Abstract
Background
Optimal management of vestibular schwannoma (VS) is still debated and thus international consensus has not been achieved. Treatment options are observation, radiotherapy, and surgery. Knowledge on the natural history of tumor growth is essential for choice of treatment modality. The aim is to present intra-/extrameatal tumor growth and management data from a prospective, unselected national cohort of patients diagnosed with VS during the period 1976–2015.
Methods
Since 1976, all data from patients diagnosed with sporadic VS in Denmark have been referred to our national treatment center, where they have been entered prospectively into the national database. Data on tumor localization, growth, and treatment were retrieved. Growth definition: >2 mm by linear measurement, in accordance with the Tokyo 2001 consensus-meeting recommendations.
Results
3637 cases of VS were diagnosed, in which 1304 patients had surgery and 21 received radiotherapy post diagnosis. 2312 patients were observed with mean follow-up of 7.33 years. Of these, 434(19%; 102 intra-and 332 extrameatal tumors) changed to active treatment during the observation period due to tumor growth. 5 years after diagnosis, 21% of the intrameatal tumors exhibited growth during observation, whereas 37% of extrameatal tumors had grown, increasing to 25% intrameatal and 42% extrameatal after 10 years. Following growth, the intrameatal tumors were mostly observed further and the extrameatal mostly underwent surgery. Tumor growth occurred mainly within the first 5 years post diagnosis.
Conclusion
This natural history study documents the growth occurrence of both intra-and extrameatal VS during the first 12 years after diagnosis and should be used in patient counseling, management, and treatment decision making.
Keywords: acoustic neuroma, conservative management, intrameatal, extrameatal, observation, tumor-growth/-size
Key Points.
Approximately 75% of intrameatal vestibular schwannomas do not grow 10 years after diagnosis.
Approximately 60% of extrameatal vestibular schwannomas do not grow 10 years after diagnosis.
Vestibular schwannoma growth may occur during the first 12 years of observation after diagnosis, predominantly within the initial 5 years.
Importance of the Study.
Long-term data are inherently difficult to obtain. The present study reports 40-year prospective data of 3637 patients with VS in Denmark. It demonstrates that the majority of tumors do not exhibit growth during a mean observation period of more than 7 years.
Copenhagen is the national treatment center of VS in Denmark, thus the 40-year data are not confounded by referral or selection bias as the cohort encompasses all patients nationwide. To our knowledge, the cohort is the largest ever reported in literature on observation of VS and contributes to the continuing debate on optimal management of especially intrameatal and small extrameatal tumors. As many treatment centers worldwide still treat these tumors actively immediately upon diagnosis with either radiotherapy or surgery, we believe it is essential that the natural history of tumor growth is known to all clinicians in order to practice evidence-based medicine and provide patients the best possible treatment.
Vestibular schwannomas (VS) are benign nerve sheath tumors arising from Schwann cells of the VIIIth cranial nerve and comprise approximately 80% of cerebellopontine angle (CPA) tumors and 8% of all intracranial tumors.1 VS exceedingly rarely undergo malignant transformation, although several case reports have documented the occurrence with or without prior radiotherapy, including histologic and molecular investigations confirming the change in tumor biology post radiation.2,3 The cause of VS is unknown, although some reports suggest viral etiology4,5 or environmental reasons such as occupation, traffic, and mobile phones,6–8 others have suggested tumor growth association (although conflicting) with aspirin/nonsteroidal anti-inflammatory drugs.9,10 The annual incidence has been reported to be increasing over many years by several centers,11 reaching approximately 34 VS/million inhabitants.12 The spontaneous course of the disease remains unpredictable, as some tumors continue to grow after diagnosis, whereas others senesce and some even shrink during observation.13 As continuous tumor growth may cause life-threatening brainstem compression, the main task of managing the condition is tumor control, secondarily preservation of cranial nerve function (ie facial nerve function, hearing, and balance). Reported tumor growth percentages vary from 12% to 76%, partly due to varying lengths of observation and growth criteria.14 Factors influencing the choice of treatment include tumor size, hearing level, and, as noted, risk of tumor growth. Thus, knowledge on the natural history of VS growth is essential in order to provide an evidence-based treatment recommendation for the patient. Whereas surgery remains undisputed in large and giant tumors,15 three treatment modalities are available for small- and medium-sized tumors: observation (wait-and-scan)/conservative management, surgery, and radiotherapy. As a significant proportion of tumors do not exhibit growth after diagnosis and a large proportion of the patients preserve good or serviceable hearing spontaneously, the trend in management has shifted toward conservatism during the recent years.16–18 In order to strengthen the evidence base on the risk of tumor growth and conservative management, this paper provides data on more than 2300 observed VS patients with a mean of almost 8 years.
Methods
Data from every patient in Denmark with a sporadic unilateral VS have since 1976 been referred to our national treatment center, at which they have been entered prospectively into a national VS database. Hence the data are not subjected to referral bias or selection bias. Several parameters were included in data, eg, the tumor size, the tumor localization, the initial treatment strategy, sex and age of the patient at diagnosis. This study reports the data on tumor localization and growth patterns as well as chosen treatment during the 40-year period from January 1, 1976 to January 1, 2016. A total of 3637 patients with sporadic unilateral VS were registered and included in the database during this period. The population in Denmark was 5.1 million in 1976, increasing to 5.7 million as of January 1, 2016.
The tumors were categorized as either intrameatal (if purely intracanalicular) or extrameatal (intra- and extrameatal). The size of an intrameatal VS was registered as 0 mm extrameatal and the size of extrameatal tumors was determined as the largest extrameatal diameter (not including a potential intrameatal portion) by linear measurement on high-resolution MR imaging (slice thickness ≤1 mm), conducted by the two senior authors: S.E.S. and P.C.T. This classification follows the Tokyo 2001 consensus meeting recommendations on reporting size of VS published by Kanzaki et al in 2003.19 Patients allocated to observation/conservative management entered an MRI follow-up scheme with an MRI scan yearly after diagnosis for 5 years, subsequently every other year for 5 years, followed by a scan every 5 years. Local radiology departments assisted in performing the MRI scans and sending the images to our treatment center. The criterion for growth of intrameatal VS was growth to extrameatal extension (into CPA) and extrameatal tumor growth was defined as an increase of more than 2 mm to rule out inter- and intraobserver measurement variability. If the VS exhibited growth, the patient would be offered a clinical appointment for discussion of further management which would be either continued observation or active treatment (radiotherapy or surgery). Since 1989, when MRI scans became an option for imaging and follow-up, almost all patients have had MRI scans for tumor growth control. Thirty-seven patients allocated to repetitive CT scans and patients diagnosed with Neurofibromatosis 2 were excluded from the material.
Ethics Statement
Institutional review board and the Danish Data Protection Agency approved the collection of patient data for the national VS database.
Results
3637 patients were diagnosed with a sporadic unilateral VS during the period 1976–2015. Female n = 1804 and male n = 1833, and the mean age at diagnosis was 57 years (range, 15–93 y). The patients are listed in Table 1 according to management and tumor localization. In total, 1095 patients had an intrameatal VS and 2509 had an extrameatal (intra- and extrameatal) VS. No data on localization were available for 33 patients (18 female and 15 male), who all had surgery as the initial treatment. The initial treatment was observation for 2312 patients (64%). During a mean of 7.33 years of observation (range, 1–37 y), 372 (16%) of these patients underwent surgery (89 intrameatal tumors; 283 extrameatal tumors) and 62 (3%) received radiotherapy (13 intrameatal tumors; 49 extrameatal tumors), of which 3 patients (5%) with extrameatal tumors had subsequent surgery as well. Thus, 1878 patients (81%) were purely observed and conservative management failed in 19% of the initially observed patients, due to growth of the tumor and subsequent active treatment (radiotherapy or surgery). The majority of the initially surgically treated VS were extrameatal (1243; 99%) and 10 patients (0.8%) received additional radiotherapeutic treatment, all of them extrameatal tumors. Eighteen patients (0.5%) had radiotherapy as the initial treatment, of which 3 had subsequent surgical treatment (17%), all being extrameatal tumors.
Table 1.
All patients diagnosed with a VS in the 40-year period 1976–2015 according to management and subdivided in tumor localizations
| Treatment | Initial Treatment Only | Additional Irradiation | Additional Operation | All VS Patients | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | E | N | I | E | N | I | E | N | I | E | N | Total | |
| Observed | 973 | 905 | 0 | 13 | 49* | 0 | 89 | 283 | 0 | 1075 | 1237 | 0 | 2312 |
| Operated | 18 | 1243 | 33 | 0 | 10 | 0 | 18 | 1253 | 33 | 1304 | |||
| Irradiated | 2 | 16 | 0 | 0 | 3 | 0 | 2 | 19 | 0 | 21 | |||
| Total | 993 | 2164 | 33 | 13 | 59 | 0 | 89 | 286 | 0 | 1095 | 2509 | 33 | 3637 |
Abbreviations: I = intrameatal, E = extrameatal, N = no data.
*The 3 patients were observed initially and subsequently received radiotherapy and surgery.
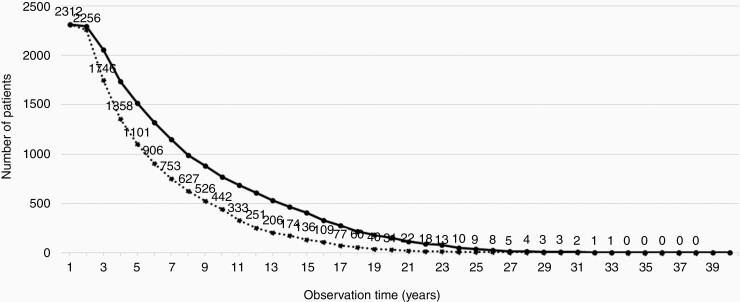
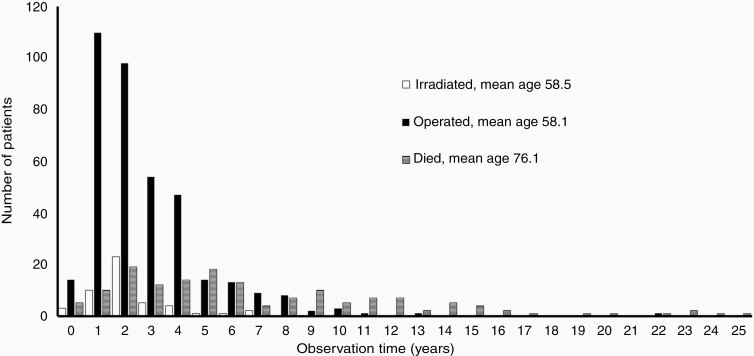
An ideal cohort and observation length could not be obtained, as it would require all the conservatively managed patients not to change to active treatment or dying during the 40-year observation period. Such patients were considered lost to follow-up. Thus, the difference between ideal and actual observations lengths was due to failure of conservative management/loss of patients to active treatment (n = 434; 19%) and deaths (n = 147; 6%); see Figure 1. In addition, optimal follow-up for the remaining and still observed patients would require all of them to have the last MRI scan performed on the day of data extraction for analysis (December 31, 2015). As this was not the case for nearly all patients, the majority had a censored period (ie, the period from their last scan to December 31, 2015) in which growth in principle could have occurred, and adding to the difference between ideal and actual observation lengths. Thus, the cumulated actual observation was 70% of the ideal observation. Figure 2 reflects part of the difference in observation lengths illustrated in Figure 1 by demonstrating the number of patients receiving active treatment after initial observation, and showing when and which treatment was given. Inevitably, some of the patients died during the 40-year observation period and are also depicted in the figure. None of the deaths were related to VS. Most patients received the active treatment within the first 5 years, predominantly surgery. No patients received radiotherapy after 7 years. The latest active treatment was performed after 22 years observation (one patient with 3 mm growth) and in total 3 patients (0.1%) were treated after 10 years observation.
Fig. 1.
Ideal and actual observation time for patients initially allocated to conservative management. The solid curve is the ideal observation and the dotted curve is the actual observation during the 40-year period. Cumulated actual observation was 70% of the ideal. Definitions explained in the Results section.
Fig. 2.
Active treatment for patients initially allocated to observation (n = 434) and patients who died during the observation period (n = 147). The black columns represent the number of patients receiving surgery, the white columns represent radiotherapy-treated patients and the horizontally lined columns represent the patients who died.
Growth and Management of Intrameatal Tumors
Growth data were available for 868 patients with intrameatal VS (Table 2), which corresponds to 81% of all observed patients with intrameatal VS (1075 patients; see Table 1). Of the 868 patients with intrameatal VS, 769 (89%) were purely observed, whereas 86 (10%) underwent surgery and 13 (1%) received radiotherapy. Of the intrameatal VS, 78% did not exhibit growth, and of these 680 patients with nongrowing VS, 98% were only observed. Tumor growth occurred in 188 of the 868 patients, corresponding to 22%, of which 63% grew 3–10 mm and 35% grew 11–20 mm. About half (106; 56%) of the growing intrameatal VS were further observed and 75 (40%) underwent surgery. Seven (3.5%) of the 188 patients with tumor growth received radiotherapy (Table 2).
Table 2.
Growth and management of intrameatal and extrameatal VS, from diagnosis to last follow-up
| Treatment | No Growth I / E | Growth I / E | Total I / E | |
|---|---|---|---|---|
| Growth | Observed I / E | Operated I / E | Irradiated I / E | Total I / E |
| Observed | 663 / 618 | 106 / 156 | 769 / 774 | |
| Operated | 11 / 48 | 75 / 222 | 86 / 270 | |
| Irradiated | 6 / 11 | 7 / 36 | 13 / 47 | |
| Total | 680 / 677 | 188 / 414 | 868 / 1091 | |
| 3‒10 mm | 81 / 143 | 34 / 195 | 4 / 34 | 119 / 372 |
| 11‒20 mm | 24 / 13 | 39 / 26 | 3 / 1 | 66 / 40 |
| >20 mm | 1 / 0 | 2 / 1 | 0 / 1 | 3 / 2 |
| Total | 106 / 156 | 75 / 222 | 7 / 36 | 188 / 414 |
Abbreviations: I = intrameatal, E = extrameatal.
*Intrameatal and extrameatal numbers are separated by a slash. The bottom part of the table represents the 188 growing intrameatal tumors and 414 growing extrameatal tumors.
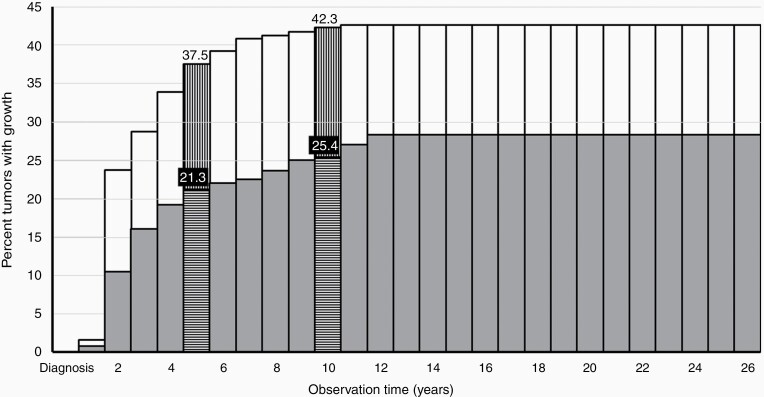
The risk of intrameatal VS growth increases during the first 12 years of observation and is depicted in Figure 3. The difference in observation periods (numerous censored data) warrants the use of a Nelson–Aalen survival plot, in this case presented as an actuarial survival rate curve, showing the cumulated risk of tumor growth, based on the observation length for all the patients in the study. In the majority of cases, tumor growth occurs within the first 3–5 years, but growth did occur up to 12 years after diagnosis. The curve and hence the cumulated risk of growth plateaus at 28.4%. When looking at the cumulated risk during the observation in actuarial rates, 21.3% of the intrameatal tumors exhibited growth 5 years after diagnosis and 25.4% had grown 10 years after diagnosis (Figure 3).
Fig. 3.
Actuarial “survival” curve of cumulated risk of growth in intrameatal and extrameatal VS with increasing observation length. The gray columns represent the risk for intrameatal tumors. The extension from the gray columns depicted by white columns represent the risk of growth in extrameatal tumors. The horizontally (intrameatal) and vertically (extrameatal) lined columns mark the 5 and 10 year actuarial rates, respectively.
Growth and Management of Extrameatal Tumors
Growth data were available for 1091 (88%) of the 1237 observed patients diagnosed with an extrameatal VS (Tables 1 and 2). Of the 1091 patients with extrameatal VS, 774 (71%) were purely observed, 270 (25%) underwent surgery, and 47 (4%) received radiotherapy, compared with the patients with intrameatal VS, where a larger proportion (89%) were purely observed. 677 (62%) of the extrameatal VS did not exhibit growth and 91% of these patients were only observed (Table 2). 414 of the 1091 patients displayed tumor growth, corresponding to 38%. The vast majority of these (90%) exhibited 3–10 mm tumor growth. In contrast to the intrameatal VS, the majority of the growing extrameatal VS received active treatment upon growth (62%). Of the 414 patients with growing extrameatal VS, 222 (54%) underwent surgery, 36 (9%) received radiotherapy, and 156 (38%) were continuously observed.
As shown in Figure 3, growth occurs primarily within the first 5 years after diagnosis, rarely thereafter, and no growth occurred after 11 years of observation. The data depiction is based on the cumulated risk of growth, in consideration of the varying lengths of observation, with the curve plateauing at 42.6%. In actuarial rates, 37.5% of the extrameatal tumors exhibited growth 5 years after diagnosis and 42.3% had grown 10 years after diagnosis (Figure 3).
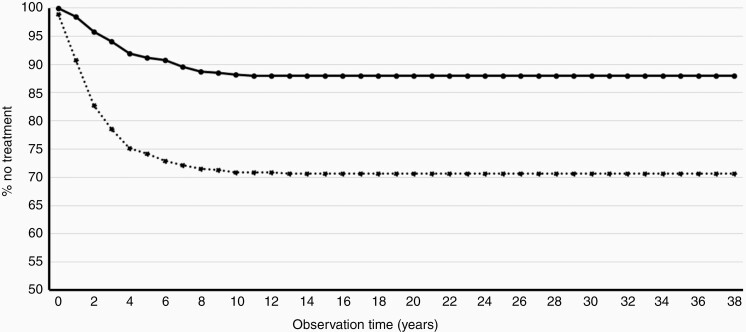
Figure 4 displays Kaplan–Meier curves of nontreatment “survival” for patients diagnosed with either an intrameatal or an extrameatal VS, showing that 88% of the intrameatal tumors and 71% of the extrameatal tumors were treated by observation only.
Fig. 4.
Kaplan–Meier ”survival” curve of nontreatment in intrameatal and extrameatal VS, depicting the risk of receiving active treatment. The solid curve shows the percentage of purely observed intrameatal tumors during the period and the dotted curve the extrameatal tumors.
Discussion
As the incidence rate of VS is rising steadily,11,12 it is increasingly important to establish the spontaneous course of disease progression (ie, tumor growth). Apart from large tumor size at diagnosis, tumor growth is the most important indication for active treatment, either radiotherapy or surgery. This report presents an update on the prospective, unselected national cohort of all patients diagnosed with a sporadic unilateral VS in Denmark during the 40-year period 1976–2015. The database currently includes 3637 consecutively registered patients, of whom 2312 were observed initially, thus representing the largest cohort of observed patients with VS reported in the literature. Compared with the previously published data from our treatment center, we now report an increased growth rate. We previously reported growth occurrence in 17% of intrameatal and 29% of extrameatal tumors,20 based on a mean follow-up of 3.6 years. The current numbers of 22% and 38%, respectively, are based on a mean follow-up of 7.33 years, which explains the increases. The numbers also illustrate that growth rarely occurs after 5 years of observation (see Figure 3). No correlation was found between tumor growth and sex or age, which is consistent with other major reports also including a variety of other potential predictors, of which initial tumor size and disequilibrium may be associated with tumor growth.10,21
The strength of the study is that it is not confounded by patient selection bias and especially referral bias, which is a potential problem in other cohort studies,10,21 as patients may be referred by selection, or managed conservatively due to either advanced age, medical comorbidity, or patient preference.14 The setup in Copenhagen may be considered close to ideal in terms of demonstrating the true epidemiology and spontaneous course of the disease. Simultaneously, this could affect the generalizability of the management in a global setting. Most tertiary or major treatment centers (particularly in North America) get patients referred after either observation or active treatment at a local hospital, which complicates data acquisition and management, leading to more heterogeneous datasets, subsequently complicating patient management. Therefore, the reason for underlining the importance of knowledge on the natural history of VS tumor growth is because regardless of tumor size, patients with sporadic unilateral VS continue to undergo active treatment immediately upon diagnosis at a number of centers around the world, even though the majority of tumors do not grow after diagnosis. The treatment given depends highly on local traditions within countries, hospitals, and departments, as the management of VS varies, particularly for intrameatal and small- to medium-sized tumors.22 Although, for several years the tendency has shifted toward conservative management and less surgery.17 Some treatment centers mostly perform surgery,15,23 others radiotherapy,24,25 and lack of knowledge on the natural history of tumor growth is likely to skew the clinical decision making toward active treatment as opposed to initial observation, especially when conflicting reports continuously are being published with different views and points of interest. The reports often argue either for tumor control being superior26,27 or for hearing deterioration being less,28,29 irrespective of which active treatment modality, and there is a lack of comparative studies. Increasingly more studies focus on quality of life (QoL) rather than tumor control or cranial nerve function, which is part of a general development in healthcare toward patient-reported outcome measures. Studies on QoL show better30 or equal outcome for conservatively managed VS compared with initial surgery or radiotherapy,31,32 or to active treatment in failed conservative management.30 However, a recent systematic review showed conflicting results regarding choice of treatment and confirmed that no large, randomized prospective studies exist on radiotherapy. According to this review, 8 studies have been published on QoL in radiated patients, of which only the study by Di Maio and Akagami was prospective.31,33 From a healthcare system perspective, conservative management is more cost-effective than active treatment, and a recent study has reported radiotherapy being less costly than surgery.34
The current 40-year prospective dataset on more than 2300 patients demonstrates the spontaneous course of VS progression, documenting that approximately 75% of intrameatal and approximately 60% of extrameatal tumors do not exhibit growth 10 years after diagnosis, as demonstrated by actuarial rates. As several studies report conflicting results regarding superiority of initial treatment modality and QoL studies show no difference between available treatment options, we believe that active treatment of intrameatal and small- to medium-sized tumors as an initial step leads to overtreatment and unnecessary risk-taking in the majority of cases.
The continuing debate on the assessment of tumor growth is a particular point to be addressed. Previous studies have shown that assessing VS size and tumor growth implicates an intra- and interobserver measurement error of up to 2 mm,35,36 which is why several studies must be read and interpreted critically, as criteria for the occurrence of growth are either not defined or defined to be less than 2 mm. In order to limit problems associated with inter-individual measurement error, the current study is based on tumor size assessment by the two senior authors S.E.S. and P.C.T., using linear measurement of the largest extrameatal tumor diameter and growth defined by an increase of at least 2 mm, which is in accordance with the majority of other authors and the Tokyo 2001 consensus meeting recommendations.19 Volumetric evaluation of tumor size is more sensitive to tumor growth compared with linear measurements, and during recent years publications have used volumetric assessments increasingly. Tumors seemingly unchanged by linear measurement may display growth by volumetric assessment and hence earlier. The growth rates by volumetric assessment are generally reported higher, as in the recent papers by Lees et al21 and Schnurman et al,37 reporting 69% (median observation, 1.1 y) and 66% growing tumors (mean observation, 25.4 mo). In 2006, our center published a volumetric intrameatal tumor growth rate of 45% (mean observation, 4.4 y),38 which is in accordance with other studies on volumetric tumor growth.9,39 It is often argued that volumetric assessment is better for irregular/complex configurated tumors (ie, geometrical characteristics not captured on a linear measurement or tumors that are not perfectly spherical), assessment in medical or radiotherapeutic treatment of tumors, and that it is more meaningful to acknowledge what may be perceived as more true growth rates by volumetric measurements, as several papers have demonstrated higher growth rates in studies directly comparing volumetric with linear measurement.21,40,41 Often, these papers report superiority in the smallest detectable difference, in reliability, and in inter- and intraobserver agreement, although not 100% consistent.40–42 However, volumetric tumor increase has also varied in previous publications, spanning from 15% to 73%,40 and although the cutoff is chosen at 20% volume increase in recent studies,9,21,37,43 this is problematic for intrameatal and small extrameatal tumors, as the measurement error is greater and smallest detectable difference higher for these cases. This may also be problematic for larger/giant tumors, where a 20% volume increase potentially could be dangerous. As for the challenge of establishing consensus regarding the definition of linear growth, the lack of consensus on a definition of significant tumor volume increase continues. Several papers often refer to the need for RANO/RECIST-like guidelines for VS. Essentially, some argue that linear assessments underestimate increases in small tumors, while other papers argue that volumetric assessment overestimates the value of this increase (eg, that a 20% spherical volume increase only constitutes 6% in one axis),37 whereas a 50% increase in one axis (eg, from 2 to 3 mm intrameatally) would result in a 300% volume increase, although not, or unlikely to be, clinically relevant.43 We definitely support the need for consensus on defining significant volume increase and clinical relevance before applying volumetric evaluation into routine clinical use. Another aspect, also acknowledged in previous studies, is that volumetric assessment methods (eg, manually segmented or semi-automated) also carry a risk of human error. Finally, the software for volumetric evaluation (eg, BrainLab) is not available in all departments, thus for technical reasons and due to time consumption, the practice is not established as a standard procedure in most centers. More importantly, it could be argued that if growth cannot be detected on linear measurements, it is hardly clinically relevant. A counter-argument may be that hearing deterioration may be associated with an increase in tumor volume which is not appreciated with linear measurements. Studies using linear measurements have shown either an insignificant increase in tumor size with progression of hearing loss or a lack of association.10 Only a few studies exist on tumor volume and hearing loss, but 2 papers have reported that an increased tumor volume is associated with hearing loss, although the first report included only a small number of patients and the other did not find an association between tumor volume increase and speech discrimination.44,45 However, a recent study did not demonstrate significant volumetric increase in tumor size and progression of hearing loss,43 which corroborates an earlier study showing no correlation between volumetric tumor growth and audiologic deterioration.39 It can hence be concluded that conflicting reports have been published. One could also argue that hearing deterioration should be the main assessment factor, rather than tumor size or volume increase, when evaluating disease progression and that the follow-up scheme should be patient tailored. The complexity of the issue of hearing and tumor growth is further stressed by reported hearing deterioration despite linear measured tumor regression.46 Hearing status in our current cohort will be reported in a separate publication. To the best of our knowledge, no papers have addressed tumor progression and affection of other cranial nerves apart from the 8th, eg, long-term observed tumor size and facial nerve function, which may be explained by the fact that facial nerve function is very rarely affected during observation.16,32,47 The facial nerve function is on the other hand frequently addressed in papers on surgical outcome, showing poor outcome associated with tumor size and type, surgical approach and finally, the degree of resection.15,16,48,49 The ability to assess tumor growth, especially in intrameatal VS, improves with enhanced imaging technology and growth rates may thus increase in the future, as continuously improving technology allows more accurate measurements. In the present study, difficulties in determining tumor size changes lead to exclusion of 37 CT scanned patients from the early part of the 40-year registration period.
From a clinical perspective, monitoring of patients with repeated MRI is feasible, provided that the follow-up is reliable and realistic, and a variety of algorithms have been proposed.20 Potential problems performing follow-up MRIs is an argument often stated by authors advocating initial active treatment, as a tight follow-up is required and not necessarily possible or feasible. Thus, patients risk getting lost in a suboptimal follow-up scheme and may potentially present years later with a large tumor and increased risks of treatment. Hopefully, future studies will identify precise predictors of growth, such as by MRI detectable markers of growth including aforementioned studies into the etiopathogenesis of VS. Studies on tumor gene expression have demonstrated association between growth and expression of certain genes and activation of specific molecular pathways, but have not accounted for age-related tumor aggressiveness.50
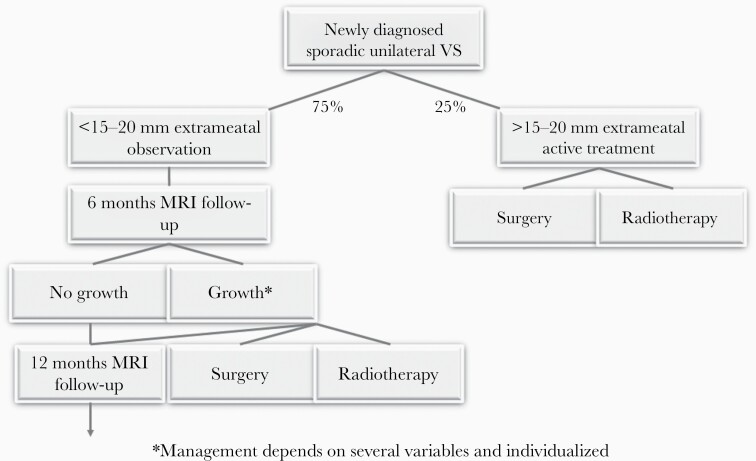
The treatment algorithm currently used in our center is illustrated in Figure 5, noting that the treatment is always based on individual factors and counseling and may deviate from the algorithm, due to comorbidity, poor contralateral hearing, or persisting vestibular problems.
Fig. 5.
VS treatment algorithm during the first 5 years, subsequently increasing to every 2 years and finally every 5 years.
Conclusion
With a mean follow-up of 7.33 years, overall growth during observation occurs in 22% of intrameatal tumors and 38% of extrameatal tumors. In actuarial rates, 21% of the intrameatal tumors have grown 5 years after diagnosis, whereas 37% of the extrameatal tumors have exhibited growth. Ten years after diagnosis the rates are 25% and 42%, respectively. The natural history of VS growth entails occurrence of tumor growth during the first 12 years after diagnosis in both intra- and extrameatal tumors, although primarily within the initial 5 years, which should be applied in patient counseling, management, and treatment strategy.
Acknowledgments
Profound gratitude and sincere thanks are due to the late Prof J. Thomsen and the late Prof M. Tos for initiating the database and to all those contributing to its maintenance over the years.
Funding
No funding was received.
Conflict of interest statement. None of the authors have any conflicts of interest to declare.
Authorship statement. Design: MR, SE-S, PC-T; data collection and analysis: MR, MMBSP, NW, SE-S, PC-T; manuscript draft, revision and final approval: MR, MMBSP, NW, SE-S, PC-T.
References
- 1. Mahaley MS Jr, Mettlin C, Natarajan N, Laws ER Jr, Peace BB. Analysis of patterns of care of brain tumor patients in the United States: a study of the brain tumor section of the AANS and the CNS and the Commission on Cancer of the ACS. Clin Neurosurg. 1990;36:347–352. [PubMed] [Google Scholar]
- 2. Bashir A, Poulsgaard L, Broholm H, Fugleholm K. Late malignant transformation of vestibular schwannoma in the absence of irradiation: case report. J Neurosurg. 2016;125(2):372–377. [DOI] [PubMed] [Google Scholar]
- 3. Shin M, Ueki K, Kurita H, Kirino T. Malignant transformation of a vestibular schwannoma after gamma knife radiosurgery. Lancet. 2002;360(9329):309–310. [DOI] [PubMed] [Google Scholar]
- 4. Burkhart CG. Herpes and acoustic neuromas: is there a cause and effect to observe? Med Hypotheses. 2010;74(6):1013–1014. [DOI] [PubMed] [Google Scholar]
- 5. Håvik AL, Bruland O, Aarhus M, et al. Screening for viral nucleic acids in vestibular schwannoma. J Neurovirol. 2018;24(6):730–737. [DOI] [PubMed] [Google Scholar]
- 6. Prochazka M, Feychting M, Ahlbom A, et al. Occupational exposures and risk of acoustic neuroma. Occup Environ Med. 2010;67(11):766–771. [DOI] [PubMed] [Google Scholar]
- 7. Schüz J, Steding-Jessen M, Hansen S, et al. Long-term mobile phone use and the risk of vestibular schwannoma: a Danish nationwide cohort study. Am J Epidemiol. 2011;174(4):416–422. [DOI] [PubMed] [Google Scholar]
- 8. Roswall N, Stangerup SE, Cayé-Thomasen P, et al. Residential traffic noise exposure and vestibular schwannoma—a Danish case-control study. Acta Oncol. 2017;56(10):1310–1316. [DOI] [PubMed] [Google Scholar]
- 9. Kandathil CK, Cunnane ME, McKenna MJ, Curtin HD, Stankovic KM. Correlation between aspirin intake and reduced growth of human vestibular schwannoma: volumetric analysis. Otol Neurotol. 2016;37(9):1428–1434. [DOI] [PubMed] [Google Scholar]
- 10. Hunter JB, Weidenheim KM, Lee AY, Dinces E. Intratemporal intraneural perineurioma of the facial nerve. Otol Neurotol. 2016;37(10):e414–e416. [DOI] [PubMed] [Google Scholar]
- 11. Marinelli JP, Modzeski MC, Lane JI, et al. Primary skull base lymphoma: manifestations and clinical outcomes of a great imitator. Otolaryngol Head Neck Surg. 2018;159(4):643–649. [DOI] [PubMed] [Google Scholar]
- 12. Reznitsky M, Petersen MMBS, West N, Stangerup SE, Cayé-Thomasen P. Epidemiology of vestibular schwannomas—prospective 40-year data from an unselected national cohort. Clin Epidemiol. 2019;11:981–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tikka T, Yiannakis CP, Stapleton E, et al. Spontaneous vestibular schwannoma regression: a case-control study. Otol Neurotol. 2018;39(10):e1118–e1124. [DOI] [PubMed] [Google Scholar]
- 14. Zanoletti E, Mazzoni A, Martini A, et al. Surgery of the lateral skull base: a 50-year endeavour. Acta Otorhinolaryngol Ital. 2019;39(Suppl. 1):S1–S146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Samii M, Matthies C, Tatagiba M. Management of vestibular schwannomas (acoustic neuromas): auditory and facial nerve function after resection of 120 vestibular schwannomas in patients with neurofibromatosis 2. Neurosurgery. 1997;40(4):696–705; discussion 705. [DOI] [PubMed] [Google Scholar]
- 16. Stangerup SE, Caye-Thomasen P. Epidemiology and natural history of vestibular schwannomas. Otolaryngol Clin North Am. 2012;45(2):257–2 68, vii. [DOI] [PubMed] [Google Scholar]
- 17. Carlson ML, Habermann EB, Wagie AE, et al. The changing landscape of vestibular schwannoma management in the United States—a shift toward conservatism. Otolaryngol Head Neck Surg. 2015;153(3):440–446. [DOI] [PubMed] [Google Scholar]
- 18. Reznitsky M, Cayé-Thomasen P. Systematic review of hearing preservation in observed vestibular schwannoma. J Neurol Surg B Skull Base. 2019;80(2):165–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kanzaki J, Tos M, Sanna M, Moffat DA, Monsell EM, Berliner KI. New and modified reporting systems from the consensus meeting on systems for reporting results in vestibular schwannoma. Otol Neurotol. 2003;24(4):642–64 8; discussion 648. [DOI] [PubMed] [Google Scholar]
- 20. Stangerup SE, Caye-Thomasen P, Tos M, Thomsen J. The natural history of vestibular schwannoma. Otol Neurotol. 2006;27(4):547–552. [DOI] [PubMed] [Google Scholar]
- 21. Lees KA, Tombers NM, Link MJ, et al. Natural history of sporadic vestibular schwannoma: a volumetric study of tumor growth. Otolaryngol Head Neck Surg. 2018. doi: 10.1177/0194599818770413. [DOI] [PubMed] [Google Scholar]
- 22. Carlson ML, Glasgow AE, Grossardt BR, Habermann EB, Link MJ. Does where you live influence how your vestibular schwannoma is managed? Examining geographical differences in vestibular schwannoma treatment across the United States. J Neurooncol. 2016;129(2):269–279. [DOI] [PubMed] [Google Scholar]
- 23. Saliba J, Friedman RA, Cueva RA. Hearing preservation in vestibular schwannoma surgery. J Neurol Surg B Skull Base. 2019;80(2):149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boari N, Bailo M, Gagliardi F, et al. Gamma Knife radiosurgery for vestibular schwannoma: clinical results at long-term follow-up in a series of 379 patients. J Neurosurg. 2014;121 Suppl:123–142. [DOI] [PubMed] [Google Scholar]
- 25. Johnson S, Kano H, Faramand A, et al. Long term results of primary radiosurgery for vestibular schwannomas. J Neurooncol. 2019;145(2):247–255. [DOI] [PubMed] [Google Scholar]
- 26. Kondziolka D, Mousavi SH, Kano H, Flickinger JC, Lunsford LD. The newly diagnosed vestibular schwannoma: radiosurgery, resection, or observation? Neurosurg Focus. 2012;33(3):E8. [DOI] [PubMed] [Google Scholar]
- 27. Chiluwal A, Rothman A, Svrakic M, Dehdashti A. Surgical outcome in smaller symptomatic vestibular schwannomas: is there a place for surgery as first option? J Neurol Surg B. 2017. doi: 10.1055/s-0037-1600545 [DOI] [PubMed] [Google Scholar]
- 28. Golfinos JG, Hill TC, Rokosh R, et al. A matched cohort comparison of clinical outcomes following microsurgical resection or stereotactic radiosurgery for patients with small- and medium-sized vestibular schwannomas. J Neurosurg. 2016;125(6):1472–1482. [DOI] [PubMed] [Google Scholar]
- 29. Peng KA, Wilkinson EP. Optimal outcomes for hearing preservation in the management of small vestibular schwannomas. J Laryngol Otol. 2016;130(7):606–610. [DOI] [PubMed] [Google Scholar]
- 30. Sandooram D, Grunfeld EA, McKinney C, Gleeson MJ. Quality of life following microsurgery, radiosurgery and conservative management for unilateral vestibular schwannoma. Clin Otolaryngol Allied Sci. 2004;29(6):621–627. [DOI] [PubMed] [Google Scholar]
- 31. Di Maio S, Akagami R. Prospective comparison of quality of life before and after observation, radiation, or surgery for vestibular schwannomas. J Neurosurg. 2009;111(4):855–862. [DOI] [PubMed] [Google Scholar]
- 32. Carlson ML, Tveiten OV, Driscoll CL, et al. Long-term quality of life in patients with vestibular schwannoma: an international multicenter cross-sectional study comparing microsurgery, stereotactic radiosurgery, observation, and nontumor controls. J Neurosurg. 2015;122(4):833–842. [DOI] [PubMed] [Google Scholar]
- 33. Tsao MN, Sahgal A, Xu W, et al. Stereotactic radiosurgery for vestibular schwannoma: International Stereotactic Radiosurgery Society (ISRS) Practice Guideline. J Radiosurg SBRT. 2017;5(1):5–24. [PMC free article] [PubMed] [Google Scholar]
- 34. Schnurman Z, Golfinos JG, Epstein D, Friedmann DR, Roland JT, Kondziolka D. Comparing costs of microsurgical resection and stereotactic radiosurgery for vestibular schwannoma. J Neurosurg. 2018:1–10. doi: 10.3171/2018.5.JNS18508 [DOI] [PubMed] [Google Scholar]
- 35. Marshall AH, Owen VM, Nikolopoulos TP, O’Donoghue GM. Acoustic schwannomas: awareness of radiologic error will reduce unnecessary treatment. Otol Neurotol. 2005;26(3):512–515. [DOI] [PubMed] [Google Scholar]
- 36. Hougaard D, Norgaard A, Pedersen T, Bibby BM, Ovesen T. Is a redefinition of the growth criteria of vestibular schwannomas needed? Am J Otolaryngol. 2014;35(2):192–197. [DOI] [PubMed] [Google Scholar]
- 37. Schnurman Z, Nakamura A, McQuinn MW, Golfinos JG, Roland JT, Kondziolka D. Volumetric growth rates of untreated vestibular schwannomas. J Neurosurg. 2019:1–7. doi: 10.3171/2019.5.JNS1923 [DOI] [PubMed] [Google Scholar]
- 38. Caye-Thomasen P, Hansen S, Dethloff T, Stangerup S-E, Thomsen J. Sublocalization and volumetric growth pattern of intracanalicular vestibular schwannomas. Laryngoscope. 2006. doi: 10.1097/01.MLG.0000217528.37106.2D [DOI] [PubMed] [Google Scholar]
- 39. van de Langenberg R, de Bondt B-J, Nelemans PJ, Dohmen AJC, Baumert BG, Stokroos RJ. Predictors of volumetric growth and auditory deterioration in vestibular schwannomas followed in a wait and scan policy. Otol Neurotol. 2011;32(2):338–344. [DOI] [PubMed] [Google Scholar]
- 40. Walz PC, Bush ML, Robinett Z, Kirsch CFE, Welling DB. Three-dimensional segmented volumetric analysis of sporadic vestibular schwannomas: comparison of segmented and linear measurements. Otolaryngol Head Neck Surg. 2012;147(4):737–743. [DOI] [PubMed] [Google Scholar]
- 41. MacKeith S, Das T, Graves M, et al. A comparison of semi-automated volumetric vs linear measurement of small vestibular schwannomas. Eur Arch Otorhinolaryngol. 2018;275(4):867–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van de Langenberg R, de Bondt BJ, Nelemans PJ, Baumert BG, Stokroos RJ. Follow-up assessment of vestibular schwannomas: volume quantification versus two-dimensional measurements. Neuroradiology. 2009;51(8):517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Patel NS, Huang AE, Dowling EM, et al. The influence of vestibular schwannoma tumor volume and growth on hearing loss. Otolaryngol Head Neck Surg. 2020. doi: 10.1177/0194599819900396. [DOI] [PubMed] [Google Scholar]
- 44. Massick DD, Welling DB, Dodson EE, et al. Tumor growth and audiometric change in vestibular schwannomas managed conservatively. Laryngoscope. 2000;110(11):1843–1849. [DOI] [PubMed] [Google Scholar]
- 45. Caye-Thomasen P, Dethloff T, Hansen S, Stangerup S-E, Thomsen J. Hearing in patients with intracanalicular vestibular schwannomas. Audiol Neurootol. 2007;12(1):1–12. [DOI] [PubMed] [Google Scholar]
- 46. Stipkovits EM, Graamans K, Van Dijk JE. Vestibular schwannoma: negative growth and audiovestibular features. Eur Arch Otorhinolaryngol. 2001;258(9):467–471. [DOI] [PubMed] [Google Scholar]
- 47. Kaltoft M, Stangerup S-E, Caye-Thomasen P. Facial nerve function after vestibular schwannoma surgery following failed conservative management. Neurosurgery. 2012;70(2):278–282. [DOI] [PubMed] [Google Scholar]
- 48. Falcioni M, Fois P, Taibah A, Sanna M. Facial nerve function after vestibular schwannoma surgery. J Neurosurg. 2011;115(4):820–826. [DOI] [PubMed] [Google Scholar]
- 49. Strickland BA, Ravina K, Rennert RC, et al. Intentional subtotal resection of vestibular schwannoma: a reexamination. J Neurol Surg B Skull Base. 2020;81(2):136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sass HCR, Hansen M, Borup R, Nielsen FC, Cayé-Thomasen P. Tumor miRNA expression profile is related to vestibular schwannoma growth rate. Acta Neurochir (Wien). 2020. doi: 10.1007/s00701-020-04238-4. [DOI] [PubMed] [Google Scholar]