Abstract
Background
Recent international sequencing efforts have allowed for the molecular taxonomy of lower-grade gliomas (LGG). We sought to analyze The Cancer Genome Atlas (TCGA, 2015) gene expression datasets on molecularly defined oligodendrogliomas (IDH-mutated and 1p/19q-codeleted) patients treated with adjuvant radiation or those observed to discover prognostic markers and pathways.
Methods
mRNA expression and clinical information of patients with oligodendroglioma were taken from the TCGA “Brain Lower Grade Glioma” provisional dataset. Transcription factor network reconstruction and analysis were performed using the R packages “RTN” and “RTNsurvival.” Elastic net regularization and survival modeling were performed using the “biospear,” “plsRCox,” “survival” packages.
Results
From our cohort of 137 patients, 65 received adjuvant radiation and 72 were observed. In the cohort that received adjuvant radiotherapy, a transcription factor activity signature, that correlated with hypoxia, was associated with shorter disease-free survival (DFS) (median = 45 months vs 108 months, P < .001). This increased risk was not seen in patients who were observed (P = .2). Within the observation cohort, a transcription factor activity signature was generated that was associated with poor DFS (median = 72 months. vs 143 months., P < .01).
Conclusions
We identified a transcription factor activity signature associated with poor prognosis in patients with molecular oligodendroglioma treated with adjuvant radiotherapy. These patients would be potential candidates for treatment intensification. A second signature was generated for patients who were more likely to progress on observation. This potentially identifies a cohort who would benefit from upfront adjuvant radiotherapy.
Keywords: adjuvant radiotherapy, biomarkers, oligodendroglioma
Key Points.
Transcription factor activities are predictive bio-markers in oligodendrogliomas.
Signature developed that predicts recurrence risk following adjuvant radiotherapy.
Second signature developed for oligodendrogliomas who would benefit from radiotherapy.
Importance of Study.
Currently adjuvant therapy for molecular oligodendrogliomas is driven by clinical characteristics, and can include adjuvant chemotherapy, radiation, chemo-radiation, or observation. We describe efforts to integrate clinical genomics to discover predictive bio-markers that would inform adjuvant treatment decisions in oligodendrogliomas. Leveraging a bio-informatic tool to infer transcription factor activity from RNA expression, we generated a transcription factor network from the TCGA “Lower Grade Glioma” dataset for whom there was adjuvant treatment information. This resulted in a transcription factor activity signature of prognosis in patients treated with adjuvant radiation, which would represent possible resistance mechanisms and signal the need for treatment intensification. A second prognostic signature for patients treated with observation alone was also developed, representing a predictive bio-marker for patients who would benefit from adjuvant radiotherapy. This research would be of interest to the field as it represents the first predictive bio-marker signature that may guide adjuvant treatment decisions for oligodendrogliomas.
Biomarkers have transformed the current approach to diagnose, classify and treat brain gliomas.1 According to the most recent World Health Organization classification guidelines oligodendrogliomas are defined as tumors with mutations in isocitrate dehydrogenase (IDH) 1 or IDH2 genes with concurrent losses in chromosome arms 1p and 19q (1p/19q co-deletion).1 The majority of these gliomas also present with mutations in FUBP1, CIC, and the Telomerase reverse transcriptase (TERT) promoter.2,3
Oligodendrogliomas are classified as low grade (grade I or II) or anaplastic (grade III) but regardless of grade, oligodendrogliomas tend to be less aggressive, more sensitive to systemic therapy, and are associated with improved overall survival compared to their astrocytic counterparts.4 Retrospective series and prospective studies confirmed that the presence of 1p/19q co-deletion in gliomas were both associated with longer survival and predictive of response to alkylator chemotherapy.5,6 However, for these tumors, the impact of TERT promoter mutations remains unclear.7
Despite the described molecular advances in the understanding of glioma biology, there remains no completely accepted standard of care in the treatment of oligodendrogliomas, molecularly defined as having mutations in IDH1 or IDH2 with concurrent losses in chromosome arms 1p and 19q (1p/19q co-deletion).1,8 Trials are limited by the rarity of oligodendrogliomas and long follow-up times. For anaplastic oligodendrogliomas (grade 3), a combination of radiation and/or chemotherapy is generally recommended; however, the most effective chemotherapy regimen and the timing of radiation remain debated. For grade I or II oligodendrogliomas treatment options are myriad including surgery, observation, chemotherapy, radiotherapy, or chemo-radiotherapy.8 Therefore, we analyzed The Cancer Genome Atlas (TCGA) data on oligodendroglioma patients treated with adjuvant radiotherapy or observation to define molecular sub-groups at a higher risk of recurrence, or with better prognosis following adjuvant treatment.
Materials and Methods
Data Source
Our cohort consists of patients with molecular oligodendroglioma (ie IDH mutated and 1p/19q co-deleted) in the TCGA database based upon data generated by the TCGA Research Network: http://cancergenome.nih.gov/. RNA expression and clinical information was taken from the TCGA “Brain Lower Grade Glioma” provisional dataset (accessed through http://www.cbioportal.org/).9,10 Validation cohort was taken from the “Chinese Glioma Genome Atlas” (accessed through http://cgga.org.cn/).11,12
Statistical Analyses
RNA expression data from the molecular oligodendroglioma cohort was used to reconstruct transcription factor networks using the R packages “RTN” and “RTNsurvival”.13,14 The list of human transcription factors and co-factors were taken from Schmeier et al.15 Univariate Cox proportional hazard models were fitted for each transcription factor using the R packages: “plsRCox” and “survival”.16–18 Top scoring transcription factors (univariate P < .05; Cox proportional hazards interaction P with RT < .1) for adjuvant radiotherapy (RT) or observation cohorts were selected. Elastic net regularization of the top scoring transcription factors was performed using the “biospear” package to generate a prognostic signature.19 Re-sampling cross-validation of the signature was performed using the “rms” package.20 R version 3.6.1.21 “R-studio” version 1.2.5033.22
Results
From 530 potential “Brain Lower Grade Glioma” (LGG) dataset patients, 164 were included in our analysis with both IDH-mutation and 1p/19q-codeletion (Fig. 1A). Out of our cohort of 164 patients, 137 had documentation of treatment, with 65 receiving adjuvant radiation (median dose: 5940 cGy) and 72 observed. From the included cohort of 137, 21% of patients who received radiation also received adjuvant chemotherapy (93% Temozolomide). 8% of patients who were initially observed received salvage chemotherapy following recurrence.
Fig. 1.
(A) Tree diagram showing the subsets from The Cancer Genome Atlas (TCGA) Lower-Grade Glioma cohort: molecular oligodendrogliomas (IDH-mutant, 1p/19q-co-deleted), treated with observation or adjuvant radiotherapy. (B) Clinical features of our two subsets: observation and adjuvant radiotherapy. Grade 3 tumors were more likely to receive radiotherapy (adj. P < .001). P-values were adjusted with a Bonferroni correction. GTR: Gross Total Resection. STR: Sub-Total Resection. Unk: Unknown. KPS: Karnofsky Performance Status. * P < .05.
In our cohort, patients receiving RT or observation were well-balanced in terms of age, receipt of chemotherapy, extent of resection, and Karnofsky performance score (Fig. 1B). However, patients who received adjuvant RT were more likely to have tumors of grade 3 oligodendroglioma (adj. P < .001).
The previously described “RTN” method13 was used to reconstruct an oligodendroglioma-specific transcriptional factor network consisting of 2070 transcription factors and co-factors, 16 436 targets, and 56 013 edges. A Cox Proportionate Hazard (Cox-PH) model was fit to each transcription factor with respect to progression-free survival or overall survival. Elastic-net regularization reduced top-scoring transcription factors (univariate P < .05 and interaction P-value with RT < .1) to a prognostic signature.
A transcription factor activity signature of 5 transcription factors (HIF3A, PAXBP1, PLAG1, SIX5, ZBTB7B) was associated with worse progression-free survival (Hazard Ratio (HR) = 4.0, log-rank P < .001, C-index = .87) for the cohort who received adjuvant radiation (Fig. 2A), but not for the observation cohort (Fig. 2B). The effect was still significant on multivariable analysis with extent of resection and age (ANOVA P < .05) (Fig. 2C,D). The signature was also significant when stratified by grade (Supplemental Figure 3a).
Fig. 2.
A transcriptional activity signature was generated which significantly correlated with progression-free survival in patients treated with adjuvant RT (univariate Hazard Ratio (HR) = 4, log-rank P < .001) (A) but not the observation cohort (P = .2) (B). The signature was prognostic for PFS on multivariable Cox regression analysis (multivariate HR = 3.4, ANOVA P < .05). Kaplan-Meier survival curves of the signature divided by extent of resection (C) and age (D). The HIF transcriptional signature was also prognostic for progression-free survival in patients treated with adjuvant RT (univariate HR = 9.1, log-rank P < .05) (E). See Supplemental Figure 1. C-index: Concordance Index. Cox-PH: Cox proportional hazards. ANOVA: Analysis of Variance. * P < .05. + positive. – negative. GTR: Gross Total Resection. STR: Sub-Total Resection.
HIF3A is a member of the hypoxia pathway, which is strongly associated with radiation resistance.23 Combined transcription activity of hypoxia pathway members, HIF1A and HIF3A, was shown to be associated with decreased progression-free survival (HR = 9.1, log-rank P < .01, C-index = .74) (Fig. 2E).
In an independent cohort, from the “Chinese Glioma Genome Atlas,” 127 molecular oligodendroglioma's were treated with adjuvant radiotherapy.11,12 We found that combined expression of HIF1A and HIF3A was significantly prognostic for overall survival in patients treated with adjuvant radiotherapy (HR = 3.3, log-rank P = .003) (Supplemental Fig. 5).
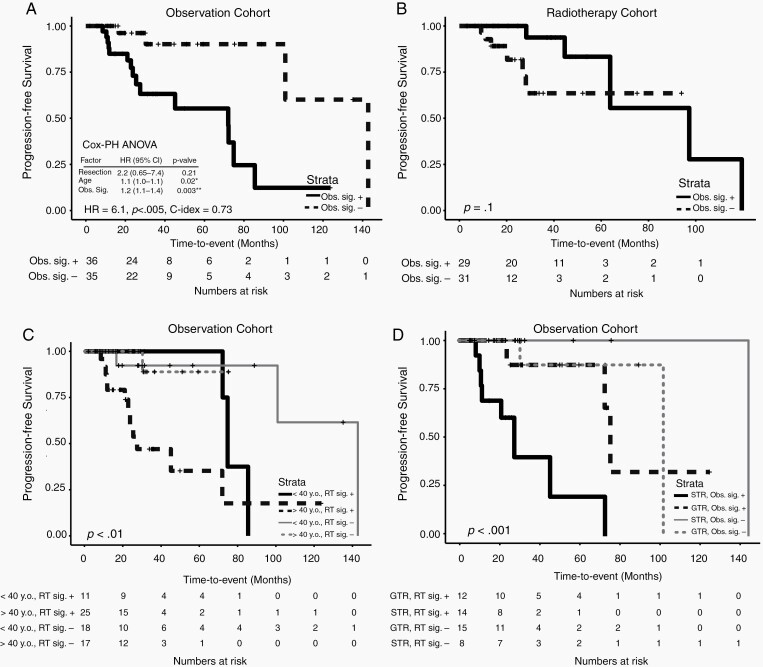
For the patients initially observed, a 19-transcription factor activity signature (ARNTL2, ASH2L, BCL3, CBX6, CREB3L3, CREBBP, CSRNP2, CTR9, DNAJA3, EBF2, ETS2, MED12, PAX5, SIM2, SOX7, TFB2M, ZFP69B, ZNF329, and ZNF436) was associated with worse progression-free survival (HR = 6.1, log-rank P < .005, C-index = .73) (Fig. 3A). It remained significantly associated in multivariable analysis with extent of resection and age (ANOVA P < .01) (Fig. 3C,D). The signature was also significant when stratified by grade (Supplemental Figure 3b). The risk was apparently abrogated in the adjuvant radiotherapy cohort (HR = .32, log-rank P = .1) (Fig. 3B). The transcription factor activity signature was also significantly predictive of overall survival even as the cohort also included salvage radiotherapy (14% of patients) (HR = 10.1, log-rank P < .01, C-index = .76) (Fig.3E).
Fig. 3.
A transcriptional activity signature was generated which significantly correlated with progression-free survival in patients observed post-resection (univariate HR = 6.1, log-rank P < .005) (A) but not the adjuvant RT cohort (log-rank P = .1) (B). The signature was prognostic for PFS on multivariable Cox regression analysis (multivariate HR = 1.2, ANOVA P < .005). Kaplan-Meier survival curves of the signature divided by age (C) and extent of resection (D). The signature was also significantly correlated with overall survival in the observation cohort (univariate HR = 10.1, log-rank P < .01) (E). The Circadian transcriptional signature was also prognostic for progression-free survival in patients observed post-resection (univariate HR = 4.4, log-rank P < .05) (F). See Supplemental Figure 2. * P < .05. ** P < .005. + positive. – negative. GTR: Gross Total Resection. STR: Sub-Total Resection.
ARNTL2 is a member of the circadian rhythm pathway, and CREBBP is known to co-activate the circadian CLOCK-BMAL1 complex.24 Therefore, we investigated whether other members of the circadian clock pathway would also be prognostic in the observation cohort. A transcription factor activity signature with circadian rhythm pathway members ARNTL2, ARNTL, and CLOCK was prognostic for progression-free survival (HR = 4.4, log-rank P < .05, C-index = .62) independent of extend of resection and age (multivariable P < .01) (Fig. 3F).
Discussion
Transcription factors represent master regulators of cell signaling state and tumor plasticity, and can be used to consolidate gene expression into signatures and pathways.13 We show that transcriptional activity of members of the hypoxia pathway was associated with decreased progression-free survival only for patients treated with adjuvant radiation. During normal oxygen tension, HIF transcription factors are rapidly degraded.25 However, in the hypoxic microenvironment of tumors, HIF proteins are stabilized and translocate to the nucleus to initiate gene expression programs involved in cell survival during stresses, such as DNA damage.25 In glioma's, HIF activity is associated with glioma stem cell maintenance (see Supplemental Figure 4), and Kessler et al. have shown that its down-regulation by siRNA in vitro sensitizes to radiation.26,27 In addition, oxygen is required to “set” or make irreversible the DNA double-strand break produced by ionizing radiation.28 This leads to 2.5–3 times more radiation dose required for the same effect in a variety of different primary histologies.29 For a given dose, the cell kill may be as high as several orders of magnitude less in hypoxic tumors.29 Therefore, HIF transcriptional program signature may represent direct radiation resistance from the lack of oxygen or indirect through pro-survival HIF target genes.
The circadian clock consists of self-sustaining oscillations of a transcription factor network with period ~24 h, governing a diverse set of processes necessary for metazoans.30 The pathway (Fig. 4) has been implicated in the pathogenesis and prognosis of a growing number of cancers.31 Li et al. have shown that expression of the CLOCK gene is increased with higher glioma grade, and promotes glioma proliferation and migration.32 Here, we show that increased transcriptional activity of the circadian complex was associated with poor prognosis for patients who were observed. However, this risk was abrogated in patients receiving adjuvant RT, which points to its potential use as a predictive biomarker.
Fig. 4.
(A) Diagram depicting the hypoxia signaling pathway. In normoxic conditions, HIF1/3 becomes prolyl hydroxylated which leads to dimer formation with VHL that shuttles the complex to proteasomal degradation. In hypoxia, HIF1/3 is more stable and translocates to the nucleus to initiate transcription of target genes with ARNT as a co-factor. (B) Diagram depicting the circadian pathway in this study of oligodendrogliomas. ARNT and ARNTL heterodimerize with CLOCK to initiate transcription of genes. Genes PER1/2, CRY1/2, and REV-ERBs are transduced and inhibit the CLOCK complex in a negative feedback loop. CREB3L3 is targeted by the ARTNL/CLOCK complex, which acts as a transcription factor to upregulate its own target genes. Figure generated using BioRender.
One limitation of this retrospective study is the unknown size of residual post-operative tumor, an important risk factor for recurrence, and predictive factor for use of adjuvant treatment.33 In addition, the two treatment groups were imbalanced by grade, with grade 3 oligodendrogliomas more likely to receive adjuvant RT. Overall survival time was confounded by the fact that some patients who were initially treated with observation received salvage RT upon progression.
Conclusion
In this study, we utilized transcriptomic data to infer transcription factor activity in the TCGA Lower-Grade Glioma cohort. By integrating with treatment information, we generated prognostic signatures for patients treated with adjuvant radiation or observation. We sought to identify the subsets which would benefit from adjuvant radiotherapy or further treatment intensification. Additional study is required to validate these signatures in an independent, prospective cohort.
Supplementary Material
Abbreviations
- TCGA
The Cancer Genome Atlas
- LGG
Lower Grade Glioma
- WHO
World Health Organization
Funding
None to disclose.
Conflict of interest statement. None to disclose.
Authorship statement. Study design and conception: J.S., Y.M., and F.M.; statistical analysis: J.S.; writing and revisions: J.S., Y.M., G.Z., K.M., and F.M.
References
- 1. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 2. Cahill DP, Louis DN, Cairncross JG. Molecular background of oligodendroglioma: 1p/19q, IDH, TERT, CIC and FUBP1. CNS Oncol. 2015;4(5):287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Masui K, Cloughesy TF, Mischel PS. Review: molecular pathology in adult high-grade gliomas: from molecular diagnostics to target therapies. Neuropathol Appl Neurobiol. 2012;38(3):271–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cairncross JG, Ueki K, Zlatescu MC, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90(19):1473–1479. [DOI] [PubMed] [Google Scholar]
- 5. Cairncross G, Wang M, Shaw E, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol. 2013;31(3):337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Van Den Bent MJ, Brandes AA, Taphoorn MJB, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013;31(3):344–350. [DOI] [PubMed] [Google Scholar]
- 7. Pekmezci M, Rice T, Molinaro AM, et al. Adult infiltrating gliomas with WHO 2016 integrated diagnosis: additional prognostic roles of ATRX and TERT. Acta Neuropathol. 2017;133(6):1001–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nabors LB, Portnow J, Ammirati M, et al. NCCN guidelines insights: central nervous system cancers, version 1.2017. J Natl Compr Canc Netw. 2017;15(11):1331–1345. [DOI] [PubMed] [Google Scholar]
- 9. Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang Y, Qian T, You G, et al. Localizing seizure-susceptible brain regions associated with low-grade gliomas using voxel-based lesion-symptom mapping. Neuro Oncol. 2015;17(2):282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu X, Li Y, Qian Z, et al. A radiomic signature as a non-invasive predictor of progression-free survival in patients with lower-grade gliomas. NeuroImage Clin. 2018;20(August):1070–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Castro MA, de Santiago I, Campbell TM, et al. Regulators of genetic risk of breast cancer identified by integrative network analysis. Nat Genet. 2016;48(1):12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fletcher MNC, Castro MAA, Wang X, et al. Master regulators of FGFR2 signalling and breast cancer risk. Nat Commun. 2013;4(May):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schmeier S, Alam T, Essack M, Bajic VB. TcoF-DB v2: update of the database of human and mouse transcription co-factors and transcription factor interactions. Nucleic Acids Res. 2017;45(D1):D145–D150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Therneau TM, Grambsch PM.. Modeling Survival Data: Extending the Cox Model. New York: Springer; 2000. [Google Scholar]
- 17. Therneau TM. A Package for Survival Analysis in S. Version 3.2; 2020. http://cran.r-project.org/package=survival.
- 18. Bastien P, Bertrand F, Meyer N, Maumy-Bertrand M. Deviance residuals-based sparse PLS and sparse kernel PLS regression for censored data. Bioinformatics. 2015;31(3):397–404. [DOI] [PubMed] [Google Scholar]
- 19. Ternès N, Rotolo F, Michiels S. biospear: an R package for biomarker selection in penalized Cox regression. Bioinformatics. 2018;34(1):112–113. [DOI] [PubMed] [Google Scholar]
- 20. Harrell FE Jr. rms: Regression Modeling Strategies. R package version 5.0-0; 2016. http://cran.r-project.org/package=rms.
- 21. R Core Team . R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2020. http://www.R-project.org/. [Google Scholar]
- 22. RStudio Team . RStudio: Integrated Development for R. Boston, MA: RStudio, PBC; 2020. http://www.rstudio.com/. [Google Scholar]
- 23. Semenza GL. Intratumoral hypoxia, radiation resistance, and HIF-1. Cancer Cell. 2004;5(5):405–406. [DOI] [PubMed] [Google Scholar]
- 24. Lee Y, Lee J, Kwon I, et al. Coactivation of the CLOCK-BMAL1 complex by CBP mediates resetting of the circadian clock. J Cell Sci. 2010;123(Pt 20):3547–3557. [DOI] [PubMed] [Google Scholar]
- 25. Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40(2):294–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li Z, Bao S, Wu Q, et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell. 2009;15(6):501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kessler J, Hahnel A, Wichmann H, et al. HIF-1α inhibition by siRNA or chetomin in human malignant glioma cells: effects on hypoxic radioresistance and monitoring via CA9 expression. BMC Cancer. 2010;10:605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ewing D. The Oxygen fixation hypothesis. Am J Clin Oncol. 1998;21(4):355–361. [DOI] [PubMed] [Google Scholar]
- 29. Brown JM. The hypoxic cell: a target for selective cancer therapy – eighteenth Bruce F. Cain Memorial Award lecture. Cancer Res. 1999;59(23):5863–5870. [PubMed] [Google Scholar]
- 30. Ueda HR, Hayashi S, Chen W, et al. System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nat Genet. 2005;37(2):187–192. [DOI] [PubMed] [Google Scholar]
- 31. Sulli G, Lam MTY, Panda S. Interplay between circadian clock and cancer: new frontiers for cancer treatment. Trends Cancer. 2019;5(8):475–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li A, Lin X, Tan X, et al. Circadian gene clock contributes to cell proliferation and migration of glioma and is directly regulated by tumor-suppressive miR-124. FEBS Lett. 2013;587(15):2455–2460. [DOI] [PubMed] [Google Scholar]
- 33. Xia L, Fang C, Chen G, Sun C. Relationship between the extent of resection and the survival of patients with low-grade gliomas: a systematic review and meta-analysis. BMC Cancer. 2018;18(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.