Abstract
Background
Supratotal resection is advocated in lower-grade gliomas (LGGs) based on theoretical advantages but with limited verification of functional risk and data on oncological outcomes. We assessed the association of supratotal resection in molecularly defined LGGs with oncological outcomes.
Methods
Included were 460 presumptive LGGs; 404 resected; 347 were LGGs, 319 isocitrate dehydrogenase (IDH)–mutated, 28 wildtype. All patients had clinical, imaging, and molecular data. Resection aimed at supratotal resection without any patient or tumor a priori selection. The association of extent of resection (EOR), categorized on volumetric fluid attenuated inversion recovery images as residual tumor volume, along with postsurgical management with progression-free survival (PFS), malignant (M)PFS, and overall survival (OS) assessed by univariate, multivariate, and propensity score analysis. The study mainly focused on IDH-mutated LGGs, the “typical LGGs.”
Results
Median follow-up was 6.8 years (interquartile range, 5–8). Out of 319 IDH-mutated LGGs, 190 (59.6%) progressed, median PFS: 4.7 years (95% CI: 4–5.3). Total and supratotal resection obtained in 39% and 35% of patients with IDH1-mutated tumors. In IDH-mutated tumors, most patients in the partial/subtotal group progressed, 82.4% in total, only 6 (5.4%) in supratotal. Median PFS was 29 months (95% CI: 25–36) in subtotal, 46 months (95% CI: 38–48) in total, while at 92 months, PFS in supratotal was 94.0%. There was no association with molecular subtypes and grade. At random forest analysis, PFS strongly associated with EOR, radiotherapy, and previous treatment. In the propensity score analysis, EOR associated with PFS (hazard ratio, 0.03; 95% CI: 0.01–0.13). MPFS occurred in 32.1% of subtotal total groups; 1 event in supratotal. EOR, grade III, previous treatment correlated to MPFS. At random forest analysis, OS associated with EOR as well.
Conclusions
Supratotal resection strongly associated with PFS, MPFS, and OS in LGGs, regardless of molecular subtypes and grade, right from the beginning of clinical presentation.
Keywords: lower-grade gliomas, malignant progression-free survival, overall survival, progression-free survival, supratotal resection
Key Point.
1. Supratotal resection prolongs PFS and OS and reduces malignant transformation in LGGs, regardless of molecular subtypes and tumor grade.
Importance of the Study.
Supratotal resection is advocated in LGGs based on theoretical advantages, but available data on the oncological impact are limited or missing. For the first time, in a large series of LGGs we assessed the association of supratotal resection with oncological outcomes, using a univariate, multivariate, and propensity score analysis. In molecularly defined LGGs, supratotal resection was associated with a decreased rate of progression and a prolonged PFS, regardless of molecular subtypes and tumor grade. Similarly, supratotal resection was associated with a reduced rate of malignant transformation and a longer OS. Our data support the use of extensive resection outside of MRI-visible margins in LGGs, regardless of molecular subtypes and tumor grade, right from the beginning of clinical presentation.
Low-grade gliomas are intrinsic brain tumors, often accompanied by seizures in young patients, appearing on conventional magnetic resonance as highly infiltrative non-enhancing masses, well visible in fluid attenuated inversion recovery (FLAIR) images, involving one to multiple lobes, and occasionally presenting small enhancing nodules inside the tumor mass.1,2 More than 90% of these tumors have isocitrate dehydrogenase 1 (IDH1) mutation and share a common biological behavior and similar clinical prognosis. They include diffuse low-grade (grade II) and intermediate-grade (grade III) gliomas, and have been recently defined as lower-grade gliomas (LGGs), a term that has replaced low grade in the current clinical practice. Although there are no randomized studies providing definitive evidence for a strict relationship between extent of resection (EOR) and survival in LGGs, “an imaging (FLAIR) complete resection of a suspected LGG is the currently favored approach, when feasible” 3–8; massive resection improves survival also after correcting for molecular status.7,8 However, the goal of complete resection must be balanced against potential damage, due to the strong infiltrative behavior of LGGs and their frequent involvement of highly functional cortical and subcortical regions. To achieve a maximal safe resection, the use of brain mapping techniques and a “functional approach” are beneficial, combining an increase in the percentage of total/near total resection and a decrease in the incidence of postoperative permanent deficits.2,9–11 In the functional approach, resection is performed until functional boundaries are encountered,12,13 which can be found within, at, or outside FLAIR-visible tumor borders.14 In the first case only a partial, or subtotal, removal is possible. In the second, a gross total resection is achieved. But, when functional boundaries are found outside the tumor area, and part of apparently normal brain parenchyma is removed along with the tumor itself, a supratotal resection is achieved.14,15 The rationale for a supratotal resection stands on the finding that LGGs infiltrate the parenchyma far beyond conventional MR abnormalities, suggesting that their relapse after surgery is due to undetected glioma cells growing beyond MRI-defined abnormalities.16 However, to date, the reports on resection going beyond the imaging targets as a strategy for surgical treatment of LGGs are very few, with limited verification of functional risk or survival benefits. Feasibility in clinical routine and global safety by the functional approach has been recently reported by our group, with very limited morbidity and comparable neuropsychological and quality of life impact to total resection.14 Although limited encouraging studies suggest an effect of supratotal resection on tumor growth control and histological transformation,17 the oncological impact of this approach on a large scale is still unknown and many important questions are still open. Its impact on rate and time of recurrence and of malignant transformation needs to be investigated in a larger sample of patients, to possibly reduce selection bias; the influence exerted by the different histomolecular subtypes, tumor grades, or previous treatments is still not known, nor is the effect of the association with adjuvant treatments.
In this study, all patients with a presumptive radiological diagnosis of LGGs consecutively admitted to our care in a 5-year period were studied. In all of them, surgery was performed according to the functional boundaries, aiming at achieving a supratotal resection whenever possible. These patients were followed for a long time (median, 6.8 y; interquartile range [IQR], 5–8) with the primary aim of assessing whether extending resection outside the MR-visible tumor border was associated with PFS; moreover, the association of supratotal resection with the occurrence and time of malignant transformation along with OS was investigated as well.
Materials and Methods
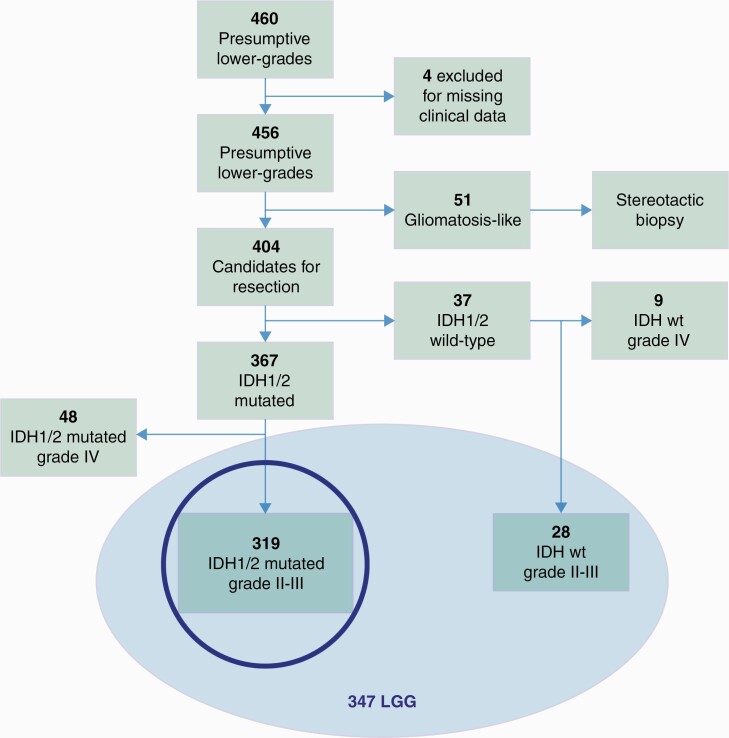
We included all patients admitted from May 2009 to April 2014 harboring a radiological diagnosis of presumptive LGGs, and candidates for resective surgery. According to the radiological definition of lower-grade gliomas, we included intrinsic brain tumors appearing on conventional magnetic resonance as highly infiltrative non-enhancing masses, well visible in FLAIR images, involving one to multiple lobes, occasionally presenting small enhancing nodules inside the tumor mass. Patients harboring a FLAIR lesion involving multiple lobes, possibly of both hemispheres (gliomatosis-like) were excluded (Fig. 1). Patients gave informed consent to the procedures which followed the Declaration of Helsinki for human experiments and were approved by Ethical Committee 1299. All patients included in the study have full preoperative, postoperative, and at follow-up clinical, imaging, and tumor data. Patients without full materials were excluded.
Fig. 1.
Data flow diagram for the retrospective cohort; the study mainly focuses on IDH-mutated LGGs (highlighted with a circle).
MR protocol includes volumetric FLAIR and T1, pre and post gadolinium weighted images, performed preoperatively, postoperatively (48 h), 1 month, and every 4 months during the first 4 years, and every 6 months afterward (eMethods 1).
Resection was accomplished according to functional boundaries aiming at achieving a supratotal resection whenever possible, without any patient or tumor (size/location/extension) a priori selection. Brain Mapping and Monitoring techniques,12,14,18 in asleep-awake-asleep anesthesia, were used to locate functional boundaries. Resection continued till functional boundaries were reached, independent of where they located to MR-visible tumor borders.
Patients were submitted to clinical, neuropsychological, and MR imaging follow-up every 4 months during the first 4 years, and every 6 months afterward. Clinical, neuropsychological, and imaging data for each follow-up time point are available for each patient.
EOR was calculated on postoperative (2 mo) volumetric FLAIR and classified on the basis of residual tumor volume (RTV) as total (RTV = 0), subtotal (0 < RTV ≤ 5 mL), and partial (RTV > 5 mL). Tumor volumes were computed onto volumetric preoperative FLAIR with manual segmentation using iPlanCranial software (BrainLab) by 5 blinded investigators (M.R., M.R., L.G., T.S., M.C.N.). A supratotal resection was defined as the complete removal of any signal abnormalities, with the volume of the postoperative cavity larger than preoperative tumor volume.18 The degree of supratotal resection is the ratio between the volume of the surgical cavity and of the tumor (as a percentage).14
PFS was calculated from the date of first surgery till the occurrence of true progression. Progression was defined (according to Response Assessment in Neuro-Oncology criteria) by either of the following: (i) a 25% increase of the T2/ FLAIR non-enhancing lesion on stable or increasing doses of steroids or (ii) development of new lesions or the appearance of enhancement (suggesting radiological evidence of malignant transformation), that require the start of a new treatment as judged by the treating physician.7,19,20 Malignant (M)PFS was defined as the time between initial surgery and demonstration of malignant progression.6 Malignant progression was based on biopsy confirmation of a high(er) grade (in case of surgery) or new or enhanced contrast enhancement on imaging with a multidisciplinary (board) consensus opinion that the findings represented progression to a higher grade.6 No deaths were observed without progression or MPFS. OS was calculated from the date of first surgery until death.
Among factors possibly associated with PFS, MPFS, and OS, the following were investigated: (i) factors relating to the patient (age, sex, clinical history duration, incidental discovery, epilepsy history, handedness, dominance); (ii) factors related to the tumor that were deductible by evaluating preoperative conventional MR (location, volume, side, involvement of functional sites at cortical or subcortical level; involvement of corpus callosum); (iii) immediate and permanent postoperative deficits; (iv) previous treatments (biopsy or surgery, preoperative chemotherapy); (v) integrated molecular/histological diagnosis, histological grade; (vi) EOR, categorized as partial, subtotal, total, supratotal; (vii) postsurgical management: wait and see, chemotherapy, radiotherapy (for definition, see eMethods 1).
The type of postsurgical management was evaluated by a multidisciplinary board: adjuvant treatments were reserved to grade II‒III tumors which underwent to subtotal or partial resection; observation to grade II tumors underwent to total or supratotal resection; grade III tumors underwent to supratotal resection were submitted to observation or to adjuvant treatment depending on the histomolecular profile and preoperative tumor volume; generally only large size tumors (with limited degree of supratotal resection) were submitted to postsurgical treatment. Chemotherapy included temozolomide (standard regimen) or less frequently procarbazine/lomustine/vincristine; radiotherapy included 54 Gy in 2 Gy per fractions (eMethods 1, Supplementary Table 5).
IDH1 status was determined by immunohistochemistry with mouse monoclonal anti-IDH1 p.R132H (DIA-H09, Dianova); in case of negative expression by mutational analysis (sequencing for alternate mutations in IDH1 or IDH2). Codeletion was determined by fluorescence in situ hybridization; alpha thalassemia/mental retardation syndrome X-linked loss, p53 mutation, and Ki67 by immunohistochemistry. Tumors were reviewed to confirm diagnostic classification and grading based on World Health Organization (WHO) 2016 criteria. Grade III tumors include all tumors with any sign of anaplasia, inclusive of those with small anaplastic foci embedded within a specimen of grade II tumor. According to the WHO 2016 classification, patients included in the analysis were IDH-mutated and IDH-wildtype grades II‒III tumors2 (Fig. 1); however, most of the analyses were performed on IDH-mutated LGGs, the “typical LGGs.”
Summary of Statistics
Continuous variables are summarized as median with IQR, and differences among groups were assessed by ANOVA. Categorical and ordinal variables are presented as frequencies, and percentages and differences were assessed with Fisher’s exact test. P-value was considered significant when <0.05. The association between the considered variables with time to event outcome was analyzed with univariate and multivariable Cox regression with Firth’s correction, accounting for possible problems of monotone likelihood, when needed. Significant variables in univariate analysis were included in the multivariable model. The proportionality of hazards was checked by inspection of the Schoenfeld residuals. Kaplan‒Meier was used for the analysis of PFS, MPFS, and OS as all deaths occurred after progression. In IDH-mutated LGGs, the comparison between supratotal and total resection in terms of PFS was studied with a propensity score matched analysis. For propensity score calculation, a logistic regression was used with EOR as dependent variable as a function of age, sex, duration of clinical history, reason for diagnosis, seizures, integrated diagnosis, previous treatment, location, side, volume, dominance, corpus callosum, grade, and immediate deficits. A nearest neighbor matching with 0.2 caliper width was used. A multivariable clustered Cox regression was used to account for matched data. Variable importance (VIMP) was evaluated using 2 measures computed by fitting a random forest: VIMP and minimal depth. Cutoffs used for VIMP correspond to the average level of the calculated VIMP measures over all the trees. Analysis was conducted using R software (for details, see eMethods 2).
Results
Patients
In the study period, 460 patients harboring a FLAIR lesion were admitted. Four patients were excluded because of partial follow-up data; 51 were submitted to stereotactic biopsy only because of tumor involving multiple lobes, possibly of both hemispheres; 404 presumptive LGGs patients (249 males, 155 females; Supplementary Table 1) were submitted to resection; 347 were LGGs, 319 with IDH mutation, 28 without (Fig. 1). Clinical, imaging, and histomolecular features of both series are detailed in Table 1A (IDH mutated) and Supplementary Table 2 (IDH wildtype). They recapitulate all the clinical and imaging features of LGGs. All patients and tumors were treated with a standard functional approach, aimed at achieving a supratotal resection, with no patient or tumor (location/size/extension) “a priori” selection. Of the patients, 290 had a first and 57 a second surgery, which followed a previous treatment. Previous treatments were partial resection followed by observation (32 patients) or chemotherapy (10 patients), stereotactic biopsy followed by observation (9 patients) or chemotherapy (6 patients). Median time interval between previous diagnosis and second surgery was 13.4 months (4–38 mo). In the IDH-mutated LGGs, a partial resection was achieved in 10 patients (3%), a subtotal in 74 (23%), a total in 125 (39%), and a supratotal in 110 (35%) (for clinical, imaging, histomolecular, and postsurgical management details of EOR classes, see Supplementary Table 3A). In the wildtype group, a subtotal was obtained in 3 (10.7%), a total in 13 (46.4%), and a supratotal in 12 (42.8%) (for clinical, imaging, histomolecular, and postsurgical management details of EOR classes, see Supplementary Table 3B).
Table 1A.
Clinical, imaging, and histomolecular features of the 319 lower grade gliomas (IDH-mutated grades II and III) patients included in the study
| Clinical Features | |||
|---|---|---|---|
| Sex | Seizures | ||
| Male | 195 (61.1%) | Focal | 72/306 (23.5%) |
| Female | 124 (38.9%) | Generalized | 234/306 (76.4%) |
| Age | Handedness | ||
| Mean (SD) | 38.9(11.8) | Left | 316 (99.1%) |
| Median [Min Max] | 38 [18–75] | Right | 3 (0.9%) |
| Duration of Clinical History | Previous Treatments* | ||
| <6 months | 90 (28.2%) | No | 263 (82.4%) |
| >6 months | 229 (71.8%) | Yes | 56 (17.6%) |
| Reason For diagnosis | Dominance | ||
| Incidental | 13 (4.1%) | Left | 185 (58.0%) |
| Symptoms | 306 (95.9%) | Right | 134 (42.0%) |
| Preoperative imaging Features | |||
| Location | Side | ||
| Frontal | 140 (43.9%) | Left | 195 (61.1%) |
| Temporal | 56 (17.6%) | Right | 124 (38.9%) |
| Parietal | 45 (14.1%) | ||
| Insular | 78 (24.5%) | ||
| Involvement of eloquent sites | Involvement of CC | ||
| Close | 299 (93.7%) | Yes | 84 (26.3%) |
| Distant | 20 (6.3%) | No | 235 (73.7%) |
| Volume (cm3) | |||
| Mean (SD) | 65.2 (61) | ||
| Median [Min Max] | 46 [0.39–386] | ||
| Histomolecular and Tumor Grade Features | |||
| Integrated Diagnosis | Tumor Grade | ||
| Astrocytoma | 130 (40.8%) | II | 248 (77.7%) |
| Oligodendroglioma | 189 (59.2%) | III | 71 (22.3%) |
| Post operative Neurological Conditions | |||
| Immediate Deficits | Permanent Deficits | ||
| No | 26 (8.2%) | No | 313 (98.1%) |
| Yes | 293 (91.8%) | Yes | 6 (1.9%) |
Dominance: indicates if the tumor was located in the dominant hemisphere for language; involvement of eloquent sites considers the distance between the tumor margins and the location of cortical or subcortical sites anatomically related to main neurological functions (motor, language, visual, visuospatial) as visible in preoperative volumetric FLAIR; involvement of CC = involvement of corpus callosum as detectable in preoperative volumetric FLAIR; previous treatments includes partial resection followed by observation (31 cases) or chemotherapy (10), stereotactic biopsy followed by observation (9) or chemotherapy (6). Immediate deficits (at 5 days postsurgery); permanent deficits (at 1 month). For details see suppl.Methods.1.
Progression-Free Survival and Associated Factors
As of February 2020, median follow-up was 6.8 years (IQR: 5–8 y); out of 404 patients included, 257 (63.6%) progressed; median PFS was 4 years (IQR: 3.7–4.7). Analyzing only the 319 IDH-mutated LGGs (“typical LGGs”), 190 (59.6%) progressed, median PFS: 4.7 years (95% CI: 4–5.3 y). All deaths were observed after progression. Clinical and imaging features of progressions are in Table 1B. Most progression occurred in cases with previous treatments and were located within the previous surgical cavity. We present here data on IDH-mutated tumors; data on IDH-wildtype are in Supplementary File R1 and Supplementary Figure 11.
Table 1B.
Clinical and imaging features of progression in the 319 IDH-mutant LGGs
| Total Population N = 319 | Progression N = 190 | |
|---|---|---|
| Gender | ||
| Male | 195 | 119 (61.0%) |
| Female | 124 | 71 (57.0%) |
| Age (mean; median) | ||
| Previous Treatment | 39; 38 | 40.2; 39 |
| Yes | 56 | 53 (94.7%) |
| No | 263 | 137 (53.6%) |
| Location | ||
| Frontal | 140 | 70 (50.0%) |
| Temporal | 56 | 29 (51.7%) |
| Parietal | 45 | 34 (75.6%) |
| Insular | 78 | 57 (73.1%) |
| Side | ||
| Left | 195 | 127 (65.1%)6 |
| Right | 124 | 63 (50.8%) |
| Involvement of eloquent sites | ||
| Close | 299 | 184 (61.5%) |
| Distant | 20 | 6 (30.0%) |
| Astro II | 89 | 61 (68.5%) |
| Oligo II | 159 | 77 (48.4%) |
| Astro III | 41 | 28 (68.3%) |
| Oligo III | 30 | 24 (80.0%) |
| Histolog. Confirmation | 74 (38.9%) | |
| Site of Progression | ||
| Cavity | 182 (95.8%) | |
| Distant | 8 (4.2%) |
Involvement of CC = involvement of corpus callosum.. Astro = astrocytoma; Oligo = oligodendroglioma; Histolog. Confirmation = indicates the cases in which transformation was confirmed by histological analysis subsequent to second surgery. Site of progression indicates the location of the progression in respect to the preexisting surgical cavity; Cavity = when the progression is within or at the border of the cavity; Distant = when the progression is far away from the preexisting resection cavity. We did not observe any leptomeningeal spread.
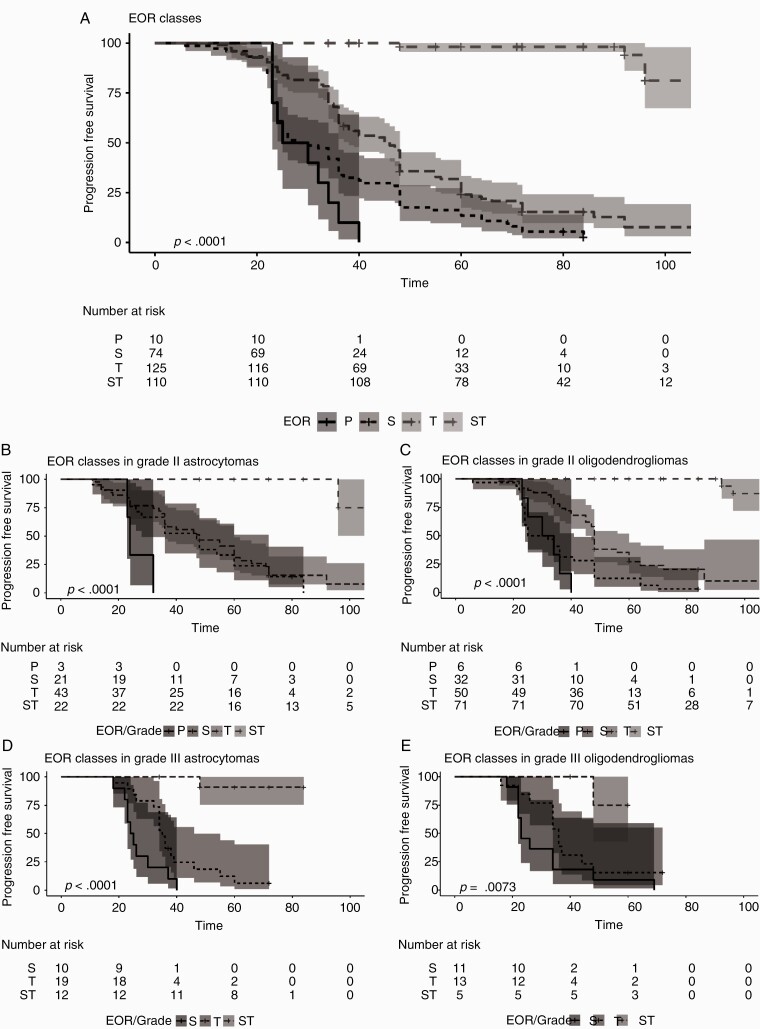
In the 319 IDH-mutated LGGs, at univariate analysis (Supplementary Figure 1), among preoperative clinical features, only duration of clinical history >6 months, reasons for diagnosis, dominance, and previous treatments associated with PFS, while sex, age at diagnosis, seizure history, and handedness were not; among imaging features, tumor location, side, vicinity to eloquent cortical/subcortical sites, corpus callosum involvement, and tumor volume >44 mL were associated with PFS (Supplementary Figure 2). There was no association with occurrence of immediate deficits while permanent postoperative deficits were associated (condition related to 6 patients only) (Supplementary Figure 3). Among histomolecular factors, integrated diagnosis (oligodendroglioma better than astrocytoma), tumor grade as a whole (grade II better than grade III), and molecular subtypes were associated with PFS (Supplementary Figure 4). In the IDH-mutated LGGs, at univariate analysis, EOR was strongly associated with PFS. All patients (100%) in the partial group progressed, 71 (95.9%) in the subtotal group, 103 (82.4%) in the total group, and only 6 (5.4%) in the supratotal (Supplementary Table 4). Median PFS was 27.5 months (95% CI: 23 NA) in partial group, 29 months (95% CI: 25–36) in subtotal, 46 months (95% CI: 38–48) in total, while at 92 months, PFS in the supratotal group was 94% (95% CI: 86.2% 100%) (Fig. 2A). The association was independent of molecular subtypes and tumor grade (Fig. 2B‒E) (Supplementary Figure 5). As for adjuvant treatment, about 90% of the patients in the partial or subtotal groups were submitted to adjuvant treatments. In total and supratotal groups, 68% and 86.4% of the patients were not submitted to any therapy, respectively. In the total group, 93.8% of patients with a grade III received chemotherapy or radiotherapy; 41.2% of patients with a grade III in the supratotal group were not submitted to any treatment (Supplementary Tables 3A, 5). When all variables (clinical, imaging, treatments) were considered together by a random forest analysis, PFS was strongly associated with treatments, particularly EOR and radiotherapy (Fig. 3A).
Fig. 2.
Kaplan Meier curves for PFS for EOR classes, and in molecular and tumor grade defined subtypes. (A) Kaplan‒Meier curves and number at risk for PFS and EOR classes. The analysis includes all 319 IDH-mutated LGGs. Time is expressed as months. Patients were categorized according to EOR classes. In Partial (N = 10) (black line) median PFS was 27.5 (95% CI: 23 NA) months (univariable HR, 1[reference]); in Subtotal (N = 74) (black small dotted line) was 29(95% CI: 29–25) months (univariable HR, 0.51 [95% CI: 0.26–1]; P = 0.05), in Total (N = 125) (black medium dotted line) was 46 (95% CI: 38–48) months (univariable HR, 0.27 [95% CI: 0.14–0.54]; P < 0.001); in supratotal (N = 110) (black large dotted lined) at 92 months 94% of patients had no recurrences (univariable HR, 0.01 [95% CI: 0.00–0.02]; P < 0.001). Shadings are the 95% confidence intervals. (B) Kaplan‒Meier curves and number at risk for PFS and EOR classes in IDH-mutated grade II astrocytomas. Only grade II IDH mutated astrocytomas were included (N = 89). Time is expressed as months. Patients were categorized according to EOR classes. In Partial (N = 3) (black line) median PFS was 24 (95% CI: 23 NA) months (univariable HR, 1[reference]); in Subtotal (N = 21) (black small dotted line) was 47 (95% CI: 28–72) months (univariable HR, 0.26 [95% CI: 0.07–0.94]; P = 0.04), in Total (N = 43) (black medium dotted line) was 48 (95% CI: 36–60) months (univariable HR, 0.21 [95% CI: 0.06–0.73]; P = 0.01); in supratotal (N = 22) (black large dotted lined) at 80 months 100% of patients had no recurrences months (univariable HR, 0.01 [95% CI: 0.00–0.06]; P < 0.001). Shadings are the 95% confidence intervals. (C) Kaplan‒Meier curves and number at risk for PFS and EOR classes in IDH-mutated grade II oligodendrogliomas. Only grade II IDH-mutated oligodendrogliomas were included (N = 159). Time is expressed as months. Patients were categorized according to EOR classes. In Partial (N = 6) (black line) median PFS was 32 (95% CI: 25 NA) months (univariable HR, 1[reference]); in Subtotal (N = 32) (black small dotted line) was 28.5 (95% CI: 24–41) months (univariable HR, 0.60 [95% CI: 0.25–1.48]; P = 0.27), in Total (N = 50) (black medium dotted line) was 48 (95% CI: 45–60) months (univariable HR, 0.24 [95% CI: 0.10–0.60];P = 0.002); in supratotal (N = 71) (black large dotted lined) at 80 months 100% of patients had no recurrence, at 92 months 93.8% had no recurrence months (univariable HR, 0.004 [95% CI: 0.00–0.02]; P < 0.001). Shadings are the 95% confidence intervals. (D) Kaplan‒Meier curves and number at risk for PFS and EOR classes in IDH mutated grade III astrocytomas. Only grade III IDH mutated astrocytomas were included (N = 41). Time is expressed as months. Patients were categorized according to EOR classes. In Subtotal (N = 10) (black line) was 24.5 (95% CI: 23 NA) months (univariable HR, 1 [reference]), in Total (N = 19) (black small dotted line) was 35(95% CI: 34–46) months (univariable HR, 0.35 [95% CI: 0.15–0.81]; P = 0.01); in supratotal (N = 12) (black large dotted lined) at 48 months 90% of patients had no recurrence (univariable HR, 0.01 [95% CI: 0.00–0.11]; P < 0.001). Shadings are the 95% confidence intervals. (E) Kaplan Meier curves and number at risk for PFS and EOR classes in IDH mutated grade III oligodendrogliomas. Only grade III IDH mutated oligodendrogliomas were included (N = 30). Time is expressed as months. Patients were categorized according to EOR classes. In Partial (1 case, not shown in figure and in the analysis) PFS was 23 months, in Subtotal (N = 11) (black dotted line) median PFS was 23 (95% CI: 22 NA) months (univariable HR, 1[reference]), in Total (N = 13) (black small dotted line) was 36 (95% CI: 34 NA) months (univariable HR, 0.53 [95% CI: 0.23–1.25]; P = 0.15); in supratotal (N = 5) (black large dotted lined) at 48 months 75% of patients had no recurrence (univariable HR, 0.07[95% CI: 0.01–0.58]; P = 0.01). Shadings are the 95% confidence intervals.
Fig. 3.
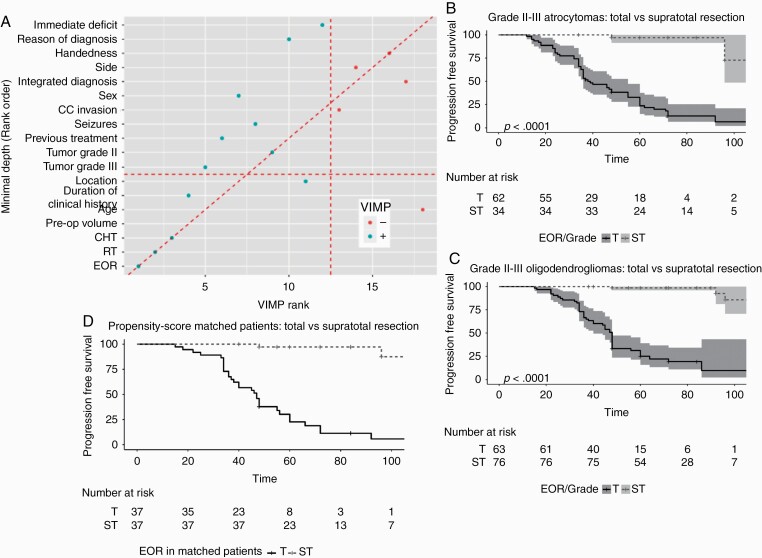
(A) Variable importance for PFS using Random.Forest in the IDH mutant LGGs. All the clinical, imaging, histomolecular and treatments (EOR, radiotherapy, chemotherapy) were considered together. VIMP and Minimal Depth are two criteria proposed in the context of random forests algorithms to evaluate variable importance in explaining the PFS. The variables on the diagonal red line are those ranked equally by the two methods. The vertical line divides variables with positive VIMP (left) from those with negative VIMP (right; unimportant). The horizontal line indicates the minimal depth threshold: important variables are below the line. PFS was mainly associated to treatments, particularly EOR and radiotherapy. The VIMP rank is reported in x-axis. The Minimal Depth (Rank Order) is in y-axis. (B) Kaplan‒Meier curve and number at risk for IDH mutated grade II III astrocytomas underwent to total vs supratotal resection (N = 96). Time is expressed as months. In Total (N = 62) (black line) was 38(95% CI: 36–55) months (univariable HR, 1 [reference]); in Supratotal (N = 34) (black dotted line) at 48 and 96 months, 97% and 72.7% respectively, of patients had no recurrence (univariable HR, 0.05 [95% CI: 0.02–0.16]; P < 0.001). Shading is the 5–95% range across distribution. (C) Kaplan‒Meier curve and number at risk for IDH-mutated grades II–III oligodendrogliomas underwent to total vs supratotal resection (N = 139). Time is expressed as months. In Total (N = 63) (black line) was 48 (95% CI: 40–48) months (univariable HR, 1 [reference]); in Supratotal (N = 76) (black dotted lined) at 48 and 96 months, 98.6% and 85.6% respectively, of patients had no recurrence (univariable HR, 0.02 [95% CI: 0.01–0.07]; P < 0.001). Shadings are the 95% confidence intervals. (D) Kaplan‒Meier curve and number at risk for the 74 IDH mutant LGGs patients of the propensity score matched group. Time is expressed as months. In Total (N = 37) (black line) was 55 (95% CI: 40–72) months (univariable HR, 1 [reference]); in Supratotal (N = 37) (black dotted line) at 96 months 87.5% of patients had no recurrence (univariable HR, 0.01 [95% CI: 0.00–0.11]; P < 0.001).
Progression-Free Survival and Supratotal Resection
To investigate whether extending resection beyond the MR-visible margins was associated with PFS we further restricted the analysis to IDH-mutated LGG patients belonging to the EOR classes total and supratotal. These 2 groups were different for some preoperative clinical and imaging factors, and integrated diagnosis. The adjusted Cox regression considered EOR (total, supratotal), integrated diagnosis (astrocytoma, oligodendroglioma), grade at first diagnosis (III vs II), and the factors that resulted significant in the univariate analysis, namely duration of clinical history, previous treatments, location, side, volume, dominance, corpus callosum. Other variables were not considered, as they involved a too restricted number of patients. EOR and grade III at first diagnosis resulted as associated with PFS also after adjusting for the other variables (Table 1C). PFS was significantly associated with EOR in each molecular subgroup. Specifically, PFS was significantly longer in astrocytoma or oligodendroglioma patients who received a supratotal resection in comparison to those who had a total resection only (Fig. 3B, C), and the association was observed in both grade II or III tumors, for each molecular subgroup (Supplementary Figure 6). In each resection group, PFS didn’t differ according to molecular subgroups (Supplementary Figure 7A‒F).
Table 1C.
Adjusted Cox regression analysis for PFS in Total and Supratotal groups. The adjusted analysis considered Extent of Resection (Supratotal ST vs Total T), integrated diagnosis (astrocytoma Astro vs oligodendroglioma Oligo) and grade at first diagnosis (III vs II). The model was adjusted for the factors that resulted significant in the univariate analysis, namely duration of clinical history, previous treatments, location, side,volume, Dominance, corpus callosum. Other variables were not considered as they involved a too restricted number of patients, namely reason for diagnosis, eloquent and permanent deficits. EOR and grade III at first diagnosis resulted significantly associated with PFS also after adjusting for the other variables.
| HR | 95% CI | P value | |
|---|---|---|---|
| ST vs T | 0.04 | 0.02–0.09 | <0.01 |
| Grade III vs Grade II | 1.94 | 1.19–3.14 | 0.01 |
| Integr.diagn. oligo vs astro | 0.74 | 0.48–1.14 | 0.18 |
| Duration clinical history >6 vs < 6 months | 0.66 | 0.42–1.03 | 0.07 |
| Previous Treats. | 1.93 | 1.19–3.13 | 0.01 |
| Location T. vs F. | 0.74 | 0.40–1.38 | 0.34 |
| Location P. vs F. | 1.07 | 0.61–1.86 | 0.82 |
| Location Ins. vs F | 1.05 | 0.62–1.79 | 0.84 |
| Righ vs Left | 0.70 | 0.38–1.31 | 0.27 |
| Volume mL | 1.00 | 0.99–1.00 | 0.30 |
| Dominant no vs yes | 0.73 | 0.38–1.38 | 0.33 |
| CC no vs yes | 0.77 | 0.49–1.22 | 0.27 |
ST, supratotal; T, Total; Integr.Diagn., integrated diagnosis; Oligo, oligodendroglioma; Astro, astrocytoma; Previous Treats., previous treatments; Location: T, temporal, F, frontal, P, parietal, Ins., insular. Dominant, dominance; CC, corpus callosum involvement.
Previous treatment was also significantly associated with PFS (Supplementary Figure 7G, H). To further explore this, the adjusted Cox regression analysis was repeated in patients who were submitted to primary surgery (no previous treatment) alone (Supplementary Table 6). Again, PFS was strongly associated with EOR and grade III, independent of molecular factors. In patients who underwent a previous treatment, the association was still evident, again independent of molecular factors and tumor grade.
The comparison of supratotal vs total resection on PFS was further investigated by a propensity score matched analysis. As in fact, patients in the total and supratotal groups have different distributions for many risk factors, and by using a propensity score matched analysis, 2 groups with similar distributions were created (Supplementary Table 7). In the 74 matched IDH-mutated patients, the standardized differences were reduced without any significant difference between groups: >90% of patients have a clinical history >6 months, with seizures, left handedness, 95% are grade II, median tumor volume of 32.1 mL (range: 2.4–240) (supratotal) and 33.9 mL (range: 2.73–259) (total). Median follow-up was 6 years (IQR, 4.5–8), median PFS 8 years (IQR, 3.8–8). According to Cox regression with robust standard errors, the HR was 0.03 (P < 0.001). The result was maintained after adjusting for age (HR, 0.03; 95% CI: 0.01–0.13) (Fig. 3D).
Supratotal Resection and Postsurgical Management
To investigate the association between supratotal resection and the access to further treatments, we looked at differences in the number and type of post surgical treatments in IDH-mutated grade III tumor subgroup (Supplementary Table 5). Globally considered, EOR associated with further treatment. Of patients with a grade III tumor in the supratotal group, 41.2% were not submitted to any treatment, in comparison to 6.2% in the total group. In the latter, 75% of patients were submitted to radiotherapy and chemotherapy, and 18.8% to chemotherapy only; in the supratotal, only 17.6% of patients received radiotherapy and chemotherapy, and 41.2% chemotherapy only. According to molecular subtypes, all patients with a grade III astrocytoma in the total group received chemotherapy, 21.1% alone, whereas 78.9% radiotherapy followed by chemotherapy; in the supratotal group, 41.7% of grade III astrocytoma received no treatment, 41.7% chemotherapy alone, and 16.7% radiotherapy followed by chemotherapy. Out of patients with a grade III oligodendroglioma in the total resection group, 15.4% refused treatment, 84% had chemotherapy (15.4% alone), and 69.2% combined radiotherapy and chemotherapy; in grade III oligodendrogliomas in the supratotal group, 40% had no treatment, 40% chemotherapy only, and 20% radiotherapy and chemotherapy.
Malignant Transformation and Supratotal Resection
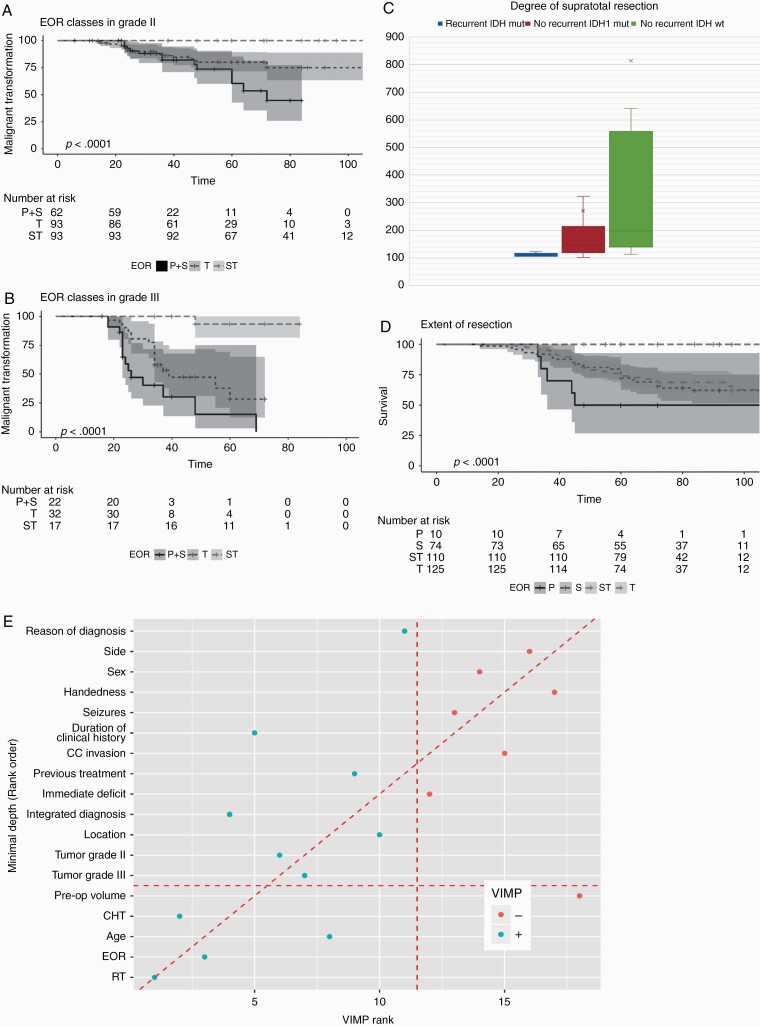
Radiological and/or histological findings of malignant transformation was documented in 63 (33.2%) out of 190 patients who progressed in the IDH mutated LGGs group (Table 2A). To investigate the association between EOR and MPFS, we performed a multivariate analysis in which MPFS was analyzed considering the effect of preoperative clinical, imaging and molecular factors. EOR, grade III at first diagnosis, integrated diagnosis (astrocytoma), and previous treatments resulted significantly associated with MPFS (Table 2B); EOR was strongly associated in both grade II and grade III tumors (Fig. 4A, B). While malignant transformation occurred in 34.6% of IDH-mutated LGGs in the subtotal/partial resection groups and in 33% of those in the total group, no events were recorded in grade II tumors that underwent to supratotal resection, and only 1 in grade III tumors (Fig. 4A, B). The association was evident in astrocytomas (independent of grade) while only in grade II oligodendrogliomas was significant (Supplementary Figure 8A‒D). While in the total resection groups, MPFS associated with tumor grade in those who underwent to supratotal was independent (Supplementary Figure 8E, F).
Table 2A.
IDH-mutated LGGs: MPFS features according to EOR classes
| Partial | Subtotal | Total | Supratotal | |
|---|---|---|---|---|
| N = 10 | N = 74 | N = 125 | N = 110 | |
| Progression | 10/10 (100.0%) | 71/74 (95.9%) | 103/125 (82.4%) | 6/110 (5.5%) |
| No malignant Transformation | 4/10 (40.0%) | 49/71 (69.1%) | 69/103 (67.0%) | 5/6 (83.4%) |
| Malignant Transformation | 6/10 (60.0%) | 22/71 (30.9%) | 34/103 (33.0%) | 1/6 (16.6%) |
| Gd Enhancing | 6/6 (60.0%) | 18/22 (25.3%) | 32/34 (94.1%) | 1/1 (100.0%) |
| Histological confirmation | 4/6 (40.0%) | 18/22 (25.3%) | 26/34 (76.4%) | 1/1 (100.0%) |
| Both | 4/6 (66.7%) | 14/22 (63.6%) | 24/34 (70.6%) | 1/1 (100%) |
The table reports for each EOR class, the number of progression, the number of progression without and with malignant transformation, the number of progression with gadolinium (GD) enhancement (radiological sign of malignant transformation), and the cases in which the transformation was confirmed by histological analysis subsequent to second surgery; the number of cases with Gd enhancement followed by histological confirmation is indicated as both.
Table 2B.
IDH-mutated LGGs: Cox regression analysis for the association of MPFS with preoperative clinical, imaging and molecular factors: EOR, grade III at first diagnosis, integrated diagnosis (astrocytoma), and previous treatment were significantly associated to MPFS
| HR | 95% CI | Pr(>|z|) | |
|---|---|---|---|
| EOR S vs P | 0.53 | 0.12–2.41 | 0.41 |
| EOR ST vs P | 0.01 | 0.00–0.09 | <0.01 |
| EOR T vs P | 0.25 | 0.05–1.17 | 0.08 |
| Grade III vs Grade II | 3.89 | 2.20–6.88 | <0.01 |
| Integr.diagn. Oligo vs Astro | 0.51 | 0.29–0.92 | 0.02 |
| Duration clinical history >6 vs <6 months | 1.03 | 0.54–1.96 | 0.94 |
| Previous Treat. yes vs no | 2.58 | 1.43–4.63 | <0.01 |
| Location T vs F | 0.84 | 0.34–2.11 | 0.72 |
| Location P vs F | 1.29 | 0.62–2.69 | 0.50 |
| Location Ins. vs F | 1.43 | 0.76–2.70 | 0.26 |
| Right vs Left | 0.44 | 0.17–1.10 | 0.08 |
| Volume mL | 1.00 | 0.99–1.00 | 0.16 |
| Dominant no vs yes | 1.42 | 0.55–3.68 | 0.47 |
| CC no vs yes | 0.63 | 0.32–1.24 | 0.18 |
S, Subtotal; P, Partial; ST, supratotal; T, Total; Integr.Diagn., integrated diagnosis; Oligo, oligodendroglioma; Astro, astrocytoma; Previous Treats., previous treatments; Location: T, temporal, F, frontal, P, parietal, Ins., insular. Dominant, dominance; CC, corpus callosum involvement.
Fig. 4.
Malignant PFS in 319 IDH mutated LGGs patients, and degree of supratotal resection. (A) Kaplan‒Meier curve and number at risk for MPFS in IDH mutated grade II tumors according to EOR classes. Grade II tumors (N = 248) are inclusive of grade II astrocytomas and grade II oligodendrogliomas. Partial and Subtotal resection groups were merged for the analysis. Time is expressed as months. In Partial+Subtotal resection group (N = 62) (black line) median PFS was 72 (95% CI: 60 NA) months (univariable HR, 1 [reference]), in Total (N = 93) (black small dotted line) at 48 and 72 months, 80% and 75% of patients had no transformation (univariable HR, 0.59 [95% CI: 0.29–1.21]; P = 0.15); in supratotal (N = 93) (black large dotted lined) no events were recorded during the observation period (univariable HR, 0.01 [95% CI: 0.00–0.08]; P < 0.001). Shadings are the 95% confidence intervals. B) Kaplan‒Meier curve and number at risk for MPFS in IDH-mutated grade III tumors according to EOR classes. Grade III tumors (N = 71) are inclusive of grade III astrocytomas and grade III oligodendrogliomas. Partial and Subtotal resection groups were merged for the analysis. Time is expressed as months. In Partial+Subtotal resection group (N = 22) (black line) median PFS was 26 (95% CI: 23 NA) months (univariable HR, 1 [reference]), in Total (N = 32) (black small dotted line) was 39 (95% CI: 34 NA) months (univariable HR, 0.46 [95% CI: 0.22–0.94]; P = 0.03); in supratotal (N = 17) (black large dotted line) only one event at 48 months (grade III oligodendroglioma; the patient refused postsurgical treatments) was recorded during the observation period (univariable HR, 0.03 [95% CI: 0.00–0.21]; P = 0.001). Shadings are the 95% confidence intervals. (C) Degree of supratotal resection in patients who underwent progression (N = 6, IDH mutated) and in those who didn’t recur, in the IDH mutated group (N = 104) and in the IDH wt group (N = 12). Data are reported as mean and median. The degree of supratotal was lower in recurrent patients (mean 111.3%, median 107.41%;) than in those who didn’t recur (IDH mutated: mean 271.11%,median 148.775%; IDH wt: mean 818.20%,median 197.08%). (D) Kaplan‒Meier curve and number at risk for OS in IDH mutated tumors according to EOR classes. Time is expressed as months. In Partial resection group (N = 10) (black line) median OS was 45 (95% CI: 36 NA) months (univariable HR, 1 [reference]), in Subtotal (N = 74) (black small dotted line) and in Total (N = 125) (black large dotted line) at 45 months, 83.8% (95% CI: 0.75–0.92) and 83.2% (95% CI: 0.76–0.90) of patients were alive; in supratotal (N = 110) (black large dotted lined) no deaths were recorded during the observation period (univariable HR, 0.01 [95% CI: 0.00–0.08]; P < 0.001). Shadings are the 95% confidence intervals. (E) Variable importance for OS using Random Forest in the IDH mutated LGGs. All the clinical, imaging, histomolecular and treatments (EOR, radiotherapy, chemotherapy) were considered together. VIMP and Minimal Depth are two criteria proposed in the context of random forests algorithms to evaluate variable importance in explaining the OS. The variables on the diagonal red line are those ranked equally by the two methods. The vertical line divides variables with positive VIMP (left) from those with negative VIMP (right; unimportant). The horizontal line indicates the minimal depth threshold: important variables are below the line. OS was mainly associated to treatments, particularly radiotherapy and EOR. The VIMP rank is reported in x-axis. The Minimal Depth (Rank Order) is in y-axis.
Recurrences and Degree of Supratotal Resection
Of the 6 IDH-mutated LGG patients in the supratotal group who progressed, 4 had previous treatments (partial surgery followed by chemotherapy); most had tumors in the frontal lobe, of large volume. The mean degree of supratotal resection in all 110 cases was 262.98%, median 143%. The degree of supratotal was lower in the 6 recurrent patients (mean 111.3%, median 107.41%) than in the 104 cases who didn’t recur (mean 271.11%, median 148.775%) (Fig. 4C). The mean degree in the 12 IDH wildtype LGGs who didn’t recur was 818.20%, median 197.08% (Fig. 4C).
Overall Survival and Supratotal Resection
In the 319 IDH-mutated LGGs the median OS at 72 months (median follow-up duration) was 0.77 (95% CI: 0.72–0.83). At univariate analysis, among preoperative clinical features, only age, duration of clinical history >6 months, reasons for diagnosis and previous treatments were associated with overall survival, while for gender, seizure history, and handedness there was no evidence of association, as for occurrence of immediate and permanent deficits. Among imaging features, only tumor volume was associated. Among histomolecular factors, tumor grade as a whole (grade II better than grade III), and molecular subtypes (oligodendroglioma better than astrocytoma) were associated (Supplementary Figure 9A, B). EOR was strongly associated with OS; at 72 months, PFS in the subtotal and total group was 65.8% (95% CI: 55.5% 78%) and 68.5% (95% CI: 60.4% 78.3%) respectively, while no events were recorded in grade II III tumors underwent to supratotal resection (Fig. 4D). OS was significantly associated with EOR in each molecular subgroup (Supplementary Figure 9C, D; Supplementary Figure 10A‒D). When all the variables were considered together by random forest analysis, OS was mainly associated with treatments, radiotherapy and EOR (Fig. 4E).
Discussion
This is the first study evaluating the association of supratotal resection with PFS, MPFS, and OS in a large series of LGGs. To reduce selection bias constitutively present in each retrospective surgical study, we included in the analysis all patients harboring a radiological diagnosis of presumptive LGGs consecutively admitted in our care in the study period. The population studied was large and representative in term of clinical and imaging features of LGGs; only patients with a diffuse large tumor involving multiple lobes and both hemisphere were excluded. This reduced the influence of tumor size, location and delineation on decision to operate and resectability.3,7 Most patients were at first diagnosis; all patients were submitted, without any tumor patient a priori selection, to a functional surgical approach aimed to achieve a supratotal resection.12,14,18 In all, the resection was pushed until functional boundaries were encountered, independent of their location to FLAIR-visible tumor margins. 85.9% were LGGs, 90.8% had IDH-mutation, of different molecular subtypes. EOR was categorized into volumetric FLAIR images, according to RTV.7
In the IDH-mutated LGGs the “typical LGGs,” PFS was associated with very few preoperative clinical factors, such as long duration of clinical history, tumor volume, or previous treatments. EOR, with postsurgical adjuvant treatments (radiotherapy), was strongly associated. In particular, the number of patients who progressed was very limited (5.4%) in the group in whom a supratotal resection was achieved, compared with that observed in the total (82.4%), subtotal (95.9%), and partial (100%) groups. The association of EOR with PFS was maintained in molecular defined subtypes and according to tumor grade. It could be argued that these results are mitigated by the possible intrinsic patients selection, with those undergoing subtotal-partial resection carriers of unfavorable tumors, and those in supratotal with more easily resectable tumors. For this reason, we focused the analysis on the low-risk group patients, those in whom a complete resection was always achieved.3,7 In fact, it is exactly in this low-risk group of patients that the question “Why stop resection when a supratotal is feasible?” is of relevance. The benefit of extending removal outside an MR-visible border was confirmed; the supratotal group was characterized by a very low number of late progression, a figure confirmed for each molecular subtype and tumor grade. The association was then finally demonstrated in the same groups by the propensity score analysis: supratotal resection not only decreased the number of progression but also postponed progression over time. The impact of supratotal resection was higher in primary tumors than in those previously treated, confirming the benefit of performing an aggressive extensive treatment right from the beginning of clinical history or diagnosis.
In IDH-mutated LGGs, malignant transformation was associated with grade III at first diagnosis, molecular subtypes (astrocytoma), and EOR; EOR was a strong determinant. While 33.7% (average) of tumors in partial/subtotal total groups transformed, no event was recorded in grade II belonging to supratotal group, and only one was documented in grade III tumors. This suggests that resecting the apparently normal tissue at the tumor periphery by decreasing the number of possibly remaining tumor cells, strongly decreases their propensity to acquire and progress toward a malignant phenotype.7,15–17
The impact of supratotal resection was also evaluated in term of OS. Although considering the median duration of the follow-up period, OS associated with molecular subtypes (oligodendroglioma better than astrocytoma), tumor grade (grade II better than grade III), tumor volume and EOR; at random forest analysis, EOR was a strong determinant, supporting the benefit of supratotal resection.
This study was mainly focused on IDH-mutated tumors, the “typical LGGs.” The 2016 WHO classification includes within the LGGs also IDH-wildtype tumors; however, recent studies suggested that these are completely different entities, which deserve a separate management.21 Interestingly, EOR was associated with PFS also in this small group of LGGs: median PFS was 34 months in total resection group, and no events were recorded in the supratotal group.
It is of note that the achievement of a supratotal resection was associated with a different choice of postsurgical treatment. Interestingly, while most of grade III tumors in the total group received an adjuvant treatment (radio or chemo, or a combination), 42% of those belonging to the supratotal group didn’t receive any treatment. In addition, in all the other cases, chemotherapy was the treatment mainly administered.
The measurement of the degree of supratotal resection links to the question of how much the resection should be extended outside the MR-visible margin to impact on PFS. Analyzing the patients who underwent progression in the supratotal group, the degree of supratotal resection reached in these patients was lower than those achieved in those who didn’t recur14,18; in addition most of these patients had a previous treatment and large volume tumor. This suggests that particularly in the presence of previous treatment, if the degree of supratotal doesn’t exceed a certain value, the benefit in terms of PFS is minimal to total resection.
The removal of apparently normal tissue outside the MR-visible borders raises the issue of safety. We addressed these issues in a previous work, analyzing the functional impact of supratotal resection in a series of 449 LGGs operated on by the same functional surgical approach: supratotal resection was safe and characterized by a very low functional risk, as demonstrated by the detailed neurological, neuropsychological, and quality of life long-term examinations.14 Some of the patients of that analysis were included in this study. When extended to the remaining part, the analysis of neuropsychological and quality-of-life profiles didn’t show any differences in patients submitted to total or supratotal resection, further confirming the global safety of the procedure (Supplementary Table 8).
This study has limitations. It is retrospective in nature, and therefore conclusions should be confirmed in further larger independent series. In addition, these data are originated from a single neuro-oncological network in Italy, strongly supporting the policy of early maximal resection.8 Therefore, it is unclear whether these results are generalizable to all institutions given possible differences in patient referral and populations, treatment selection policy, and posttreatment management plans. Furthermore, the association between degree of supratotal and PFS opens to the assessment of number of infiltrative tumor cells or of tumor cell-free borders.15,16 This raises new questions, all outside the scope of the study.
In conclusion, this study confirms the association of EOR with PFS, MPFS, and OS in LGGs4,6,7,22–25; furthermore, this is the first study to our knowledge to explore the outcome of supratotal resection in a large cohort of LGGs, in light of clinical and molecular information. This study supports extensive resection outside of MR-visible margins, regardless of molecular subtypes, right from the beginning of clinical presentation.
Supplementary Material
Acknowledgment
We thank Dr Paola Borroni for the linguistic review of the manuscript.
Funding
The work was supported by funds 18482 from AIRC (Associazione Italiana Ricerca sul Cancro) to LB.
Conflict of interest statement. The authors have declared no conflicts of interest.
Authorship statement: Marco Rossi: conception, design, collection of the data; data analysis; manuscript writing; Lorenzo Gay: conception, design, collection of the data; data analysis; manuscript writing; Federico Ambrogi: statistical analysis, manuscript writing and reviewing. Tommaso Sciortino: data collection, analysis, manuscript writing; Marco Conti Nibali: data collection, analysis, manuscript writing; Guglielmo Puglisi: data collection, analysis, manuscript writing and reviewing; Antonella Leonetti: data collection, analysis, manuscript writing and reviewing; Manuela Caroli: data collection, manuscript reviewing. Cristina Mocellini: data collection, manuscript reviewing; Susanna Cordera: data collection, manuscript reviewing; Andrea Pace: data collection, manuscript reviewing; Matteo Simonelli: data collection, manuscript reviewing; Federico Pessina: manuscript reviewing. Piera Navarria: data collection, manuscript reviewing; Riccardo Soffietti: manuscript writing and reviewing; Roberta Rudà: data analysis, manuscript writing and reviewing; Marco Riva: data collection, data analysis, manuscript reviewing; Lorenzo Bello: conception and design of the study, data collection and analysis, manuscript writing and reviewing.
References
- 1. Reuss DE, Mamatjan Y, Schrimpf D, et al. IDH mutant diffuse and anaplastic astrocytomas have similar age at presentation and little difference in survival: a grading problem for WHO. Acta Neuropathol. 2015;129(6):867–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Buckner J, Giannini C, Eckel-Passow J, et al. Management of diffuse low-grade gliomas in adults—use of molecular diagnostics. Nat Rev Neurol. 2017;13(6):340–351. [DOI] [PubMed] [Google Scholar]
- 3. Schiff D, Van den Bent M, Vogelbaum MA, et al. Recent developments and future directions in adult lower-grade gliomas: Society for Neuro-Oncology (SNO) and European Association of Neuro-Oncology (EANO) consensus. Neuro Oncol. 2019;21(7):837–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jakola AS, Myrmel KS, Kloster R, et al. Comparison of a strategy favoring early surgical resection vs a strategy favoring watchful waiting in low-grade gliomas. JAMA. 2012;308(18):1881–1888. [DOI] [PubMed] [Google Scholar]
- 5. Jakola AS, Skjulsvik AJ, Myrmel KS, et al. Surgical resection versus watchful waiting in low-grade gliomas. Ann Oncol. 2017;28(8):1942–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith JS, Chang EF, Lamborn KR, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26(8):1338–1345. [DOI] [PubMed] [Google Scholar]
- 7. Wijnenga MMJ, French PJ, Dubbink HJ, et al. The impact of surgery in molecularly defined low-grade glioma: an integrated clinical, radiological, and molecular analysis. Neuro Oncol. 2018;20(1):103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rudà R, Angileri FF, Ius T, et al. ; SINch Neuro-Oncology Section, AINO and SIN Neuro-Oncology Section . Italian consensus and recommendations on diagnosis and treatment of low-grade gliomas. An intersociety (SINch/AINO/SIN) document. J Neurosurg Sci. 2020;64(4):313–334. [DOI] [PubMed] [Google Scholar]
- 9. Aghi MK, Nahed BV, Sloan AE, Ryken TC, Kalkanis SN, Olson JJ. The role of surgery in the management of patients with diffuse low grade glioma: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2015;125(3):503–530. [DOI] [PubMed] [Google Scholar]
- 10. Duffau H. A new philosophy in surgery for diffuse low-grade glioma (DLGG): oncological and functional outcomes. Neurochirurgie. 2013;59(1):2–8. [DOI] [PubMed] [Google Scholar]
- 11. De Witt Hamer PC, Robles SG, Zwinderman AH, Duffau H, Berger MS. Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis. J Clin Oncol. 2012;30(20):2559–2565. [DOI] [PubMed] [Google Scholar]
- 12. Bello L, Riva M, Fava E, et al. Tailoring neurophysiological strategies with clinical context enhances resection and safety and expands indications in gliomas involving motor pathways. Neuro Oncol. 2014;16(8):1110–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bello L, Fava E, Carrabba G, Papagno C, Gaini SM. Present day’s standards in microsurgery of low-grade gliomas. Adv Tech Stand Neurosurg. 2010;35:113–157. [DOI] [PubMed] [Google Scholar]
- 14. Rossi M, Ambrogi F, Gay L, et al. Is supratotal resection achievable in low-grade gliomas? Feasibility, putative factors, safety, and functional outcome. J Neurosurg 2019;17:1–14. [DOI] [PubMed] [Google Scholar]
- 15. Yordanova YN, Moritz-Gasser S, Duffau H. Awake surgery for WHO Grade II gliomas within “noneloquent” areas in the left dominant hemisphere: toward a “supratotal” resection. Clinical article. J Neurosurg. 2011;115(2):232–239. [DOI] [PubMed] [Google Scholar]
- 16. Pallud J, Varlet P, Devaux B, et al. Diffuse low-grade oligodendrogliomas extend beyond MRI-defined abnormalities. Neurology. 2010;74(21):1724–1731. [DOI] [PubMed] [Google Scholar]
- 17. Duffau H. Long-term outcomes after supratotal resection of diffuse low-grade gliomas: a consecutive series with 11-year follow-up. Acta Neurochir (Wien). 2016;158(1):51–58. [DOI] [PubMed] [Google Scholar]
- 18. Rossi M, Sani S, Nibali MC, Fornia L, Bello L, Byrne RW. Mapping in low-grade glioma surgery: low- and high-frequency stimulation. Neurosurg Clin N Am. 2019;30(1):55–63. [DOI] [PubMed] [Google Scholar]
- 19. van den Bent MJ, Wefel JS, Schiff D, et al. Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 2011;12(6):583–593. [DOI] [PubMed] [Google Scholar]
- 20. Wen PY, Chang SM, Van den Bent MJ, Vogelbaum MA, Macdonald DR, Lee EQ. Response assessment in neuro-oncology clinical trials. J Clin Oncol. 2017;35(21):2439–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weller M, Weber RG, Willscher E, et al. Molecular classification of diffuse cerebral WHO grade II/III gliomas using genome- and transcriptome-wide profiling improves stratification of prognostically distinct patient groups. Acta Neuropathol. 2015;129(5):679–693. [DOI] [PubMed] [Google Scholar]
- 22. Choi J, Kim SH, Ahn SS, et al. Extent of resection and molecular pathologic subtype are potent prognostic factors of adult WHO grade II glioma. Sci Rep 2020;10:2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sanai N, Chang S, Berger MS. Low-grade gliomas in adults. J Neurosurg. 2011;115(5):948–965. [DOI] [PubMed] [Google Scholar]
- 24. Capelle L, Fontaine D, Mandonnet E, et al. ; French Réseau d’Étude des Gliomes . Spontaneous and therapeutic prognostic factors in adult hemispheric World Health Organization Grade II gliomas: a series of 1097 cases: clinical article. J Neurosurg. 2013;118(6):1157–1168. [DOI] [PubMed] [Google Scholar]
- 25. Tom MC, Cahill DP, Buckner JC, Dietrich J, Parsons MW, Yu JS. Management for different glioma subtypes: are all low-grade gliomas created equal? Am Soc Clin Oncol Educ Book. 2019;39:133–145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.