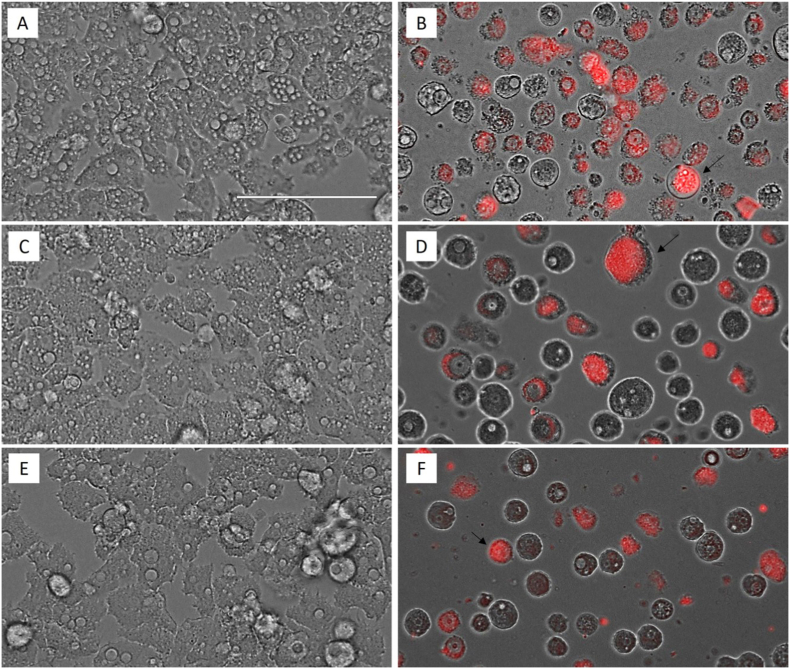
In order to evidence the chromatin condensation, A. castellanii Neff (Fig. 2, B), A. griffini (D) and A. polyphaga (F) trophozoites were incubated with the IC90 of Matrix Ocular for 24 h and the double-stain Hoechst 33342/PI was performed. The intense blue and the scarce red fluorescence indicates an early phase of the apoptotic process, while the intense blue and red fluorescence evidences a late apoptosis process since the propidium iodide has reach to the nuclei material. A. castellanii Neff (Fig. 2, A), A. griffini (C) and A. polyphaga (E) trophozoites were incubated in fresh PYG medium as negative control, showing the total absence of fluorescence.
Fig. 2.
Effect of the IC90 concentration of Matrix Ocular (B, D and F: A. castellanii Neff, A. griffini and A. polyphaga respectively) on the chromatin regarding the negative control (A, C and E, respectively) at 24 h. All images (40x) are based on Live Cell Imaging Microscope EVOS FL Cell Imaging System (Scale Bar 100 μm).
3.2.2. Plasma membrane permeability
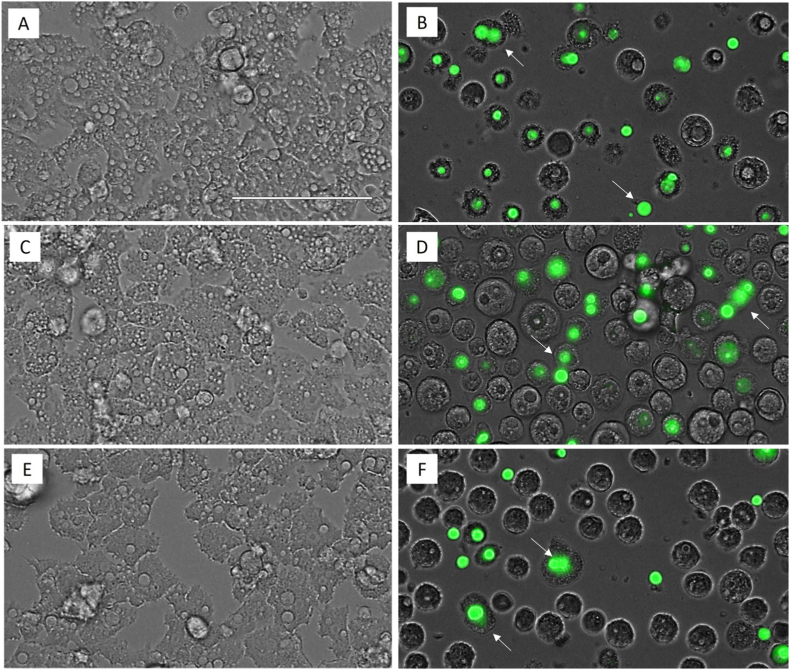
An important evidence of the lack of a cellular necrotic process is the maintenance of the plasmatic membrane integrity. In Fig. 3, even though the plasmatic membrane permeability has been altered, the cell integrity was maintained, avoiding the emptying of the cellular content to the cytosol. The permeabilization of the A. castellanii Neff (Fig. 3, B), A. griffini (D) and A. polyphaga (F) trophozoites plasmatic membrane has been evidenced by the use of SYTOX® Green dye and it is due to the effect of the IC90 of Matrix Ocular after 24 h of incubation when comparing with each negative control (A, c and E, respectively).
Fig. 3.
Permeabilization of the Acanthamoeba spp. trophozoites plasmatic membrane evidenced by the SYTOX® Green dye due to the effect of the IC90 of Matrix Ocular (B, D and F: A. castellanii Neff, A. griffini and A. polyphaga respectively) in relation to the negative control (A, C and E, respectively) for 24 h. All images (40x) are based on Live Cell Imaging Microscope EVOS FL Cell Imaging System (Scale Bar 100 μm). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.2.3. Analysis of mitochondrial membrane potential
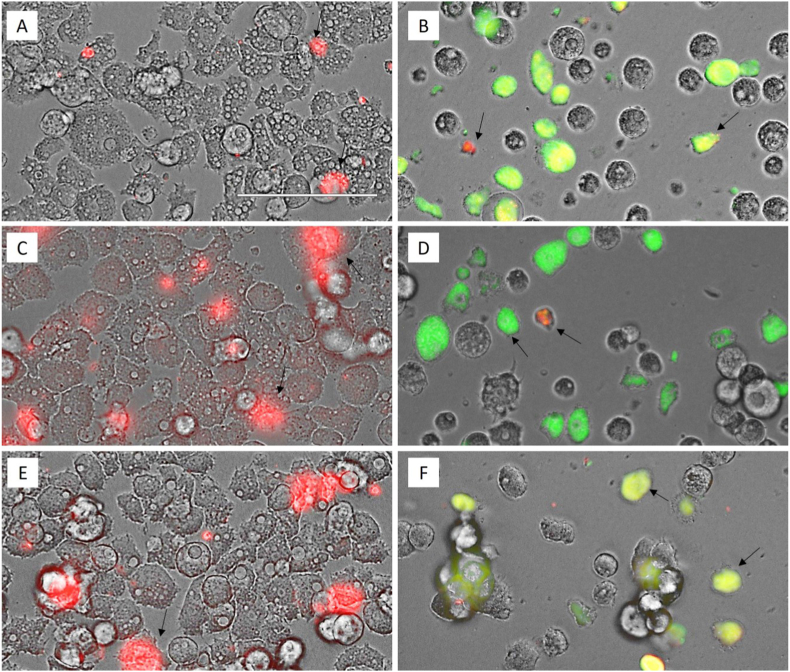
Fig. 4, shows that A. castellanii Neff, A. griffini and A. polyphaga trophozoites treated with IC90 of Matrix Ocular exhibit higher green fluorescence (B, D and F, respectively) comparing with each negative control (A, C and E). The green fluorescence indicates a decrease of the mitochondrial membrane potential, while in the negative control with an unaltered mitochondrial membrane potential we can observe red fluorescence caused by JC-1 dimers.
Fig. 4.
Effect on the mitochondrial potential evidenced by the JC-1 assay kit in trophozoites treated with the IC90 of Matrix Ocular (B, D and F: A. castellanii Neff, A. griffini and A. polyphaga respectively) in relation to the negative control (A, C and E, respectively) for 24 h. All images (40x) are based on Live Cell Imaging Microscope EVOS FL Cell Imaging System (Scale Bar 100 μm).
3.2.4. Measurement of ATP
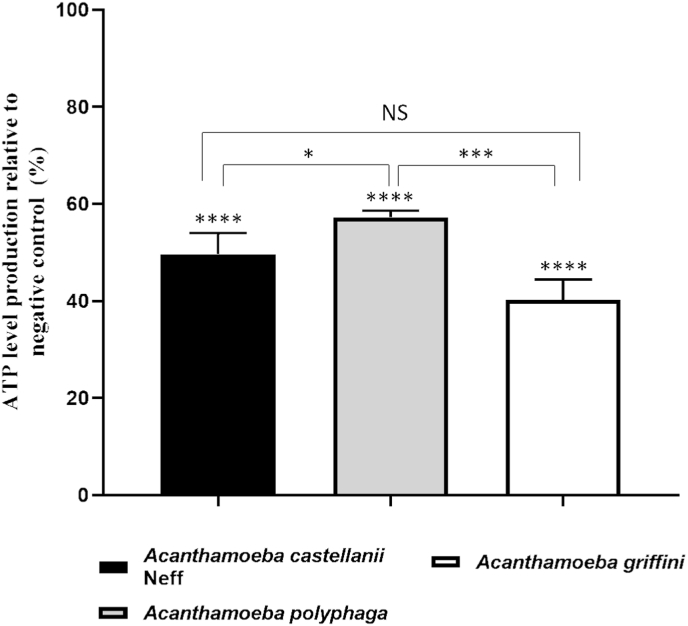
The correct mitochondrial functioning depends on a suitable mitochondrial membrane potential, as well as an appropriate level of ATP. The measuring of the ATP level generated after the treatment with the IC90 Matrix Ocular for 24 h, showed a highly significant decrease compared to the negative control (Fig. 5). A One Way ANOVA test was carried out to test the statistical differences between means, p ˂ 0.0001 (****). Moreover, while there were significantly differences between A. castellanii Neff and A. polyphaga, p < 0.05 (*) and A. polyphaga and A. griffini, p < 0.001 (***), there was no difference between A. castellanii Neff and A. griffini. This last strain has demonstrated to present the most affected ATP level due to the effect of Matrix Ocular (<40% when comparing to the negative control).
Fig. 5.
ATP level decreasing in the three evaluated Acanthamoeba spp. A One Way ANOVA test was carried out to test the statistical differences between means. (NS [non significance]); p < 0.05 [*]; p < 0.001 [***]; p ˂ 0.0001 [****]).
3.2.5. Detection of reactive oxygen species (ROS)
The staining with the CellROX® Deep Red has demonstrated that the trophozoite treatment with IC90 of Matrix Ocular generated intracellular ROS after 24 h in the three evaluated strains (Fig. 6).
Fig. 6.
Detection of reactive oxygen species (ROS) in trophozoites treated with the IC90 of Matrix Ocular (B, D and F: A. castellanii Neff, A. griffini and A. polyphaga respectively) in relation to the negative control (A, C and E, respectively) for 24 h. All images (40x) are based on Live Cell Imaging Microscope EVOS FL Cell Imaging System (Scale Bar 100 μm).
4. Discussion
The number of Acanthamoeba Keratitis (AK) cases has increased due to contact lens wearers rise in both developed and developing countries (Maycock and Jayaswal, 2016, Brown et al., 2018). Even though this protozoa infection is rare in the general population (estimated incidence: 1.4 cases/million people/year), it is highly frequent in contact lens wearers population (Khan 2009; Trabelsi et al., 2012; Lorenzo-Morales et al., 2015). An early and effective treatment is crucial to avoid side effects such as blindness or eye removal (Lorenzo-Morales et al., 2015). One of the most common problems in the AK treatment is the cyst form of Acanthamoeba, which is highly resistant to therapy. Therefore, a long-term treatment with different drug combinations is necessary to remove both cysts and trophozoites (Lim et al., 2008; Mirjalali et al., 2013). Despite of the recommended treatment regimen for AK that includes a biguanides such as chlorhexidine (Martín-Navarro et al., 2008, 2013), there is no totally effective treatment against AK until now. Most of the already commercialized ophthalmic solutions present different preservatives such as Propylene Glycol or benzalkonium chloride (BAK), whose have demonstrated to be effective against the two life cycle stages of Acanthamoeba spp. (Sifaoui et al., 2017, 2018). Nevertheless, it is important to highlight that the AK most common treatment Chlorhexidine, presents a higher toxicity in corneal cells than the BAK (Sapozhnikov et al., 2020).
In the present study we have evaluated the anti-Acanthamoeba effect of nine different ophthalmic solutions. Only ASDA Dry Eyes and Systane® Complete present preservatives (PHMB 0,0001% and POLYQUAD® (polyquaternium-1) 0.001%, respectively) (Table 2). However, the other seven analysed compounds do not present any preservative. Interestingly, ASDA Dry Eyes has presented the highest IC50, only preceded by Ectodol®, which was totally ineffective against A. castellanii Neff since the recommended concentration of PHMB to treat AK is 0.02% (Martín-Navarro et al., 2008, 2013). On the other hand, Systane® Complete contains 0.001% of POLYQUAD®, which has demonstrated to damage A. castellanii trophozoites and cysts at 0.05% and 0.005% of BAK (Sunada et al., 2014). However, in 1998 Niszl and Markus, presented a study where they demonstrated that 0.004% of BAK results inefficacious against Acanthamoeba cysts (Niszl and Markus, 1998). Therefore, taking into account these previous studies, our work remark the Systane® Complete anti-Acanthamoeba activity, independently of the BAK concentration.
Related to the other seven ophthalmic solutions without preservatives, Matrix Ocular® demonstrated to be the most effective against A. castellanii Neff trophozoites. Thus, we tested its efficacy against the two life cycle phases of A. castellanii, A. polyphaga and A. griffini, as well as to evaluate the induction of cell death mechanisms in these strains (Table 1). While the IC50 values against the trophozoite stage are similar in the three tested strains, A. castellanii Neff presented the lowest IC50 value against cysts, becoming the least resistant strain. Both A. polyphaga and A. griffini clinical strains have presented similar IC50 against the cyst stage (Table 1). There are different reports whose demonstrate the efficacy of the Chlorhexidine against these two Acanthamoeba stages (Martín-Navarro et al., 2008, 2013) and despite it has been described as one of the most effective treatments against AK (Lorenzo-Morales et al., 2015), we have to highlight that Chlorhexidine presents a high toxicity in corneal cells (Sapozhnikov et al., 2020). Matrix Ocular® is an already commercialized ophthalmic solution with a lubrication effect as main purpose and it is medically prescribed to protect the cornea and the conjunctiva after surgeries or epithelial damage. Matrix Ocular® has been developed from a natural polysaccharide named Arabinogalactan, which has been isolated from the wood of the softwood plant Larix spp. This genus can be found widely distributed across regions of China, Russia, Canada, Central Europe, and other cool, temperate regions of the northern hemisphere (Mason and Zhu, 2014). Arabinogalactan (5% w/w) has been reported as a novel mucoadhesive polysaccharide which can be used for the treatment of dry eyes and corneal wounds and to heal the dry spots on cornea (Burgalassi et al., 2007). Moreover, as it has been commented previously, Matrix Ocular® does not present any preservatives, whose could present toxic effects in corneal cells, such as Propylene Glycol or BAK (Sapozhnikov et al., 2020). Novel polymers, like arabinogalactan, have demonstrated the potential to safely deliver drugs at a controlled rate in different ophthalmic formulations (Pahuja et al., 2012). A recent study has demonstrated the beneficial effect of the synergetic formulation of Arabinogalactan and hyaluronic acid on the mitigation of the inflammatory process in the worsening of dry eye syndrome (Silvani et al., 2020). Besides, in the present research we have evidenced the programmed cell death induced by Matrix Ocular®. The absence of a necrotic cell death to avoid an excessive inflammatory response in the host, is one of the key to develop new therapies against Acanthamoeba infections. Additionally, there is a need to increasing the search of new molecules which produce programmed cell death (PCD) or apoptosis-like processes. Therefore, taking into account this fact and the mitigation effects of the inflammatory process in the dry eye syndrome, our study proposes Matrix Ocular® to continue with further studies to elucidate new treatments against AK.
5. Conclussions
In the present work, we have demonstrated that Matrix Ocular® is effective against the two life cycle stages of Acanthamoeba spp., inducing programmed cell death mechanisms in the three evaluated pathogenic strains. Therefore, Matrix Ocular® is an interesting starting point for further studies in order to develop a novel and effective treatment against AK, with an important mitigation of the inflammatory process.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Aknowledgements
This work was funded by Project PI18/01380 from Instituto de Salud Carlos III, Spain and RICET [RD16/0027/0001 project, from Programa Redes Temáticas de Investigación Cooperativa, FIS (Ministerio Español de Salud, Madrid, Spain), FEDER. R.L.R.-E. was funded by a grant from the Agencia Canaria de Investigación, Innovación y Sociedad de la Información cofunded by FEDER. M.R.-B. was funded by RICET. IS was funded by the Agustín de Betancourt Programme (Cabildo de Tenerife, TFinnova Programme supported by MEDI and FDCAN funds).
Contributor Information
M. Reyes-Batlle, Email: mreyesba@ull.edu.es.
J.E. Piñero, Email: jpinero@ull.edu.es.
J. Lorenzo-Morales, Email: jmlorenz@ull.edu.es.
References
- Brown A.C., Ross J., Jones D.B., Collier S.A., Ayers T.L., Hoekstra R.M., Backensen B., Roy S.L., Beach M.J., Yoder J.S., Acanthamoeba Keratitis Investigation Team Risk Factors for Acanthamoeba Keratitis-A Multistate Case-Control Study, 2008-2011. Eye & contact lens. 2018;44(Suppl 1):S173–S178. doi: 10.1097/ICL.0000000000000365. [DOI] [PubMed] [Google Scholar]
- Burgalassi S., Nicosia N., Monti D., Falcone G., Boldrini E., Chetoni P. Larch arabinogalactan for dry eye protection and treatment of corneal lesions: investigations in rabbits. J. Ocul. Pharmacol. Therapeut. 2007;23(6):541–550. doi: 10.1089/jop.2007.0048. [DOI] [PubMed] [Google Scholar]
- Cabello-Vílchez A.M., Martín-Navarro C.M., López-Arencibia A., Reyes-Batlle M., Sifaoui I., Valladares B., Piñero J.E., Lorenzo-Morales J. Voriconazole as a first-line treatment against potentially pathogenic Acanthamoeba strains from Peru. Parasitol. Res. 2014;113:755–759. doi: 10.1007/s00436-013-3705-8. [DOI] [PubMed] [Google Scholar]
- González-Robles A., Salazar-Villatoro L., Omaña-Molina M., Reyes-Batlle M., Martín-Navarro C.M., Lorenzo-Morales J. Morphological features and in vitro cytopathic effect of Acanthamoeba griffini trophozoites isolated from a clinical case. J. Parasitol. Res. 2014 doi: 10.1155/2014/256310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi R. Generation of multiple ocular lineages from human pluripotent stem cells and its application to regenerative medicine. Yakugaku Zasshi. 2021;141(1):55–60. doi: 10.1248/yakushi.20-00177-4. [DOI] [PubMed] [Google Scholar]
- Kaczanowski S., Sajid M., Reece S.E. Evolution of apoptosis-like programmed cell death in unicellular protozoan parasites. Parasites Vectors. 2011;4:44. doi: 10.1186/1756-3305-4-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N.A. vol. 424. Caister Academic Press; 2009. (Biology and Pathogenesis). [Google Scholar]
- Kinnunen T., Koskela M. Antibacterial and antifungal properties of propylene glycol, hexylene glycol, and 1, 3-butylene glycol in vitro. Acta Derm. Venereol. 1991;71:148–150. [PubMed] [Google Scholar]
- Lim N., Goh D., Bunce C., Xing W., Fraenkel G., Poole T.R., Ficker L. Comparison of polyhexamethylene biguanide and chlorhexidine as monotherapy agents in the treatment of Acanthamoeba keratitis. Am. J. Ophthalmol. 2008;145(1):130–135. doi: 10.1016/j.ajo.2007.08.040. [DOI] [PubMed] [Google Scholar]
- Lorenzo-Morales J., Kliescikova J., Martinez-Carretero E., De Pablos L.M., Profotova B., Nohynkova E., Osuna A., Valladares B. Glycogen phosphorylase in Acanthamoeba spp.: determining the role of the enzyme during the encystment process using RNA interference. Eukaryot. Cell. 2008;7(3):509–517. doi: 10.1128/EC.00316-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo-Morales J., Khan N.A., Walochnik J. An update on Acanthamoeba keratitis: diagnosis, pathogenesis and treatment. Parasite. 2015;22:10. doi: 10.1051/parasite/2015010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Navarro C.M., Lorenzo-Morales J., Cabrera-Serra M.G., Rancel F., Coronado-Álvarez N.M., Piñero J.E., Valladares B. The potential pathogenicity of chlorhexidine-sensitive Acanthamoeba strains isolated from contact lens cases from asymptomatic individuals in Tenerife, Canary Islands, Spain. J. Med. Microbiol. 2008;57:1399–1404. doi: 10.1099/jmm.0.2008/003459-0. PMID: 18927419. [DOI] [PubMed] [Google Scholar]
- Martín-Navarro C.M., Lorenzo-Morales J., Machin R.P., López-Arencibia A., García-Castellano J.M., de Fuentes I., Loftus B., Maciver S.K., Valladares B., Piñero J.E. Inhibition of HMG-CoA reductase and the application of statins as a novel effective therapeutic approach against Acanthamoeba infections. Antimicrob. Agents Chemother. 2013;57:375–381. doi: 10.1128/AAC.01426-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason W.L., Zhu J.J. In: Fenning T., editor. Vol. 81. Springer; Dordrecht: 2014. (Challenges and Opportunities for the World’s Forests in the 21st Century. Forestry Sciences). [DOI] [Google Scholar]
- Maycock N.J., Jayaswal R. Update on Acanthamoeba Keratitis: diagnosis, treatment, and outcomes. Cornea. 2016;35(5):713–720. doi: 10.1097/ICO.0000000000000804. [DOI] [PubMed] [Google Scholar]
- McBride J., Ingram P.R., Henriquez F.L., Roberts C.W. Development of colorimetric microtiter plate assay for assessment of antimicrobials against Acanthamoeba. J. Clin. Microbiol. 2005;43(2):629–634. doi: 10.1128/JCM.43.2.629-634.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirjalali H., Niyyati M., Abedkhojasteh H., Babaei Z., Sharifdini M., Rezaeian M. Pathogenic assays of Acanthamoeba belonging to the t4 genotype. Iran. J. Parasitol. 2013;8(4):530–535. PMID: 25516733. [PMC free article] [PubMed] [Google Scholar]
- Montiel F., Martín-Navarro C.M., Alió J.L., López-Vélez R., Martínez-Carretero E., Valladares B., Piñero J.E., Lorenzo-Morales J. Successful monitoring and treatment of intraocular dissemination of Acanthamoeba. Arch. Ophthalmol. 2012;130:1474–1475. doi: 10.1001/archophthalmol.2012.2376. [DOI] [PubMed] [Google Scholar]
- Nalawade T.M., Bhat K., Sogi S.H. Bactericidal activity of propylene glycol, glycerine, polyethylene glycol 400, and polyethylene glycol 1000 against selected microorganisms. J. Int. Soc. Prev. Community Dent. 2015;5:114. doi: 10.4103/2231-0762.155736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niszl I.A., Markus M.B. Anti-Acanthamoeba activity of contact lens solutions. Br. J. Ophthalmol. 1998;82(9):1033–1038. doi: 10.1136/bjo.82.9.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira-Ferreira C., Leuzinger-Dias M., Tavares-Ferreira J., Torrão L., Falcão-Reis F. Microbiological profile of infectious keratitis in a Portuguese tertiary centre. J. Ophthalmol. 2019;6328058 doi: 10.1155/2019/6328058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahuja P., Arora S., Pawar P. Ocular drug delivery system: a reference to natural polymers. Expet Opin. Drug Deliv. 2012;9(7):837–861. doi: 10.1517/17425247.2012.690733. [DOI] [PubMed] [Google Scholar]
- Paimela T., Ryhanen T., Kauppinen A Marttila L., Salminen A., Kaarniranta K. The preservative polyquaternium-1 increases cytoxicity and NF-kappaB linked inflammation in human corneal epithelial cells. Mol. Vis. 2012;18:1189–1196. PMCID: PMC3351434. [PMC free article] [PubMed] [Google Scholar]
- Reyes-Batlle M., Mura-Escorche G., Sifaoui I., Otero-Ruiz A., Alfaro-Sifuentes R., López-Arencibia A., Rocha-Cabrera P., Chiboub O., Rizo-Liendo A., Zamora-Herrera J., Bethencourt-Estrella C.J., Rodríguez-Expósito R.L., Nicolás-Hernández D.S., Piñero J.E., Lorenzo-Morales J. In vitro evaluation of combined commercialized ophthalmic solutions against Acanthamoeba strains. Pathogens. 2019;8(3):109. doi: 10.3390/pathogens8030109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha-Cabrera P., Reyes-Batlle M., Martín-Navarro C.M., Dorta-Gorrín A., López-Arencibia A., Sifaoui I., Martínez-Carretero E., Piñero J.E., Martín-Barrera F., Valladares B., Lorenzo-Morales J. Detection of Acanthamoeba on the ocular surface in a Spanish population using the Schirmer strip test: pathogenic potential, molecular classification and evaluation of the sensitivity to chlorhexidine and voriconazole of the isolated Acanthamoeba strains. J. Med. Microbiol. 2015;64:849–853. doi: 10.1099/jmm.0.000103. [DOI] [PubMed] [Google Scholar]
- Sapozhnikov S.V., Sabirova A.E., Shtyrlin N.V., Druk A.Y., Agafonova M.N., Chirkova M.N., Kazakova R.R., Grishaev D.Y., Nikishova T.V., Krylova E.S., Nikitina E.V., Kayumov A.R., Shtyrlin Y.G. Design, synthesis, antibacterial activity and toxicity of novel quaternary ammonium compounds based on pyridoxine and fatty acids. Eur. J. Med. Chem. 2020;211:113100. doi: 10.1016/j.ejmech.2020.113100. [DOI] [PubMed] [Google Scholar]
- Seino S., Takada Y., Saika S. Effects of benzalkonium chloride in ophthalmic eyedrop medications on corneal epithelium. Yakugaku Zasshi. 2021;141(1):35–39. doi: 10.1248/yakushi.20-00177-1. [DOI] [PubMed] [Google Scholar]
- Siddiqui R., Khan N.A. Biology and pathogenesis of Acanthamoeba. Parasites Vectors. 2012;5:6. doi: 10.1186/1756-3305-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sifaoui I., Rodríguez-Talavera I., Reyes-Batlle M., Rodríguez-Expósito R.L., Rocha-Cabrera P., Piñero J.E., Lorenzo-Morales J. In vitro evaluation of commercial foam Belcils® on Acanthamoeba spp. Int J Parasitol Drugs Drug Resist. 2020;14:136–143. doi: 10.1016/j.ijpddr.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sifaoui I., Capote Yanes E.C., Reyes-Batlle M., Rodríguez-Expósito R.L., Piñero J.E., Lorenzo-Morales J. Combined amoebicidal effect of atorvastatin and commercial eye drops against Acanthamoeba castellanii Neff: in vitro assay based on mixture design. Pathogens. 2020;9(3):219. doi: 10.3390/pathogens9030219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sifaoui I., Reyes-Batlle M., López-Arencibia A., Chiboub O., Rodríguez-Martín J., Rocha-Cabrera P., Valladares B., Piñero J.E., Lorenzo-Morales J. Toxic effects of selected proprietary dry eye drops on Acanthamoeba. Sci. Rep. 2018;8:8520. doi: 10.1038/s41598-018-26914-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sifaoui I., Reyes-Batlle M., López-Arencibia A., Wagner C., Chiboub O., De Agustino Rodríguez J., Rocha-Cabrera P., Valladares B., Piñero J.E., Lorenzo-Morales J. Evaluation of the anti-Acanthamoeba activity of two commercial eye drops commonly used to lower eye pressure. Exp. Parasitol. 2017;183:117–123. doi: 10.1016/j.exppara.2017.07.012. [DOI] [PubMed] [Google Scholar]
- Silvani L., Bedei A., De Grazia G., Remiddi S. Arabinogalactan and hyaluronic acid in ophthalmic solution: experimental effect on xanthine oxidoreductase complex as key player in ocular inflammation (in vitro study) Exp. Eye Res. 2020;196:108058. doi: 10.1016/j.exer.2020.108058. [DOI] [PubMed] [Google Scholar]
- Sunada A., Kimura K., Nishi I., Toyokawa M., Ueda A., Sakata T., Suzuki T., Inoue Y., Ohashi Y., Asari S., Iwatani Y. In vitro evaluations of topical agents to treat Acanthamoeba keratitis. Ophthalmology. 2014;121(10):2059–2065. doi: 10.1016/j.ophtha.2014.04.013. [DOI] [PubMed] [Google Scholar]
- Trabelsi H., Dendana F., Sellami A., Sellami H., Cheikhrouhou F., Neji S., Makni F., Ayadi A. Pathogenic free-living amoebae: epidemiology and clinical review. Pathol. Biol. 2012;60(6):399–405. doi: 10.1016/j.patbio.2012.03.002. [DOI] [PubMed] [Google Scholar]