Abstract
The outbreak of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has presented a global public health emergency. Although predominantly a pandemic of acute respiratory disease, corona virus infectious disease-19 (COVID-19) results in multi-organ damage that impairs cardiopulmonary (CP) function and reduces cardiorespiratory fitness. Superimposed on the CP consequences of COVID-19 is a marked reduction in physical activity that exacerbates CP disease (CPD) risk. CP exercise testing (CPET) is routinely used in clinical practice to diagnose CPD and assess prognosis; assess cardiovascular safety for rehabilitation; and delineate the physiological contributors to exercise intolerance and exertional fatigue. As such, CPET plays an important role in clinical assessments of convalescent COVID-19 patients as well as research aimed at understanding the long-term health effects of SARS-CoV-2 infection. However, due to the ventilatory expired gas analysis involved with CPET, the procedure is considered an aerosol generating procedure. Therefore, extra precautions should be taken by health care providers and exercise physiologists performing these tests. This paper provides recommendations for CPET testing during the COVID-19 pandemic. These recommendations include indications for CPET; pre-screening assessments; precautions required for testing; and suggested decontamination protocols. These safety recommendations are aimed at preventing SARS-CoV-2 transmission during CPET.
Keywords: COVID-19, Gas exchange, Stress testing
Abbreviations: CDC, Centers for Disease Control and Prevention; COVID-19, Corona virus infectious disease-19; CP, Cardiopulmonary; CPD, Cardiopulmonary disease; CPET, Cardiopulmonary exercise testing; CRF, Cardiorespiratory fitness; CV, Cardiovascular; ERS, European Respiratory Society; HEPA, High efficiency particulate air; PA, Physical activity; PCR, Polymerase chain reaction; PPE, Personal and protective equipment; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2
Introduction
The outbreak of the novel, highly contagious, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, has presented a global public health emergency. Within a year of the first reported cases in the United States, more than 25 million Americans have been infected with the virus and over 400,000 deaths have been reported.1 Although predominantly defined as a pandemic of acute respiratory disease, corona virus infectious disease-19 (COVID-19) has since been shown to result in multi-organ damage resulting in a wide spectrum of clinical manifestations. Importantly, cardiovascular (CV) complications that demonstrate a pathophysiology that is unique to COVID-19 are consistently reported.2 , 3 In this respect, inflammatory mediated thrombotic disease, cardiac and vascular injury are all highly prevalent in COVID-19 patients and associated with hospitalization and death.3, 4, 5, 6 Moreover, even after COVID-19 recovery, a large proportion of individuals demonstrate cardiac dysfunction, myocardial inflammation, endothelial dysfunction and increased arterial stiffness.7 , 8 As such, beyond the acute period of COVID-19 related illness, some individuals report persistent symptoms that are marked by shortness of breath and extreme fatigue.9 , 10 These prolonged clinical symptoms have been termed ‘long-COVID-19’ syndrome and pose a growing heath concern with an undefined pathophysiology.9
Superimposed on the cardiopulmonary (CP) consequences of COVID-19 is a marked reduction in physical activity (PA) levels that are secondary to ‘work from home’ and ‘shelter in place’ public health efforts to reduce the transmission of the SARS-CoV-2.11 It is well established that reduced PA levels are a known contributor to the development and progression of CV diseases.12 In addition, leading a more sedentary lifestyle increases the risk of an exercise induced myocardial infarction.13 Prior to the COVID-19 pandemic, a larger proportion of the global population presented with a sedentary phenotype, a phenotype that may permanently worsen as we recover from the current global health crisis.14
Cardiorespiratory fitness (CRF) portends a wealth of information related to health status and prognosis. In fact, the prognostic value of CRF is so robust it is now considered a vital sign; regardless of health status at the time of assessment (i.e., apparently healthy, at risk for chronic disease, or diagnosed with one or more chronic conditions), individuals with a higher CRF have an improved prognosis.15 As such, improving CRF, through lifestyle, pharmacologic, or surgical interventions, is a clinical imperative. CP exercise testing (CPET) is the gold-standard approach to assessing CRF, both cross-sectionally and longitudinally, and is routinely employed in clinical practice and research for 1) diagnostic indications to further confirm suspected CP disease (CPD) or gauge disease severity if confirmed; 2) for prognostic risk assessment; 3) to ascertain the safety of taking part in exercise following a sedentary lifestyle and develop individually tailored exercise prescriptions; and 4) to delineate the pathophysiological contributors to exercise intolerance and exertional fatigue.16 Therefore, due to the CP consequences of COVID-19, the recent further increase in sedentary behavior in the general population, and the unknown contributors of Long-COVID-19 related fatigue, there is an increased need for CPET in clinical practice and research. In fact, current clinical guidelines in the United Kingdom now call for a CPET in the clinical work up for long-COVID-19.9
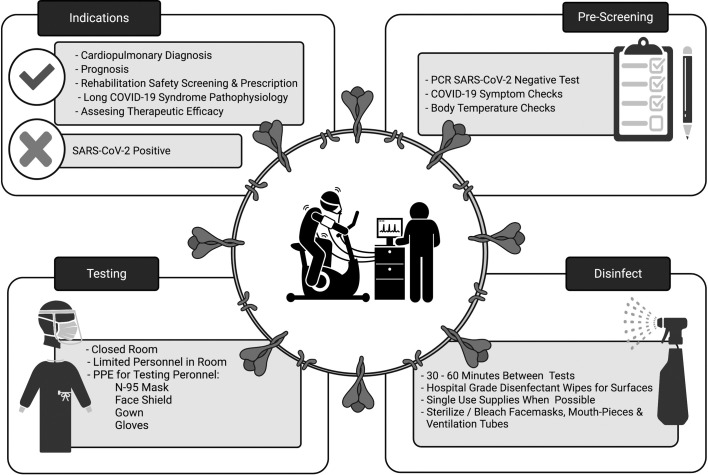
Due to the breath-by-breath ventilatory expired gas analysis involved with CPET, the procedure is considered an aerosol generating procedure.17 Additionally, the increased exhalation pressure and volume associated with exercise may increase the span and number of pathogen-bearing droplets.18 For these reasons, to prevent the transmission of SARS-CoV-2, additional safety precautions are necessary when performing CPET. We therefore propose a brief overview for clinical and research professionals performing CPETs during the COVID-19 pandemic (Fig. 1 ).
Fig. 1.
An overview of CPET procedures and precautions during the COVID-19 pandemic.
PCR, polymerase chain reaction; PPE, personal and protective equipment.
Indications for CPET and pre-screening
For patients with COVID-19 or suspected COVID-19, CPET is generally not indicated, other than for patients with resolved infection to estimate the long-term non-infectious consequences of the disease. Consultation with infectious disease and/or epidemiology may be also be needed. For patients without COVID-19, standard indications for CPET should be followed.19 It is preferable for patients be outside of the window of infectiousness. For these patients, requirements for a negative polymerase chain reaction (PCR) test varies given the time from first sign/symptom of infection, time from initial positive screening, and severity of illness.
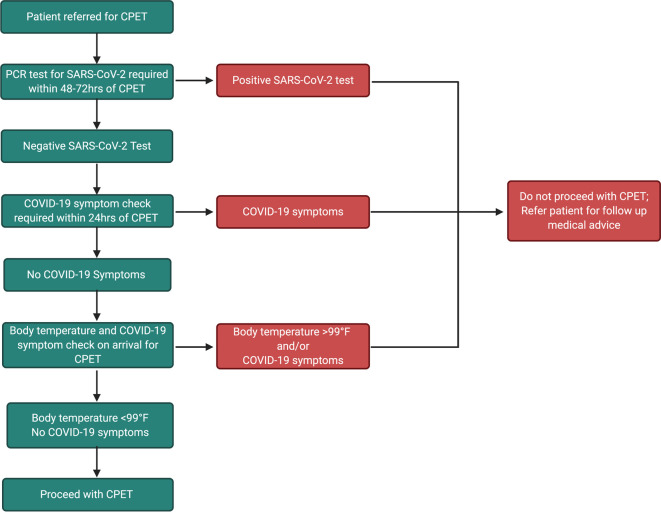
All patients or participants referred for a CPET should undergo comprehensive COVID-19 pre-screening to mitigate the chances of performing a test on a patient with active SARS-CoV-2 infection. Pre-screening should involve a PCR SARS-CoV-2 test within 48–72 h of CPET, as well as comprehensive COVID-19 symptom and body temperature checks within 24 h that are repeated immediately prior to the test. The recommended pre-screening procedure is detailed in the algorithm shown in Fig. 2 . Rapid testing assays are not recommended due to the high prevalence of false-negative in asymptomatic individuals.20 Patients previously diagnosed with COVID-19 can test positive for the infection up to 3 months after symptom onset.21 These patients are no longer considered infectious and it is appropriate to deescalate their case following the guidance set forth by the Center for Disease Control and Prevention (CDC).22 All patients that are outside the 3 month window of their first positive test should be retested. Patients who had a mild case of COVID-19 should be 10 days out from their first test and symptom onset, and free of all symptoms experienced during the initial phase of their infection.22 Patients who were severely ill, and immunocompromised patients, should also be symptom free and at least 20 days out from the first positive test and symptom onset.23 It should be acknowledged that even with a negative test, subjects should still be treated as if they are positive due to the increased risk of transmission during CPET, and all testing staff should adhere to appropriate personal protective equipment (PPE) guidelines set forth by their institution, which may include the use of a N95 mask and face shield, gown and gloves.
Fig. 2.
Recommended pre-screening algorithm for CPET during the COVID-19 pandemic.
CPET, cardiopulmonary exercise test; PCR, polymerase chain reaction.
Pre-test considerations
As per routine practice, testing equipment should be turned on and calibrated for quality control. It is best practice to ensure the temperature and humidity of the room is within recommended ranges. All testing equipment should be inspected for wear and tear, replacing damaged or worn-out pieces when necessary. In efforts to minimize the spread of respiratory droplets, surfaces should be clear of items not necessary for CPET. In line filters are recommended for pulmonary function testing, but not recommended for CPET.24 Prior to retrieving subject for test preparation, the test administrator should ensure that everything needed for the test is in the room to avoid having to leave the room after applying appropriate PPE.
The patient should not remove their mask until the test administrator has applied the proper PPE for the test. If the patient needs to remove their mask for any reason prior to applying the mouth-piece or face mask, a level 3 surgical mask and face shield is appropriate. However, it is recommended to fully don following the recommendations in the following section to avoid excessive removing and reapplying PPE after the patient has already removed their mask.
PPE requirements for patients and test personnel
PPE guidelines for patients and testing personnel during aerosolizing procedures have been set forth by the CDC25 and are outlined in Table 1 . In brief, the patient should remain in their mask until the test administrator has properly donned and has instructed the patient to remove their mask. Prior to the removal of the patient's mask, a level 3 surgical mask and a face shield is recommended while in the same room as the patient. During preparation the test administrator will be in physical contact with the patient and it is important due to the close proximity of the two individuals as well as the duration of pre-test procedures (>15 min), that both parties maintain appropriate protection from one another.
Table 1.
Recommended PPE requirements during CPET preparation, testing, and decontamination procedures outlined by the Center for Disease Prevention and Control.25
| Activity | Personnel/Patient | Activity | PPE requirement |
|---|---|---|---|
| Test preparation | Test personnel | Test preparation | Level 3 surgical mask |
| Face shield/eye protection | |||
| Patient | Face mask | ||
| CPET | Test personnel | CPET | Gown |
| N-95 mask | |||
| Face shield/eye protection | |||
| Gloves | |||
| Patient | None | ||
| Post test | Test personnel | Post test | Gown |
| N-95 mask | |||
| Face shield/eye protection | |||
| Gloves | |||
| Patient | Face mask | ||
| Decontamination | Test personnel | Gown | |
| N-95 mask | |||
| Face shield/ eye protection | |||
| Gloves |
CPET, cardiopulmonary exercise testing; PPE, personal protective equipment.
Donning should be done in the follow order: 1) hand hygiene; 2) gown; 3) N95 respirator mask; 4) eye protection; and 5) gloves. Before donning, hands should be washed with soap or an alcohol-based solution or hand sanitizing gel. To avoid touching the face, eyes or mouth, a plastic gown should be secured first, followed by a N95 respirator mask and then face shield. Gloves should be placed on last and changed between adjusting the face mask and or mouthpiece on the patient and the start of the test. To minimize exposure, the number of personnel in the room during the test should only include essential personnel required for test administration and safety. All personnel required to be in the room during the test should adhere to these recommendations when entering the room. The door to the room should be securely closed and remain closed until the test is completed.
After completion of the test, the test administrator can remove the mask or mouth-piece from the patient and should instruct the patient to put on their mask that was removed for testing. Once the patient has replaced their mask they can leave the room.
Post-test decontamination
Decontamination of the testing room should immediately follow the test. Surfaces that come in contact with patients should be cleaned after every test. Visible debris should be removed with warm water and a liquid detergent prior to the use of disinfecting wipes. Hospital grade disinfectant wipes are recommended to disinfect all surfaces in the testing laboratory. Table 2 outlines recommended single use, single patient use, and products that can be decontaminated, sterilized, and reused. For reusable products, it is important to check after every use and sterilization that the product has not been damaged or warped. Check for cracks, stains, and parts not fitting together appropriately and replace when necessary.
Table 2.
Equipment use and decontamination recommendations.
| Equipment | Use | Decontamination |
|---|---|---|
| Flow sensors | Reusable (discard after 10 uses) | Wash with soap and water. Submerge in neutral-pH enzymatic solution. Sterile processing recommended. |
| Mouthpiece | Reusable | Wash with soap and water. Soak for 60 min in 10% bleach solution and rinse with cold water; OR Submerge in neutral-pH enzymatic solution and send for sterile processing. |
| Nose clip | Single use | Discard after use; OR wash with soap and water. Soak for 60 min in 10% bleach solution and rinse with cold water. |
| Face mask and coupler | Reusable | Wash with soap and water. Soak for 10 min in 10% bleach solution and rinse with cold water; OR Submerge in neutral-pH enzymatic solution and send for sterile processing. |
| Neoprene masks | Single patient usea | Wash with warm soap and water. Rinse with cold water. Air dry. |
| Pulse oximetry band/ head band | Single patient usea | Wash with warm soap and water. Rinse with cold water. Air dry. |
Can be reused for same patient and discard when tests are discontinued.
Single use items should be discarded after every test. Single patient items can be washed in warm water and detergent, dried, placed in an appropriately labeled bag for the patient, and stored for future uses. If a patient uses a neoprene mask and does not plan a test again, the mask can be considered single use and discarded with the other single use items.
Multi-use items should be cleaned with warm water and a detergent solution to remove visible biological matter. If available, items should then be submerged in a neutral-pH enzymatic solution to prevent the adherence of any present biological materials before sending for sterile processing. If sterile processing is unavailable, submerging the items in 10% bleach solution for 60 min, followed by a cold water rinse is recommended. Room temperature or low-pressure air drying is appropriate. Drying chambers are acceptable to use so long as the temperature does not exceed 49 °C.
Lastly, it is important to provide adequate time in between tests to allow the testing environment to air out. The European Respiratory Society (ERS) recommends rearranging test schedules to allow for at least 30–60 min between tests.24 High efficiency particulate air (HEPA) filtration systems have been considered as an extra precautionary measure for post-test aeration, however it is unclear if this is best practice due to these systems collecting aerosolized material creating viral colonization. Therefore, the ERS does not recommend the use of HEPA filters during lung function testing. 24
Considerations regarding SARS-CoV2 immunity
There are many questions regarding SARS-CoV2 immunity with the ongoing campaign for vaccination in the healthcare providers and the community, the significant number of cases who have already experienced the disease, and a number of cases who have received passive immunization by means of convalescent plasma or monoclonal antibodies directed against the SARS-CoV2 proteins. While the SARS-CoV-2 vaccines appear to be highly effective in clinical trials, complete protection cannot be assumed, and therefore the same degree of precautions applies to both the providers and patients independent of vaccination status.
For those patients or providers who have recovered from COVID-19, immunity from re-infection cannot be assumed and therefore, the same degree of precautions applies independent of whether or not a prior infection has occurred. Similarly, the use of passive immunization may confer immunity, yet the efficacy and duration are unknown and therefore, as before, the same degree of precautions apply.
CP function in convalescent COVID-19 patients
Due to the respiratory and cardiac manifestations of COVID-19, it would be expected that CRF is markedly reduced during the period of acute infection. However, cardiopulmonary function in convalescent patients is largely unknown, particularly in severely infected patients experiencing long-COVID-19 syndrome. Recent evidence from both non-severe and hospitalized COVID-19 patients show that these individuals have a peak oxygen consumption (VO2) below age-predicted levels three months after discharge or convalescence.10 , 26 Towards understanding the pathophysiology of long-COVID-19 syndrome, comprehensive assessments of CP function and the oxygen transport chain are warranted. In this respect, lasting pulmonary damage27 , 28 could potentially alter oxygen diffusion from the lungs to the circulation, resulting in hypoxemia. Impairments in CV function that are evident in convalescent patients8 stand to hamper oxygen delivery to meet the metabolic demand of active skeletal muscle. Finally, some recent evidence suggests that dynapenia is a substantial contributor to reduced CRF in convalescent COVID-19 patients,26 , 29 thus implicating a peripheral limitation to exercise tolerance in this population. The long-term consequences of diminished skeletal muscle quality and function and the consequential effects on oxygen utilization warrant investigation. In this respect, CPET is a powerful tool to help uncover the physiological contributors to CRF and fatigue.
Conclusions
Guidelines for aerosolizing procedures may vary by institution, however, each institution should incorporate the guidance set forth by the CDC and ERS for optimal safety[ 24 , 25 ]. The value of CPETs for diagnostic and prognostic evaluation of cardiovascular diseases, as well as assessing interventional efficacy, justifies the importance of continuing these tests during the SARS-CoV-2 pandemic. Testing personnel should be prepared and familiar with the unique risks that come with CPET during the SARS-CoV-2 pandemic and should proceed with caution during its conduction. The risks and benefits of CPET should be weighed on a case by case basis, and institutions should highly consider cancelling or postponing tests that do not provide greater benefit than risk.
Declaration of Competing Interest
None to declare.
Acknowledgements
Danielle Kirkman is supported by a Career Development Award 19CDA34740002 from the American Heart Association. This work is supported by CTSA award UL1TR002649 to Virginia Commonwealth University.
References
- 1.Centers for Disease Control and Prevention CDC COVID Data Tracker. 2021. https://covid.cdc.gov/covid-data-tracker/#cases_casesper100klast7days
- 2.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5:831–840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 3.Bonow R.O., O’Gara P.T., Yancy C.W. Cardiology and COVID-19. J. Am. Med. Assoc. 2020;324:1131–1132. doi: 10.1001/jama.2020.15088. [DOI] [PubMed] [Google Scholar]
- 4.Shi S., Qin M., Shen B., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan. China JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dixon D.L., Van Tassell B.W., Vecchié A., et al. Cardiovascular considerations in treating patients with coronavirus disease 2019 (COVID-19) J. Cardiovasc. Pharmacol. 2020;75:359–367. doi: 10.1097/FJC.0000000000000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bikdeli B., Madhavan M.V., Jimenez D., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puntmann V.O., Carerj M.L., Wieters I., et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ratchford S.M., Stickford J.L., Province V.M., et al. Vascular alterations among young adults with SARS-CoV-2. Am. J. Physiol. Heart Circ. Physiol. 2021;320:H404–h410. doi: 10.1152/ajpheart.00897.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Venkatesan P. NICE guideline on long COVID. Lancet Respir. Med. 2021;9:129. doi: 10.1016/S2213-2600(21)00031-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raman B., Cassar M.P., Tunnicliffe E.M., et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine. 2021;31:100683. doi: 10.1016/j.eclinm.2020.100683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer J., Herring M., McDowell C., et al. Joint prevalence of physical activity and sitting time during COVID-19 among US adults in April 2020. Prev. Med. Rep. 2020;20:101256. doi: 10.1016/j.pmedr.2020.101256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elagizi A., Kachur S., Carbone S., Lavie C.J., Blair S.N. A review of obesity, physical activity, and cardiovascular disease. Curr. Obes. Rep. 2020;9:571–581. doi: 10.1007/s13679-020-00403-z. [DOI] [PubMed] [Google Scholar]
- 13.Franklin B.A. Preventing exercise-related cardiovascular events: is a medical examination more urgent for physical activity or inactivity? Circulation. 2014;129:1081–1084. doi: 10.1161/CIRCULATIONAHA.114.007641. [DOI] [PubMed] [Google Scholar]
- 14.Hall G., Laddu D.R., Phillips S.A., Lavie C.J., Arena R. A tale of two pandemics: how will COVID-19 and global trends in physical inactivity and sedentary behavior affect one another? Prog. Cardiovasc. Dis. 2020;64:108–110. doi: 10.1016/j.pcad.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ross R., Blair S.N., Arena R., et al. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134:e653–e699. doi: 10.1161/CIR.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 16.ASCM's Guidelines for Exercise Testing and Prescription. 10th ed. Wolters Kluwer; Philadelphia, USA: 2018. [Google Scholar]
- 17.Tran K., Cimon K., Severn M., Pessoa-Silva C.L., Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: potential implications for reducing transmission of COVID-19. J. Am. Med. Assoc. 2020;323:1837–1838. doi: 10.1001/jama.2020.4756. [DOI] [PubMed] [Google Scholar]
- 19.ATS/ACCP statement on cardiopulmonary exercise testing. Am. J. Respir. Crit. Care Med. 2003;167:211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 20.Prince-Guerra J.L., Almendares O., Nolen L.D., et al. Evaluation of Abbott BinaxNOW rapid antigen test for SARS-CoV-2 infection at two community-based testing sites — Pima County, Arizona, November 3–17, 2020. Morb. Mortal. Wkly Rep. 2021;70 doi: 10.15585/mmwr.mm7003e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korea Centers for Disease Control and Prevention Findings From Investigation and Analysis of Re-positive Cases. 2021. https://www.cdc.go.kr/board/board.es?mid=a30402000000&bid=0030&act=view&list_no=367267&nPage=1
- 22.Balady G.J., Arena R., Sietsema K., et al. Clinician’s guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:191–225. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention Discontinuation of Transmission-Based Precautions for Patients with Confirmed SARS-CoV-2 Infection. https://www.cdc.gov/coronavirus/2019-ncov/hcp/disposition-hospitalized-patients.html
- 24.European Respiratory Society . 2021. Recommendation from ERS Group 9.1 (Respiratory function technologists/Scientists) Lung function testing during COVID-19 pandemic and beyond.https://ers.app.box.com/s/zs1uu88wy51monr0ewd990itoz4tsn2h [Google Scholar]
- 25.Centers for Disease Control and Prevention Interim Infection Prevention and Control Recommendations for Healthcare Personnel During the Coronavirus Disease 2019 (COVID-19) Pandemic. 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html#print
- 26.Clavario P., De Vincenzo M., Lotti R., et al. Assessment of functional capacity with cardiopulmonary exercise testing in non-severe COVID-19 patients at three months follow-up. MedRxiv. 2020 [Google Scholar]
- 27.Zhao Y.M., Shang Y.M., Song W.B., et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25:100463. doi: 10.1016/j.eclinm.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van den Borst B., Peters J.B., Brink M., et al. Comprehensive health assessment three months after recovery from acute COVID-19. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blokland I.J., Ilbrink S., Houdijk H., et al. Exercise capacity after mechanical ventilation because of COVID-19: Cardiopulmonary exercise tests in clinical rehabilitation. Ned. Tijdschr. Geneeskd. 2020;164 [PubMed] [Google Scholar]