Graphical abstract
Keywords: COVID-19, SARS-CoV-2, COVID-19 vaccines, Vaccine immune responses, Intranasal COVID-19 vaccines, Intramuscular COVID-19 vaccines
Abbreviations: ACE2, Angiotensin-converting enzyme 2; ADCC, Antibody-dependent cell-mediated cytotoxicity; APC, Antigen presenting cell; BALT, Bronchus-associated lymphoid tissue; COVID-19, Coronavirus disease 2019; CTLs, Cytotoxic T lymphocytes; EUA, Emergency Use Authorisation; FFU, Focus-forming units; GALT, Gut-associated lymphoid tissue; Ifu, Infectious units; IN, Intranasal; IL-5, Interleukin-5; IL-6, Interleukin-6; IM, Intramuscular; MALT, Mucosa-associated lymphoid tissue; M Cells, Microfold cells; MERS, Middle Eastr Respiratory Syndrom; NALT, Nasopharynx-associated lymphoid tissue; NKC, Natural killer cells; PFU, Plague-forming units; RBD, receptor-binding domain; RdRp, RNA-dependent RNA polymerases; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; TCR, T-cell receptor; Th2, T helper 2 cells; TLR, Tall-like receptors
Abstract
It is striking that all marketed SARS-CoV-2 vaccines are developed for intramuscular administration designed to produce humoral and cell mediated immune responses, preventing viremia and the COVID-19 syndrome. They have a high degree of efficacy in humans (70–95%) depending on the type of vaccine. However, little protection is provided against viral replication and shedding in the upper airways due to the lack of a local sIgA immune response, indicating a risk of transmission of virus from vaccinated individuals.
A range of novel nasal COVID-19 vaccines are in development and preclinical results in non-human primates have shown a promising prevention of replication and shedding of virus due to the induction of mucosal immune response (sIgA) in upper and lower respiratory tracts as well as robust systemic and humoral immune responses. Whether these results will translate to humans remains to be clarified. An IM prime followed by an IN booster vaccination would likely result in a better well-rounded immune response, including prevention (or strong reduction) in viral replication in the upper and lower respiratory tracts.
1. Introduction
Many human pathogens enter the human organism via a mucosal site such as the gastrointestinal mucosa (e.g., poliovirus, Vibrio cholerae, HIV-1), genital mucosa (e.g., human papilloma virus (HPV), HIV-1) and respiratory mucosa (e.g., influenza virus, Mycobacterium tuberculosis, coronavirus, adenovirus, rhinovirus, respiratory syncytial virus (RSV) (Belyakov and Ahlers, 2009). Some mucosal pathogens can spread to systemic sites by entering the blood circulation, whereas others only develop the disease at a local site such as for HIV-1.
The innate mucosal immune system present in humans has evolved to protect humans from invading pathogens, by specifically recognizing and eliminating harmful species. The innate mucosal immune system comprises a variety of recognition receptor molecules (e.g., TLRs, NOD-like receptors), which after activation can effectively recognize invading pathogens and generate an immune response that prevents or limits pathogen entry and neutralises any adverse reactions such as tissue damage. Furthermore, it regulates the adaptive response in cases of severe infection and also helps generate a memory response (Aich and Dwivedy, 2011, Belyakov and Ahlers, 2009). A comprehensive review (Poland et al., 2020) discusses in detail the innate immune response in patients infected with the coronavirus SARS-CoV-2 and the effect of age, sex, ethnicity and disease severity on the human humoral and cellular immune responses. It has been shown that patients infected with the virus develop IgM, IgA and IgG antibody responses together with CD4 + and CD8 + T-cells responses within 1–2 weeks after infection, the longevity of which are dependent on the factors listed above.
In humans, the airways are highly prone to the risk of viral infections which can be the cause of seasonal epidemics or even pandemics and thereby pose a severe health risk to the world’s population, especially those with underlying medical conditions or those of certain ethnicities. For example, one of the most widespread viral infections is caused by the influenza virus which exists as four types, A, B, C and D. It is, however, the influenza virus A and B that are the cause of seasonal epidemics every year and only influenza A virus is known to cause flu pandemics. Pandemics generally occur when a variant influenza A virus emerges that is highly infective and with the ability to efficiently transmit between people (Rose et al., 2012).
Influenza A viruses are normally characterized by two proteins on the surface of the virus: hemagglutinin (H) and neuraminidase (N) with 18 different hemagglutinin subtypes and 11 different neuraminidase subtypes. Subtypes of influenza A viruses seasonally circulating in people include: A(H1N1) and A(H3N2). The A(H1N1) viruses appeared in the spring of 2009 causing a flu pandemic with a morbidity of about 200,000 people around the world. This virus, called the “A(H1N1)pdm09 virus”, or in common terms “2009 H1N1”, has since continued to circulate in the population and has undergone relatively limited genetic changes and changes to their antigenic properties that affect immunity over time.
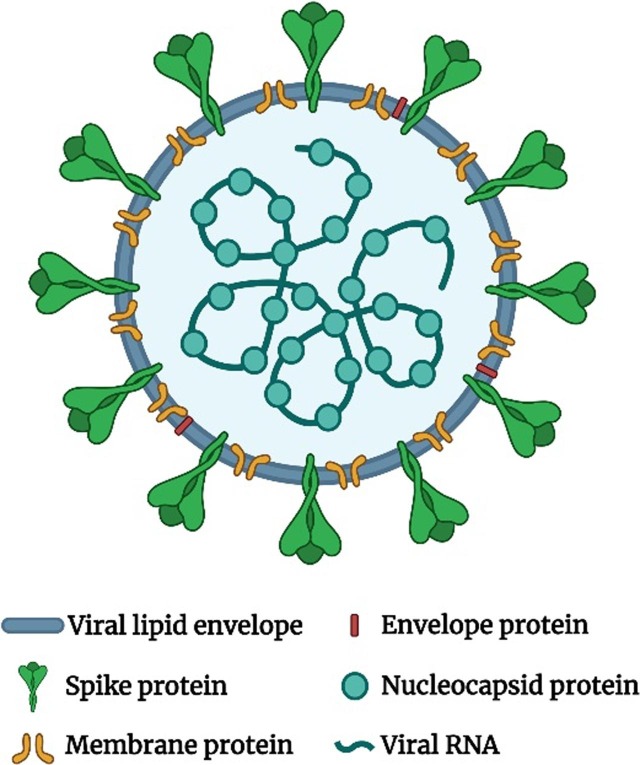
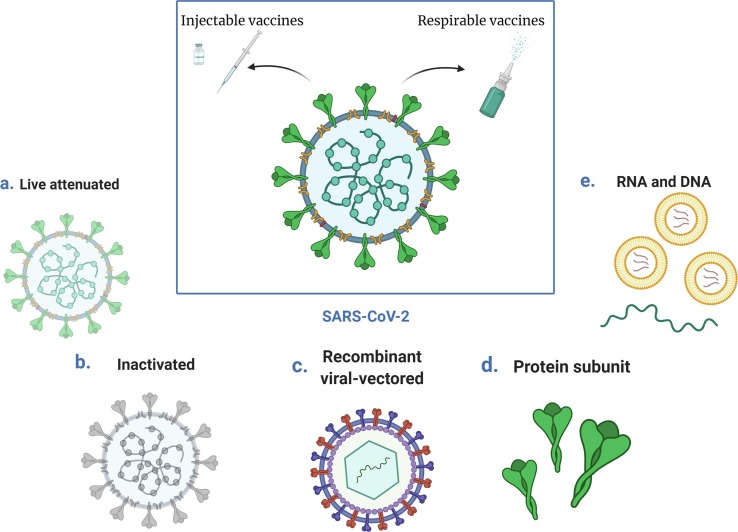
The COVID-19 pandemic, that started in Wuhan, China in the end of 2019, was caused by the transmission of “severe acute respiratory syndrome coronavirus 2” the so-called SARS-CoV-2 virus. SARS-CoV-2 is a member of the coronavirus family which can cause common colds and the more fatal Middle East respiratory syndrome (MERS). The SARS-CoV-2 is a positive-sense single-stranded RNA (+ssRNA) virus with a single linear RNA segment. The genome of CoV is the largest RNA genome (26.4–31.7 kilobases) of all known RNA viruses (Woo et al., 2009). Each virion is from 50 to 200 nm in diameter and comprises four different structural proteins, namely S (spike), E (envelope), M (membrane) and N (nucleocapsid), where the N protein surrounds the RNA genome and the S, E and M proteins form the viral envelope (Fig. 1 ).
Fig. 1.
The structure of SARS-CoV-2 virion.
The S protein (a glycoprotein) forms homo trimeric spikes on the virion and is responsible for the ability of the virus to attach to and fuse with the membrane of the host cell, engaging the cell surface receptor angiotensin-converting enzyme 2 (ACE2), and thereby allowing it cell entry (“Coronaviruses - a general introduction”; Letko et al., 2020, Wu et al., 2020). SARS-CoV-2 is efficiently transmitted from person to person and therefore rapidly spread across all continents. The transmission of the virus occurs via respiratory droplets from cough and sneezes, from speaking and also at least indoors with air flow, suggesting that the virus may be airborne (“239 Experts With One Big Claim: The Coronavirus Is Airborne - The New York Times”, “Talking is worse than coughing for spreading COVID-19 indoors | Live Science”). It has been shown that the nasal epithelium has the highest concentration of ACE2 and the lowest is found in the alveoli (Hou et al., 2020). Hence, it is to be expected that the replication of the virions mostly takes place in nasal mucosa (Sims et al., 2005) and furthermore in the salivary gland ducts that also are rich in the expression of ACE2 (Liu et al., 2011).
The SARS-CoV-2 has a high mutation rate because of the error prone RdRp (RNA-dependent RNA polymerases) of the virus which is responsible for the duplication of genetic information. Hence, the virus is prone to create variants of the virus, of which the most prominent at present are a) the UK (or Kent) variant known as B.1.1.7, which show several mutations and especially one in the S protein that causes the virus to bind more tightly to the ACE2 receptor; b) the South African variant known as B.1.351, also with mutations in the S protein and c) the Danish variant appearing in minks and mink farmers with four changes in the spike protein which makes the virus moderately resistant to neutralizing antibodies, d) the Brazilian virus, known as P1, that is feared to be more contagious than the original virus and very recently the Indian variant that appears to have two mutations (“Science Brief: Emerging SARS-CoV-2 Variants | CDC”, “WHO | SARS-CoV-2 mink-associated variant strain – Denmark”).
In order to combat such viral infections, developed countries at least, have immunization programmes for yearly vaccination, for example against influenza, with most emphasis on vaccination of the older part of the population. This is also reflected in the current situation with the COVID-19 pandemic where at least the developed countries presently are competing to vaccinate as quickly as possible their most vulnerable subjects. For example, the UK has managed to vaccinate more than 30 million people over a period of 4 months (Jan-April 2021) which has taken planning, co-ordination and investment of a magnitude only previously seen in wartime. So far, all the approved vaccines are by intramuscular (IM) injection only, although different research institutions are working on development of an intranasal (IN) SARS-CoV-2 vaccine. Ideally a vaccine, at least against mucosal pathogens, should induce not only systemic but also mucosal immune responses and while until recently it has been the general understanding that parenteral vaccines are poor inducers of mucosal immunity, and hence would be expected to be less effective against mucosal antigens, this concept has now been challenged. It has become evident through numerous studies for at least some mucosal pathogens (e.g., influenza virus and poliovirus) that vaccines can induce mucosal immune responses after systemic vaccination (especially if an effective vaccine formulation is developed) showing high titres of neutralising antibodies capable of preventing disease (Clements and Freytag, 2016, De Haan et al., 2001, Herremans, et al., 1999). However, whether this is the case for the present IM COVID-19 vaccines has not been fully evaluated.
In general, for mucosally transmitted infections, such as for influenza and SARS-CoV-2 viruses, it is considered highly attractive to administer vaccines via the nasal route, since this route has the advantage of inducing both a systemic and a strong local mucosal immune response. Furthermore, for IN administration there is no requirement for specialised medical personal to administer the dose, hence the product should have a higher patient compliance. This is beneficial especially in less developed countries and hence nasal immunisation is a more cost effective and efficient means of delivering vaccines in a time of pandemics. So far, the intranasal influenza vaccines Fluenz Tetra™, licensed in EU for children between 2 and 18 years of age, and FluMist Quadrivalent, licensed in USA and Canada for persons between 2 and 49 years, are tetravalent cold adapted live attenuated influenza vaccines produced by Medimmune/AstraZeneca, UK, respectively (FluMist Quadrivalent | FDA [WWW Document]. URL https://www.fda.gov/vaccines-blood-biologics/vaccines/flumist-quadrivalent (accessed 3.22.21)). The yearly vaccine strains are based on recommendation from the WHO, but basically contains two A strains and two B strains. The IN spray is applied with 0.1 mL of liquid vaccine in each nostril. Furthermore, a similar live attenuated (trivalent) nasal flu vaccine (Nasovac-S) has been developed and marketed in India by CiplaMed in collaboration with the Serum Institute of India (Kulkarni and Raut, 2013).
Several reviews have in the last twenty years dealt with nasal versus injectable vaccines in general and the correspondent immune responses, among others, Van Ginkel et al. (2000); Davis, 2001, Jabbal-Gill, 2010, Borges et al., 2010, Rose et al., 2012, Kraehenbuhl and Neutra, 2013, Yusuf and Kett, 2017; Mato (2019); Hellfritzsch and Scherließ (2019). Few has dealt in particular with the SARS-CoV-2 virus, to mention Isho, et al., 2020, Ludwig and Zarbock, 2020, Jeyanathan et al., 2020, Dong et al., 2020.
The present review sets out to evaluate IN vaccination as an alternative to IM administration of vaccines particularly related to the current SARS-CoV-2 pandemic and the existing SARS-CoV-2 vaccines either already marketed or in the pipeline for approval within the foreseeable future. Of special interest is the difference in immune responses between IN and IM SARS-CoV-2 vaccines and any potential toxicological issues for IN vaccines. The review will also discuss formulation aspects of such vaccines and touch upon the immune system of the upper respiratory tract and the immune response versus that after an IM injection of the vaccine.
1.1. The mucosal immune system
Humans should theoretically be protected against pathogens entering the body through mucosal membranes by the mucosal immune system, also called the mucosa-associated lymphoid tissue (MALT) which is situated in the mucosal tissues of the nose, lungs, gastrointestinal tract, vagina, and rectum. The MALT encompasses proximal structures that, dependent on the location, are named the e.g., nasopharynx-associated lymphoid tissue (NALT), the bronchus-associated lymphoid tissue (BALT) and the gut-associated lymphoid tissue (GALT) (Brandtzaeg et al., 2008). Therefore, mucosal immunity often is best induced by administration of vaccines by a mucosal route since mucosal immunisation generally, if an optimal vaccine formulation is developed, will result in both a mucosal and a systemic immune response (Borges et al., 2010). Of the various routes of mucosal administration, the nasal and the oral routes are the most acceptable and accessible, but due to the hostile gastrointestinal environment, where the antigen can potentially be degraded or denaturated, and the dilution by intestinal content requiring high doses of antigenic material and specialised vaccine formulations, the nasal route is preferential to the oral.
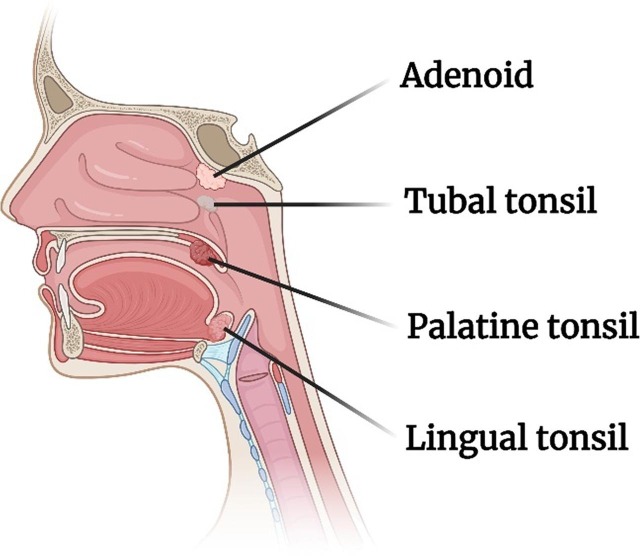
1.1.1. Nasal associated lymphoid tissue (NALT)
In humans the nasal lymphoid tissue is situated in the oropharynx and described as a ring of tissues (Waldeyer’s ring), comprising the nasopharyngeal adenoids (or tonsils), the paired tubal tonsils and the paired palatine and lingual tonsils (Fig. 2 ).
Fig. 2.
Pharyngeal lymphoid tissue of Waldeyer’s ring.
The adenoids are similar to the Peyer’s patches in the intestines in that they contain aggregates of lymphoid tissue. The NALT is strategically placed in the nasopharynx and oropharynx areas so that they can be exposed not only to airborne antigens but also alimentary antigens. Furthermore, the epithelial surface of the NALT invaginates into valleys, the so-called crypts that increases the area for antigen interaction and for retainment. M−like cells (or microfold cells) are located in these crypts (Brandtzaeg, 2011, Cesta, 2006). It should also be noted that the epithelial cells are covered with mucus that acts as a barrier to invasion of pathogens and cilia that through the mucociliary clearance mechanism may quickly transport the pathogens down the esophagus.
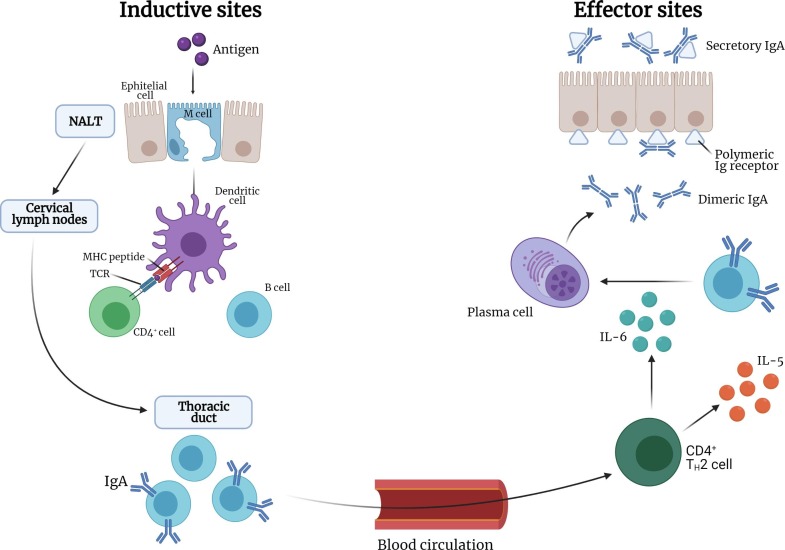
Antigens reaching the nasal mucosa can be transported to the NALT. Soluble antigens can penetrate between epithelial cells and reach the antigen-presenting cells (APC) such as macrophages and dendritic cells whereas particulate antigens are transported across the epithelium via M−like cells (or microfold M−cells) that are present in the epithelial cell layer overlying the NALT. The APC process and present the antigen to the T cells e.g., CD4 + T cells in the lymphoid tissue that can then induce IgA-committed B-cell development in the lymphoid follicle. The B-cells migrate from the NALT to the regional cervical lymph nodes via the efferent lymphatics and then the antigen specific CD4 + cells and IgA + B cells migrate to the nasal passage through the thoracic duct and the blood circulation. The IgA + B cells then, in the presence of cytokines (e.g., IL-5 and IL-6 produced by T helper cells), differentiate into IgA producing plasma cells that create dimeric forms of IgA which subsequently become secretory IgA by binding to polymeric Ig receptors present on the epithelial mucosal cells. This secretory IgA is then released into the nasal mucosal surface. Specific neutralising IgG (antibodies) are also present within the mucosal tissues derived from local plasma cells or from blood by diffusion from local fenestrated epithelia (Fig. 3 ) (Kiyono and Fukuyama, 2004).
Fig. 3.
Antigen processing pathway of the NALT.
Hence, as has been reported by some researchers, after an appropriate antigen stimulation of the NALT, both a potent humoral and cellular immune response is normally elicited both at a mucosal and systemic level (Rose et al., 2012; Van Ginkel et al., 2000a). The antigens reaching the NALT are met with two different defence mechanisms involving antibodies namely the production of secretory IgA which helps in preventing further viral infection and IgG antibodies which can neutralize viruses that are generated in the mucosa.
As indicated above, secretory IgA is an important effector molecule for protecting the mucosal surface, however, the contribution of the cellular immune system in this defence should not be underestimated. A cell-mediated immune response has a strategic advantage, as opposed to an antibody-mediated immune response, in that T cells can recognize peptides from the core proteins of for example influenza virus and that the core proteins are normally expressed and presented earlier during infection than proteins that are targeted for neutralising antibodies, as for example is the case for hemagglutinin and neuraminidase of influenza virus (Van Ginkel et al., 2000a). Two mechanisms are involved in the killing of infected cells that entail specific cytotoxic T lymphocytes (CTLs) or antibody-dependent cell-mediated cytotoxicity (ADCC), a collaboration between natural killer (NK) cells and antibodies. It should be noted that vaccination by a mucosal route such as the nasal can induce generalized mucosal immune responses, not only at the nasal mucosa but also at distant mucosal effector sites (Belyakov and Ahlers, 2009).
2. Vaccine design approaches
2.1. SARS-CoV-2 antigen selection
The SARS-CoV-2 S protein binds primarily to the ACE2 receptors to mediate viral entry, in the upper and lower respiratory tracts. The mature S protein is a trimeric class I fusion protein located on the surface of the virion. It possesses two fragments, the S1 containing the receptor binding domain (RBD) and the S2 containing the fusion peptide. Different studies with monoclonal antibodies have demonstrated that infected humans develop robust neutralizing antibodies against the S protein and in particular against the S1 fragment with the receptor-binding domain (RBD) of the SARS-CoV-2 (Baum et al., 2020, Hansen et al., 2020, Ju et al., 2020). In early studies for SARS-CoV-2 vaccines, the N protein was also evaluated for effectiveness but, using in vivo models, N-based vaccines resulted in no protection. Furthermore, they showed an exacerbation of the infection due to increased pulmonary eosinophilic infiltration (Deming et al., 2006). M and E proteins are of less interest as vaccine targets due to lower immunogenicity (Du et al., 2008).
2.2. Vaccine platforms
Advances in virology, molecular biology and immunology have created many alternatives to traditional vaccine approaches. More than 100 vaccine candidates against the SARS-CoV-2 virus are currently in development (“Vaccines – COVID19 Vaccine Tracker”), based on several different platforms (Fig. 4 ). These platforms can be divided into “traditional” approaches (i.e., live attenuated or inactivated virus vaccines) and “innovative approaches” such as RNA or DNA vaccines and recombinant viral-vectored vaccines.
Fig. 4.
Vaccine platforms under development against SARS-CoV-2.
2.2.1. Live attenuated viral vaccines
Live attenuated vaccines derive directly from the pathogenic viruses that still possess the ability to infect cells and replicate but are treated in order to cause no or only very mild disease. The attenuation can be completed by growing the virus at unfavourable conditions such as at non-optimal temperature or by rational modification of the virus genome (e.g., codon de-optimization, removal of genes responsible for counteracting innate immune recognition (Broadbent et al., 2016, Talon et al., 2000)). However, these techniques are time-consuming and technically challenging, resulting in a difficult and long development. Being nearly identical to the natural virus causing the infection, a live attenuated virus usually creates a strong and long-lasting humoral and cell-mediated immune response after a prime/boost vaccination regimen. Moreover, since the virus is replicating after the vaccination, the immune response is targeting both structural and non-structural viral proteins, widening the humoral and cellular immune responses without the use of adjuvants since these vaccines already contain naturally occurring adjuvants (Lee and Nguyen, 2015). This type of vaccine can be given intranasally to induce a mucosal immune response such as in the case of the quadrivalent influenza vaccine against A(H1N1), A(H3N2) and two influenza B viruses available in the market with the brand name FluMist Quadrivalent (“FluMist Quadrivalent | FDA”). It is easily administered as 0.2 mL suspension supplied in a single-dose pre-filled intranasal spray device to be divided approximately one-half into each nostril.
2.2.2. Inactivated viral vaccines
In inactivated viral vaccines the whole disease-causing virus or a part of it (where the genetic material has been wrecked) is usually present. Compared to live attenuated viral vaccines, they are considered safer and more stable and although their genetic material has been destroyed, they still contain many antigenic proteins and hence, as in the case for coronaviruses (e.g. SARS-CoV-2), the immune responses are likely to target many different proteins such as the S but also M, E, and N. Inactivated vaccines only stimulate antibody-mediated responses, which can be weaker and less long-lived, as compared to live attenuated vaccines, and hence, inactivated vaccines are often administered alongside adjuvants and also booster doses may be required. The vaccine production requires biosafety level 3 facilities in which the virus is grown in a cell culture (usually Vero cells) followed by the inactivation. The productivity of the virus in cell culture could affect the final production yield (Yadav et al., 2021). This type of vaccine has proven to be safe and effective in the prevention of diseases like polio and influenza (https://www.who.int/vaccine_safety/initiative/tech_support/Part-2.pdf - accessed March 22, 2021).
2.2.3. Recombinant viral-vectored vaccines
Viral vector-based vaccines (in the form of a modified harmless version of an alternative virus) use a modified virus (the vector) to deliver the genetic code (RNA or DNA) for an antigen, (e.g., in the case of COVID-19 the S protein) into human cells which then will produce the antigen. Infecting the cells and instructing them to produce the antigen, this type of vaccine mimic a natural viral infection in order to generate the requested immune response (Rollier et al., 2011). This mechanism induces a strong cellular immune response by T cells as well the production of antibodies by B cells. The viral vectors are grown in cell lines and their production is quick and easy (Sebastian and Lambe, 2018).
Viral vectors can be replicating and non-replicating. Replicating viral vectors possess the ability to replicate and thus they can produce new viral particles providing a continuous source of vaccine antigens for prolonged periods. This results in a stronger immune response with a single dose compared to the non-replicating viral vectors. Replicating viral vectors are selected so that the virus cannot cause a disease whilst infecting the host. They typically derive from attenuated viruses engineered to express the specific antigen protein such as the S protein for COVID-19 vaccine. On the other hand, non-replicating viral vectors do not retain the ability to make new viral particles because the key viral genes for the replication have been previously removed. The most common approaches of this vaccine type are based on an adenovirus delivered intramuscularly. As an advantage of viral vectored vaccines, their production does not require the use of live pathogen viruses, the vectors can be easily produced in large quantities showing a good stimulation of both B and T cell responses in vivo (Zhu et al., 2020a). As a disadvantage, pre-existing vector immunity can neutralize the vaccine efficacy. However, this problem can be easily avoided by using vectors that are rare in humans (Mercado et al., 2020), derived from animals (Folegatti et al., 2020) or viruses that do not generate much immunity. Moreover, as vector immunity can be problematic during the second dose in a prime-boost regimen, the use of two different viral vectors during the two doses can help avoiding this problem. Nevertheless, in this case, vaccine antigen can only be produced as long as the initial vaccine remains in infected cells, resulting in a generally weaker immune response. Booster doses are likely to be required.
An example of a viral vector vaccine is the recombinant, replication-competent rVSV-ZEBOV vaccine against Ebola (Marzi et al., 2011) approved by FDA in 2019. It consists of vesicular stomatitis virus (VSV) genetically modified to express the main glycoprotein from the Zaire ebolavirus. It is a suspension administered intramuscularly with a single dose (https://www.fda.gov/media/133748/download - accessed March 22, 2021).
2.2.4. Protein subunit vaccines
Protein subunit vaccines (also called acellular vaccines) do not contain any whole virus, but instead purified antigenic fragments such as isolated proteins (e.g., the S protein on the SARS-CoV-2 virus) specifically selected because of their capacity to stimulate the immune system.
Many different antigens can be selected to develop acellular vaccines such as specific isolated proteins from viral or bacterial pathogens, chains of sugar molecules (polysaccharides) found in the cell walls of some bacteria or a carrier protein binding a polysaccharide chain in order to boost the immune response. Acellular vaccines are generally considered very safe since they cannot cause the disease. The immune response usually is not as robust as for live attenuated vaccines, hence, booster doses are most often required. A possible disadvantage of this type of vaccine is that isolated proteins could be denatured and thus bind to different antibodies than the protein of the pathogen. In the case of SARS-CoV-2, the antigenic proteins used are the S protein or the RBD. The advantage of this type of vaccine is that live virus is not handled. Commonly used protein subunit vaccines are the acellular pertussis (aP) vaccines that contain the inactivated pertussis toxin detoxified either by treatment with a chemical or by using molecular genetic techniques (https://www.who.int/vaccine_safety/initiative/tech_support/Part-2.pdf - accessed March 22, 2021). To improve the efficacy of this vaccine, alum is added as adjuvant to promote a stronger antibody response. (Allen and Mills, 2014). Another acellular vaccine is against Hepatitis B containing the hepatitis B virus surface antigen (HBsAg) produced with recombinant technology. Even this vaccine contains aluminium phosphate or aluminium hydroxide as adjuvant to boost the immune response after the administration (https://www.who.int/vaccine_safety/initiative/tools/Hep_B_Vaccine_rates_information_sheet.pdf - accessed March 22, 2021).
2.2.5. RNA and DNA vaccines
Nucleic acid-based vaccines follow a different strategy compared to the other vaccines. Instead of directly providing the protein antigen to the body, they deliver the genetic code of the antigen to the cells in the body instructing the cells to produce the antigen that then will stimulate an immune response. This type of vaccines is quick and easy to develop and are the most promising vaccines for the future. They are divided into RNA- and DNA-based vaccines. RNA vaccines use messenger RNA (mRNA) or self-replicating RNA normally formulated in a particulate carrier such as a lipidic bilayer membrane (liposome). This formulation protects the mRNA when it first enters the body and helps cell internalization (Pardi et al., 2015). Higher doses are required for mRNA than for self-replicating RNA, which amplifies itself. When the mRNA is inside the cells, it can be translated into the antigen protein by ribosomes to start the stimulation of the immune response. Then the mRNA is naturally broken down and removed by the body. A main advantage of this technology is that the vaccine can be produced completely without the use of cell cultures, however, the long-term storage stability is challenging since it requires frozen storage. RNA-based vaccines are usually administered by injection and are therefore unlikely to induce strong mucosal immunity (Pardi et al., 2018).
Being more stable than mRNA/RNA, DNA do not require to be formulated in particulate carriers. They are based on plasmid DNA that can be produced at large scale in bacteria. The DNA contains mammalian expression promoters and the specific gene that encodes for the antigen (e.g., the spike protein) produced after the uptake in the cells of the vaccinated person. To be delivered, they usually need delivery strategies such as electroporation that help the DNA cellular uptake. Both these technologies based on nucleic acids are the latest frontier of vaccination and up till now two different mRNA vaccines have been approved for human use (i.e., Moderna and Pfizer/BioNTech (Baden et al., 2021, Polack et al., 2020)) meanwhile the most advanced DNA vaccine so far is the INO-4800 from Inovio that has entered Phase 2/3 clinical trials (“Safety, Immunogenicity, and Efficacy of INO-4800 for COVID-19 in Healthy Seronegative Adults at High Risk of SARS-CoV-2 Exposure - Full Text View - ClinicalTrials.gov”).
2.3. Adjuvants
Many vaccine formulations contain an adjuvant or adjuvants combinations that enhance the immune response to the vaccination. The word “adjuvant” means “to help/aid”, and initially adjuvants were used only to increase the immunogenic potential of purified antigens. Not all the types of vaccines need an adjuvant such as the live attenuated virus that possess naturally occurring adjuvants. In recent years, by knowing and understanding the immunology of vaccination, the role of adjuvants has expanded (Pasquale et al., 2015).
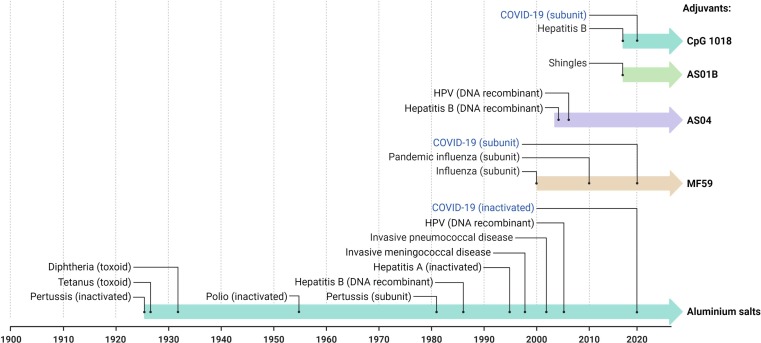
The first adjuvants authorized (nearly 70 years ago) for human use were aluminium salts (e.g., aluminium hydroxide, aluminium phosphate, aluminium potassium sulphate (alum)). They are still the most widely used because of their wide-spectrum ability to strengthen immune responses and their safety. They act primarily to increase antibody production with an immune mechanism that remains incompletely understood (Lee and Nguyen, 2015).
Newer adjuvants have been developed to target specific components of the body’s immune response such as the tall-like receptors (TLR) that, when triggered, stimulate the production of pro-inflammatory cytokines/chemokines and type I interferons that increase the host’s ability to eliminate the pathogen. Adaptive immunity is developed immediately after the innate immune response so that the protection against disease is stronger and lasts longer (Steinhagen et al., 2011).
Among new adjuvants already licensed, AS04 (Didierlaurent et al., 2009) is a mixture of monophosphoryl lipid A that act as TLR4 agonist and aluminium salt, MF59 (Liang et al., 2020) is an oil in water emulsion composed of squalene that act by improving antigen uptake, recruiting immune cells and promoting the migration of activated APS, AS01B (Alving et al., 2012) is a liposomal combination of monophosphoryl lipid A and a natural compound extracted from the Chilean soapbark tree (i.e., QS-21), and Cytosine phosphoguanine (CpG) (Liang et al., 2020) that is a synthetic form of DNA that mimics bacterial and viral genetic material acting as TLR9 agonist. Different examples of vaccines that uses adjuvants are reported in Fig. 5 .
Fig. 5.
Timeline of the main adjuvants used in human vaccines.
3. Marketed injectable SARS-CoV-2 vaccines
So far, at the time of writing this review, ten SARS-CoV-2 vaccines have been fully approved or approved under Emergency Use Authorisation (EUA) (or similar) by the regulatory authorities and distributed for use in various countries such as EU, UK, Russia, USA, India and China. The marketed injectable vaccines are listed in Table 1 .
Table 1.
Approved injectable COVID-19 vaccines.
| Developer name | Code name | Vaccine type | ImmunisationSpecifics | Efficacy | Storage Conditions |
|---|---|---|---|---|---|
| Moderna/NIAID, USA | mRNA-1273 | mRNA (Lipid nanoparticles) | Expressing S protein - Dose and booster dose IM | After 2nd dose 95.6% in 18–65 year group and 86.4% in over 65 year group.Overall 94.1% | −25 to −15 °COpened vials: 2 – 25 °C for 6 h |
| BioNTech/Pfizer, Germany/USA | BTN162b2/Comirnaty | mRNA (Lipid nanoparticles) | Expressing S protein - Dose and booster dose IM | 95% after 2nd dose | −80 to−60 °CApplication to FDA to change to −25 to −15 °C |
| AstraZeneca/Oxford Jenner Inst., UK | AZD1222 | Non-replicating viral vector (ChAdOx1) | Expressing S proteinDose and booster dose IM | 70% an average from two different dosing regimens, against severe/critical about 100 % | 2 – 8 °C for up to 6 months |
| Gamaleya Research Institute, Russia | Sputnik V/Gam-COVID-Vac | Non-replicating viral vector (Ad26/Ad5) | Heterologous Ad26 prime/Ad5 boost doses IM | Greater than 90% Full trial results not published | Suspension at − 18 °C / Lyophilised at 2 °C – 8 °C |
| Johnson & Johnson/Janssen Pharmaceuticals, USA/Belgium | Ad26.COV2.S | Non-replicating viral vector (Ad26) | Expressing S protein.Single dose IM | Against moderate -severe/critical COVID-19 at 28 days, 66% and against severe/critical at 28 days 85.4% | 2 – 8 °C |
| CanSino Biological/Beijing Institute of Biotechnology/Academy of Military Medical Sciences, China | Ad5-nCoV | Non-replicating viral vector (Ad5) | Expressing S protein.Single dose IM | Against moderate -severe/critical COVID-19, 65.7% and against severe/critical 74.8% | 2 – 8 °C |
| Sinopharm CNBG/Beijin Institute of Biological Products, China | BBIBP-CorV | Inactivated SARS-CoV-2 virus | Multiple viral antigens -Dose and booster dose IM | Phase 3 studies not publishedSinopharm: 79%UAE: 86% | 2 – 8 °C |
| Bharat Biotech/Indian Council Medical Res./National Institute of Virology, India | Covaxin®/BBV152 | Inactivated SARS-CoV-2 virus | Multiple viral antigens - Dose and booster dose IM | 80.6%Interim Phase 3 data Full trial data not published | 2 – 8 °C |
| Sinovac Biotech, China | CoronaVac® | Inactivated SARS-CoV-2 virus | Multiple viral antigens - Dose and booster dose IM | 78% for mild cases but later changed to 50% | 2 – 8 °C |
| Anhui Zhifei Longcom Biopharm/Chinese Academy of Medical Sciences, China | ZF2001 | Protein subunit | SARS-CoV-2 RBD-dimer – 3 doses | Data not published | 2 – 8 °C |
3.1. Pfizer/BioNTech COVID-19 vaccine
The BioNTech/Pfizer COVID-19 vaccine was the first vaccine to be approved by regulatory authorities (in the Western world) 2nd December 2020, first in the UK as a temporary marketing authorisation, then in US with an Emergency Use Authorisation (EUA) (11th December 2020) and then in EU with a conditional marketing authorisation (21st December 2020) for active immunisation by IM injection to prevent coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in individuals 16 years of age and older. The BioNTech/Pfizer vaccine is a nucleic acid-based vaccine supplied as a frozen suspension in a multiple dose vial (5–6 doses) stored at between −80 °C and −60 °C. Before use, each vial is diluted with 1.8 mL of sterile 0.9% sodium chloride injection, USP and can be stored at between 2 °C and 25 °C for no more than 6 h (FDA full emergency use authorisation (EUA) prescribing information, 2021). Data for storage at −25 °C and −15 °C have been submitted to the FDA and if approved will allow storage at this temperature range for a total of two weeks (“Pfizer and BioNTech Submit COVID-19 Vaccine Stability Data at Standard Freezer Temperature to the U.S. FDA Nasdaq:BNTX”). The vaccine, code-named BTN 162b2, is administered IM as a series of two doses (0.3 mL each) three weeks apart.
Each dose contains 30 mg of a nucleoside-modified messenger RNA (mRNA) encoding the trimerized receptor-binding domain (RBD) of the viral full-length spike (S) glycoprotein of SARS-CoV-2 and is formulated in a lipid nanoparticle formulation (Walsh et al., 2020).
The approval of the vaccine is based on a range of preclinical studies and Phase 1, 2 and 3 clinical studies comprising formulation, dose range, and age group efficacy studies. For example, in a phase 1 and 2 clinical study it was found that the vaccine induced robust S protein-specific antibody and CD4 + and CD8 + T cell responses after two repeated vaccine injections (Mulligan et al., 2020, Sahin, et al., 2020). In a Phase 2/3 clinical study, approximately 44,000 volunteers of 12 years and older were given two doses of the BTH162b2 vaccine 21 days apart or a saline placebo injection and assessed for safety and efficacy of the vaccine. The age groups were 12–15 years (0.3%), 16–17 years (0.4%), 16–64 years (77.9%), 65–74 years (17.4%) and more than 75 years (4.4%), and similar distribution for the placebo group. In terms of vaccine efficacy measured as first COVID-19 occurrence from day 7 after the second vaccine dose, it was found that in all subjects, the occurrence of infection in the treatment group was 9 subjects out of 19,965 and in the placebo group 169 subjects out of 20,172, giving a similar vaccine efficacy of 94.6% in all age groups. The safety profile of the vaccine was characterised by short-term, mild-to-moderate pain at the injection site, fatigue and headache. The occurrence of serious side effects was low and similar to the placebo group (Fact sheet for healthcare providers administering vaccine (Vaccine Providers)), n.d.; Polack et al., 2020).
3.2. AstraZeneca/Oxford Jenner Inst COVID-19 vaccine
The AstraZeneca/Oxford Jenner Institute COVID-19 vaccine was approved the 30th December 2020 as a conditional marketing authorisation (CMA) by the MHRA in the UK and as a CMA in the EU by EMA the 29th January 2021 for active immunisation to prevent coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 in individuals 18 years of age and older. Approval in the USA is pending. The vaccine (ChAdOx1-S) is supplied as a ready-made aqueous suspension for IM injection. Each multidose vial contain 8 × 0.5 mL doses with not less than 2.5 × 108 infectious units and can be stored for six months at 2 °C to 8 °C and when opened for no more than 48 h at the same temperature. The vaccination regimen is two separate doses of 0.5 mL each with an interval of 4–12 weeks between doses.
The AstraZeneca COVID-19 vaccine works by delivering the genetic code of the SARS-CoV-2 spike protein to the body’s cells, that will produce the antigen (i.e., the S-glycoproteins). It is a monovalent vaccine comprising a single recombinant replication-deficient chimpanzee adenovirus vector encoding the full-length SARS-CoV-2 spike glycoprotein gene (DNA), where the immunogen in the vaccine is expressed in the trimeric pre-fusion conformation. After administration, the S glycoprotein is expressed locally and able to stimulate the production of neutralising antibody (humoral response) and cellular immune responses.
The conditional approval of the COVID-19 vaccine was based on a range of preclinical and phase 1, 2 and 3 clinical studies evaluation safety and efficacy of the vaccine of which some results are described here. A recently reported phase 1/2 clinical study in 5,258 healthy volunteers of age 18–55 years were administered either ChAdOx1 nCoV-19 at a dose of 5 × 1010 viral particles or the meningitis vaccine control (MenACWY) as a single IM injection whereas ten participants also received a booster dose 28 days after the first ChAdOx1 nCoV-19 dose. There were no serious adverse events related to ChAdOx1 nCoV-19. It was found that the vaccine induced a spike-specific T-cell responses that peaked on day 14, whereas a potent anti-spike IgG response rose by day 28 and were augmented following a second dose. The trial did not show to what extent both CD4 + and CD8 + T cell subsets were activated (Folegatti et al., 2020). Vaccine efficacy was found to be 62.6% in subjects receiving two recommended doses with any dose interval between 3 and 23 weeks with no cases of COVID-19 hospitalisation in subjects who received two doses of the COVID-19 vaccine as compared to eight in the control.
A single blind, randomised, controlled phase 2/3 clinical in healthy volunteers of 18 years and older were divided in age groups of 18–55 years, 56–69 years and 70 years and older. In a low-dose cohort subjects received either IM ChAdOx1 nCoV-19 (2.2 × 1010 virus particles) or a control vaccine (MENACWY) using a complicated block randomisation and stratified by age and dose group and study site. Secondly, subjects were recruited to the standard dose cohort (3.5 × 1010 virus particles) and a similar randomisation procedure. The specific aim of the study was to assess the safety and humoral and cellular immunogenicity of single-dose and double-dose regimen in subjects older than 55 years. In subjects who received two doses of vaccine the median anti-spike SARS-CoV-2 IgG response were similar in all age groups at 28 days after the booster dose. By 14 days after the booster dose, 99% of the boosted subjects had neutralising antibody responses. The T-cell responses peaked at 14 days after a single standard dose. It was also concluded that the ChAdOx1 nCoV-19 vaccine was better tolerated in older subjects than in younger but had a similar immunogenicity across all age groups (Ramasamy et al., 2020).
Recently, Voysey et al. (2021) published an interim analysis of four randomised controlled trials (phase 1/2/3) pooling results from studies COV001 (UK), COV002 (UK), COV003 (Brazil) and COV005 (South Africa). Pooling all results, the mean efficacy was 70.4%. But remarkably, in subjects who received a low dose (LD) followed by a standard dose (SD) the efficacy was 90.0%. There were ten subjects hospitalised due to COVID-19 but these were all in the control group. The duration of the protection was not determined. On the 22nd March AstraZeneca announced that a US phase 3 trial (two doses 4 weeks apart) showed a statistically significant vaccine efficacy of 79% at preventing symptomatic COVID-19 and 100% efficacy at preventing severe disease and hospitalisation. Notably in subjects aged 65 years and over the vaccine efficacy was found to be 80%. The study was based on 32,449 subjects, with a 2:1 randomisation of vaccine to placebo and accruing 141 symptomatic cases of COVID-19 (“AZD1222 US Phase III trial met primary efficacy endpoint in preventing COVID-19 at interim analysis”).
It should be noted that in an earlier study in non-human primates, although the rhesus macaques showed a reduced viral load in the bronchoalveolar lavage (BAL) fluid after IM vaccination there was no difference in nasal viral shedding between vaccinated and control SARS-CoV-2 infected macaques (van Doremalen et al., 2020).
The Oxford Vaccine Group published a study (yet to be peer reviewed) in Lancet on February 4th 2021 that analysed the efficacy of ChAdOx1 nCoV-19 against a novel variant B.1.1.7 of SARS-CoV-2 and showed that the efficacy was similar to that against other lineages of the virus. Furthermore, the vaccination resulted in a reduction in viral load and duration of shedding. This could impact on the transmission of disease (Emary et al., 2021). Finally, recently scientists from Scotland evaluated data from people who had received, either the Pfizer/BioNTech or the ChAdOx1 nCoV-19 vaccine and found that four weeks after receiving the initial dose, the AstraZeneca/Oxford vaccine appeared to reduce the risk of hospitalisation of infected patients by 94% whereas for the Pfizer/BioNTech vaccine the reduction in risk of hospitalisation 28–34 days after the first dose was reduced by 85%. These were very encouraging results in terms of life saving vaccinations (“COVID-19: Single vaccine jab linked to 85% and 94% drop in risk of coronavirus hospital admissions in Scotland, study shows | UK News | Sky News” URL https://news.sky.com/story/covid-19-vaccine-rollout-linked-to-85-and-94-drop-in-coronavirus-hospital-admissions-in-scotland-study-shows-12225532 (accessed 3.22.21).).
3.3. Moderna COVID-19 vaccine
The Moderna COVID-19 Vaccine was developed through a collaboration between Moderna Inc. and The National Institute of Allergy and Infectious Diseases (NIAID) and was given EUA approval by the FDA in the US the 18th December 2020, in Canada on the 23rd December 2020 under an Interim Order, in the EU (URL https://news.sky.com/story/covid-19-vaccine-rollout-linked-to-85-and-94-drop-in-coronavirus-hospital-admissions-in-scotland-study-shows-12225532 (accessed 3.22.21).) and in the UK on the 6th and the 8th January 2021, respectively, as CMAs for active immunisation to prevent COVID-19 cases by SARS-CoV-2 in individuals aged 18 and over. The Moderna COVID-19 is a mRNA-based vaccine (mRNA-1273) comprising a sequence mRNA encoding the spike glycoprotein encapsulated in lipid nanoparticles similar to the Pfizer/BioNTech COVID-19 vaccine. It is supplied in multiple-dose vials as a frozen suspension that needs to be stored at −25 °C to −15 °C, but can be stored thawed at between 2 °C and 8 °C for up to 30 days prior to first use. Hence, this vaccine is easier to handle and distribute at storage temperature than the Pfizer/BioNTech COVID-19 vaccine. Each 0.5 mL IM prime/boost dose of Moderna COVID-19 vaccine contains 100 µg of nucleoside-modified messenger RNA (mRNA) encoding the prefusion stabilized spike glycoprotein (S) of SARS-CoV-2 virus (Corbett et al., 2020a) in lipid nanoparticles (66–107 nm in diameter; Hassett et al., 2019). (“COVID-19 Vaccine Moderna | European Medicines Agency”).
A phase 1, dose escalation (25 mg, 100 µg and 250 µg), open-label clinical trial included 45 healthy subjects 18–55 years of age (15 subjects in each group), receiving to doses of mRNA-1273 vaccine 28 days apart. After the first vaccination neutralising antibodies were detected in less than half the subjects. A dose response effect was seen with antibody responses, highest for the 250 µg dose group. After the booster vaccination the higher responses in the 100 µg and 250 µg vaccination groups were similar in magnitude. Adverse effects occurred in more than half of the subjects and included fatigue, chills, headache, myalgia and pain at injection site. Systemic adverse effects more commonly occurred after the second vaccination in particular with the 250 µg dose (Jackson et al., 2020).
In an expansion of the Phase 1, dose-escalating, open-label clinical of the mRNA-1273 vaccine described above, 40 older subjects (56–70 or more than 70 years of age) were recruited and received two doses of either 25 µg or 100 µg 28 days apart. Interestingly, by day 57 the anti-S-2P geometric mean titre was higher among subjects of more than 70 years than of subjects between 56 and 70 years of age. It was also confirmed that the 100 µg dose of vaccine induced higher binding and neutralising antibody titres than the 25 µg dose, supporting the use of the 100 µg dose in the Phase 3 study (Anderson et al., 2020). In a further “correspondence paper” the authors reported that serum neutralizing antibodies continued to be detected (with a slight expected decline in titres of binding and neutralising antibodies) in all participants at day 119 and that, although correlates of protection against SARS-CoV-2 infection in humans have not been established, the mRNA-1273 had the potential to provide durable humoral immunity (Widge et al., 2021).
A Phase 3, randomised, placebo controlled blinded clinical efficacy and immunogenicity trial of Moderna COVID-19 Vaccine in subjects of 18 years and older is presently ongoing in the USA with 14,134 subjects receiving the vaccine and 14,073 subjects the placebo injection (type unknown), two doses 1 month apart. The median age was 53 years (range 18–95 years), 25.3% of the subjects were 65 years or older and 18.5% of the subjects were considered at increased risk of severe COVID-19 due to pre-existing medical conditions. The study found a median % vaccine efficacy of 94.1%. In the subgroup analyses, the efficacy in the 18–65 years group was found to be 95.6% whereas, in the over 65 years group, it was 86.4%. No cases of severe COVID-19 were reported in the Moderna COVID-19 vaccine group compared to 30 cases in the placebo group (www.modernatx.com/covid19vaccine - accessed March 22, 2021). As far as the authors of the present review are aware the Phase 3 study results have as yet not been published.
3.4. Gamaleya Research Institute COVID-19 vaccine (Sputnik V)
The Gamaleya Research Institute has developed a vaccine comprising two vector-based components, i.e., recombinant adenovirus type 26 (rAD26-S) and type 5 (rAd5-S) that both carry the gene for SARS-CoV-2 full length spike glycoprotein. The vaccine received regulatory approval in Russia by the Ministry of Health of the Russian Federation on the 11th August 2020, before the availability of Phase 2/3 clinical trials data. The vaccine is given as two separate component vaccines, rAD26-S as the prime IM injection and rAd5-S as the booster injection administered 21 days apart. Each dose contains 1.0 × 1011 viral particles. The vaccines are produced both as frozen vaccines (Gam-COVID-Vac) for large scale use with a volume of 0.5 mL (storage at – 18 °C) and in a lyophilised form (GAM-COVID-Vac-Lyo; storage at 2 °C to 8 °C) (to be reconstituted in 1.0 mL of sterile water for injection before use) for delivery to distant regions of Russia (Logunov et al., 2020). (https://roszdravnadzor.gov.ru/i/upload/files/Hoвocти/Фaйлы/28.12.2020/инcтpyкцияпo пpимeнeнию ЛC.pdf - accessed March 22, 2021).
The two-component vaccine was evaluated for safety and immunogenicity in two separate open, non-randomised phase 1/2 clinical studies in 76 healthy subjects, planned to be aged 18–60 years of age (although the authors declared that the “volunteers were fairly young”). In the first stage of the study (36 subjects) the subjects were given either; a single dose of rAd26-S or rAd5-S (either frozen or lyophilised) and assessed for safety for 28 days. In Stage 2 of the studies 40 subjects were given a prime dose of rAd26-S and on day 21 a booster dose of the rAd5-S. Both vaccine formulations were safe and well tolerated and most adverse effects were mild, and no serious adverse events were found. All subjects in both studies were, according to the authors, found to have seroconverted at day 21 showing RBD-specific (neutralising) IgGs with titres observed equal to or higher than those seen in patients recovered from COVID-19. Furthermore, T cell responses (CD4 + and CD8 + ) were detected in all subject at day 28 (Logunov et al., 2020).
An interim analysis of a controlled phase 3 clinical trial, initiated September 7th 2020, evaluating the safety and efficacy of the rAd26-S or rAd5-S heterologous vaccine, was published February 2nd, 2021 (Logunov et al., 2021). The study was randomised, double-blind and placebo controlled and took place at 25 hospitals or polyclinics in Moscow. The primary outcome was the proportion of subjects confirmed with COVID-19 infection 21 days after receiving the first dose. Secondary outcomes were the severity of COVID-19 infections, changes in antibody levels against the spike protein S and N protein, changes in neutralising antibody titres and changes in antigen specific cellular immunity levels. 19,866 subjects received either two doses of vaccine or placebo and were included in the analysis. From day 21, 0.1% of the vaccination group subjects and 1.3% of the placebo group subjects, were found to have contracted COVID-19. The vaccine efficacy was calculated to be 91.6%. No serious side effects were considered to be associated with vaccination. RBD-specific IgG was detected in 98% of the samples with a seroconversion rate of 98.25%, whereas, the data for the placebo samples were 15% and 14.9%, respectively. In terms of neutralising antibodies, on day 42 after first vaccination, the GMT was 44.5 and the seroconversion was 95.83%, compared to 1.6 and 7.14%, respectively, in the placebo group. The cellular immune response was highest in the vaccine group (expressed as IFN-g secretion 28 days after the first vaccination. The tolerability profile of the vaccine in subjects aged 18 and older was good. Studies are ongoing to investigate a single dose regimen of vaccination (Logunov et al., 2021).
Warnings were published from the Paul-Ehrlich Institute in Germany, together with the WHO, on the 11th August 2020 against the limited transparency of the regulatory approval of the Sputnik V vaccine, when at that time no data from phase 2/3 clinical trials with thousands of subjects (or even interim data) had been released (“Paul-Ehrlich-Institut - Homepage - Statement: Regulatory Approval in Russia of a COVID-19 Vaccine Developed by Gamaleya Institute”). Another concern, in our opinion, is that the vaccine was approved for subjects over 18 but the mean age of the volunteers was between 25.3 years and 31.4 years which (as was also admitted by the authors) would (taking into account the standard deviations), mean very few if any volunteers were over 40 years of age. (https://cattiviscienziati.com/2020/09/07/note-of-concern/ - accessed March 22, 2021).
3.5. Johnson & Johnson/Janssen Pharmaceuticals COVID-19 vaccine
The Johnson & Johnson COVID-19 vaccine was developed in collaboration with its subsidiary, Janssen Pharmaceuticals, in Belgium. The vaccine was authorised by the FDA the 27th February 2021 for use under an EUA for active immunisation to prevent COVID-19 caused by SARS-CoV-2 in subjects 18 years of age and older. The vaccine is a recombinant, replication-incompetent adenovirus type 26 (Ad26) (previously used in J & J’s Ebola vaccine) that encodes the full-length SARS-CoV-2 S protein in a stabilized conformation. The vaccine is in the form of a administered IM, as a single dose vaccine (0.5 mL), stored frozen (-20 °C, two years stability) at the manufacturer and then shipped and stored at 2 °C to 8 °C (3 months only) at the end user. After puncture of the vial, it can be stored for 6 h at 2 °C to 8 °C .
The interim results from a phase 1-2a multicentre, placebo controlled clinical trial of the Ad26.COV2.S COVID-19 vaccine, in subjects between the ages of 18–55 years and those 65 years or older, was published by Sadoff et al. (2021). The trial will eventually consist of 3 cohorts, but initially the younger group of subjects was divided into cohort 1a (target 375 subjects) and cohort 1b (target 25 subjects for in-depth analysis of immunogenicity) and the older group in cohort 3 (target 375 subjects). Enrolment to Cohort 2, comparing longer term data on single dose versus prime/boost dose regimens, started 4 month later and are not discussed in the publication. Cohort 1 and 3 received Ad26COV2.S at low dose (5 × 1010 viral particles per mL), high dose (1 × 1011 viral particles per mL) or placebo (0.9% NaCl solution) given IM in a single dose or two-dose regimen 56 days apart. The results showed that the vaccine was safe, with only mild side effects and that it induced an immune response both in younger and in older subjects. Neutralising antibodies were detected in at least 90% of the subjects on day 29 after first vaccine dose and reached 100% on day 57. Titres remained stable at least to day 71, with a second dose providing an increase in titre. Spike binding antibody responses were similar to neutralising antibody responses. The cell mediated response was skewed towards Th1 cells, with CD4 + detected in 76–83% of the subjects on day 14 and CD8 + T cell responses were robust but lower in the older group (Cohort 3).
The safety, efficacy and immunogenicity of a single dose Ad26COV2.S vaccine is now being assessed in a Phase 3 multicentre, double-blind, randomised and placebo-controlled clinical trial (Ensemble 1) taking place in USA, South Africa, Brazil, Chile, Argentina, Columbia, Peru and Mexico, in subjects aged 18 years and older (FDA, Full Emergency Use Authorisation (EUA), prescribing information-Janssen COVID-19 vaccine. February 27th 2021) (https://www.cdc.gov/vaccines/covid-19/clinical-considerations/managing-anaphylaxis.html - accessed March 22, 2021)).
A total of 44,325 subjects were randomised into two groups, receiving either a single dose vaccine (5 × 1010 viral particles) or a placebo injection. The side effect profile of the vaccine could generally be considered as mild. A causal relationship could not be determined between severe adverse events and the vaccine. The efficacy (based on 468 cases of symptomatic COVID-19 among 43,783 subjects) of the vaccine against moderate to severe/critical COVID-19, 14 days post injection, was found to be 63.7% in the 18–59 year group and 76.3% in 60 years and older group, and at 28 days post injection 66.1% and 66.2%, respectively, for the same groups. The efficacy against severe/critical COVID-19, in all subjects at day 14, was 76.7% and at 28 days post injection 85.4%, respectively. The efficacy subgroup analyses from USA, Brazil and South Africa, against moderate to severe/critical and severe/critical, were not significantly different to the efficacies obtained for analysis of the whole cohort (http://www.physics.emory.edu/faculty/weeks/lab/papers/bogner-micron07.pdf - accessed March 17, 2021) (February 26, 2021). A second phase 3 clinical trial (Ensemble 2) started its enrolment in November 2020 and subjects will receive two doses of Ad26COV2.S, separated by 56 days. It is assumed that the reason for this change from a single dose to a prime/boost dose regimen is the wish to investigate whether the efficacy and the longevity of the protective immunogenicity will increase.
3.6. CanSino Biological/Beijing Institute of Biotechnology/Academy of Military Medical Sciences COVID-19 vaccine
The Ad5-nCoV COVID-19 vaccine has been developed in a collaboration between CanSino Biological, Beijing institute of Biotechnology and the Academy of Military Medical Sciences and contains the information that codifies for the SARS-CoV-2 full-length S protein delivered in the human adenovirus serotype 5 vector (Ad5). The vaccine has been approved for emergency use in China (February 2021), Mexico (February 2021), Pakistan (February 2021), and Hungary (March 2021) (“China approves two more domestic COVID-19 vaccines for public use | Reuters”, “Mexico approves China’s CanSino and Sinovac COVID-19 vaccines | Reuters” (Mexico approves China’s, xxxx), “Pakistan approves Chinese CanSinoBIO COVID vaccine for emergency use | Reuters”, “UPDATE 2-China’s CanSino Biologics COVID-19 vaccine receives emergency use approval in Hungary | Reuters”).
Preliminary Phase 1 safety and immunogenicity data obtained from 108 participants (18–60 years old) showed an acceptable safety and immunogenicity profile with two doses of 5 x1010 and 1 × 1011 viral particles (Zhu et al., 2020b). The results from the double blind, randomised placebo-controlled phase 2 trials were performed with the two selected doses (5 x1010 and 1 × 1011 viral particles) on a total of 508 volunteers, 18–83 years of age. Both dose groups elicited anti-RBD antibodies in more than 95% of the participants after 28 days. Moreover, around 90% of the vaccinated participants showed the activation of specific T-cell responses. No serious adverse reactions were reported, meanwhile less than 10% of participants reported severe adverse reactions and 72% reported mild adverse effects (Zhu et al., 2020b).
Two Phase 3 efficacy trials are ongoing (Clinical Trial Identifier: NCT04526990 and NCT04540419) with the enrolment of 40,000 and 500 volunteers respectively in Argentina, Chile, Mexico, Pakistan, and Russia to evaluate the protection from the incidence of severe COVID-19.
3.7. Sinopharm CNBG/Beijin Inst. Biological Products COVID-19 vaccine
Sinopharm CNBG’s COVID-19 vaccine was developed as a collaboration between Sinopharm CNBG and Beijing Institute of Biological Products which comprises the inactivated SARS-CoV-2 whole virus in combination with the adjuvant, alum. The National Medical Products Administration (NMPA) granted a conditional market approval to the vaccine on the 30th December 2020, but was already approved ahead of Phase 3 clinical trials for emergency use in China (China Approves Sinopharm’s Covid-19 Vaccine as it Moves to Inoculate Millions - The New York Times [WWW Document]. URL https://www.nytimes.com/2020/12/30/business/china-vaccine.html (accessed 3.22.21), the United Arab Emirates (UAE), Bahrain, Egypt and Jordan and reportedly was administered in hundreds of thousands of people (China Injects Hundreds of Thousands With Experimental Covid-19 Vaccines - WSJ [WWW Document]. URL https://www.wsj.com/articles/china-injects-hundreds-of-thousands-with-experimental-covid-19-vaccines-11599834029?tesla=y (accessed 3.16.21)). There also seems to be a second similarly produced vaccine developed in a collaboration between Sinopharm and Wuhan Institute of Biological Products. Studies with both of these vaccines are described below.
As described above, the use of inactivated whole virus has been a standard method of development of vaccines against a range of viral infections such as influenza, polio and hepatitis and often need coadministration with an adjuvant in order to induce efficient immunogenicity (Murdin et al., 1996, Vellozzi et al., 2009). Sinopharm’s COVID-19 vaccines are cultivated in a qualified Vero cell line and the supernatant of the infected cells inactivated twice with β-propiolactone. The inactivated viruses are adsorbed onto 0.5 mg of alum, dispersed in 0.5 mL sterile phosphate buffered saline and packed into prefilled syringes (Xia et al., 2020).
Phase 1 and phase 2 studies have been published by the same first author, but it seems that the first phase 1/2 study was performed on the Wuhan vaccine, whereas, the second phase 1/2 study related to the Beijing vaccine BBIBP-CorV. The first published clinical study showed the results of an interim analysis of two randomised placebo-controlled trials (phase 1/2) that evaluated the effect of the inactivated vaccine against SARS-CoV-2 on safety and immunogenicity. The phase 1 study, comprising 96 subjects (mean age 41.2 years), were assigned to one of three vaccine dose groups (2.5, 5 and 10 µg/dose) and a control group that received the alum adjuvant only (24 in each group) received three IM injections on days 0. 28 and 56. The phase 2 study had 224 subjects enrolled (mean age 43.5 years) that were randomised to a 5 µg dose given in one group on day 0 and day 14 and in the other group on days 0 and 21, and a control group receiving alum only. The inactivated vaccine was well tolerated in all dose groups and no serious vaccine related side effects, were seen. The vaccine induced neutralising antibodies, the titres of which was higher for the vaccine given with a longer interval between prime and boost dose. The authors claimed that in general the titres were similar to those produced by other COVID-19 vaccines. The authors also reported that no notable changes were found in the lymphocyte subset distribution or various cytokines (e.g., T helper 2 cells, IL-4, IL-5 and IL-10), indicating that a cellular response had not been induced by the vaccine (Xia et al., 2020).
As explicitly stated in the paper, the second safety and immunogenicity phase 1/2 study of inactivated SARS-CoV-2 vaccine used the BBIBP-CorV vaccine. The study was randomised, double blind and placebo controlled and divided up in two stages. Phase 1 enrolled 192 healthy subjects age 18–80 years, negative for serum specific IgM/IgG antibodies against SARS-CoV-2. The subjects were separated into two age groups 18–59 years and more than 60 years of age and randomised to receive a two-dose regimen of vaccine or placebo of 2, 4 or 8 µg on days 0 and 28. In Phase 2 of the study, 448 subjects (18–59 years of age) were enrolled and assigned randomly to receive vaccine or placebo on a single dose regimen of 8 µg on day 0, or on a two dose regimen of 4 µg on days 0 and 14, 0 and 21 or 0 and 28. Participants in each cohort were allocated 3:1 to receive vaccine or placebo, respectively. The vaccine was well tolerated, and the adverse reactions were mild to moderate. No serious side effects were reported within day 28 of vaccination. Humoral immunogenicity responses were induced in all vaccine recipients on day 42 after first vaccination. The prime/boost vaccination of 4 mg vaccine on day 0 and 21 or 0 and 28 achieved the higher neutralising antibody titres surpassing those from a single dose of 8 µg or 4 µg dose on day 0 and 14. Consistent with the results from the first publication of results from vaccination with a similar vaccine (Xia et al., 2020), the present study did not find any noticeable changes in lymphocyte subsets or cytokines, indicating no cellular immunity was induced. It should be noted that a seroconversion rate of 100% was reached earlier in the 18–59 years age group compared to the group aged 60 and over and more over that the titres of neutralising antibodies were lower in the older group (Xia et al., 2021).
As far as we are aware, results from Phase 3 studies have not been published, but it has been reported by UAE that interim results showed that the BBIBP-CorV vaccine had an 86% efficacy rate, 99% seroconversion rate of neutralising antibody and 100% effectiveness in preventing moderate to severe cases of COVID-19. However, Sinopharm announced that its internal data showed an efficacy rate of 79% (“China Approves Sinopharm’s Covid-19 Vaccine as it Moves to Inoculate Millions - The New York Times”, “UAE: Ministry of Health announces 86 per cent vaccine efficacy | Health – Gulf News”).
3.8. Bharat Biotech/Indian Council Medical Res./National Inst Virology COVID-19 vaccine
Bharat Biotech’s Covaxin® is developed in collaboration with the Indian Council of Medical Research (ICMR) and the National Institute of Virology (NIV). The vaccine is similar to the Sinovac and the Sinopharm COVID-19 vaccines in that it is based on well-established vaccine technology i.e., whole b-propiolactone-inactivated SARS-CoV-2 virions cultivated in a qualified Vero cell line. After inactivation, the vaccine is adjuvated with an imidazoquinoline (IMDG) class molecule (TLR7 and TLR8 agonist) chemisorbed on alum (Algel) (Algel-IMDG). Imidazoquinoline molecules have been shown to induce cell-mediated immune responses both in vitro and in vivo (Philbin et al., 2012, Smith et al., 2016). The IM prime/boostvaccine (28 days apart) is in a liquid form presented in multidose vials, with storage required at 2 °C – 8 °C. The Covaxin® was granted approval for emergency restricted use in India by the Drug Controller General of India - Central Drugs Standard Control Organization (DCGI-CDSCO) January 3rd 2021.
Ella et al. (2020) reported (interim) results from a phase 1 clinical trial in 375 subjects on the safety and immunogenicity of the inactivated SARS-CoV-2 BBV152 vaccine from Bharat Biotech. The study was a double-blind, randomised and controlled study carried out at 11 hospitals across India in healthy subjects 18–55 years of age. Subjects were randomised to receive one of three vaccine formulations i.e., 3 µg/dose with Algel-IMDG, 6 µg/dose with Algel-IMDG, 6 µg/dose with Algel or an Algel only control. The vaccines were administered IM on days 0 and 14. All solicited adverse events were mild (69%) or moderate (31%) and most frequent after the first dose. One serious side effect was not related to the vaccine. The study found IgG titres to all epitopes (S protein, receptor-binding domain, nucleocapsid protein) increased rapidly after administration of both doses. Further, the seroconversion rates (after second dose, day 28) were found to be 87.9% for 3 µg/dose with Algel-IMDG, 91.9% for 6 µg/dose with Algel-IMDG, and 82.8% for 6 µg/dose with Algel. The responses were similar to those observed in the convalescent serum collected from 41 patients who had recovered from COVID-19, and similar to those induced by other SARS-CoV-2 inactivated vaccines. Notably, samples analysed at 104 days showed seroconversions of 73.5%, 81,1% and 73.1%, respectively. CD3+, CD4 + and CD8 + T cell responses were detected in a subset of 16 patients in both the Algel-IMDG-vaccine groups, whereas minimal levels were detected in subjects vaccinated with the Algel-vaccine formulation.
The phase 2 clinical trial was a double-blind, randomised, multicentre study in India to evaluate the immunogenicity and safety of the BBV152 vaccine. Healthy subjects (380; 12 – 65 years) were randomised to receive, either a 3 µg/dose with Algel-IMDG vaccine, or a 6 µg/dose with Algel-IMDG vaccine by IM injection four weeks apart. There was no control vaccination. The study was conducted across nine states in India. The plaque reduction neutralization test (PRNT50) seroconversion rates of neutralising antibodies, found at day 56, were 92.9% and 98.3%, respectively, for the 3 µg/dose and 6 µg/dose vaccinations, which were higher than those measured in the Phase 1 study. This could possibly be due to the longer time between doses. For both vaccine groups the ratio of Th1/Th2 cytokines was biased towards a Th1 response (IFN-g + TNF-a + IL-2) rather than a Th2 response (IL-5, IL10, IL13) both at day 42 and day 56. The majority of the adverse events were mild and resolved within 24 h and according to the authors, the safety profile of BV152 was noticeably lower than for other SARS-CoV-2 vaccine platforms (Ella et al., 2020). These results have been published in MedRxiv and have yet to undergo peer review. The publication notes its preliminary status and that the manuscripts should not be considered for clinical application, nor relied upon as established information for news reporting. It should be noted that no efficacy data are available from the two published clinical trials. However, according to Bharat Biotech’s website, a phase 3 clinical trial that will enrol 25,800 subjects is under way (Bharat Biotech to begin clinical trial of COVID-19 intranasal vaccine next week [WWW Document], n.d. URL https://www.businesstoday.in/sectors/pharma/bharat-biotech-to-begin-clinical-trial-of-covid-19-intranasal-vaccine-next-week/story/432938.html (accessed 3.22.21).), and interim results were announced by Bharat Biotech on the 3rd March 2021. An efficacy estimate based on 43 cases, where 36 cases of COVID-19 were observed in the placebo group and 7 cases in the vaccinated group, resulted in an efficacy of 80.6% (https://www.bharatbiotech.com/images/press/covaxin-phase3-efficacy-results.pdf - accessed March 22, 2021).
3.9. Sinovac COVID-19 vaccine
Similar to Sinopharm, Sinovac Biotech Ltd in China has developed a COVID-19 vaccine that comprises SARS-CoV-2 whole virus cultivated in Vero cells and inactivated with β-propiolactone. The inactivated virus is injected IM in combination with the adjuvant, alum in phosphate buffered saline (0.5 mL) (Gao et al., 2020).
The 8th February 2021, the National Medical Products Administration (NMPA) of China granted market approval for the vaccine. Furthermore, the vaccine was already approved for emergency use in China in July 2020, ahead of the initiation of Phase 3 clinical trials (“Sinovac Covid-19 vaccine granted approval in China”) and in Indonesia by BPOM on the 11th January 2021 (Indonesia green lights China’s Sinovac COVID-19 vaccine [WWW Document]. URL https://www.biopharma-reporter.com/Article/2021/01/11/Indonesia-green-lights-China-s-Sinovac-COVID-19-vaccine (accessed 3.16.21)).
Zhang et al. (Zhang et al., 2021) reported results from a safety, tolerability and immunogenicity phase 1/2 clinical trial in healthy adults 18–59 years of age. The study was randomised, double blind and placebo controlled, and as for the Sinopharm studies, the clinical trial was separated in a phase 1 and a phase 2 study. 144 subjects were enrolled in the phase 1 study and separated into two vaccination regimen cohorts, i.e., vaccination at day 0 and 14 and vaccination at day 0 and 28. Also, within each of these cohorts, using block randomisation, the first 36 subjects were assigned to a low dose of CoronaVac (3 µg per 0.5 mL of alum diluent per dose) and the other 36 subjects to a high dose of CoronaVac (6 µg per 0.5 mL of alum diluent per dose). Furthermore, within each block, the subjects were given either two doses of CoronaVac or of placebo (aluminium hydroxide in phosphate buffered saline). For the phase 2 study 600 subjects were enrolled and separated into two vaccination regimen cohorts, i.e., vaccination at day 0 and 14 and vaccination at day 0 and 28, as for the phase 1 study. The subjects were randomly assigned (2:2:1) using block randomisation to receive two doses of either low-dose or high-dose CoronaVac vaccine or the placebo.
No serious adverse effects were recorded for any of the subjects in the two studies. For the phase 1 part of the study, seroconversion for neutralising antibodies was seen in 83% in the 3 µg group, 79% in the 6 µg group and 4% in the placebo group. For the phase 2 study, the seroconversion for neutralising antibodies, was 92% in the 3 µg group, 98% in the 6 µg group and 3% in the placebo group at day 14 in the days 0- and 14-day dosing regimen, whereas at day 28, in the days 0 and 28 day dosing regimen, seroconversion was higher, with the respective results of 97%, 100% and 0%. Importantly, the induced humoral immune responses (neutralising antibodies) were significantly higher in the younger subjects (18–39 years of age) than in the older (40–59 years of age). The study did not assess whether the vaccine induced cellular immune responses (T cell responses) in the subjects (Zhang et al., 2021).
Zhang et al. (2021) states that three phase 3 studies are ongoing in Brazil, Indonesia and Turkey evaluating the low vaccine dose of 3 µg CoronaVac in 0.5 mL of diluent, with a 0- and 14-day vaccination regimen. Future phase 3 trials will also evaluate the 0- and 28-day dosing regimen. Further, the study in Brazil will also assess the T cell responses in the subjects.
No formal scientific papers have been published describing the outcome of the various Phase 3 studies. However, in a press release on the 5th February 2021, Sinovac announced Phase 3 results from its CoronaVac vaccine (“Sinovac Announces Phase III Results of Its COVID-19 Vaccine-SINOVAC - Supply Vaccines to Eliminate Human Diseases”). The Press release first states that Phase 3 trials started July 21, 2020 in Brazil, Turkey, Indonesia and Chile and that a total of 25,000 subjects have been enrolled across those four countries. All studies were randomised, double blind and placebo controlled and followed a vaccination regimen on days 0 and 14. The dose given was, as seen above, 3 µg CoronaVac in 0.5 mL of diluent including alum. The press release goes on to state that as of December 2020, 12,396 health workers of more than 18 years of age were enrolled, presumably in Brazil only (Palacios et al., 2020). The vaccine efficacy against SARS-CoV-2 was 50.65% for all cases, but 83.7% for cases requiring medical treatment and 100% for hospitalized, severe and fatal cases. The press release then describes the outcome of the Turkish two stage study (first health workers and then those from the general population) as of December 23, 2020 with all subjects (7,371) ranging from 18 −59 years. The study found an efficacy for prevention of COVID-19 injection of 91.25%. In a separate press release (“Indonesia green lights China’s Sinovac COVID-19 vaccine”) data from the Indonesian trial showed a 65.3% efficacy, with no information given on whether this efficacy data was the combined overall result.
3.10. Anhui Zhifei Longcom Biopharm/Chinese Academy of Medical Sciences COVID-19 vaccine
The Anhui Zhifei Longcom Biopharmaceutical COVID-19 protein subunit vaccine developed in China contains the RBD-dimeric antigen adjuvanted with aluminium hydroxide. At the time of writing, the vaccine has received approval for use in China (March 2021) and Uzbekistan (March 2021) (China IMCAS’s COVID-19 vaccine obtained emergency use approval in China | Reuters [WWW Document]. URL https://www.reuters.com/article/health-coronavirus-china-vaccine-idUSL4N2LD3BZ (accessed 3.24.21), “Uzbekistan approves Chinese-developed COVID-19 vaccine | Reuters”).
In a phase 1 study, 50 healthy adults aged 18–59 years were enrolled and randomly allocated to three groups to receive three times two different doses of vaccine (25 μg or 50 μg RBD-dimer with adjuvant) or the placebo (adjuvant-only) intramuscularly, 30 days apart. Systemic adverse reactions were absent or mild in most participants without severe adverse effects. After three doses, neutralizing antibodies were detected in serum samples of all the participants receiving either the 25 μg or 50 μg dose of the vaccine. The SARS-CoV-2-neutralizing geometric mean titres (GMTs) were 94.5 for the 25 μg group and 117.8 for the 50 μg group (Yang et al., 2020).
In a phase 2 study, 900 healthy adults aged 18–59 years were enrolled and randomly allocated to six subject groups to receive vaccine (25 μg or 50 μg RBD-dimer, with adjuvant) or placebo (adjuvant-only) intramuscularly, with the first 3 groups given two doses of 25 μg vaccine, 50 μg vaccine or placebo 30 days apart and the latter 3 groups given three doses of 25 μg vaccine, 50 μg vaccine or placebo 30 days apart. Systemic adverse reactions were absent or mild in most participants without severe adverse effects. After three doses, neutralizing antibodies (RBD-binding IgG) were detected in the serum of 97% (the 25 μg group) and 93% (the 50 μg group) of participants. The SARS-CoV-2-neutralizing GMTs were 102.5 for the 25 μg group and 69.1 for the 50 μg group after three doses, exceeding the level of a panel of COVID-19 convalescent samples (GMT, 51). Vaccine induced balanced TH1 and TH2 responses. The 50 μg group did not show enhanced immunogenicity compared with the 25 μg group (Yang et al., 2020).
A phase 3 clinical study started in the end of 2020 in China, Ecuador, Indonesia, Pakistan, and Uzbekistan (Clinical Trial Identifier: NCT04646590; Registration number: ChiCTR2000040153) enrolling 29,000 volunteers.
4. Nasal versus intramuscular vaccination
4.1. General advantages and disadvantages of nasal vaccination
As mentioned above, many human pathogens such as influenza virus and SARS-CoV-2 enter the human body via the respiratory tract and hence, it is a natural progression to investigate and exploit the possibility of developing nasal vaccines to combat such infections. Nasal vaccines offer an attractive alternative to injectable vaccines in that it may be possible to use a lower dose than for IM/SC injection, the vaccine can be delivered to the appropriate site, namely the NALT, nasal vaccines do not necessarily require to be administered by a health-care person, and it is a better alternative for vaccination of children who generally are not keen on injections. Furthermore, nasal vaccines can be delivered in simple low-bioburden single/or bi-dose nasal devices, avoiding the need for a sterile environment during administration, which is of great benefit for vaccination programmes in third world countries. Also, dry powder nasal vaccines have been developed that can avoid the cold-chain production which is cost saving.
The nasal epithelium, especially at Waldeyer’s ring in the nasopharynx, encloses follicle-associated lymphoid tissue, the NALT, that is important for creating (local and disseminated) mucosal immune responses. As discussed further below, nasal vaccines have been shown to induce both humoral and cell mediated immune responses and furthermore both serum IgG and local nasal neutralizing mucosal IgA protecting against colonization by invading pathogens. Moreover, intranasal immunization has been reported to enable the induction of cross-reactive antibodies that could be indicative of cross-protection (Jang et al., 2012). As further discussed below, after IM/SC injection of vaccines systemic replication of virus is prevented, but only limited mucosal protection in the form of IgG transudation to airways surfaces are induced.
In order to induce the required immune response and provide long term immunity after nasal vaccination it is of essence to select an optimal delivery system for the specific nasal vaccine, since depending on the type of vaccine formulation e.g live-attenuated vaccines, inactivated viral vaccines, MRA/ DNA encoded particulate systems, subunit or purified antigens with or with/out the use of adjuvants, different immune responses may be induced. Furthermore, it is important that the nasal vaccine remains in the nasal cavity/nasopharynx at sufficient time to enable the vaccine to reach the NALT. This can generally be achieved as necessary with liquid or powder bio-adhesive vaccine formulations, that to some extent is able to overcome the mucociliary clearance system. The potential problem of toxicological effects of nasally applying vaccines will be discussed below.
4.2. Mucosal immune response - injectable vaccines
A natural infection by a respiratory virus induces both systemic IgG antibodies, T cell responses and mucosal antibody responses in the form of secretory immunoglobulin A (SIgA) (Hagenaars et al., 2008, Isho, et al., 2020). The upper respiratory tract, such as the nasal cavity, is suggested to mainly be protected by the SIgA, and the lower respiratory tract, by IgG (Spiekermann et al., 2002). IM injected vaccine prevents systemic replication of the virus but induces only limited mucosal protection through IgG transudation to airway surfaces, such as in the lungs. It is the general perception that, whereas mucosal (e.g., nasal) vaccination results in high titres of protective secretory IgA antibodies at the mucosal site with lower systemic IgG antibodies and cell-mediated immunity, the opposite is the case for parenteral vaccination (Krammer, 2020, MacPherson et al., 2008, Su et al., 2016; Van Ginkel et al., 2000b).
Matsuda et al. (2021) also state that there are many examples of a failure to protect against respiratory virus infections when using IM non-replicating vaccines, for example RSV, parainfluenza virus type 3, Ad4, rotavirus and measles vaccines. It is possible that IM vaccines against respiratory viruses induce disease-preventing or disease-attenuating immunity but does not lead to “sterilizing” immunity (Krammer, 2020).
Experimental DNA vaccines have been shown to induce significant protection against a pathogen challenge, where for example a DNA vaccine, encoding the fusion gene of bovine respiratory syncytial virus (BRSV), was administered IM to calves and induced antigen specific IgG and IgA responses in sera and BAL fluids (Taylor et al., 2005). However, the protection against BRSV infection was not as high as that induced by a prior BRSV infection. For influenza vaccines, the administration of either IN (30 µg) or IM (2 × 10 µg) inactivated influenza virus vaccine elicited antibody secreting cells in the bone marrow and dispersion of memory B cells to organised lymphoid tissue, however, the IgG response was strongest after IM injection, whereas IgA production was only prominent after IN vaccination. The authors suggested that the widespread dispersion of IgG memory B cells to secondary lymphoid tissues, including Peyer’s patches and the NALT, after the IM vaccination, would ensure prompt activation in the event of an influenza infection (Joo et al., 2010).
In another example, rabbits were immunised with an HPV 6bL1 DNA vaccine against human papillomavirus by IM and vaginal administration. The mucosal administration induced 6bL1 virus specific IgA antibodies in the vaginal secretions, showing neutralising activity in a hemagglutination assay, for up to 14 weeks after vaccination. No mucosal immune response was detected in vaginal secretions after IM vaccination (Schreckenberger et al., 2000).
Furthermore, a study evaluated the immunological effect of a novel inactivated whole trivalent influenza virus vaccine, given IN as a prime/boost vaccine 21 days apart in 21 elderly subjects, compared with a single dose (22 subjects) of a commercial IM influenza vaccine. Serum IgG and IgM antibodies and nasal IgA were determined by a hemagglutination inhibition test and ELISA, respectively. The mucosal IgA response was found to be 47.6–71.4% and 18.1–31.8% for subjects given IN and IM vaccinations, respectively, whereas the detected serum antibody response was similar for the two routes of administration, 20.0–61.9% and 18.2–72.7%, respectively. On study completion, 57.1, 65.0 and 50.0% of the IN vaccinated subsets were seroprotected to A/Beijin, A/Sydney and B/Harbin, respectively, and similarly 68.1, 77.2 and 54.5% were immune after IM vaccination. The authors concluded that the IN vaccination was significantly more effective than the IM vaccine in inducing a mucosal IgA response, which they further suggested, may prevent influenza at its early stages and contribute to the reduction of morbidity and complications in the elderly (Muszkat et al., 2003).
In a study published by Samdal et al. (2005), an inactivated whole virus (A/New Caledonia/20/99(h1N1)-like re-assortant IVR116) influenza vaccine, either in saline, mixed with formaldehyde inactivated Bordetella pertussis or in a thixotropic vehicle, were given to 3 groups of subjects for IN immunisation, as four doses, with one-week intervals. All vaccinated groups developed significant IgG and IgA antibody responses after four doses, and 6 weeks after the immunisation 80% of the subject reached hemagglutination inhibition titres of more than 40, which was considered to be protective. In addition, significant increases in CD4 + T-cell proliferation and cytotoxic T-cells were detected. However, no additive effect was found for the addition of B. pertussis or for the thixotropic formulation, that probably was added to evaluate the effect of a prolonged residence in the nasal cavity.
Recently, Matsuda et al. (2021) reported on a study in subjects vaccinated with a replication-competent, Ad4-based vaccine carrying a full-length HA gene from the influenza AH5N1 virus (A/Vietnam/1194/2004) (Ad-4-H5-Vtn recombinant vaccine). The vaccine was given, either orally (1010 vp), directly to the tonsils (103 –108 vp) or nasally (103 –108 vp). Viral shedding, from nose, mouth and rectum, together with H5 specific IgG and IgA antibodies and T cell responses, were detected. It was found that Ad-4-H5-Vtn DNA was shed from most subjects immunised in the upper respiratory tract. The vaccine induced increases in the H5, specific CD4 + and CD8 + T cells in the peripheral blood, as well as increases in IgG and IgA in nasal, cervical and rectal secretions and high levels of serum neutralising antibodies against H5 that remained stable for 26 weeks. The authors concluded that the Ad4 vaccine platform showed considerable promise for vaccines designed to stimulate B cell response to viral surface glycoproteins.
Hence, as seen above, the literature does describe examples of studies where mucosal immune responses, to some extent, are induced after IM injection of a respiratory virus vaccine and that complete or partial protection against such a virus is attainable. However, it is also evident, that for some virus antigens mucosal strategies, including specific adjuvant formulations and a combination of antigens that activate multiple arms of the immune system, would be necessary in order to generate a robust up-front protective immunity.
It has been suggested by Bleier et al. (2021) that, although the IM injected COVID-19 vaccines against SARS-CoV-2 virus presently available on the market are designed to produce an IgG response, preventing viremia and the COVID-19 syndrome, they generally provide little protection against viral replication and shedding in the airways, since such protection requires the presence of a local secretory IgA response. The authors state that preclinical studies of both adenovirus (Ad26) and mRNA (mRNA-1273) IM vaccines demonstrated “persistent virus in nasal swabs although the animals were protected against COVID-19” and refer to two publications by Mercado et al., 2020, Corbett et al., 2020b. Furthermore, the authors state that vaccinated subjects may still become infected and transmit live virus from the upper airways, although they are themselves asymptomatic.
In the study by Corbett et al. (2020b), non-human primates (rhesus macaques) were vaccinated IM at week 0 and 4, with 10 µg or 100 µg mRNA-1273 SARS-CoV-2 vaccine from Moderna and compared to a control (IM saline). Four weeks after the second vaccination all animals were challenged with a total of 7.6 × 105 SARS-Cov-2 plaque forming units (PFU) intranasally (0.5 mL per nostril) and by the intratracheal route (3 mL). The vaccine induced S-specific antibodies and neutralising activity, together with Th1 helper cells and predominantly CD4 + T cell responses with low or undetectable Th2 or CD8 + responses. Only one in eight of the vaccinated animals, in each of the 10 µg and 100 µg dose vaccine groups, showed viral replication (subgenomic RNA) in the BAL fluid by day 2 after the virus challenge, compared to all eight animals in the control group. However, in nasal swab (NS) samples, none of the animals in the 100 µg dose group, showed viral replication, whereas in the 10 µg dose group, five out of eight animals and six out of eight in the control group did.
Mercado et al. (2020) studied a single dose of AD26 vector-based IM vaccines expressing SARS-CoV-2 S protein in non-human primates against a sham control. The rhesus macaques were challenged with SARS-CoV-2 virus (1.0 × 105 TCID50 ~ 1.2 × 108 RNA copies) by the intratracheal and the nasal routes at six weeks. One of the six vaccine variants tested, comprising an Ad26 vector encoding a prefusion stabilised S immunogen (S.PP), induced a robust neutralising antibody response and a Th1-biased T cell response. Furthermore, all animals that received the Ad 26-S.PP vaccine variant, demonstrated no detectable virus in BAL fluid and one showed a low amount of virus in the nasal swab (NS) sample, compared to sham animals that showed a medium peak both in BAL fluid and NS. The animals, that received other vaccine variants, generally demonstrated reduced viral loads in NS compared with controls, although protection was not as good as for the Ad26-S.PP vaccine variant, which became the marketed Johnson & Johnson SARS-CoV-2 vaccine, Ad26.COV2.S.
In a similar study in non-human primates (rhesus macaques), van Doremalen et al. (2020) found that animals vaccinated IM with the ChAdOx1nCov-19 vaccine encoding for the S protein of SARS-CoV-2, using either a single dose or a prime-boost regimen, induced a balanced humoral and cellular immune response (Th1/Th2 T helper cells). The animals were challenged with 2.6 × 106 TCDID50 SARS-CoV-2 virus to both the upper and the lower respiratory tract 28 days after vaccination. Compared with control animals, a significantly reduced viral load in the BAL fluid and lung tissue was observed in vaccinated animals, whereas, no difference in nasal shedding of SARS-CoV-2 virus was found between vaccinated and control animals in the NS. These results suggest that the IM vaccination prevented replication of virus in the lower respiratory tract, but not in the nasal cavity. It should be noted that no evidence of immune enhanced disease was found after viral challenge in the vaccinated SARS-CoV-2 infected animals.
The study by Voysey et al (2021) discussed above, included a phase 2/3 study in the UK with the IM AZD1222 vaccine, that also assessed the possibility of asymptomatic spread of SARS-CoV-2 through vaccinated subjects. Each subject swabbed their nose and throat every week and asymptomatic infections were detected in 0.9% (29 subjects) in the vaccine group and 1.2% (40 subjects) in the control group, indicating an efficacy of 27.3% against asymptomatic SARS-CoV-2, and hence potentially against transmission.
The amount of SARS-CoV-2 virus that is required for efficient human transmission is presently not known, however, it is known that the amount of virus found in the upper airways of subjects just after infection, is in the order of 106 RNA copies per nasal swab, which is close to the challenge doses given in the challenge studies discussed above. Presently, it is also unclear whether the detection of viral shedding in the upper airways in non-human primate translates directly to humans.
4.3. Toxicological aspects
Recently, there has been an intensive discussion about a possible link between the first dose of in particular the AstraZeneca vaccine and recently the Johnson & Johnson vaccine, both given by IM injection, and the occurrence of extremely rare type of blood clots occurring alongside a low level of platelets. For the AstraZeneca vaccine the risk of developing this side effect after vaccination is overall roughly 1 in 250,000 subjects. The risk is highest in younger people (20–29 years of age) with 1.1 cases per 100,000 vaccinations compared to 0.2 cases per 100,000 in older people (60–69 years of age). This should be compared to the risk of hospital admission in ICU with Covid-19 infection of 0.8 per 100,000 (hppts://fullfact.org; 8th April 2021). Both the AstraZeneca and the Johnson & Johnson vaccines are based on the use of replication-deficient adenovirus encoded for the S-protein. It is now recommended in some countries that these two vaccines are only administered to the older population. Cases of apparent secondary immune thrombocytopenia (ITP) have been reported (17 cases in 20 million vaccinated subjects) after vaccination with the Pfizer/BioNtech and the Moderna vaccines (Lee et al., 2021). Both these vaccines are based on mRNA encoding the S protein encapsulated in a lipid nanoparticle formulation. The authors conclude that at present it cannot be excluded that the IM vaccines have the potential to trigger ITP, albeit very rarely. The AstraZeneca vaccine is (as described below) presently in development for nasal vaccination but, as far as we are aware, no information has been published concerning potential serious side effects after using these vaccines for nasal administration.
Relative few vaccines have been licensed for nasal application including Fluenz TetraTM (EU)/ FluMist Quadravalent (US, Can) which are tetravalent cold-adapted live-attenuated influenza vaccines (LAIV) produced by Medimmune/AstraZeneca and Nasovac® which is a similar trivalent influenza vaccine produced in India by CiplaMed in collaboration with the Serum Institute of India Ltd. For the latter vaccine a post marketing study reported that the vaccine was safe in that 90% of all events were non-serious and mild and 10% moderate in severity with no event lasting more than 4 days. No deaths, life threatening events, permanent disability or hospitalisation were reported (Kulkarni and Raut, 2013). Similarly, the Fluenz TetraTM/FluMist Quadravalent nasal vaccines were found to be safe (Lycke, 2012). However, due to the involvement of eggs in the production process, LAIV have been reported to have particular allergic side effects in that it causes significant wheezing for up till 42 days after nasal administration. Hence, the vaccine is precluded for administration to asthmatic patients with unstable asthma (Vasu et al., 2008).
The addition of adjuvants to a vaccine can be necessary for the enhancement of the immune response especially for vaccines comprising purified antigens. As outlined above a range of adjuvants are available including alum, chitosan and also bacterial toxins such as cholera toxin (CT) or heat-labile enterotoxin (LT), or inactivated viral envelopes such as recombinant adenoviruses. Some side effects have been reported after the use of bacterial toxins in vaccines given nasally (Van Ginkel et al., 2000a). It is therefore necessary to consider both the potential toxicity as well as the protective immunity conferred by the selected adjuvant. For example, an inactivated viral subunit influenza vaccine (Nasalflu, Berna Biotech) was adjuvanted with E. coli heat-labile toxin and found after intranasal vaccination to increase the risk of Bell’s palsy. The licence for the vaccine was revoked and is no longer available (Mutsch et al., 2004). It was suggested that the toxin may have been transported from the nasal cavity to the CNS in the same way as was shown for the adjuvant CT (Van Ginkel et al., 2000a; Fujihashi et al., 2002).
The SARS-CoV-2 virus enter cells by engaging the spike protein with an ACE2 receptor. The ACE2 receptors are present throughout the body especially with high levels in the small intestine, testis, kidneys, heart, thyroid, and adipose tissue, while blood, spleen, bone marrow, brain, blood vessels, and muscle had the lowest ACE2 expression levels and medium expression was found in the lungs, colon, liver, bladder, and adrenal gland. Hence theoretically it is possible for the SARS-CoV-2 virus to enter all of these tissue since the S-protein is attached to the surface of the virus and therefore available for interaction with the ACE2 receptors. However, at least for most of the nasal vaccines described below that is under development the carrier e.g. adenovirus and lentiviral vectors are encoded for the S protein and hence is not accessible for interaction with the ACE2 receptor before the S protein has entered a cell and is being produced. The exception is the nasal vaccine in development by University of Houston that comprise liposomes with surface adsorbed S protein
It has been suggested that the SARS-CoV-2 virus can reach the brain by using the transneural route of entry into the olfactory epithelium (where ACE2 is expressed) and transsynaptic routes to spread further into the brain (Butowt and Bilinska, 2020). However, whether the recombinant vectors carrying the encoding for the S-protein are able to enter the olfactory tissue either by paracellular or transcellular transport and potentially result in adverse effects of the vaccine, has, as far as we are aware, not been investigated. Adenovirus has been used for nose to brain delivery of drugs, but it was not shown that the adenovirus itself actually entered the brain, only that the drug did (Ma et al., 2016). Using the paracellular route to enter the olfactory tissue, through the tight junctions, particles should be less than 20 nm whereas using the transcellular route either into olfactory tissue cells or olfactory neural cells the particle size should ideally be less than 100 nm (Illum, 2007, Illum, 2015). The adenovirus vectors used for many of the nasal Covid-19 vaccines under investigation are about 90 nm in diameter, the lentiviral vector about 80–100 nm (similar to the SARS-CoV-2 virus of about 100 nm) and the Newcastle Disease virus is between 150 and 400 nm. It should be noted that the endocytosis process is dependent not only on particle size but also particle characteristics such as charge and surface properties. Furthermore, there is a difference in deposition of the SARS-CoV-2 virus particles in the nose that has entered the nasal cavity by normal inhalation and vaccine particles that are sprayed with stronger force into the cavity. Therefore, whether a nasal spray will reach the olfactory region (2.5% of nasal surface area) positioned in the top of the nasal cavity, is highly unlikely unless a specialised nasal delivery device is used. Hence, it is difficult to predict whether the vaccine carriers could enter the olfactory epithelium, whether any toxic events would result from such entry and hence toxicological studies will need to be done in line with normal regulatory demands before the nasal SARS-CoV-2 vaccines are licensed for marketing.
5. Nasal vaccines in development against SARS-CoV-2 virus infection
As discussed above, it is important that a COVID-19 vaccine should protect humans against a later SARS-CoV-2 viral infection by creating the necessary humoral and cell mediated responses, to include neutralising antibodies, not only in the blood, but also at the upper respiratory tract, such as the nasal mucosal membrane, together with the lower respiratory tract i.e., the lungs.
Furthermore, it is also of importance that vaccinated subjects are not prone to asymptomatic nasal viral shedding and therefore potential transmission of disease to other subjects. Hence, there is presently a great interest in the development of nasal COVID-19 vaccines, although at the time of writing no mucosal vaccine has been approved by regulatory authorities.
The following discussion only includes developments where at least preclinical studies have been published. It should be noted that many of the publications discussed below have been preliminarily published on-line in non-peer review publications such as “www.BioRxiv.org”. However, taken together the papers still give a good overview and information of the potential benefits of nasal COVID-19 vaccines as compared to the IM vaccines.
5.1. Altimmune Inc
Altimmune Inc. is developing a nasally administered, single dose, COVID-19 vaccine, AdCovid™, based on a replication-deficient adenovirus type 5 (Ad5)-vectored vaccine encoding for the receptor binding domain (RBD) of the SARS-CoV-2 spike (S) protein. A preclinical study in mice, tested the immunogenicity of AdCOVID™ after intranasal administration of one of three doses of vaccine 3.35 × 108 ifu (high-dose), 6 × 107 ifu (mid-dose) or 6 × 106 ifu (low-dose) given in a volume of 50 μL, or a control in the form of buffer. The vaccine demonstrated a strong IgG serum neutralising activity, several fold higher than the titre recommended by the FDA, and a potent mucosal immunity with a 29-fold increase in mucosal IgA in the respiratory tract as measured in the BAL fluid. Furthermore, a potent stimulation of the cell mediated immunity, in the form of antigen specific CD8 + killer T cells, was found in the lungs as early as 10 days after vaccination. No nasal samples were collected for identification of secretory nasal IgA. The authors concluded that their AdCOVID™ vaccine generated both humoral and cellular responses at both systemic and mucosal sites, particularly within the lungs, which is a major site for infection and disease (King et al., 2020). A Phase 1 clinical trial is ongoing which will evaluate the safety and immunogenicity of a single dose of AdCOVID™ in up to 180 healthy adult volunteers between 18 and 55 years of age. AdCOVID™ will be administered to subjects at one of three dose levels as a nasal spray. In addition to the primary study endpoint, the immunogenicity of AdCOVID™ will be evaluated by serum IgG binding and neutralizing antibody titres, mucosal IgA antibody levels from nasal samples, and T cell responses. The study was approved by the FDA on the 25th February 2021 (“Altimmune Commences Enrollment in Phase 1 Clinical Trial of AdCOVIDTM -- a Needle-Free, Single-Dose Intranasal COVID-19 Vaccine Candidate – Altimmune”).
5.2. Washington University School of Medicine
Washington University School of Medicine (in collaboration with other institutions) has developed a SARS-CoV-2 vaccine (ChAd-SARS-CoV-2-S) based on chimpanzee adenovirus (simian AD-36) that encodes a prefusion stabilised S protein. The immune response in mice, after IM and IN vaccination, was evaluated. The animals were immunised with 1010 viral particles of ChAd-SARS-CoV-2-S or ChAdV-empty (empty vectored adenovirus, control) in 50 μL PBS via IM injection or IN inoculation. A subset group of vaccinated animals were given a booster immunization at four weeks. To express transiently the human ACE2 receptor in the mice, the vaccinated mice were given a single intraperitoneal injection of 2 mg anti-lfnar1 mAb one day before IN administration of 108 PFU (plaque-forming-units) of Hu-ADV5-hACE2. The mice were challenged five days later with an IN inoculation of 4 × 105 FFU (focus-forming-units) of SARS-CoV-2. The IM vaccination induced strong systemic humoral, and cell mediated immune responses (but no S-or RBD specific IgA in serum), but a minimal mucosal immune response. The IM vaccine did protect against lung infection, inflammation and pathology in the challenged animal model, however, the IM vaccination did not completely protect against the SARS-CoV-2 infection, since substantial levels of viral RNA were still detected in the lungs. In contrast, a single dose IN inoculated vaccine induced high levels of neutralising antibody (anti-SARS-CoV-2 IgA) and showed complete protection in upper and lower airways after the viral challenge (Hassan et al., 2020).
Recently, the ChAd-SARS-CoV-2-S vaccine was also tested in 12 non-human primates (rhesus macaques) that were immunised with a single IN dose of the vaccine or a ChAd control. One month later, the animals were challenged with SARS-CoV-2 virus, by the intranasal and intrabronchial routes. The immunisation (as opposed to the control) induced anti-S, anti-RBD IgG and neutralising antibodies as well as T cell responses and after challenge with SARS-CoV-2 virus (1 × 106 TCID50), prevented or considerably limited appearance of infection in nasal swabs at days 1–7, in BAL fluids (5 of 6 animals) and lung tissues. At later time points, infectious virus was not found in nasal swabs of vaccinated animals. An inverse relationship was found between viral RNA levels in BAL fluids from three days after the SARS-CoV-2 challenge, and neutralising antibody titres. The authors concluded, that an IN immunisation with ChAd-SARS-CoV-2-S, could potentially control nasal infection and hence prevent both viruses induced disease and also transmission (Hassan et al., 2021). Business Today (“Bharat Biotech to begin clinical trial of COVID-19 intranasal vaccine next week”) (10th March 2021) disclosed that Bharat Biotech is in collaboration with the Washington University team for the further development of the ChAd-SARS-CoV-2-S vaccine (also called BBV154) and that a phase 1/2 clinical trial in 175 subjects should start the week of the 15th March 2021.
5.3. Codagenix Inc.
Codagenix Inc. has developed an intranasal vaccine against SARS-CoV-2 (COVI-VAC) based on a live attenuated whole virus platform, which uses “synthetic biology” to re-code the genes of viruses into a safe and stable vaccine. The Codagenix COVI-VAC “de-optimised” virus can be grown easily in cell culture. As far as the present authors are aware, results from preclinical studies have not been published and the information available is from a news review (“First patient dosed with intranasal COVID-19 vaccine candidate”). However, a phase 1 clinical study, to evaluate the safety and immune responses of intranasally administered COVI-VAC in 48 healthy young subjects (18–30 years of age), is presently ongoing in the UK. The subjects, divided into three groups, will receive either two doses of COVI-VAC, 28 days apart, two doses of placebo (saline) or one dose of COVI-VAC and 1 dose of placebo. The dose is administered by drops (no information of number of drops) into each nostril. Each subject will record any symptoms and oral temperature daily for 14 days. Blood samples and intranasal samples will be collected to assess the immune response. The study plan was approved by the MHRA on the 22nd December 2020. The first subject was dosed on the 12th January 2021 (“First patient dosed with intranasal COVID-19 vaccine candidate”).
5.4. AstraZeneca/Oxford Jenner Inst.
AstraZeneca/Oxford Jenner Inst. (who developed ChAdOx1 nCoV-19/AZD1222 for intramuscular injection as discussed above) have also evaluated the same vaccine administered nasally in hamsters and in non-human primates (van Doremalen et al., 2021). After IM injection of the vaccine in rhesus macaques, the animals were protected against pneumonia but no reduction in sub-genomic and genomic viral shedding (RNA) from the nasal cavity was found, with the shedding being similar to that from control animals, indicating replicating virus in the upper respiratory tract.
Three groups of 10 Syrian hamsters were given either a single IN dose (2.5 × 108 virus particles) of ChAdOx1 nCoV-19 (50 µL), the same dose of vaccine given IM (100 µL) or an IM control vaccine. In a challenge study 28 days after vaccination the animals were given 40 µL of 104 TCDID50 SARS-Cov-2/human virus intranasally. In a transmission experiment, vaccinated animals were housed with non-vaccinated donor animals and left for 4 h. Vaccination via both routes, resulted in high IgG titres with no significant difference between the titres. Neutralising antibodies were significantly higher in IN vaccinated animals. Viral RNA was detected in nasal swabs from all animals, but was significantly reduced in IN vaccinated animals compared to controls on days 1–3 and 6–7. A significant reduction in viral RNA, from oropharyngeal swabs from IM vaccinated animals compared to control, was only seen 7 days after vaccination. For infectious virus, there was a significant difference in amount of virus in the oropharyngeal swabs for IN vaccinated compared to control animals, whereas there was no difference in amount of viral RNA nor infectious virus for IM vaccinated animals as compared to control. Furthermore, viral RNA or infectious virus could not be detected in lung tissue from IN vaccinated animals (van Doremalen et al., 2021).
In the non-human primate studies, four rhesus macaques were vaccinated IN with a dose of 2.5 × 1010 virus particles ChAdOx1 nCoV-19 in a prime/boost regimen and compared with four control animals. Blood, nasal swabs and BAL fluid samples were also collected throughout the studies. Animals were challenged with 106 SARS-Cov-2/human virus particles both intratracheally and nasally. Higher fractions of IgA to total Ig antibodies were found in the nasal swabs compared to BAL fluid and serum samples. S and RBD -specific IgG antibodies was found in serum and nasal swabs but not in BAL fluid at day seven after the prime vaccination (at −49 days post infection ~ DPI). Higher IgG titres were found after the booster vaccination (-28 DPI). SARS-CoV-2 specific IgA titres were low after the prime vaccination, but higher after the booster vaccination, and also detected in BAL fluid 7 days after the booster vaccination. Serum neutralising antibodies were found in vaccinated animals at titres similar to those found in previous studies after IM vaccination. After challenge, the nasal swabs in control animals contained genomic and sub-genomic RNA and infectious virus. Viral RNA was found in nasal swabs of vaccinated animals but at a lower level and in fewer animals. Genomic and sub-genomic RNA was detected in BAL fluid of all control animals. Genomic RNA was found in all four vaccinated animals at early time points whereas sub-genomic RNA was only found in one animal at low levels. No infectious virus could be detected in BAL fluids from vaccinated animals and the viral load in the lungs was significantly lower for vaccinated than for control animals. However, no difference in viral load in the nasal cavity was found after IN vaccination. Hence, IN vaccination resulted in reduced shedding and a reduction in viral load in the BAL fluid and in the lower respiratory tract tissue (van Doremalen et al., 2021).
5.5. Lancaster University (UK)/ Biomedical research institute Texas (US).
The Division of Biomedical and Life Sciences, Lancaster University has engineered a COVID-19 vaccine based on a live attenuated and vectored Newcastle Disease virus (NDV) encoding a human codon-optimised S glycoprotein gene of SARS-CoV-2, that is administered by the intranasal route. The NDV vaccine platform has been shown in preclinical models and in humans to be safe and effective against a range of other viruses including influenza. In a published study, Park et al. (Park et al., 2021) evaluated the immunogenicity and safety of the rNDV-S based live attenuated virus vaccine in mice and the protective efficacy in hamsters. Groups of 12 BALB/c mice were inoculated with 106 PFU of the test vaccine in a prime/booster regimen 7 days apart, rNDV-S, a wild type NDV (rNDV-WT) or with phosphate buffered saline. The rNDV-S induced robust systemic humoral (S protein specific IgG and anti-RBD specific IgG) and cell-mediated immune responses in the lungs and in serum in mice, where CD4 + T cell IFNg and NK T-cell TNF + were significantly increased only for the rNDV-S vaccinated animals. The vaccine also appeared to be safe, since no clinical disease signs were observed throughout the experiments nor was any adverse pathology found in the tissues examined (Park et al., 2021).
In a further study, a total of 8 Syrian hamsters in each group were vaccinated IN with 1 × 106 PFU of rNDV-WT, rNDT-S or a mock control once or twice with two weeks interval. To assess protection efficacy of the rNDT-S vaccine, hamsters immunised (prime or boosted) were challenged IN with 2 × 104 PFU of SARS-Cov-2 virus. Hamsters that received prime/booster of the vaccine were protected against the SARS-CoV-2 viral challenge from lung infection, inflammation and pathological lesions. Furthermore, four days after vaccination, both a single and a double dose of the vaccine totally blocked the viral shedding in the nasal cavity and in the lungs with the potential of preventing clinical disease and transmission from vaccinated subjects (Park et al., 2021).
5.6. University of Houston, department of chemical and biomolecular engineering
An et al. (2020) from University of Houston, Texas reported a study on a single dose intranasal vaccine in BALB/c mice evaluating a subunit vaccine containing a trimeric or monomeric S protein from the SARS-CoV-2 virus and using a liposomal, stimulator of interferon genes (STING), as an adjuvant. The vaccine was prepared by mixing the trimeric or monomeric S protein with a suspension of the STING encapsulated in negatively charged liposomes to allow adsorption of the S protein on the liposomes. The mean particle diameter of the resultant liposomes was 105 nm. BALB/c mice (groups of four) were administered a single dose intranasally in one of the following formulations, a) adjuvant only – liposome-STING, b) control – protein only, c) trimeric-STING liposomes and d) monomeric-STING liposomes. Sera were collected at day 7 and 15 after vaccination, and nasal wash, BAL fluid, NALT, lungs and spleen were harvested 15 days after injection. The trimeric-STING-liposome vaccine seroconverted and showed robust anti-S IgG levels in serum that was also detected in BAL fluid at day 7 and 15. Furthermore, robust splenic T cell responses were also detected. Mice immunised with the trimeric-STING-liposome vaccine showed IgA responses in the BAL fluid and in the NALT and an increase in the number of total IgA secreting and S-specific IgA antibody secreting cells (ASCs) was also detected in the spleen compared to control. The T and B cell responses were further activated within the NALT confirming its role as an inductive site.
5.7. Institut Pasteur-TheraVectys Joint Laboratory
Institut Pasteur-TheraVectys Joint Laboratory published studies recently in two preclinical models, mice (with induced expression of the human SARS-CoV-2 receptor, hACE2) and hamsters. They evaluated a novel IN COVID-19 vaccine candidate based on a lentiviral vector eliciting neutralising antibodies against the S glycoprotein of SARS-CoV-2. The mice studies included prime/boost (1 × 107/1 × 107 transduction units (TU)) intraperitoneal (IP) injections and prime/target (1 × 107/3 × 107 TU) IP/IN administration of vaccine compared to control, together with challenge studies (0.3 × 105 TCID50 of SARS-CoV-2). The prime/boost injection of the vaccine resulted in very high serum neutralising IgG against the S protein together with cellular immunity. Furthermore, partial protection was observed after the challenge test, with lung viral load significantly reduced for both prime/boost (10-fold) and prime/target (1000-fold) in vaccinated animals, whereas, IgA was detectable in the upper respiratory tract only in the prime/target vaccinated animals. The authors concluded, from this part of the study, that local IgA in the upper respiratory tract is necessary for full protection against a challenge with SARS-CoV-2 virus. The study regimen was repeated in golden hamsters, which are naturally permissive to SARS-CoV-2 replication. Strong and comparable anti S IgG were detected in the sera of animals, from both the prime/boost and prime/target groups. Neutralising activity was found to be highest in the prime/target animals and comparable to those seen in COVID-19 cases in humans. After challenge with SARS-CoV-2 virus, the viral lung loads were significantly lower than in control for both vaccination groups and the prime/target vaccination strategy induced almost full protection. The authors concluded that the studies provided evidence of the substantial prophylactic effects of vaccination with the lentiviral based vaccine against SARS-CoV-2 and showed intranasal immunisation as a powerful means to combat COVID-19 infection (Ku et al., 2021).
6. Conclusions and perspectives
In a recent study, the acute humoral responses to a SARS-CoV-2 virus infection, such as antibody secreting cells and the presence of virus specific neutralising antibodies in saliva, BAL fluid and serum, were measured in 159 patients with COVID-19 (Sterlin et al., 2021). It was found that the early humoral immune responses to the viral infection were dominated by IgA antibodies, and that SARS-CoV-2 neutralisation was more closely correlated with IgA than IgM or IgG. One month after onset of the symptoms from a SARS-CoV-2 infection, the serum IgA concentrations decreased notably, whereas neutralising IgA in saliva were detectable for up to 73 days after onset of symptoms. It has also been shown that the dimeric form of IgA, found in the mucosa, is more potent against SARS-CoV-2 than both IgA and IgG monomers (Wang et al., 2021). The authors concluded from the study, that IgA mediated mucosal immunity could be the most critical defence mechanism against SARS-CoV-2 and may reduce viral shedding and transmission of the virus from person to person (Sterlin et al., 2021). Likewise, Butler et al. (2021) found that robust neutralisation was only apparent in nasal wash samples from convalescent subjects with varying severity of COVID-19. Serum neutralisation and effector functions correlated with the magnitude of a SARS-CoV-2 -specific IgG response, whereas mucosal neutralisation was associated with IN SARS-CoV-2 -specific IgA in the nasal mucosa. This has important implications for understanding of the protection against SARS-CoV-2 virus afforded by prior infection by the virus and also importantly, when considering the development of a vaccine for protection against COVID-19. An ideal vaccine candidate must not only protect the subject against the disease but also prevent the subject from acting as an asymptomatic vector and transmitting the virus to other people. Furthermore it is of importance to ensure that the vaccines are storage stable and that the vaccine after application does not induce severe side effects as compared to injectable sars-CoV-2 vaccines.
It is striking that all COVID-19 vaccines against SARS-CoV-2, presently approved by regulatory authorities, are administered by intramuscular injections. These IM injected COVID-19 vaccines against SARS-CoV-2 virus are predominantly designed to produce an IgG and cell mediated responses, preventing viremia and the COVID-19 syndrome. They have been shown to have a high degree of efficacy in humans (70–95%). However, as preclinical studies and as a recent clinical study have shown, they generally provide little protection against viral replication and shedding in the upper airways (38%–49% less likelyhood of passing on the virus compared to unvaccinated people), since such protection requires the presence of a local sIgA immune response. As Bleier et al. (2021) have stated, preclinical studies of both adenovirus (Ad26) and mRNA (mRNA-1273) IM vaccines demonstrated “persistent virus in nasal swabs although the animals were protected against COVID-19”. As discussed above, studies in hamsters and rhesus macaques with intranasal vaccines generally showed induced mucosal immune responses (such as secretory IgA), not only in the portals of entry of the virus, such as the nasal cavity, but also in the lower respiratory tract and prevented or provided a significant reduction in viral shedding and therefore, also transmission between animals.
From the results in the preclinical studies on intranasal vaccines, it is likely that a similar protective efficacy seen in the IM COVID-129 vaccines in humans, will be found with the IN COVID-19 vaccine candidates. Results from the first clinical studies should be available in second quarter of 2021. However, whether these IN vaccines will also afford a strong prevention (or reduction) of viral replication in the nasal cavity and lungs and hence prevent transmission of virus by asymptomatic subjects, will only be clarified when viral titre endpoints are incorporated into vaccine clinical trials. It is likely that a combination of an IM prime vaccination and an IN-booster vaccination (IM/IN) would provide a viable alternative to the IM/IM prime/booster vaccines, with a better well-rounded humoral and cell mediated immune response. Presently, the longevity of the immune responses created by the vaccines is not known and hence a further development could be that (as is the case for flu vaccination) a yearly vaccination will be needed against SARS-CoV-2. Such a booster could be given as a IN vaccine.
CRediT authorship contribution statement
Mattia Tiboni: Conceptualization, Writing - original draft, Writing - review & editing. Luca Casettari: Conceptualization, Writing - original draft, Writing - review & editing. Lisbeth Illum: Conceptualization, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors acknowledge Fabio de Belvis for the design of the graphical abstract. Images created with Biorender.com.
References
- Experts With One Big Claim: The Coronavirus Is Airborne - The New York Times [WWW Document]. URL https://www.nytimes.com/2020/07/04/health/239-experts-with-one-big-claim-the-coronavirus-is-airborne.html (accessed 3.16.21).
- Aich P., Dwivedy Importance of innate mucosal immunity and the promises it holds. Int. J. Gen. Med. 2011;4:299. doi: 10.2147/ijgm.s17525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, A.C., Mills, K.H.G., 2014. Improved pertussis vaccines based on adjuvants that induce cell-mediated immunity. Expert Rev. Vaccines. DOI: 10.1586/14760584.2014.936391. [DOI] [PubMed]
- Altimmune Commences Enrollment in Phase 1 Clinical Trial of AdCOVIDTM -- a Needle-Free, Single-Dose Intranasal COVID-19 Vaccine Candidate – Altimmune [WWW Document]. URL https://ir.altimmune.com/news-releases/news-release-details/altimmune-commences-enrollment-phase-1-clinical-trial-adcovidtm (accessed 3.22.21).
- Alving, C.R., Peachman, K.K., et al., 2012. Adjuvants for human vaccines. Curr. Opin. Immunol. DOI: DOI: 10.1016/j.coi.2012.03.008. [DOI] [PMC free article] [PubMed]
- An, X., Martinez-Paniagua, M., et al., 2020. Single-dose intranasal vaccination elicits systemic and mucosal immunity against SARS-CoV-2. bioRxiv Prepr. Serv. Biol. 2020.07.23.212357. DOI: 10.1101/2020.07.23.212357. [DOI] [PMC free article] [PubMed]
- Anderson E.J., Rouphael N.G., et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N. Engl. J. Med. 2020;383:2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AZD1222 US Phase III trial met primary efficacy endpoint in preventing COVID-19 at interim analysis [WWW Document]. URL https://www.astrazeneca.com/media-centre/press-releases/2021/astrazeneca-us-vaccine-trial-met-primary-endpoint.html (accessed 3.24.21).
- Baden L.R., El Sahly H.M., et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum A., Fulton B.O., et al. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science (80-. 2020;). 369:1014–1018. doi: 10.1126/science.abd0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyakov I.M., Ahlers J.D. What Role Does the Route of Immunization Play in the Generation of Protective Immunity against Mucosal Pathogens? J. Immunol. 2009;183:6883–6892. doi: 10.4049/jimmunol.0901466. [DOI] [PubMed] [Google Scholar]
- Bharat Biotech to begin clinical trial of COVID-19 intranasal vaccine next week [WWW Document], n.d. URL https://www.businesstoday.in/sectors/pharma/bharat-biotech-to-begin-clinical-trial-of-covid-19-intranasal-vaccine-next-week/story/432938.html (accessed 3.22.21).
- Bleier B.S., Ramanathan M., et al. COVID-19 Vaccines May Not Prevent Nasal SARS-CoV-2 Infection and Asymptomatic Transmission. Otolaryngol. - Head Neck Surg. (United States). 2021 doi: 10.1177/0194599820982633. [DOI] [PubMed] [Google Scholar]
- Borges, O., Lebre, F., et al., 2010. Mucosal vaccines: Recent progress in understanding the natural barriers. Pharm. Res. DOI: 10.1007/s11095-009-0011-3. [DOI] [PubMed]
- Brandtzaeg P. Potential of nasopharynx-associated lymphoid tissue for vaccine responses in the airways. Am. J. Respir. Crit. Care Med. 2011 doi: 10.1164/rccm.201011-1783OC. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P., Kiyono H., et al. Terminology: Nomenclature of mucosa-associated lymphoid tissue. Mucosal Immunol. 2008;1:31–37. doi: 10.1038/mi.2007.9. [DOI] [PubMed] [Google Scholar]
- Broadbent A.J., Santos C.P., et al. Evaluation of the attenuation, immunogenicity, and efficacy of a live virus vaccine generated by codon-pair bias de-optimization of the 2009 pandemic H1N1 influenza virus, in ferrets. Vaccine. 2016;34:563–570. doi: 10.1016/j.vaccine.2015.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler S.E., Crowley A.R., et al. Distinct Features and Functions of Systemic and Mucosal Humoral Immunity Among SARS-CoV-2 Convalescent Individuals. Front. Immunol. 2021;11:3797. doi: 10.3389/fimmu.2020.618685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butowt R., Bilinska K. SARS-CoV-2: Olfaction, brain infection, and the urgent need for clinical samples allowing earlier virus detection. ACS Chem. Neurosci. 2020;11:1200–1203. doi: 10.1021/acschemneuro.0c00172. [DOI] [PubMed] [Google Scholar]
- Cesta M.F. Normal Structure, Function, and Histology of Mucosa-Associated Lymphoid Tissue. Toxicol. Pathol. 2006;34:599–608. doi: 10.1080/01926230600865531. [DOI] [PubMed] [Google Scholar]
- China Approves Sinopharm’s Covid-19 Vaccine as it Moves to Inoculate Millions - The New York Times [WWW Document]. URL https://www.nytimes.com/2020/12/30/business/china-vaccine.html (accessed 3.22.21).
- China IMCAS’s COVID-19 vaccine obtained emergency use approval in China | Reuters [WWW Document]. URL https://www.reuters.com/article/health-coronavirus-china-vaccine-idUSL4N2LD3BZ (accessed 3.24.21).
- China Injects Hundreds of Thousands With Experimental Covid-19 Vaccines - WSJ [WWW Document]. URL https://www.wsj.com/articles/china-injects-hundreds-of-thousands-with-experimental-covid-19-vaccines-11599834029?tesla=y (accessed 3.16.21).
- Clements J.D., Freytag L.C. Parenteral vaccination can be an effective means of inducing protective mucosal responses. Clin. Vaccine Immunol. 2016;23:438–441. doi: 10.1128/CVI.00214-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett K.S., Edwards D.K., et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020;586:567–571. doi: 10.1038/s41586-020-2622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett K.S., Flynn B., et al. Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates. N. Engl. J. Med. 2020;383:1544–1555. doi: 10.1056/nejmoa2024671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVID-19: Single vaccine jab linked to 85% and 94% drop in risk of coronavirus hospital admissions in Scotland, study shows | UK News | Sky News [WWW Document]. URL https://news.sky.com/story/covid-19-vaccine-rollout-linked-to-85-and-94-drop-in-coronavirus-hospital-admissions-in-scotland-study-shows-12225532 (accessed 3.22.21).
- COVID-19 Vaccine Moderna | European Medicines Agency [WWW Document]. URL https://www.ema.europa.eu/en/medicines/human/EPAR/covid-19-vaccine-moderna (accessed 3.22.21).
- Davis, S.S., 2001. Nasal vaccines. Adv. Drug Deliv. Rev. DOI: DOI: 10.1016/S0169-409X(01)00162-4. [DOI] [PubMed]
- De Haan L., Verweij W.R., et al. Nasal or intramuscular immunization of mice with influenza subunit antigen and the B subunit of Escherichia coli heat-labile toxin induces IgA- or IgG-mediated protective mucosal immunity. Vaccine. 2001;19:2898–2907. doi: 10.1016/S0264-410X(00)00556-9. [DOI] [PubMed] [Google Scholar]
- Deming D., Sheahan T., et al. Vaccine Efficacy in Senescent Mice Challenged with Recombinant SARS-CoV Bearing Epidemic and Zoonotic Spike Variants. PLoS Med. 2006;3 doi: 10.1371/journal.pmed.0030525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didierlaurent A.M., Morel S., et al. AS04, an Aluminum Salt- and TLR4 Agonist-Based Adjuvant System, Induces a Transient Localized Innate Immune Response Leading to Enhanced Adaptive Immunity. J. Immunol. 2009;183:6186–6197. doi: 10.4049/jimmunol.0901474. [DOI] [PubMed] [Google Scholar]
- Dong Y., Dai T., et al. A systematic review of SARS-CoV-2 vaccine candidates. Signal Transduct. Target. Ther. 2020 doi: 10.1038/s41392-020-00352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L., He Y., et al. Development of subunit vaccines against severe acute respiratory syndrome. Drugs of Today. 2008 doi: 10.1358/dot.2008.44.1.1131830. [DOI] [PubMed] [Google Scholar]
- Ella, R., Reddy, S., et al., 2020. Safety and immunogenicity clinical trial of an inactivated SARS-CoV-2 vaccine, BBV152 (a phase 2, double-blind, randomised controlled trial) and the persistence of immune responses from a phase 1 follow-up report. medRxiv. DOI: 10.1101/2020.12.21.20248643.
- Emary K.R.W., Golubchik T., et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) Vaccine Against SARS-CoV-2 VOC 202012/01 (B.1.1.7) SSRN Electron. J. 2021 doi: 10.2139/ssrn.3779160. [DOI] [Google Scholar]
- FluMist Quadrivalent | FDA [WWW Document]. URL https://www.fda.gov/vaccines-blood-biologics/vaccines/flumist-quadrivalent (accessed 3.22.21).
- Folegatti P.M., Ewer K.J., et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujihashi K., Koga T., et al. A dilemma for mucosal vaccination: efficacy versus toxicity using enterotoxin-based adjuvants. Vaccine. 2002;20:2431–2438. doi: 10.1016/s0264-410x(02)00155-x. [DOI] [PubMed] [Google Scholar]
- Gao, Q., Bao, L., et al., 2020. Development of an inactivated vaccine candidate for SARS-CoV-2. Science (80-.). 369, 77–81. DOI: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed]
- Hagenaars N., Mastrobattista E., et al. Head-to-head comparison of four nonadjuvanted inactivated cell culture-derived influenza vaccines: Effect of composition, spatial organization and immunization route on the immunogenicity in a murine challenge model. Vaccine. 2008;26:6555–6563. doi: 10.1016/j.vaccine.2008.09.057. [DOI] [PubMed] [Google Scholar]
- Hansen J., Baum A., et al. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science (80-. 2020;). 369:1010–1014. doi: 10.1126/science.abd0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan A.O., Feldmann F., et al. A single intranasal dose of chimpanzee adenovirus-vectored vaccine protects against SARS-CoV-2 infection in rhesus macaques. Cell Reports Med. 2021;100230 doi: 10.1016/j.xcrm.2021.100230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan A.O., Kafai N.M., et al. A Single-Dose Intranasal ChAd Vaccine Protects Upper and Lower Respiratory Tracts against SARS-CoV-2. Cell. 2020;183:169–184.e13. doi: 10.1016/j.cell.2020.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassett K.J., Benenato K.E., et al. Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines. Mol. Ther.: Nucl. Acids. 2019;15:1–11. doi: 10.1016/j.omtn.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellfritzsch, Scherließ Mucosal Vaccination via the Respiratory Tract. Pharmaceutics. 2019;11:375. doi: 10.3390/pharmaceutics11080375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herremans, T.M.P.T., Reimerink, J.H.J., et al., 1999. Induction of Mucosal Immunity by Inactivated Poliovirus Vaccine Is Dependent on Previous Mucosal Contact with Live Virus. J. Immunol. 162, 5011 LP – 5018. [PubMed]
- Hou Y.J., Okuda K., et al. SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract. Cell. 2020;182:429–446.e14. doi: 10.1016/j.cell.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illum L. Nanoparticulate systems for nasal delivery of drugs: A real improvement over simple systems ? J. Pharm. Sci. 2007;96:473–483. doi: 10.1002/jps.20718. [DOI] [PubMed] [Google Scholar]
- Illum L. The potential for nose-to-brain delivery of drugs, in “Nanoparticles in the lung: Environmental exposure and drug delivery”. CRC Press/ Taylor & Francis Group; 2015. pp. 295–321. [Google Scholar]
- Indonesia green lights China’s Sinovac COVID-19 vaccine [WWW Document]. URL https://www.biopharma-reporter.com/Article/2021/01/11/Indonesia-green-lights-China-s-Sinovac-COVID-19-vaccine (accessed 3.16.21).
- Isho, B., Abe, K.T., et al., 2020. Mucosal versus systemic antibody responses to SARS-CoV-2 antigens in COVID-19 patients. medRxiv. DOI: 10.1101/2020.08.01.20166553. [DOI] [PMC free article] [PubMed]
- Jabbal-Gill I. Nasal vaccine innovation. J. Drug Target. 2010 doi: 10.3109/1061186X.2010.523790. [DOI] [PubMed] [Google Scholar]
- Jackson L.A., Anderson E.J., et al. An mRNA Vaccine against SARS-CoV-2 — Preliminary Report. N. Engl. J. Med. 2020;383:1920–1931. doi: 10.1056/nejmoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang Y.H., Byun Y.H., et al. Cold adapted pandemic 2009 H1N1 influenza virus live vaccine elicits cross-reactive immune response agaisnt seaonal and H5 influenza a viruses. J. Virol. 2012;86:5953–5958. doi: 10.1128/JVI.07149-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyanathan, M., Afkhami, S., et al., 2020. Immunological considerations for COVID-19 vaccine strategies. Nat. Rev. Immunol. DOI: 10.1038/s41577-020-00434-6. [DOI] [PMC free article] [PubMed]
- Joo H.M., He Y., et al. Quantitative analysis of influenza virus-specific B cell memory generated by different routes of inactivated virus vaccination. Vaccine. 2010;28:2186–2194. doi: 10.1016/j.vaccine.2009.12.058. [DOI] [PubMed] [Google Scholar]
- Ju B., Zhang Q., et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020;584:115–119. doi: 10.1038/s41586-020-2380-z. [DOI] [PubMed] [Google Scholar]
- King, R.G., Silva-Sanchez, A., et al., 2020. Single-dose intranasal administration of AdCOVID elicits systemic and mucosal immunity against SARS-CoV-2 in mice. bioRxiv Prepr. Serv. Biol. 2020.10.10.331348. DOI: 10.1101/2020.10.10.331348.
- Kiyono, H., Fukuyama, S., 2004. Nalt-versus Peyer’s-patch-mediated mucosal immunity. Nat. Rev. Immunol. DOI: 10.1038/nri1439. [DOI] [PMC free article] [PubMed]
- Kraehenbuhl J.-P., Neutra M. Mucosal Vaccines: Where Do We Stand? Curr. Top. Med. Chem. 2013;13:2609–2628. doi: 10.2174/15680266113136660186. [DOI] [PubMed] [Google Scholar]
- Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020 doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- Ku M.W., Bourgine M., et al. Intranasal vaccination with a lentiviral vector protects against SARS-CoV-2 in preclinical animal models. Cell Host Microbe. 2021;29:236–249.e6. doi: 10.1016/j.chom.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni P.S., Raut S.K. A post marketing surveillance study of a human live-virus pandemic influenx=za A (H1N1) vaccine (Nasovac®) in India. Hum. Vac. Immunther. 2013;9:122–124. doi: 10.4161/hv.22317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E.-J., Cines D.B., et al. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am. J. Hematol. 2021;96:534–537. doi: 10.1002/ajh.26132. https://orcid.org/0000-0002-8340-6027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Nguyen M.T. Recent Advances of Vaccine Adjuvants for Infectious Diseases. Immune Netw. 2015;15:51. doi: 10.4110/in.2015.15.2.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letko M., Marzi A., et al. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z., Zhu H., et al. Adjuvants for Coronavirus Vaccines. Front. Immunol. 2020;11:2896. doi: 10.3389/fimmu.2020.589833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Wei Q., et al. Epithelial Cells Lining Salivary Gland Ducts Are Early Target Cells of Severe Acute Respiratory Syndrome Coronavirus Infection in the Upper Respiratory Tracts of Rhesus Macaques. J. Virol. 2011;85:4025–4030. doi: 10.1128/jvi.02292-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logunov D.Y., Dolzhikova I.V., et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396:887–897. doi: 10.1016/S0140-6736(20)31866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logunov D.Y., Dolzhikova I.V., et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–681. doi: 10.1016/s0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig S., Zarbock A. Coronaviruses and SARS-CoV-2: A Brief Overview. Anesth. Analg. 2020;93–96 doi: 10.1213/ANE.0000000000004845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lycke N. Recent progress in mucosal vaccine development: Potential and limitations. Nat. Rev. Immunlog. 2012;12:592–605. doi: 10.1038/nri3251. [DOI] [PubMed] [Google Scholar]
- Ma X., Liu P., et al. Intranasal Delivery of Recombinant AAV Containing BDNF Fused with HA2TAT: a Potential Promising Therapy Strategy for Major Depressive Disorder. Sci. Rep. 2016;6:22404. doi: 10.1038/srep22404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson A.J., McCoy K.D., et al. The immune geography of IgA induction and function. Mucosal Immunol. 2008 doi: 10.1038/mi.2007.6. [DOI] [PubMed] [Google Scholar]
- Marzi A., Ebihara H., et al. Vesicular Stomatitis Virus-Based Ebola Vaccines With Improved Cross-Protective Efficacy. J. Infect. Dis. 2011;204:S1066–S1074. doi: 10.1093/infdis/jir348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda K., Migueles S.A., et al. A replication competent adenovirus-vectored influenza vaccine induces durable systemic and mucosal immunity. J. Clin. Invest. 2021;131 doi: 10.1172/jci140794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado N.B., Zahn R., et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature. 2020;586:583–588. doi: 10.1038/s41586-020-2607-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mexico approves China’s CanSino and Sinovac COVID-19 vaccines | Reuters [WWW Document]. URL https://www.reuters.com/article/health-coronavirus-mexico-cansino/update-2-mexico-approves-chinas-cansino-and-sinovac-covid-19-vaccines-idUSL1N2KG0NO (accessed 3.24.21).
- Mulligan M.J., Lyke K.E., et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- Murdin A.D., Barreto L., et al. Inactivated poliovirus vaccine: Past and present experience. Vaccine. 1996 doi: 10.1016/0264-410X(95)00211-I. [DOI] [PubMed] [Google Scholar]
- Muszkat M., Greenbaum E., et al. Local and systemic immune response in nursing-home elderly following intranasal or intramuscular immunization with inactivated influenza vaccine. Vaccine. 2003;21:1180–1186. doi: 10.1016/S0264-410X(02)00481-4. [DOI] [PubMed] [Google Scholar]
- Mutsch M., Zhou W., et al. Use of inactivated intranasal influenza vaccine and the risk of Bell's Palsy in Switzerland. N. Engl. J. Med. 2004;350:896–903. doi: 10.1056/NEJMoa030595. [DOI] [PubMed] [Google Scholar]
- Pakistan approves Chinese CanSinoBIO COVID vaccine for emergency use | Reuters [WWW Document]. URL https://www.reuters.com/article/us-health-coronavirus-pakistan-vaccine-idUSKBN2AC1FG (accessed 3.24.21).
- Palacios R., Patiño E.G., et al. Double-Blind, Randomized, Placebo-Controlled Phase III Clinical Trial to Evaluate the Efficacy and Safety of treating Healthcare Professionals with the Adsorbed COVID-19 (Inactivated) Vaccine Manufactured by Sinovac – PROFISCOV: A structured summary of a study protocol for a randomised controlled trial. Trials. 2020 doi: 10.1186/s13063-020-04775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardi, N., Hogan, M.J., et al., 2018. mRNA vaccines-a new era in vaccinology. Nat. Rev. Drug Discov. DOI: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed]
- Pardi N., Tuyishime S., et al. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J. Control. Release. 2015;217:345–351. doi: 10.1016/j.jconrel.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J.-G., Oladunni, F.S., et al., 2021. Article Immunogenicity and Protective Efficacy of an Intranasal Live-attenuated Vaccine Against SARS-CoV-2 in Preclinical Animal Models. bioRxiv 2021.01.08.425974. DOI: 10.1101/2021.01.08.425974. [DOI] [PMC free article] [PubMed]
- Pasquale A., Preiss S., et al. Vaccine Adjuvants: from 1920 to 2015 and Beyond. Vaccines. 2015;3:320–343. doi: 10.3390/vaccines3020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul-Ehrlich-Institut - Homepage - Statement: Regulatory Approval in Russia of a COVID-19 Vaccine Developed by Gamaleya Institute [WWW Document]. URL https://www.pei.de/SharedDocs/Downloads/EN/newsroom-en/dossiers/approval-vaccine-russia.pdf (accessed 3.22.21).
- Pfizer and BioNTech Submit COVID-19 Vaccine Stability Data at Standard Freezer Temperature to the U.S. FDA Nasdaq:BNTX [WWW Document]. URL https://www.globenewswire.com/news-release/2021/02/19/2178817/0/en/Pfizer-and-BioNTech-Submit-COVID-19-Vaccine-Stability-Data-at-Standard-Freezer-Temperature-to-the-U-S-FDA.html (accessed 3.22.21).
- Philbin V.J., Dowling D.J., et al. Imidazoquinoline Toll-like receptor 8 agonists activate human newborn monocytes and dendritic cells through adenosine-refractory and caspase-1-dependent pathways. J. Allergy Clin. Immunol. 2012;130 doi: 10.1016/j.jaci.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack F.P., Thomas S.J., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland G.A., Ovsyannikova I.G., et al. SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates. Lancet. 2020 doi: 10.1016/S0140-6736(20)32137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy M.N., Minassian A.M., et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2020;396:1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollier C.S., Reyes-Sandoval A., et al. Viral vectors as vaccine platforms: Deployment in sight. Curr. Opin. Immunol. 2011;23:377–382. doi: 10.1016/j.coi.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Rose, M.A., Zielen, S., et al., 2012. Mucosal immunity and nasal influenza vaccination. Expert Rev. Vaccines. https://doi.org/10.1586/erv.12.31. [DOI] [PubMed]
- Sadoff J., Le Gars M., et al. Interim Results of a Phase 1–2a Trial of Ad26.COV2.S Covid-19 Vaccine. N. Engl. J. Med. 2021;NEJMoa2034201 doi: 10.1056/NEJMoa2034201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin, U., Muik, A., et al., 2020. Concurrent human antibody and TH1 type T-cell responses elicited by a COVID-19 RNA vaccine. medRxiv. DOI: 10.1101/2020.07.17.20140533.
- Samdal H.H., Bakke H., et al. A non-living nasal influenza vaccine can induce major humoral and cellular immune responses in humans without the need for adjuvants. Hum. Vaccin. 2005;1:85–90. doi: 10.4161/hv.1.2.1718. [DOI] [PubMed] [Google Scholar]
- Schreckenberger C., Sethupathi P., et al. Induction of an HPV 6bL1-specific mucosal IgA response by DNA immunization. Vaccine. 2000;19:227–233. doi: 10.1016/S0264-410X(00)00173-0. [DOI] [PubMed] [Google Scholar]
- Science Brief: Emerging SARS-CoV-2 Variants | CDC [WWW Document]. URL https://www.cdc.gov/coronavirus/2019-ncov/more/science-and-research/scientific-brief-emerging-variants.html (accessed 3.16.21).
- Sebastian S., Lambe T. Clinical advances in viral-vectored influenza vaccines. Vaccines. 2018 doi: 10.3390/vaccines6020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims A.C., Baric R.S., et al. Severe Acute Respiratory Syndrome Coronavirus Infection of Human Ciliated Airway Epithelia: Role of Ciliated Cells in Viral Spread in the Conducting Airways of the Lungs. J. Virol. 2005;79:15511–15524. doi: 10.1128/jvi.79.24.15511-15524.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinovac Announces Phase III Results of Its COVID-19 Vaccine-SINOVAC - Supply Vaccines to Eliminate Human Diseases [WWW Document]. URL http://www.sinovac.com/?optionid=754&auto_id=922 (accessed 3.22.21).
- Sinovac Covid-19 vaccine granted approval in China [WWW Document]. URL https://www.pharmaceutical-technology.com/news/china-approval-sinovac-vaccine/ (accessed 3.22.21).
- Smith A.J., Li Y., et al. Evaluation of novel synthetic TLR7/8 agonists as vaccine adjuvants. Vaccine. 2016;34:4304–4312. doi: 10.1016/j.vaccine.2016.06.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiekermann G.M., Finn P.W., et al. Receptor-mediated immunoglobulin G transport across mucosal barriers in adult life: Functional expression of FcRn in the mammalian lung. J. Exp. Med. 2002;196:303–310. doi: 10.1084/jem.20020400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhagen F., Kinjo T., et al. TLR-based immune adjuvants. Vaccine. 2011 doi: 10.1016/j.vaccine.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterlin D., Mathian A., et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci. Transl. Med. 2021;13:2223. doi: 10.1126/scitranslmed.abd2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su F., Patel G.B., et al. Induction of mucosal immunity through systemic immunization: Phantom or reality? Hum. Vaccin. Immunother. 2016;12:1070–1079. doi: 10.1080/21645515.2015.1114195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talking is worse than coughing for spreading COVID-19 indoors | Live Science [WWW Document]. URL https://www.livescience.com/covid-19-spread-talking-coughing-indoors.html (accessed 3.16.21).
- Talon J., Salvatore M., et al. Influenza A and B viruses expressing altered NS1 proteins: A vaccine approach. Proc. Natl. Acad. Sci. U. S. A. 2000;97:4309–4314. doi: 10.1073/pnas.070525997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G., Bruce C., et al. DNA vaccination against respiratory syncytial virus in young calves. Vaccine. 2005;23:1242–1250. doi: 10.1016/j.vaccine.2004.09.005. [DOI] [PubMed] [Google Scholar]
- UAE: Ministry of Health announces 86 per cent vaccine efficacy | Health – Gulf News [WWW Document]. URL https://gulfnews.com/uae/health/uae-ministry-of-health-announces-86-per-cent-vaccine-efficacy-1.1607490555571 (accessed 3.16.21).
- -China’s CanSino Biologics COVID-19 vaccine receives emergency use approval in Hungary | Reuters [WWW Document]. URL https://www.reuters.com/article/health-coronavirus-cansinobio-hungary-idUSL1N2LK00K (accessed 3.24.21).
- Uzbekistan approves Chinese-developed COVID-19 vaccine | Reuters [WWW Document]. URL https://www.reuters.com/article/uzbekistan-china-coronavirus-vaccine-idINS0N2IK00P (accessed 3.24.21).
- van Doremalen N., Lambe T., et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature. 2020;586:578–582. doi: 10.1038/s41586-020-2608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen, N., Purushotham, J., et al., 2021. Intranasal ChAdOx1 nCoV-19/AZD1222 vaccination reduces shedding of SARS-CoV-2 D614G in rhesus macaques. bioRxiv Prepr. Serv. Biol. 2021.01.09.426058. DOI: 10.1101/2021.01.09.426058.
- Van Ginkel F.W., Jackson R.J., et al. Cutting edge: The mucosal adjuvant cholera toxin redirects vaccine proteins into olfactory tissues. J. Immunolog. 2000;165:4778–4782. doi: 10.4049/jimmunol.165.9.4778. [DOI] [PubMed] [Google Scholar]
- Van Ginkel, F.W., Nguyen, H.H., et al., 2000. Vaccines for mucosal immunity to combat emerging infectious diseases. Emerg. Infect. Dis. DOI: 10.3201/eid0602.000204. [DOI] [PMC free article] [PubMed]
- Vasu N., Ghaffari G., et al. Adverse events associated with intranasal influenza vaccine in the United States. Ther. Adv. Res. Dis. 2008;2:193–198. doi: 10.1177/1753465808093933. [DOI] [PubMed] [Google Scholar]
- Vellozzi C., Burwen D.R., et al. Safety of trivalent inactivated influenza vaccines in adults: Background for pandemic influenza vaccine safety monitoring. Vaccine. 2009;27:2114–2120. doi: 10.1016/j.vaccine.2009.01.125. [DOI] [PubMed] [Google Scholar]
- Voysey M., Clemens S.A.C., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh E.E., Frenck R.W., et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N. Engl. J. Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Lorenzi J.C.C., et al. Enhanced SARS-CoV-2 neutralization by dimeric IgA. Sci. Transl. Med. 2021;13:eabf1555. doi: 10.1126/scitranslmed.abf1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO | SARS-CoV-2 mink-associated variant strain – Denmark [WWW Document]. URL https://www.who.int/csr/don/03-december-2020-mink-associated-sars-cov2-denmark/en/ (accessed 3.22.21).
- Widge A.T., Rouphael N.G., et al. Durability of Responses after SARS-CoV-2 mRNA-1273 Vaccination. N. Engl. J. Med. 2021;384:80–82. doi: 10.1056/nejmc2032195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo P.C.Y., Lau S.K.P., et al. Coronavirus Diversity, Phylogeny and Interspecies Jumping. Exp. Biol. Med. 2009;234:1117–1127. doi: 10.3181/0903-MR-94. [DOI] [PubMed] [Google Scholar]
- Wu C., Liu Y., et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B. 2020;10:766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S., Duan K., et al. Effect of an Inactivated Vaccine Against SARS-CoV-2 on Safety and Immunogenicity Outcomes: Interim Analysis of 2 Randomized Clinical Trials. JAMA - J. Am. Med. Assoc. 2020;324:951–960. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S., Zhang Y., et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021;21:39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav P.D., Ella R., et al. Immunogenicity and protective efficacy of inactivated SARS-CoV-2 vaccine candidate, BBV152 in rhesus macaques. Nat. Commun. 2021;12:1–11. doi: 10.1038/s41467-021-21639-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, S., Li, Y., et al., 2020. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD protein vaccine against COVID-19 in adults: Pooled analysis of two randomized, double-blind, placebo-controlled, phase 1 and 2 trials. medRxiv. DOI: DOI: 10.1101/2020.12.20.20248602. [DOI] [PMC free article] [PubMed]
- Yusuf, H., Kett, V., 2017. Current prospects and future challenges for nasal vaccine delivery. Hum. Vaccines Immunother. https://doi.org/10.1080/21645515.2016.1239668. [DOI] [PMC free article] [PubMed]
- Zhang Y., Zeng G., et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021;21:181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F.C., Guan X.H., et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396:479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F.C., Li Y.H., et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395:1845–1854. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]