Abstract
BACKGROUND
Cardiac catheterization is the gold standard for cardiopulmonary hemodynamic assessment, although its widespread use could be restricted due to its invasive nature. The aim of this study is to compare multiple echocardiography parameters, including right atrial (RA) strain, with right heart catheterization (RHC) data in patients with heart failure reduced ejection fraction (HFrEF) in the assessment of right heart hemodynamics.
METHODS
Patients with HFrEF (defined by left ventricular ejection fraction [LVEF] ≤ 35%) were enrolled prospectively in this study. All patients underwent echocardiography and RHC. RA pressure (RAP), right ventricular end diastolic pressure (RVEDP), systolic pulmonary artery pressure (sPAP) and pulmonary vascular resistance (PVR) were calculated in RHC. Right ventricular (RV) diastolic and systolic function, RAP, RA size, sPAP and PVR were also measured by echocardiography.
RESULTS
Fifty patients (36 men) aged 13–51 years with LVEF ≤ 35% were enrolled in this study. There was a significant correlation between echocardiography and catheterization data (r > 0.6, p < 0.001). The RV diastolic grading had significant relation with RA volume (p < 0.001) and RA strain (p < 0.001) in echocardiography, and with RVEDP (p = 0.01) and RAP (p < 0.001) in RHC. There were significant relations between the New York Heart Association classification and RV diastolic function grading (p < 0.001), with RA strain (p = 0.019), and size (p = 0.04).
CONCLUSIONS
RA function, as assessed by strain imaging, correlates with right heart hemodynamics in patients with HFrEF.
Keywords: Cardiac catheterization; Echocardiography; Heart atria; Heart failure; Heart failure, systolic
INTRODUCTION
Right heart catheterization (RHC) is the gold standard for cardiopulmonary hemodynamic assessment, which is a crucial step in heart transplantation.1) Although this technique has few complications, it is invasive.2),3),4),5) On the other hand, Doppler echocardiography is a non-invasive technology and can provide a comprehensive assessment of the hemodynamics.6) Although numerous studies have examined the relationship between right-sided cardiac catheterization and echocardiography variables, their correlation is still debatable.7),8) This study was conducted to investigate the correlation between echocardiography parameters obtained during assessment of the right heart and right-sided cardiac catheterization data in patients with heart failure reduced ejection fraction (HFrEF) who were waiting for heart transplantation. Right atrial (RA) strain, a new echocardiographic parameter, was also investigated to define its importance and relationship to hemodynamic data that were derived invasively.
METHODS
Patients
This cross-sectional study was conducted from March 2015 to 2017 using data derived from Doppler echocardiography and heart catheterization in a tertiary hospital. Patients with HFrEF, defined by ejection fraction (EF) ≤ 35%, who were candidates for heart transplantation were initially assessed for eligibility to be enrolled in this study. The study was approved by the Research Ethics Committee of Mashhad University of Medical Sciences, Mashhad, Iran (IR.MUMS.fm.REC.1395.201), and informed consent was obtained from each participant according to the Institutional Review Board approval prior to study entrance.
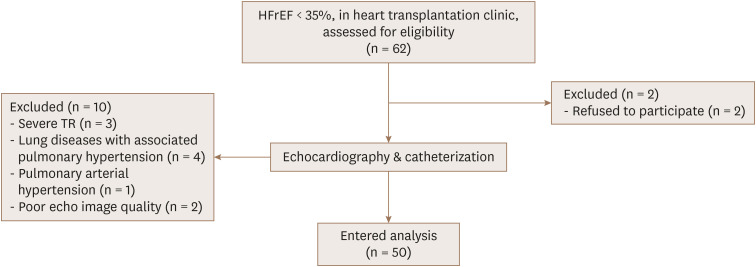
All individuals underwent echocardiography and cardiac catheterization. Echocardiography and cardiac catheterization evaluations were done within a 24-hour time interval to minimize the role of different loading conditions as a confounding factor. The exclusion criteria included the following: atrial or ventricular arrhythmias during the study course, congenital heart disease, pulmonary arterial hypertension, lung disease with secondary pulmonary hypertension, severe tricuspid regurgitation (TR; including free TR with peak velocity [TRV] < 2.5 m/sec), or poor imaging quality (Figure 1).
Figure 1. Algorithm of enrollment of the patients.HFrEF: heart failure reduced ejection fraction, TR: tricuspid regurgitation.
RHC
RHC was performed by one cardiologist with a fellowship in heart failure (HF) who was blinded to the echocardiographic data. The catheterization was based on the standard protocol and was done through the femoral vein via an A1 catheter under fluoroscopy. RA pressure (RAP), right ventricular end diastolic pressure (RVEDP), right ventricular (RV) systolic pressure, mean pulmonary artery pressure (mPAP), systolic pulmonary artery pressure (sPAP), diastolic pulmonary artery pressure and pulmonary capillary wedge pressure (PCWP) were measured. Cardiac output (CO) and pulmonary vascular resistance (PVR) were calculated by the Fick method with PVR in the wood unit.
Transthoracic echocardiography (TTE)
Comprehensive TTE was done by an echocardiologist who was blind to the catheterization data and used a Philips IE33 scanner (Philips Ultrasound, Bothell, WA, USA) and an X5-1 matrix array probe. TEE was performed with the patient in the left lateral position in accordance with the American Society of Echocardiography guidelines.9)
RA area and volume were measured in the apical four-chamber view, with the latter using the disk summation method. RAP was indirectly estimated based on the diameter and respiratory collapsibility of the inferior vena cava (IVC). The sPAP was calculated based on TRVmax2 × 4 + RAP, and mPAP was achieved from 4 × (early pulmonary regurgitation velocity)2 + RAP. Tricuspid annulus systolic velocity (S′) and tricuspid early diastolic velocity (E′) were derived from Doppler tissue imaging of the lateral side of the tricuspid annulus in the apical 4-chamber view. The RV end-systolic area (RVESA) and RV end-diastolic area (RVEDA) were also measured to calculate the RV fractional area change (%RVFAC) = (RVEDA − RVESA)/RVEDA × 100%.
PVR was determined by the following equation10):
| PVR = (TRV/RV outflow tract velocity time integral) × 10 + 0.16 |
Longitudinal strain of the right ventricle (only the free wall) and the right atrium were measured in the RV-focused apical 4-chamber view. Optimal depth and gain and proper visualization of the cavity throughout the cardiac cycle were used. For RV strain analysis, the endocardial tracing started at the lateral side of the tricuspid valve (TV) annulus and stopped at the insertion of the RV free wall in the left ventricular (LV). The average of the longitudinal strain in the 3 segments of the RV free wall was defined as RV strain. In RA strain assessment, the tracing started at the TV annulus, continued along the endocardial border of the RA lateral wall, RA roof, and RA septal wall, and ended at the opposite tricuspid annulus.
Grading of RV diastolic function was based on the right heart echocardiography guidelines by pulsed Doppler of the tricuspid inflow, tissue Doppler of the lateral tricuspid annulus, pulsed Doppler of the hepatic vein, and IVC size and its collapsibility. Diastolic state was defined as normal or with dysfunction (grade I to III). A tricuspid E/A ratio < 0.8 suggested impaired relaxation (grade 1), a tricuspid E/A ratio of 0.8 to 2.1 with an E/e′ ratio > 6, or diastolic flow predominance in the hepatic veins was in favor of pseudo-normal filling pattern abnormality (grade II), and a tricuspid E/A ratio > 2.1 with deceleration time < 120 ms suggested restrictive filling (grade III).10)
Statistical analysis
The results were shown as numbers (%) or the mean value ± standard deviation. The correlations between echocardiography and catheterization data were evaluated using Pearson's correlation. A Bland-Altman plot was used to analyze the agreement between these two methods (echocardiography and catheterization). One-way analysis of variance (ANOVA) was applied to compare the echocardiography data and catheterization variables based on RV diastolic dysfunction classification. A p-value < 0.05 was considered statistically significant.
RESULTS
Of the 62 patients with HFrEF (EF < 35%), 50 patients were included in the final analysis (Figure 1). The mean patient age was 34.24 years (range, 13–51 years), and 72% of the patients were male. The underlying etiology of HFrEF was non- ischemic in most cases (72%). Fourteen patients suffered from HF due to ischemic events, in which coronary revascularization was done in 71% of cases. The baseline clinical characteristics of the patients are shown in Table 1.
Table 1. Baseline clinical characteristics of the included patients (n = 50).
| Characteristics | Values | |
|---|---|---|
| Age (year) | 34.24 ± 10.52 | |
| Male/female | 36/14 | |
| Underlying etiology for HF | ||
| Ischemic | 14 (28) | |
| Non-ischemic | 36 (72) | |
| CRT/ICD | 0/10 | |
| Mean arterial pressure (mmHg) | 81 ± 11 | |
| Heart rate (per min) | 80 ± 15 | |
| BSA (m2) | 1.74 ± 0.19 | |
| LVEF | 21.6 ± 7.5 | |
| NYHA classification | ||
| I | 0 | |
| II | 11 (22) | |
| III | 27 (54) | |
| IV | 12 (24) | |
| Coronary revascularization | 10 (20) | |
| Previous medication | ||
| RAS blocker | 50 (100) | |
| Beta-blocker | 43 (86) | |
| CCB | 7 (14) | |
| Furosemide | 37 (62) | |
| Spironolactone | 28 (58) | |
| Statin | 29 (58) | |
| Aspirin | 14 (28) | |
All values are presented as means ± standard deviations or numbers of patients (%).
BSA: body surface area, CCB: calcium channel blocker, CRT: cardiac resynchronization therapy, HF: heart failure, ICD: implantable cardioverter defibrillator, LVEF: left ventricle ejection fraction, NYHA: New York Heart Association, RAS: renin angiotensin blocker.
Echocardiography and catheterization data
The echocardiography data are summarized in Table 2. The systolic, diastolic and mean pulmonary artery pressures, derived from catheterization, were 40.06 ± 9.80 mm Hg, 26.64 ± 7.84 mm Hg, and 31.08 ± 8.22 mm Hg, respectively. The measured mean PCWP was 21 mm Hg, and the PVR was 3.5 woods. The echocardiography and catheterization parameters of right heart hemodynamics were compared, and the correlation analysis showed significant correlation (r > 0.6, p < 0.001), with the least correlation for RAP (r = 0.62) and the best correlation for PVR (r = 0.86).
Table 2. Echocardiography parameters in all participants (n = 50).
| Parameters | Values |
|---|---|
| RA area (cm2) | 14.91 ± 7.51 |
| RA volume (cc) | 87.49 ± 56.15 |
| TV E velocity (cm/s) | 51.59 ± 20.21 |
| TV A velocity (cm/s) | 30.09 ± 14.06 |
| TV E/A ratio | 1.41 ± 0.67 |
| TA E' (cm/s) | 7.35 ± 2.56 |
| E/E' ratio | 6.75 ± 3.14 |
| LVEF (%) | 21.60 ± 7.50 |
| RVFAC (%) | 28.00 ± 13.00 |
| sPAP (mmHg) | 40.57 ± 11.47 |
| PVR (woods) | 2.48 ± 1.50 |
| RAP (mmHg) | 16.80 ± 7.62 |
| RA strain (%) | 13.06 ± 10.01 |
| RV strain (%) | −16.61 ± 8.80 |
All values are presented as means ± standard deviations.
A: tricuspid late diastolic velocity, E: tricuspid early diastolic velocity, LVEF: left ventricular ejection fraction, PVR: pulmonary vascular resistance, RA: right atrial, RAP: right atrial pressure, RV: right ventricular, RVFAC: right ventricular fractional area change, sPAP: systolic pulmonary artery pressure, TA: tricuspid annulus, TV: tricuspid valve.
RV diastolic dysfunction
Grading of RV diastolic dysfunction revealed normal diastolic function in 11 (22%) patients, mild diastolic dysfunction in 10 (20%), pseudo-normal filling pattern in 22 (44%) and restrictive filling pattern in 7 (14%) patients.
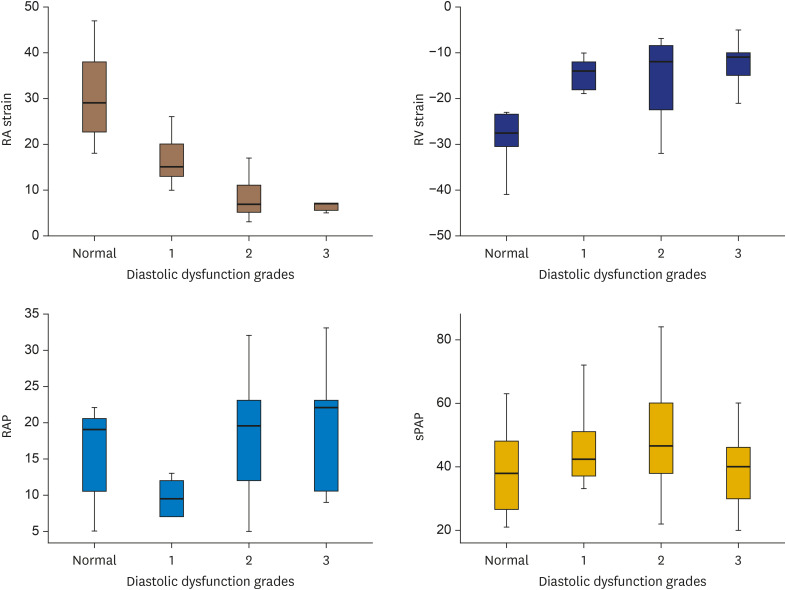
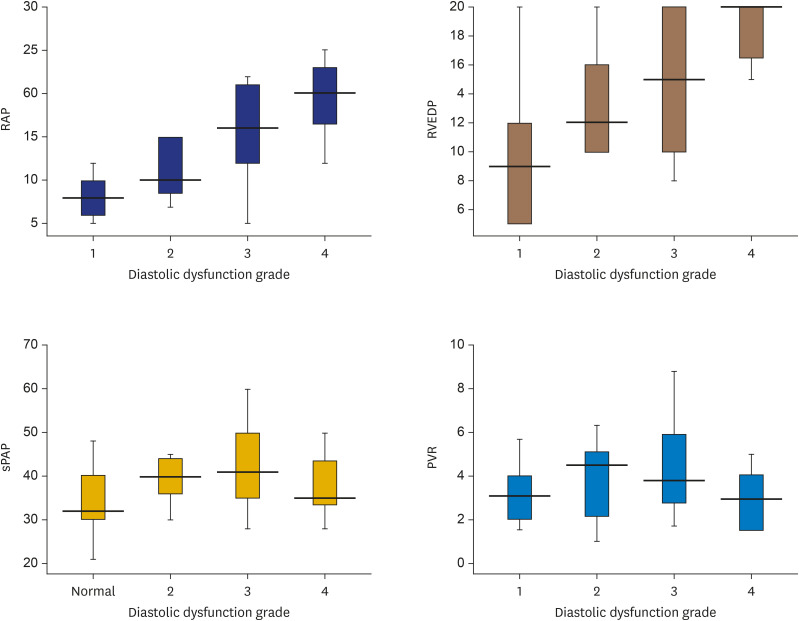
The ANOVA test was used to determine the statistically significant differences between echocardiography and catheterization hemodynamic parameters with respect to the RV diastolic dysfunction grading subgroups (Figures 2 and 3). According to an analysis of the echocardiography data, the RV diastolic grading had significant relation with RA volume (p < 0.001), RA strain (p < 0.001), RV longitudinal strain (p = 0.005) and RVFAC (p = 0.005), but no significant relation was found with PAP, EF or PVR. An analysis of the catheterization data demonstrated that RV diastolic grading showed significant relation with RVEDP (p = 0.01) and RAP (p < 0.001), but not with sPAP (p = 0.1), PVR (p = 0.4), or CO (p = 0.4).
Figure 2. Relation of echocardiography (RA and RV strain, RAP size, and sPAP) with different RV diastolic function groups.
RA: right atrial, RAP: right atrial pressure, RV: right ventricular, sPAP: systolic pulmonary artery pressure.
Figure 3. Relation of catheterisation data (RAP, RVEDP, sPAP, PVR) with different RV diastolic function groups.
PVR: pulmonary vascular resistance, RV: right ventricular, RVEDP: right ventricular end diastolic pressure, RAP: right atrial pressure, sPAP: systolic pulmonary artery pressure.
New York Heart Association (NYHA) classification and RV hemodynamic data
There was significant relation between RV diastolic function grading and the NYHA classification using the Kruskal-Wallis test (p < 0.001). Statistically significant relation was found in RA strain (p = 0.019) and RA volume (p = 0.04) among the echocardiography parameters, and only RAP (p = 0.03) showed statistically significant relation among the catheterization variables.
DISCUSSION
For many years, the right ventricle has been neglected during the evaluation of patients with left-sided HF. During the past two decades, however, several studies have shown that RV dysfunction (RVD) is common in left HF, and it also results in increased morbidity and mortality.11) RVD is now recognized as a major prognostic factor, whether in the presence of preserved (HFpEF) or HFrEF.12),13),14)
Because the most common cause of RVD is LV dysfunction, a comprehensive study of right heart hemodynamics is crucial, especially before heart transplantation, and RHC has been the gold standard method.15),16),17) However, because of its invasive nature, risk for complications and expense, RHC has been substituted with other non-invasive and valid methods. Echocardiography, alongside RHC, was used in this study to evaluate right heart hemodynamics, and the related data showed a significant correlation between echocardiography and catheterization (r > 0.6, p < 0.001). RV diastolic function was also assessed, and different grades of dysfunction were detected in 78% of enrolled HFrEF patients, which signifies that RV diastolic dysfunction is a common problem in patients with left ventricle dysfunction and is significantly correlated with the NYHA class of participants. However, the data on this aspect of RV function are lacking and its prognostic role is not completely understood. RA size and strain (measured by echocardiography) and RVEDP, and RAP (calculated by RHC), were related with the grading of diastolic dysfunction. Consequently, they could be defined as a surrogate marker for RV diastolic dysfunction. A study by Sallach et al.18) in patients with chronic systolic HF (EF < 35%) revealed that the indexed RV volume showed relation with systolic and diastolic RV function and serum brain natriuretic peptide levels, and it was defined as a predictive factor for adverse events such as death, heart transplantation and hospitalization due to HF. RA dysfunction seems to affect CO by impairing RV filling. In a study by Wright et al., the increase in RAP was associated with the decrease in RA strain.19) In another study, HFrEF, but not HFpEF, was associated with significant RA dysfunction.20) Such findings are compatible with this study, which showed the impairment of RA function in HFrEF patients with increased RAP. In addition, RA strain, compared to the echo parameters of RV systolic function, had a stronger relation with the NYHA class.
Several limitations are noted in this study. The longitudinal strain analysis for both the RV and RA was done with the same software as the LV, and it seems that more studies are needed to assess the validity of this technique. In addition, the effect of RV diastolic dysfunction grading on long term prognosis was not evaluated due to lack of patients’ follow up according to the study design. Further studies are needed to address the prognostic implication of RA strain analysis in patients with HFrEF.
In conclusion, this study suggested that the determinant factor for RV diastolic function was RA size, pressure and function (by strain), but not pulmonary artery systolic pressure or CO. Right atrium strain could be suggested as a new echocardiographic parameter in the assessment of RV hemodynamics in patients with HF.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Vakilian F, Tavallaie A, Poorzand H.
- Data curation: Vakilian F, Poorzand H.
- Formal analysis: Vakilian F, Tavallaie A, Salehi M.
- Investigation: Vakilian F, Tavallaie A, Poorzand H.
- Methodology: Salehi M.
- Project administration: Vakilian F, Tavallaie A, Poorzand H.
- Resources: Alimi H.
- Software: Alimi H.
- Supervision: Poorzand H.
- Validation: Vakilian F, Alimi H, Poorzand H, Salehi M.
- Visualization: Tavallaie A, Alimi H.
- Writing - original draft: Alimi H, Poorzand H.
- Writing - review & editing: Alimi H.
References
- 1.Hunt SA, Abraham WT, Chin MH, et al. Focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines developed in collaboration with the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1–90. doi: 10.1016/j.jacc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 2.Johnson LW, Lozner EC, Johnson S, et al. Coronary arteriography 1984–1987: a report of the registry of the society for cardiac angiography and interventions. I. Results and complications. Cathet Cardiovasc Diagn. 1989;17:5–10. doi: 10.1002/ccd.1810170103. [DOI] [PubMed] [Google Scholar]
- 3.Dorros G, Cowley MJ, Simpson J, et al. Percutaneous transluminal coronary angioplasty: report of complications from the National Heart, Lung, and Blood Institute PTCA registry. Circulation. 1983;67:723–730. doi: 10.1161/01.cir.67.4.723. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy JW. Complications associated with cardiac catheterization and angiography. Cathet Cardiovasc Diagn. 1982;8:5–11. doi: 10.1002/ccd.1810080103. [DOI] [PubMed] [Google Scholar]
- 5.Noto TJ, Jr, Johnson LW, Krone R, et al. Cardiac catheterization 1990: a report of the registry of the Society for Cardiac Angiography and Interventions (SCA&I) Cathet Cardiovasc Diagn. 1991;24:75–83. doi: 10.1002/ccd.1810240202. [DOI] [PubMed] [Google Scholar]
- 6.Mullens W, Borowski AG, Curtin RJ, Thomas JD, Tang WH. Tissue Doppler imaging in the estimation of intracardiac filling pressure in decompensated patients with advanced systolic heart failure. Circulation. 2009;119:62–70. doi: 10.1161/CIRCULATIONAHA.108.779223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karas MG, Kizer JR. Echocardiographic assessment of the right ventricle and associated hemodynamics. Prog Cardiovasc Dis. 2012;55:144–160. doi: 10.1016/j.pcad.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Sohail A, Korejo HB, Shaikh AS, et al. Correlation between echocardiography and cardiac catheterization for the assessment of pulmonary hypertension in pediatric patients. Cureus. 2019;11:e5511. doi: 10.7759/cureus.5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Ghio S, Gavazzi A, Campana C, et al. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37:183–188. doi: 10.1016/s0735-1097(00)01102-5. [DOI] [PubMed] [Google Scholar]
- 12.Bosch L, Lam CS, Gong L, et al. Right ventricular dysfunction in left-sided heart failure with preserved versus reduced ejection fraction. Eur J Heart Fail. 2017;19:1664–1671. doi: 10.1002/ejhf.873. [DOI] [PubMed] [Google Scholar]
- 13.Ghio S, Temporelli PL, Klersy C, et al. Prognostic relevance of a non-invasive evaluation of right ventricular function and pulmonary artery pressure in patients with chronic heart failure. Eur J Heart Fail. 2013;15:408–414. doi: 10.1093/eurjhf/hfs208. [DOI] [PubMed] [Google Scholar]
- 14.Ghio S, Guazzi M, Scardovi AB, et al. Different correlates but similar prognostic implications for right ventricular dysfunction in heart failure patients with reduced or preserved ejection fraction. Eur J Heart Fail. 2017;19:873–879. doi: 10.1002/ejhf.664. [DOI] [PubMed] [Google Scholar]
- 15.Lim HS, Hsich E, Shah KB. International Society of Heart and Lung Transplantation position statement on the role of right heart catheterization in the management of heart transplant recipients. J Heart Lung Transplant. 2019;38:235–238. doi: 10.1016/j.healun.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King KM, Ghali WA, Faris PD, et al. Sex differences in outcomes after cardiac catheterization: effect modification by treatment strategy and time. JAMA. 2004;291:1220–1225. doi: 10.1001/jama.291.10.1220. [DOI] [PubMed] [Google Scholar]
- 17.Roghi A, Savonitto S, Cavallini C, et al. Impact of acute renal failure following percutaneous coronary intervention on long-term mortality. J Cardiovasc Med (Hagerstown) 2008;9:375–381. doi: 10.2459/JCM.0b013e3282eee979. [DOI] [PubMed] [Google Scholar]
- 18.Sallach JA, Tang WH, Borowski AG, et al. Right atrial volume index in chronic systolic heart failure and prognosis. JACC Cardiovasc Imaging. 2009;2:527–534. doi: 10.1016/j.jcmg.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 19.Wright LM, Dwyer N, Wahi S, Marwick TH. Association with right atrial strain with right atrial pressure: an invasive validation study. Int J Cardiovasc Imaging. 2018;34:1541–1548. doi: 10.1007/s10554-018-1368-3. [DOI] [PubMed] [Google Scholar]
- 20.Chirinos JA, Satija V, Gaddam S, et al. Right atrial function in heart failure with preserved and reduced ejection fraction. J Am Coll Cardiol. 2018;71:A1604. [Google Scholar]