Graphical abstract
Keywords: SARS-CoV-2, Therapeutic approach, Gene silencing, siRNA, Nucleocapsid Gene
Abstract
SARS-CoV-2 is a single-stranded RNA (+) virus first identified in China and then became an ongoing global outbreak. In most cases, it is fatal in humans due to respiratory malfunction. Extensive researches are going to find an effective therapeutic technique for the treatment of SARS-CoV-2 infected individuals. In this study, we attempted to design a siRNA molecule to silence the most suitable nucleocapsid(N) gene of SARS-CoV-2, which play a major role during viral pathogenesis, replication, encapsidation and RNA packaging. At first, 270 complete N gene sequences of different strains in Bangladesh of these viruses were retrieved from the NCBI database. Different computational methods were used to design siRNA molecules. A siRNA molecule was built against these strains using the SiDirect 2.0 server. Using Mfold and the OligoCalc server, the siRNA molecule was tested for its secondary structure and GC material. The Clustal Omega tool was employed to evaluate any off-target harmony of the planned siRNA molecule. Herein, we proposed a duplex siRNA molecule that does not fit any off-target sequences for the gene silencing of SARS-CoV-2. To treat SARS-CoV-2 infections, currently, any effective therapy is not available. Our engineered siRNA molecule could give an alternative therapeutic approach against various sequenced SARS-CoV-2 strains in Bangladesh.
1. Introduction
A deadly bat-borne Coronaviridae family virus SARS-CoV-2 was identified first in mid-December of 2019 in Wuhan, China (Chan et al., 2020). Since then, it has become an ongoing outbreak worldwide. To this date, it has affected 213 countries and territories across the globe (Centers-of-Disease-Control-and-Prevention, 2020). In this positive sense, the single-stranded RNA virus has already infected about thirteen million people worldwide till July 2020 (Anon., 2021). Patients suffering from SARS-CoV-2 infection are usually found with complications in the respiratory tract, diarrhea, high fever, thrombocytopenia, lymphopenia, and increased C-reactive protein and lactate dehydrogenase levels within the first 3–6 days of viral exposure (Chan et al., 2020; Zhang et al., 2020). With a genome size of ∼30000 bases, one of the largest nonsegmented genomes among all RNA viruses, this virus has already compiled plentiful changes in its genome and procures more (Forni et al., 2017; Woo et al., 2009). Due to the sequencing technologies advancement, various databases preserves the available whole genome sequence of these deadly virus strains now. Researchers are looking for effective molecular therapy against the SARS-CoV-2 using these resources. Bangladeshi scientists also sequenced different SARS-CoV-2 strains, where 273 sequences were available in the NCBI public database until December 2020 (Saha et al., 2020; Moniruzzaman et al., 2020). A comparative analysis computed the origin of the various strains of this virus in Bangladesh among publicly available SARS-CoV-2 genome sequences from 27 countries. Their prediction about Bangladeshi strains based on phylogenetic analysis reported that the pathogen appeared from multiple countries (Shishir et al., 2021). This study was on 64 SARS-CoV-2 isolated genomic sequences from Bangladesh with the reference (NC_045512.2 first Chinese SARS-CoV-2 sequence) and 433 sequences of the whole world available from several countries. The sequences of different Bangladeshi strains were showed three clusters, among them 43 of the 64 sequences shared a comparable node with Germany, which carried a common ancestor with the United Kingdom. Two other clusters of Bangladesh had 4 and 5 sequences, respectively, and in both cases, they shared the same node with the sequence of SARS-CoV-2 reported from India and Saudi Arabia.
On the other hand, 12 sequences that did not belong to any clusters were found to be similar with sequences from Europe, including United Kingdom, Germany, France, Italy, and Russia, while one of these sequences was close to the sequence reported from the USA (Shishir et al., 2021; Hasan et al., 2020). The underlying link of Bangladeshi SARS-CoV-2 isolates with part of a haplotype observed high in Europe (Shishir et al., 2021; Mahmud et al., 2020). Though the virus is a mutated particle over time, the SARS-CoV-2 sequence shares 79.6 % similarity to SARS-CoV (Zhou et al., 2020; Marra et al., 2003), with the fewer mutations and higher (90 %) homology showing N gene, which is more stable and conserved over time, but the S gene has 76 % similarity (Marra et al., 2003; Drosten et al., 2003; Grifoni et al., 2020; Holmes and Enjuanes, 2003; Rota et al., 2003; Zhu et al., 2005). Nucleocapsid gene silencing Small Interfering RNA (siRNA) molecules potentiality has already been reported (Ge et al., 2003). Double-stranded 20–25 base pairs containing RNA generally work through the RNA interference (RNAi) pathway to silence a specific gene expression, where it causes mRNA breakage into the following transcription (Agrawal et al., 2003). These mRNA degrading small molecules play an important role in the post-transcriptional gene silencing (PTGS) (Hamilton and Baulcombe, 1999).
In-vitro studies have verified that siRNA can significantly repress gene expression in mammalian cells (Lee et al., 2002; Kapadia et al., 2003; Lee et al., 2003). In addition, effective in vivo silencing of the endogenous gene and transgene expression is already revealed (McCaffrey et al., 2002; Giladi et al., 2003; Sorensen et al., 2003; Tompkins et al., 2004a). The capability of small interfering RNA is already proved in a in vitro study through the inhibition of SARS-CoV N gene expression in cultured cells and mouse muscles (Zhao et al., 2005). More than 80 % blocking activity was shown utilizing chemically synthesized siRNA duplexes targeting genomic RNA of SARS-CoV (Zheng et al., 2004). Like other viruses, SARS-CoV-2 nucleocapsid(N) protein is a multifunctional protein and plays a crucial role in encapsidation, viral RNA transcription, and replication (Chang et al., 2016).
For this reason, the study of the nucleocapsid protein or N gene has become much popular as a diagnostic and therapeutic target (Liu et al., 2020; Eshaghi et al., 2005). However, some studies show a mutation in the N gene, which is not the focus of this study. Moreover, our main objective is to identify a common therapeutic target for different SARS-CoV-2 strains of Bangladesh. Targeting this sequence may cause SARS-CoV-2 viral inhibition like SARS-CoV by impeding the N gene's translation and thus preventing the RNA transcription and replication (Kapadia et al., 2003; Wu et al., 2005).
2. Materials and methods
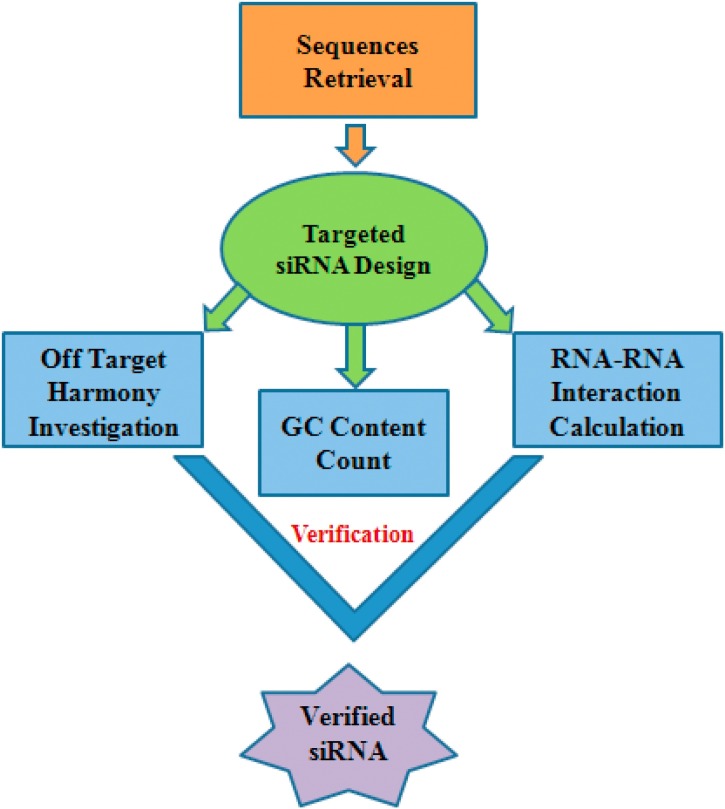
The complete workflow is summarized into the methodology (Fig. 1 )
Fig. 1.
The potential siRNA molecule prediction methodology.
2.1. Viral strain selection
The selection of SARS-CoV-2 strains and their associated information, including their genus, family, host, transmission pattern, disease pathogenicity, genome, proteome, and the available therapeutic agents against them, was identified ViralZone (http://viralzone.expasy.org/) of the Ex-PASy Bioinformatics Resource Portal.
2.2. Sequence retrieval and evolution analysis
Two hundred and seventy-three sequences of different SARS-CoV-2 (BDG) strains were collected from the viral gene bank database available at NCBI (http://www.ncbi.nlm.nih.gov/). Among them, 270 complete cds of nucleocapsid(N) protein gene was selected, and the other 3 incomplete sequences were disqualified for the next experiment. Multiple sequence alignment of the retrieved sequences was done by Clustal Omega (Sievers et al., 2011) (https://www.ebi.ac.uk/Tools/msa/clustalo/), and phylogeny was analyzed by Phylogeny analysis (http://www.phylogeny.fr/simple_phylogeny.cgi) tool.
2.3. Target prediction and allowable siRNA molecule devising
A web server siDirect 2.0 (http://siDirect2.RNAi.jp/) (Naito et al., 2009) was used for efficient and target-specific siRNA design for mammalian RNAi. It utilized Ui-Tei, Amarzguioui, and Reynolds rules combined (Ui‐Tei et al., 2004; Reynolds et al., 2004; Amarzguioui and Prydz, 2004), and as a parameter, melting temperature (Tm) below 21.5 °C for siRNA duplex was also used. Besides, these other parameters were taken on the concept of algorithms given in Table 1 .
Table 1.
Algorithms or rules for the rational design of siRNA molecules.
| No. | Rules Name | Rules |
|---|---|---|
| 1. | Ui-Tei rules |
|
| 2. | Amarzguioui rules |
|
| 3. | Reynolds rules |
|
Source: (Oany et al., 2015).
2.4. Off target harmony investigation
Blast tool (Johnson et al., 2008) (http://www.ncbi.nlm.nih.gov/blast) was used to identify off-target similarity with any sequence on whole Gene bank datasets other than the target sequence by applying expected threshold value 10 as a parameter.
2.5. GC content count and secondary structure prophecy
Oligonucleotide Properties Calculator for GC content calculation of predicted siRNA, OligoCalc (Kibbe, 2007) (http://basic.northwestern.edu/biotools/OligoCalc.html) tool was used while Mfold server (Zuker, 2003) (http://www.mfold.-rna.albany.edu/) was used for secondary structure prediction aimed to compute the free energy of folding.
2.6. Calculation of RNA-RNA interaction through thermodynamics
To study the thermodynamics of interaction between predicted siRNA and target gene RNAcofold program (Gruber et al., 2008) (http://rna.tbi.univie.ac.at/cgi-bin/RNAcofold.cgi) was used. The hybridization energy and base-pairing form of two RNA sequences was calculated by it. It functions as an extension of McCaskill's partition function algorithm to compute base-pairing probabilities, realistic interaction energies, and equilibrium concentrations of duplex structures.
2.7. Verification of the considered siRNA
Finally, the siRNAPred (Kumar et al., 2009) (http://imtech.res.in/raghava) server from Imtech was used to validate the predicted siRNA further. Here we used efficacy prediction for 21 mers. The predicted siRNA was screened against the Main 21 dataset using a binary pattern. siRNAPred score greater than 0.9 predicts very high efficacy, a score ranging 0.8−0.9 indicates high efficacy, and a score ranging 0.7−0.8 predicts moderate efficacy.
3. Results and discussion
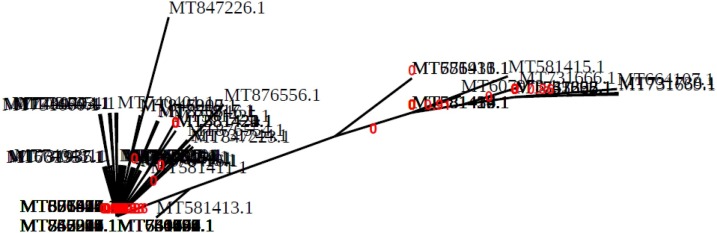
At first, N gene cds of different SARS-CoV-2 strains in Bangladesh were picked to design a siRNA molecule. The N gene sequence of the SARS-CoV-2 Bangladeshi strain (MT476385.1) was first sequenced by the child health research foundation (CHRF). The 270 complete sequences were collected from NCBI. The accession numbers and Phylogenetic tree of these complete cds were shown by date released (Supplementary file 1 and Supplementary file 2). It was stated from the phylogenetic analysis tool that all the sequences had a common predecessor and some significant harmony reserved during evolution (Fig. 2 ).
Fig. 2.
Phylogenetic analysis (treedyn) of the nucleocapsid(N) gene of different SARS-CoV-2 (BDG) strains.
Extremely fruitful small interfering RNA with maximal target specificity from the retrieved sequences computed by the siDirect web-based online software system. The proposed consensus target for siRNA with location is shown in Table 2 . The Clustal Omega server did multiple sequence alignment (MSA) among the different SARS-CoV-2 cds of the N gene (Supplementary file 3). Web server siDirect predicts siRNA by calculating the Tm of the seed target duplex using the nearest neighbor model and the thermodynamic parameters in Table 3
Table 2.
Selected siRNA and their location with the consensus target of the N gene cds.
| Total Accession | Target | Location of the target within the gene | siRNA target sequence within the gene |
|---|---|---|---|
| 270 | Consensus (270/270) | 314−336 | GTCCAAGATGGTATTTCTACTAC |
| Consensus (270/270) | 727−749 | GGCCAAACTGTCACTAAGAAATC | |
| Consensus (269/270) | 789−811 | TGCCACTAAAGCATACAATGTAA | |
| Consensus (270/270) | 892−914 | TACAAACATTGGCCGCAAATTGC |
Table 3.
Top four RNA oligo sequences against the targeting 21 nt of target sequences.
| Target sequence 21 nt target + 2 nt overhang | RNA oligo sequences 21 nt guide (5′→3′) 21 nt passenger (5′→3′) | Functional siRNA selection: Ui-Tei Reynolds Amarzguioui |
|---|---|---|
| GTCCAAGATGGTATTTCTACTAC | AGUAGAAAUACCAUCUUGGAC | U R A |
| CCAAGAUGGUAUUUCUACUAC | ||
| GGCCAAACTGTCACTAAGAAATC | UUUCUUAGUGACAGUUUGGCC | U R A |
| CCAAACUGUCACUAAGAAAUC | ||
| TGCCACTAAAGCATACAATGTAA | ACAUUGUAUGCUUUAGUGGCA | U R A |
| CCACUAAAGCAUACAAUGUAA | ||
| TACAAACATTGGCCGCAAATTGC | AAUUUGCGGCCAAUGUUUGUA | U R A |
| CAAACAUUGGCCGCAAAUUGC |
The formula for calculating the Tm is:
| Tm = {(1000 ×ΔH) / (A + ΔS + Rln (CT/4))} −273.15 + 16.6 log [Na+] | (1) |
Where ΔH (kcal/ mol) is the sum of the nearest neighbor enthalpy change, A is the helix initiation constant (-10.8), ΔS is the sum of the nearest neighbor entropy change.37 R is the gas constant (1.987 cal/deg/mol), and CT is the total molecular concentration of the strand (100 μM). [Na+] was fixed at 100 mM.
Our targeted siRNA's GC content was calculated 38 %, 40 %, 36 %, 43 % by the OligoCalc calculator, an oligonucleotide features Counter. Mfold server predicts RNA secondary structure through widely used algorithms based on a minimal free energy state (Zuker, 1989). The RNAcofold server from Vienna RNA web services calculated the hybridization energy and base-pairing pattern of the RNA sequences (Table 4 ). The siRNAPred server assessed the 21mer siRNA through the Support vector machine-based methods with high accuracy. The Main21 dataset of the siRNAPred server consists of 2182 siRNAs (21mer) derived from homogeneous experimental conditions. The binary model was chosen for the justification. These results in the top calculation score of each 21mer siRNA were 0.946, 0.861, 0.986, 0.793. This score lies within the range of very high efficacy, high efficacy, and moderate prediction score for siRNAPred server (≥0.9, very high efficacy; 0.8−0.9, high efficacy; 0.7−0.8, moderate efficacy). Potential treatment can be provided through siRNA therapeutic approaches for the diseases hereditary genetic defects, viral infectious diseases, immune disorders, and cancers caused by a particular gene set (Aagaard and Rossi, 2007). The siRNA to silence specific genes against several viruses such as hepatitis C virus (Gruber et al., 2008), HIV-1 infection (Kumar et al., 2009), and herpes simplex virus 2 infections is already approved as a potential (Palliser et al., 2006).
Table 4.
The proposed siRNA molecule with GC%, the free energy of binding with target and validity.
| Target | Predicted duplex siRNA candidate at 37 °C | GC% | Free energy of binding (Kcal/mol) | Validity (Binary) |
|---|---|---|---|---|
| Consensus target | AGUAGAAAUACCAUCUUGGAC | 38 % | −31.50 Kcal/mol | 0.946 |
| CCAAGAUGGUAUUUCUACUAC | ||||
| UUUCUUAGUGACAGUUUGGCC | 40 % | −34.54 Kcal/mol | 0.861 | |
| CCAAACUGUCACUAAGAAAUC | ||||
| ACAUUGUAUGCUUUAGUGGCA | 36 % | −30.74 Kcal/mol | 0.986 | |
| CCACUAAAGCAUACAAUGUAA | ||||
| AAUUUGCGGCCAAUGUUUGUA | 43 % | −31.61 Kcal/mol | 0.793 | |
| CAAACAUUGGCCGCAAAUUGC |
Designing a fruitful siRNA molecule to target a particular gene has a number of challenges. Finding an operative delivery manner is the main challenge; further challenges are off-target silencing, the creation of the immune reaction, and finally, the siRNA stability (Gavrilov and Saltzman, 2012). Thus, scientists have improved some models to prophesy the efficacy for a deliberate siRNA fragment before in vivo investigation– named Ui-Tei rules, Amarzguioui rules, and Reynolds rule (Taxman et al., 2006). In this study, we also follow all of these rules to designing a siRNA molecule. The proposed siRNA is covered the threshold score 6 of Reynolds rules, which indicates a proficient siRNA. The GC substance was also found to be within the supported range of 30–52 % of the proposed siRNA (Chan et al., 2009). Another important parameter for siRNA efficiency is the prediction of thermodynamics of RNA-RNA interaction which was predicted by the RNAcofold server. Here the two RNA sequences are concatenated, and an ampersand specifies the point of concatenation. The two heterodyne sequences A and B binding free energy ΔGAB can be calculated by the equation (Bernhart et al., 2006),
| ΔG binding = ΔGAB – (ΔGA + ΔGB) | (2) |
Now the binding free energy for the interaction into the selected siRNA with its consensus target is
The binding free energy (ΔGAB) of the above four siRNA meets the qualified range.
Off-target silencing ability of the proposed siRNA was checked using the NCBI blast program and there is no effects were identified. But in vivo experimentation is mandatory for the measurement of the immune response activity which is the most important challenge for siRNA designing. The MSA result showed a single code mutation in a single strain for 3rd siRNA the target locality in the N gene cds identified by siDirect server but the other three 1st, 2nd, and 4th were completely conserved within 270 different sequences (Supplementary file 2). Also, 1st siRNA showed very high efficacy, which is greater than 2nd and 4th. Its predicted GC content percentage (38 %) and free binding energy (-31.50Kcal/mol) were in the excellent range (Oany et al., 2015). On top of all the analysis, it can be decided that the proposed 1st siRNA molecule meets all the desired criteria to be considered a potential siRNA and might play an important role to combat SARS-CoV-2. These results support the possible utilization of the anticipated novel siRNA hostile to the 270 strains of Bangladesh considered in our study. Scientists are trying to show outstanding collective effort due to characterize SARS-CoV-2 and recognize effective treatment as well as therapeutic alternatives for this rapidly proliferative, highly infective and potentially death causing virus (Iacob and Iacob, 2020). Multiple tentative drugs having viral defeated potentiality have been introduced since the beginning of this virulent disease. Nevertheless, effective molecular agents is not identified currently for the treatment of the increased number of people because of their lack of an appropriate medical response (Iacob and Iacob, 2020; Chowdhury et al., 2021), especially in Bangladeshi people. Although this study is not the first molecular therapeutic approach for treating SARS-CoV-2, our predicted siRNA can be the most suitable molecular therapeutic approach after passing experimental validation.
The last years, many studies have been revealed that explain various mechanism of in vivo uses of siRNA in systemically or locally. The most significant numerous articles focus on using unchanged siRNAs administration through intravenously by hydrodynamic transfection. Intravenous and Intranasal unmodified siRNAs delivery into lung targeting nucleoprotein of Influenza virus and SARS corona virus gives relief from Influenza virus infections (Tompkins et al., 2004b) and SARS corona virus fever (Li et al., 2005) in vivo, which represents strong protection against these lethal viruses.
In recent times, application of polyethyleneimine has been extended towards the complexation and delivery of RNA molecules, especially siRNAs (Urban-Klein et al., 2005). While chemically unchanged RNA molecules are very unstable with having high degrading possibility, but the enzymatic or non-enzymatic degradation is completely protected by PEI complexation. Before or after influenza virus infection, PEI promoted siRNAs' delivery into the lungs through IV route decrease virus production (Ge et al., 2004). In vitro activity also proved for siRNA nanoplexes which prevents siRNAs from serum devastation (Schiffelers et al., 2004). Polyethyleneimine and siRNA complexes are effectively delivered into particular cells in vitro, specifically low molecular weight PEI exhibit high siRNA protection and delivery efficacies (Werth et al., 2006). Lyophilization preserves physical stability and biological activity of the PEI/RNA (siRNA or ribozyme) complexes under some conditions (Werth et al., 2006; Brus et al., 2004).
4. Conclusions
Molecular therapy is replacing conventional therapeutic approaches as a promising alternative way because of its specificity and successive nature. Potentiality of siRNAs have already afford to effectual cure for different diseases. In this study, we have anticipated that a siRNA molecule to apply as therapeutic agent to combat SARS-CoV-2. Functionality and stability of the predicted siRNA molecule is also defined using various algorithms with their suitable parameters. Molecular therapies are precise to DNA sequences that are sometimes nonfunctional against different strains. Our designed siRNA is expected to overcome this issue as it has targeted a conserved sequence found in different SARS-CoV-2 strains, which Bangladeshi scientists sequence. While proper in vitro and in vivo support is still mandatory, even so we expect that this siRNA molecule will afford a practical treatment method hostile to the targeted Bangladeshi SARS-CoV-2 strains.
Funding
No funding for this study.
Author statement
All authors are equally contributed and approved it for Publication.
Declaration of Competing Interest
None.
Acknowledgement
We thank all of them to give an outstanding support during this work.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.compbiolchem.2021.107486.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Aagaard L., Rossi J.J. RNAi therapeutics: principles, prospects and challenges. Adv. Drug Deliv. Rev. 2007;59(March (2-3)):75–86. doi: 10.1016/j.addr.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal N., Dasaradhi P.V., Mohmmed A., Malhotra P., Bhatnagar R.K., Mukherjee S.K. RNA interference: biology, mechanism, and applications. Microbiol. Mol. Biol. Rev. 2003;67(December (4)):657–685. doi: 10.1128/MMBR.67.4.657-685.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarzguioui M., Prydz H. An algorithm for selection of functional siRNA sequences. Biochem. Biophys. Res. Commun. 2004;316(April (4)):1050–1058. doi: 10.1016/j.bbrc.2004.02.157. [DOI] [PubMed] [Google Scholar]
- https://www.worldometers.info/coronavirus/countries-where-coronavirus-has-spread/.
- Bernhart S.H., Tafer H., Mückstein U., Flamm C., Stadler P.F., Hofacker I.L. Partition function and base pairing probabilities of RNA heterodimers. Algorithms Mol. Biol. 2006;1(January (1)):3. doi: 10.1186/1748-7188-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brus C., Kleemann E., Aigner A., Czubayko F., Kissel T. Stabilization of oligonucleotide-polyethylenimine complexes by freeze-drying: physicochemical and biological characterization. J. Control Release. 2004;95(1):119–131. doi: 10.1016/j.jconrel.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Centers-of-Disease-Control-and-Prevention . 2020. Confirmed 2019-nCoV Cases Globally.https://www.cdc.gov/coronavirus/2019-ncov/locations-confirmed-cases Retrieved January 31, 2020, from. [Google Scholar]
- Chan C.Y., Carmack C.S., Long D.D., Maliyekkel A., Shao Y., Roninson I.B., Ding Y. A structural interpretation of the effect of GC-content on efficiency of RNA interference. BMC Bioinformatics. 2009;10(January (S1)):S33. doi: 10.1186/1471-2105-10-S1-S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J., Xing F., Liu J., Yip C.C., Poon R.W., Tsoi H.W. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(February (10223)):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.K., Lo S.C., Wang Y.S., Hou M.H. Recent insights into the development of therapeutics against coronavirus diseases by targeting N protein. Drug Discov. Today. 2016;21(April (4)):562–572. doi: 10.1016/j.drudis.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury U.F., Shohan M.U., Hoque K.I., Beg M.A., Siam M.K., Moni M.A. A computational approach to design potential siRNA molecules as a prospective tool for silencing nucleocapsid phosphoprotein and surface glycoprotein gene of SARS-CoV-2. Genomics. 2021;113(January (1)):331–343. doi: 10.1016/j.ygeno.2020.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten C., Günther S., Preiser W., Van Der Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A., Berger A. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348(May (20)):1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- Eshaghi M., Tan W.S., Ong S.T., Yusoff K. Purification and characterization of Nipah virus nucleocapsid protein produced in insect cells. J. Clin. Microbiol. 2005;43(July (7)):3172–3177. doi: 10.1128/JCM.43.7.3172-3177.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forni D., Cagliani R., Clerici M., Sironi M. Molecular evolution of human coronavirus genomes. Trends Microbiol. 2017;25(January (1)):35–48. doi: 10.1016/j.tim.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilov K., Saltzman W.M. Therapeutic siRNA: principles, challenges, and strategies. Yale J. Biol. Med. 2012;85(June (2)):187. [PMC free article] [PubMed] [Google Scholar]
- Ge Q., McManus M.T., Nguyen T., Shen C.H., Sharp P.A., Eisen H.N., Chen J. RNA interference of influenza virus production by directly targeting mRNA for degradation and indirectly inhibiting all viral RNA transcription. Proc. Natl. Acad. Sci. U. S. A. 2003;100(March (5)):2718–2723. doi: 10.1073/pnas.0437841100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Q., Filip L., Bai A., Nguyen T., Eisen H.N., Chen J. Inhibition of influenza virus production in virus-infected mice by RNA interference. Proc. Natl. Acad. Sci. U. S. A. 2004;101(23):8676–8681. doi: 10.1073/pnas.0402486101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giladi H., Ketzinel-Gilad M., Rivkin L., Felig Y., Nussbaum O., Galun E. Small interfering RNA inhibits hepatitis B virus replication in mice. Mol. Ther. 2003;8(November):769–776. doi: 10.1016/s1525-0016(03)00244-2. [DOI] [PubMed] [Google Scholar]
- Grifoni A., Sidney J., Zhang Y., Scheuermann R.H., Peters B., Sette A. A sequence homology and bioinformatic approach can predict candidate targets for immune responses to SARS-CoV-2. Cell Host Microbe. 2020;27(April (4)) doi: 10.1016/j.chom.2020.03.002. 671-80.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber A.R., Lorenz R., Bernhart S.H., Neuböck R., Hofacker I.L. The vienna RNA websuite. Nucleic Acids Res. 2008;36(April (suppl_2)):W70–4. doi: 10.1093/nar/gkn188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton A.J., Baulcombe D.C. A species of small antisense RNA in posttranscriptional gene silencing in plants. Science. 1999;286(October (5441)):950–952. doi: 10.1126/science.286.5441.950. [DOI] [PubMed] [Google Scholar]
- Hasan M.M., Das R., Rasheduzzaman M., Hossain M.H., Muzahid N.H., Salauddin A., Rumi M.H., Rashid S.M., Siddiki A.Z., Mannan A. Global and local mutations in bangladeshi SARS-CoV-2 genomes. BioRxiv. 2020;(January) doi: 10.1016/j.virusres.2021.198390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes K.V., Enjuanes L. The SARS coronavirus: a postgenomic era. Science. 2003;300(May (5624)):1377–1378. doi: 10.1126/science.1086418. [DOI] [PubMed] [Google Scholar]
- Iacob S., Iacob D.G. SARS-coV-2 treatment approaches: numerous options, no certainty for a versatile virus. Front. Pharmacol. 2020;11(August):1224. doi: 10.3389/fphar.2020.01224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M., Zaretskaya I., Raytselis Y., Merezhuk Y., McGinnis S., Madden T.L. NCBI BLAST: a better web interface. Nucleic Acids Res. 2008;36(April (suppl_2)):W5–9. doi: 10.1093/nar/gkn201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapadia S.B., Brideau-Andersen A., Chisari F.V. Interference of hepatitis C virus RNA replication by short interfering RNAs. Proc. Natl. Acad. Sci. U. S. A. 2003;100(February (4)):2014–2018. doi: 10.1073/pnas.252783999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibbe W.A. OligoCalc: an online oligonucleotide properties calculator. Nucleic Acids Res. 2007;35(July (suppl_2)):W43–6. doi: 10.1093/nar/gkm234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Lata S., Raghava G.P. siRNApred: SVM based method for predicting efficacy value of siRNA. Proceedings of the First International Conference on Open Source for Computer Aided Drug Discovery (OSCADD); CSIR-IMTECH; 2009. Mar. [Google Scholar]
- Lee N.S., Dohjima T., Bauer G., Li H., Li M.J., Ehsani A., Salvaterra P., Rossi J. Expression of small interfering RNAs targeted against HIV-1 rev transcripts in human cells. Nat. Biotechnol. 2002;20(May (5)):500–505. doi: 10.1038/nbt0502-500. [DOI] [PubMed] [Google Scholar]
- Lee M.T., Coburn G.A., McClure M.O., Cullen B.R. Inhibition of human immunodeficiency virus type 1 replication in primary macrophages by using Tat- or CCR5-specific small interfering RNAs expressed from a lentivirus vector. J. Virol. 2003;77:11964–11972. doi: 10.1128/JVI.77.22.11964-11972.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B.-J., Tang Q., Cheng D., et al. Using siRNA in prophylactic and therapeutic regimens against SARS coronavirus in Rhesus macaque. Nat. Med. 2005;11(9):944–951. doi: 10.1038/nm1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Liu L., Kou G., Zheng Y., Ding Y., Ni W., Wang Q., Tan L., Wu W., Tang S., Xiong Z. Evaluation of nucleocapsid and spike protein-based enzyme-linked immunosorbent assays for detecting antibodies against SARS-CoV-2. J. Clin. Microbiol. 2020;58(May (6)):e00461–20. doi: 10.1128/JCM.00461-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmud A.S., Taznin T., Sarkar M.M., Uzzaman M.S., Osman E., Habib M.A., Akter S., Banu T.A., Goswami B., Jahan I., Hossain M.S. The genetic variants analysis of circulating SARS-CoV-2 in Bangladesh. BioRxiv. 2020;(January) [Google Scholar]
- Marra M.A., Jones S.J., Astell C.R., Holt R.A., Brooks-Wilson A., Butterfield Y.S., Khattra J., Asano J.K., Barber S.A., Chan S.Y., Cloutier A. The genome sequence of the SARS-associated coronavirus. Science. 2003;300(May (5624)):1399–1404. doi: 10.1126/science.1085953. [DOI] [PubMed] [Google Scholar]
- McCaffrey A.P., Meuse L., Pham T.T., Conklin D.S., Hannon G.J., Kay M.A. RNA interference in adult mice. Nature. 2002;418(July):38–39. doi: 10.1038/418038a. [DOI] [PubMed] [Google Scholar]
- Moniruzzaman M., Hossain M.U., Islam M.N., Rahman M.H., Ahmed I., Rahman T.A., Bhattacharjee A., Amin M.R., Rashed A., Keya C.A., Das K.C. Coding-complete genome sequence of SARS-CoV-2 isolate from Bangladesh by sanger sequencing. Microbiol. Resour. Announce. 2020;9(July (28)):e00626–20. doi: 10.1128/MRA.00626-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito Y., Yoshimura J., Morishita S., Ui-Tei K. siDirect 2.0: updated software for designing functional siRNA with reduced seed-dependent off-target effect. BMC Bioinformatics. 2009;10(December (1)):1–8. doi: 10.1186/1471-2105-10-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oany A.R., Hossain M.U., Ahmad S.A. Computational approach to design a potential siRNA molecule to silence the nucleocapsid gene of different nipah virus strains of Bangladesh. Biores. Commun.-(BRC) 2015;1(1):40–44. [Google Scholar]
- Palliser D., Chowdhury D., Wang Q.Y., Lee S.J., Bronson R.T., Knipe D.M., Lieberman J. An siRNA-based microbicide protects mice from lethal herpes simplex virus 2 infection. Nature. 2006;439(January (7072)):89–94. doi: 10.1038/nature04263. [DOI] [PubMed] [Google Scholar]
- Reynolds A., Leake D., Boese Q., Scaringe S., Marshall W.S., Khvorova A. Rational siRNA design for RNA interference. Nat. Biotechnol. 2004;22(March (3)):326–330. doi: 10.1038/nbt936. [DOI] [PubMed] [Google Scholar]
- Rota P.A., Oberste M.S., Monroe S.S., Nix W.A., Campagnoli R., Icenogle J.P., Penaranda S., Bankamp B., Maher K., Chen M.H., Tong S. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300(May (5624)):1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- Saha S., Malaker R., Sajib M.S., Hasanuzzaman M., Rahman H., Ahmed Z.B., Islam M.S., Islam M.S., Hooda Y., Ahyong V., Vanaerschot M. Complete genome sequence of a novel coronavirus (SARS-CoV-2) isolate from Bangladesh. Microbiol. Resour. Announce. 2020;9(June (24)) doi: 10.1128/MRA.00568-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffelers R.M., Ansari A., Xu J., et al. Cancer siRNA therapy by tumor selective delivery with ligand-targeted sterically stabilized nanoparticle. Nucleic Acids Res. 2004;32(19):e149. doi: 10.1093/nar/gnh140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishir T.A., Naser I.B., Faruque S.M. In silico comparative genomics of SARS-CoV-2 to determine the source and diversity of the pathogen in Bangladesh. PLoS One. 2021;16(January (1)) doi: 10.1371/journal.pone.0245584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers F., Wilm A., Dineen D., Gibson T.J., Karplus K., Li W., Lopez R., McWilliam H., Remmert M., Söding J., Thompson J.D. Fast, scalable generation of high‐quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011;7(1):539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen D.R., Leirdal M., Sioud M. Gene silencing by systemic delivery of synthetic siRNAs in adult mice. J. Mol. Biol. 2003;327(April):761–766. doi: 10.1016/s0022-2836(03)00181-5. [DOI] [PubMed] [Google Scholar]
- Taxman D.J., Livingstone L.R., Zhang J., Conti B.J., Iocca H.A., Williams K.L., Lich J.D., Ting J.P., Reed W. Criteria for effective design, construction, and gene knockdown by shRNA vectors. BMC Biotechnol. 2006;6(December (1)):7. doi: 10.1186/1472-6750-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins S.M., Lo C.Y., Tumpey T.M., Epstein S.L. Protection against lethal influenza virus challenge by RNA interference in vivo. Proc. Natl. Acad. Sci. U. S. A. 2004;101(June):8682–8686. doi: 10.1073/pnas.0402630101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins S.M., Lo C.-Y., Tumpey T.M., Epstein S.L. Protection against lethal influenza virus challenge by RNA interference in vivo. Proc. Natl. Acad. Sci. U. S. A. 2004;101(23):8682–8686. doi: 10.1073/pnas.0402630101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ui‐Tei K., Naito Y., Takahashi F., Haraguchi T., Ohki‐Hamazaki H., Juni A., Ueda R., Saigo K. Guidelines for the selection of highly effective siRNA sequences for mammalian and chick RNA interference. Nucleic Acids Res. 2004;32(February (3)):936–948. doi: 10.1093/nar/gkh247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban-Klein B., Werth S., Abuharbeid S., Czubayko F., Aigner A. RNAi-mediated gene-targeting through systemic application of polyethylenimine (PEI)-complexed siRNA in vivo. Gene Ther. 2005;12(5):461–466. doi: 10.1038/sj.gt.3302425. [DOI] [PubMed] [Google Scholar]
- Werth S., Urban-Klein B., Dai L., Höbel S., Grzelinski M., Bakowsky U., Czubayko F., Aigner A. A low molecular weight fraction of polyethylenimine (PEI) displays increased transfection efficiency of DNA and siRNA in fresh or lyophilized complexes. J. Control. Release. 2006;112(May (2)):257–270. doi: 10.1016/j.jconrel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Woo P.C., Lau S.K., Huang Y., Yuen K.Y. Coronavirus diversity, phylogeny and interspecies jumping. Exp. Biol. Med. 2009;234(October (10)):1117–1127. doi: 10.3181/0903-MR-94. [DOI] [PubMed] [Google Scholar]
- Wu C.J., Huang H.W., Liu C.Y., Hong C.F., Chan Y.L. Inhibition of SARS-CoV replication by siRNA. Antiviral Res. 2005;65(January (1)):45–48. doi: 10.1016/j.antiviral.2004.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N., Wang L., Deng X., Liang R., Su M., He C., Hu L., Su Y., Ren J., Yu F., Du L. Recent advances in the detection of respiratory virus infection in humans. J. Med. Virol. 2020;92(April (4)):408–417. doi: 10.1002/jmv.25674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P., Qin Z.L., Ke J.S., Lu Y., Liu M., Pan W., Zhao L.J., Cao J., Qi Z.T. Small interfering RNA inhibits SARS-CoV nucleocapsid gene expression in cultured cells and mouse muscles. FEBS Lett. 2005;579(April (11)):2404–2410. doi: 10.1016/j.febslet.2005.02.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B.J., Guan Y., Tang Q., Du C., Xie F.Y., He M.L., Chan K.W., Wong K.L., Lader E., Woodle M.C., Lu P.Y. Prophylactic and therapeutic effects of small interfering RNA targeting SARS coronavirus. Antivir. Ther. 2004;9(June (3)):365–374. [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(March (7798)):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Liu M., Zhao W., Zhang J., Zhang X., Wang K., Gu C., Wu K., Li Y., Zheng C., Xiao G. Isolation of virus from a SARS patient and genome-wide analysis of genetic mutations related to pathogenesis and epidemiology from 47 SARS-CoV isolates. Virus Genes. 2005;30(January (1)):93–102. doi: 10.1007/s11262-004-4586-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuker M. On finding all suboptimal foldings of an RNA molecule. Science. 1989;244(April (4900)):48–52. doi: 10.1126/science.2468181. [DOI] [PubMed] [Google Scholar]
- Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31(July (13)):3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.