TO THE EDITOR:
Complement has emerged as a likely driver of the immune response and end-organ damage in COVID-19. In patients with severe disease, deposition of terminal complement and microthrombosis have been observed in the lung, skin, kidney, and heart.1‐4 Recently, we demonstrated that the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein leads to amplification of the alternative pathway of complement on cell surfaces through competition with complement factor H (CFH) for binding heparan sulfate.5 Thus, in vitro, the SARS-CoV-2 spike protein can convert an inactivator surface to an activator surface on nucleated cells.
Two messenger RNA (mRNA)-based vaccines that lead to transient expression of the SARS-CoV-2 spike protein are highly efficacious in preventing severe infection.6,7 Reactions to these vaccines are generally mild; however, increased complement amplification could theoretically lead to more severe effects in diseases like paroxysmal nocturnal hemoglobinuria (PNH), where blood cells lack complement regulatory proteins.8 Here, we describe significant adverse reactions to COVID-19 vaccines in 4 patients with PNH. We also present 2 patients with PNH who received the vaccine without significant adverse effects or hemolysis.
Patient characteristics and reactions to the COVID-19 vaccines are shown in Table 1. Patients were identified based on self-report of receiving the vaccine. Patients were aged 25 to 63 years, had PNH granulocyte clones of ≥80%, and had not received transfusions in the past year. Reactions occurred from the day of administration to 5 days later and lasted 1 to 6 days. Four of 6 patients reported fever. Patients 2, 3, and 4 experienced severe hemolysis with 2 to 4 g/dL hemoglobin decrease. Patient 1 had a presumptive thrombotic manifestation. Patients 5 and 6 received both doses of the Pfizer-BioNTech BNT162b2 (Pfizer-BioNTech) COVID-19 vaccine without clinical or laboratory signs of hemolysis.
Table 1.
Characteristics of patients with PNH and COVID-19 vaccine reactions
| Patient | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Sex | Male | Male | Female | Male | Female | Female |
| Age, y | 25 | 45 | 32 | 63 | 51 | 59 |
| Age at diagnosis, y | 24 | 24 | 23 | 31 | 43 | 46 |
| Disease history | Hemoglobinuria | Aplastic anemia, transfusion dependence, microvascular small bowel thrombosis, renal failure, smooth muscle dystonia | Aplastic anemia, transfusion dependence | Transfusion dependence, hemoglobinuria, smooth muscle dystonia | Hemoglobinuria, fatigue | Hemoglobinuria, fatigue, extravascular hemolysis with transfusion dependence on C5 inhibition |
| Complement inhibitor treatment | No | Ravulizumab | Ravulizumab* | Ravulizumab | Ravulizumab | Ravulizumab Danicopan |
| PNH clone | 11% RBC, 80% granulocytes | >99% RBC, >99% granulocytes | 39% RBC, 99% granulocytes | 99% RBC, 99% granulocytes | 77% RBC, 95% granulocytes | 76% RBC, 81% granulocytes |
| Blood type | A positive | A positive | A positive | O positive | A negative | O positive |
| COVID-19 vaccine | Pfizer-BioNTech | Pfizer-BioNTech | Moderna | Moderna | Pfizer-BioNTech | Pfizer-BioNTech |
| Vaccine dose | 2 | 1 | 2 | 2 | 2 | 2 |
| Last dose ravulizumab prior to vaccination | N/A | 4 wk | 4 wk | 4 wk (dose 1) 7 wk (dose 2) |
5 wk | 3.5 wk (dose 1) 6.5 wk (dose 2) |
| Time to symptom onset postvaccine | 5 d | Same day | Same day | Same day | 1 d | N/A |
| Duration of symptoms | 5 d | 6 d | 1 d | 6 d | 1 d | N/A |
| Hemoglobin prevaccination, g/dL | 10.9 | 11.6 | 11.3 | 11.9 | 10.9 | 11.4 |
| Hemoglobin postvaccination, g/dL | 10.4 | 8.9 | 8.4 | 10.7 (dose 1) 7.1 (dose 2) |
11.1 | 11.0 (dose 1) 10.7 (dose 2) |
| LDH prevaccination, U/L | 1127 | 211 | 255 | 305 | 207 | 263 |
| LDH postvaccination, U/L | 1633 | — | — | 312 (dose 1) 342 (dose 2) |
220 | 276 (dose 1) 258 (dose 2) |
| Total bilirubin prevaccination, mg/dL | 0.8 | 1.4 | 0.7 | 2.4 | 0.6 | 1.5 |
| Total bilirubin postvaccination, mg/dL | 2.5 | — | 2.7 | 7.1 (dose 1) 3.0 (dose 2) |
— | 2.1 (dose 1) 1.2 (dose 2) |
| AST prevaccination, U/L | 70 | 18 | 19 | 26 | 17 | 32 |
| AST postvaccination, U/L | 102 | — | 25 | 24 (dose 1) 22 (dose 2) |
— | 31 (dose 1) 24 (dose 2) |
| Hospitalization/emergency department | Yes | No | Yes | No | No | No |
| Thrombotic complication | Microvascular small bowel thrombosis | No | No | No | No | No |
| Transfusion required† | No | No | 2 units RBC | No | No | No |
| Vaccine reaction | Abdominal pain, fever | Fever, myalgia, headache, fatigue, hemoglobinuria | Fever, chills | Fever, fatigue, dark urine, vomiting, diarrhea | Headache, fatigue | None |
— denotes no information is available; AST, aspartate amino transferase; LDH, lactate dehydrogenase; N/A, not applicable; RBC red blood cell.
Additionally on danicopan; however, 2 doses were missed immediately following vaccination.
None of these patients were previously transfused in the past year.
Patient 1 is a 25-year-old man diagnosed with PNH 6 months prior in the setting of hemoglobinuria and had limited disease manifestations not requiring PNH-directed therapy. Five days after receiving the second dose of the Pfizer-BioNTech vaccine, he developed abdominal pain leading to evaluation in an emergency department. Computed tomography with contrast showed peripancreatic fat stranding with normal lipase, concerning for possible small bowel microvascular thrombosis. D-dimer was elevated to 0.73 µg/mL fibrinogen-equivalent units from 0.21 µg/mL. His symptoms resolved after 5 days. He subsequently was initiated on ravulizumab, a terminal complement inhibitor.
Patient 2 is a 45-year-old man with a 20-year history of PNH. His last dose of ravulizumab was 4 weeks prior to vaccination. On the evening after his first dose of the Pfizer-BioNTech vaccine, he experienced fever, headache, myalgia, and severe fatigue, which lasted 6 days. He also developed hemoglobinuria on postvaccination days 1 and 2, associated with 2.7 g/dL hemoglobin decrease.
Patient 3 is a 32-year-old woman with a 10-year history of PNH on ravulizumab with her last dose 4 weeks prior to vaccination. She was also part of a clinical trial with danicopan, an oral complement factor D inhibitor. She missed 2 doses of danicopan immediately after her second vaccination due to concerns that the drug may interfere with vaccine potency; therefore, danicopan was not at therapeutic levels at the time of her reaction. Notably, she took danicopan throughout her first vaccination and did not experience breakthrough hemolysis. Approximately 12 hours after receiving the second dose of the Moderna mRNA-1273 COVID-19 vaccine, she had a fever (39°C) and rigors. She presented to a local hospital, where she was noted to have a 3 g/dL hemoglobin decrease and received 2 units of packed red blood cells.
Patient 4 is a 63-year-old man diagnosed with PNH 30 years ago, currently treated with ravulizumab. He experienced fatigue and darkening of his urine following his first dose of the Moderna vaccine. He had an ∼1 g/dL hemoglobin decrease on laboratory tests 3 days following his first vaccination. In addition, his total bilirubin rose to 7.1 mg/dL from baseline 2.4 mg/dL. Following his second dose, he noted fevers, diarrhea, vomiting, severe fatigue, and dark urine. Laboratory tests 1 week later, as symptoms were resolving, showed >4 g/dL hemoglobin decrease from his baseline.
Based on these observations, we sought to evaluate whether the SARS-CoV-2 spike protein directly leads to increased hemolysis. Recent data suggest the SARS-CoV-2 spike protein binds heparan sulfate on nucleated cells9 and amplifies the alternative pathway of complement through interference with the binding of CFH, an alternative pathway inhibitor.5 However, CFH primarily binds sialic acid on human erythrocytes,10 and mature erythrocytes express little heparan sulfate.11
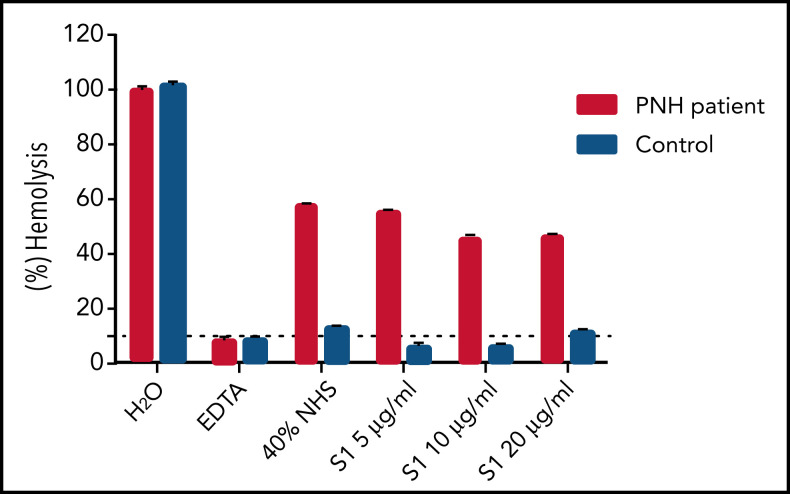
To test the effect of the SARS-CoV-2 spike protein on hemolysis, we performed erythrocyte lysis using erythrocytes from a patient with PNH and acidified normal human serum (aNHS) with addition of the SARS-CoV-2 spike protein subunit 1 (S1) (see supplemental methods, available on the Blood Web site). Briefly, type O-positive red blood cells from 1 patient with PNH and 1 control were collected. aNHS preincubated with and without S1 was added to the erythrocytes. Following incubation at 37°C for 1 hour, absorbance at 405 nm was measured in the cell-free supernatants. Hemolysis in each sample was compared with total water-induced lysis of the erythrocytes. S1 did not increase hemolysis of PNH erythrocytes as compared with aNHS alone (Figure 1). Furthermore, S1 does not appear to bind erythrocytes (data not shown). This suggests that postvaccination hemolysis is not mediated by the direct effect of the spike protein. We postulate that strong complement amplification as a byproduct of the inflammatory response is responsible for the clinically observed hemolysis, as has been reported with other vaccines, infections, and surgeries.
Figure 1.
Erythrocyte lysis with addition of the SARS-CoV-2 spike protein S1. Hemolysis is not increased with addition of the SARS-CoV-2 spike protein S1 to PNH erythrocytes. Addition of 40% aNHS with various concentrations of the spike protein to type O-positive erythrocytes from a patient with PNH (49% PNH red blood cell clone: 25% type III and 24% type II cells) and type O-positive erythrocytes from a healthy control, incubated at 37°C for 1 hour. Water (H2O) serves as a positive control. EDTA added to serum serves as an inhibitor of hemolysis. Data are shown as mean ± standard error of the mean of duplicate wells.
In patients with PNH on complement inhibition, strong complement-amplifying conditions such as infection, surgery, or pregnancy may trigger pharmacodynamic breakthrough (as previously defined by Risitano et al).12 Complement-amplifying conditions lead to C3b accumulation on the cell surface; at high densities of C3b, C5 can assume a conformational change, disrupting the ability of eculizumab to inhibit terminal complement.13,14 Hemolysis following COVID-19 vaccination, which occurred in 3 patients on ravulizumab, suggests pharmacodynamic breakthrough. Although many vaccines can lead to hemolysis and thrombosis in PNH, this effect is mitigated in most patients on complement inhibitors.15 Ravulizumab, a new C5 inhibitor with a half-life 4 times longer than eculizumab, is reported to have significantly fewer instances of pharmacokinetic breakthrough hemolysis.16 Furthermore, 3 instances of breakthrough hemolysis occurred 4 weeks from the last ravulizumab infusion, making suboptimal C5 inhibition unlikely. As seen in patients 3 and 6, a proximal complement inhibitor, such as danicopan, may prevent breakthrough hemolysis precipitated by the vaccine; however, it is equally possible that the stronger immune response after the second vaccine dose was primarily responsible for the breakthrough hemolysis in patient 3.
This study provides insight into the mechanism of pharmacodynamic breakthrough precipitated by COVID-19 vaccination in patients with PNH on ravulizumab. Similar disease flares may be anticipated in other complement-mediated disorders, such as complement-mediated hemolytic uremic syndrome, cold agglutinin disease, catastrophic antiphospholipid syndrome, and HELLP syndrome.17 As SARS-CoV-2 leads to a severe inflammatory state, the benefits of vaccinating patients with PNH likely outweigh the risks; however, clinicians and patients should be aware of this serious adverse effect, and patients should be educated to report any symptoms postvaccination. We recommend vaccination within 4 weeks of the last ravulizumab infusion and 1 week of eculizumab infusion and that patients maintain optimal hydration. Adverse reactions appear time-limited and can be managed with supportive care and transfusions as needed.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grants R01 HL 133113 (R.A.B.) and T32 HL 007525 (G.F.G.), and the Jack Levin–C. Lockard Conley Research Fellowship in Benign Hematology (G.F.G.).
Footnotes
For original data, please e-mail the corresponding author.
The online version of this article contains a data supplement.
Authorship
Contribution: G.F.G. performed research, collected and analyzed data, and wrote the first draft of the manuscript; X.Y. designed and performed research; J.Y., B.A.Y.C., E.M.B., and S.C. interpreted results and edited the paper; R.A.B. designed research, analyzed data, and wrote the manuscript; and all authors reviewed the manuscript and approved the final version.
Conflict-of-interest disclosure: R.A.B. receives research funding from Alexion. S.C. has served on advisory boards for Alexion and Sanofi-Genzyme, and her institution has received research funding on her behalf from Takeda. The remaining authors declare no competing financial interests.
Correspondence: Robert A. Brodsky, Division of Hematology, School of Medicine, Johns Hopkins University, 720 Rutland Ave, Ross Research Bldg, Room 1025, Baltimore, MD 21205; e-mail: brodsro@jhmi.edu.
REFERENCES
- 1.Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conway EM, Pryzdial ELG.. Is the COVID-19 thrombotic catastrophe complement-connected? J Thromb Haemost. 2020;18(11):2812-2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicolai L, Leunig A, Brambs S, et al. Immunothrombotic dysregulation in COVID-19 pneumonia is associated with respiratory failure and coagulopathy. Circulation. 2020;142(12):1176-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu J, Yuan X, Chen H, Chaturvedi S, Braunstein EM, Brodsky RA.. Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition. Blood. 2020;136(18):2080-2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group . Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brodsky RA. How I treat paroxysmal nocturnal hemoglobinuria. Blood. 2021;137(10):1304-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clausen TM, Sandoval DR, Spliid CB, et al. SARS-CoV-2 infection depends on cellular heparan sulfate and ACE2. Cell. 2020;183(4):1043-1057.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hyvärinen S, Meri S, Jokiranta TS.. Disturbed sialic acid recognition on endothelial cells and platelets in complement attack causes atypical hemolytic uremic syndrome. Blood. 2016;127(22):2701-2710. [DOI] [PubMed] [Google Scholar]
- 11.Vogt AM, Winter G, Wahlgren M, Spillmann D.. Heparan sulphate identified on human erythrocytes: a Plasmodium falciparum receptor. Biochem J. 2004;381(Pt 3):593-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Risitano AM, Marotta S, Ricci P, et al. Anti-complement treatment for paroxysmal nocturnal hemoglobinuria: time for proximal complement inhibition? A position paper from the SAAWP of the EBMT. Front Immunol. 2019;10:1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mannes M, Dopler A, Zolk O, et al. Complement inhibition at the level of C3 or C5: mechanistic reasons for ongoing terminal pathway activity. Blood. 2021;137(4):443-455. [DOI] [PubMed] [Google Scholar]
- 14.Harder MJ, Kuhn N, Schrezenmeier H, et al. Incomplete inhibition by eculizumab: mechanistic evidence for residual C5 activity during strong complement activation. Blood. 2017;129(8):970-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnold L, Kelly R, Munir T, et al. Thrombotic events with Neisseria meningitidis vaccination in patients with paroxysmal nocturnal hemoglobinuria, UK experience [abstract]. Blood. 2020;136(suppl 1). Abstract 1684. [Google Scholar]
- 16.Brodsky RA, Peffault de Latour R, Rottinghaus ST, et al. Characterization of breakthrough hemolysis events observed in the phase 3 randomized studies of ravulizumab versus eculizumab in adults with paroxysmal nocturnal hemoglobinuria. Haematologica. 2021;106(1):230-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gavriilaki E, Brodsky RA.. Complementopathies and precision medicine. J Clin Invest. 2020;130(5):2152-2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.