Abstract
Breast cancer is one of the most frequent malignancies. The aim of the article is to analyse the cost-utility ratio and budgetary impact of talazoparib treatment for patients with locally advanced or metastatic gBRCA + breast cancer from the perspective of the Spanish National Health System. Analyses were based on the EMBRACA clinical trial and the model was constructed according to “partitioned survival analysis”. Two scenarios were considered in order to compare talazoparib with the alternatives of capecitabine, vinorelbine and eribulin: 1. Chemotherapy in patients pre-treated with anthracyclines/taxanes and, 2. A second- and subsequent-line treatment option. Treatment types following relapse were recorded in the mentioned clinical trial. The effectiveness measure used was quality-adjusted life years (QALY). The average health cost of patients treated at 43 months with talazoparib was 84,360.86€, whilst current treatment costs were 26,683.90€. The effectiveness of talazoparib was 1.93 years of survival (1.09 QALY) relative to 1.58 years (0.83 QALY) in the treatment group. The incremental cost-utility ratio was 252,420.04€/QALY. This represents the additional cost required to earn an additional QALY when changing from regular treatment to talazoparib. Regarding budgetary impact, the number of patients susceptible to receiving treatment with between 94 and 202 talazoparib was estimated, according to scenario and likelihood. The 3-year cost difference was between 6.9 and 9 million euros. The economic evaluation conducted shows an elevated incremental cost-utility ratio and budgetary impact. Taking these results into account, the price of talazoparib would have to be lower than that taken as a reference to reach the cost-utility thresholds.
Keywords: Breast neoplasms, Healthcare costs, Poly(ADP-Ribose) polymerase inhibitors, Cost-utility analysis, Progression-free survival
Highlights
-
•
As far as the authors know, this paper is the first economic evaluation of iPARP in advanced/metastatic breast cancer.
-
•
Talazoparib does not extend the median survival time compared to capecitabine, vinorelbine and eribulin.
-
•
As for low survival improvement of talazoparib, it should be used with caution in patients with breast cancer BRCA mutation.
1. Introduction
According to the AECC Cancer Observatory, 33,307 new cases of breast cancer were diagnosed in Spain in 2019, with this representing slightly more than 30% of all tumours in females [1].
Treatment with talazoparib is indicated as a monotherapy for adult patients with germline BRCA1/2 mutations (gBRCA+), with locally advanced or metastatic HER2 negative breast cancer. Patients must have been treated previously withan anthracycline and/or a taxane in an advanced/metastatic or neoadjuvant setting, unless patients are not candidates for this treatment. Hormone receptor-positive breast cancer patients must have previously been treated with endocrine therapy, or not be considered candidates for such therapy. Talazoparib works by blocking poly-ADP-ribose-polymerases (PARP) which repair damaged DNA in cells (both normal and cancerous) during cell division [2]. Thus, damaged DNA in cancerous cells cannot be repaired, leading to death [3].
Evidence around the effectiveness of treatment with talazoparib is based on the EMBRACA clinical trial [4], which included patients with locally advanced or gBRCA + metastatic breast cancer. This phase 3 trial included a total of 431 patients. Patients were randomised according to a 2:1 ratio, with 287 being assigned to receive talazoparib (1 mg/day) and 144 being assigned to receive breast cancer treatment chosen by their doctor (TSM). Four options were available for this alternative treatment: Capecitabine, vinorelbine, gemcitabine or eribulin. Analysis revealed statistically significant benefits of talazoparib in relation to progression-free survival, with a hazard ratio (HR) of 0.54 and 3-months difference between treatment group medians. No benefit was found in relation to overall survival (provisional analysis, secondary variables).
The aim of the present report is to estimate the cost-utility ratio and budgetary impact of treatment with talazoparib relative to standard treatment in women with advanced or metastatic breast cancer with BRCA mutation. Patients had been pre-treated with anthracyclines and taxanes or were not candidates to receive it. Currently, regular treatments (capecitabine, vinorelbine, gemcitabine or eribulin) can be employed alternatively in successive lines. Eribulin was introduced in Spain for patients pre-treated with, or not candidates for capecitabine, as it is not considered to offer any significant improvement over the latter [5].
2. Material and methods
Design and structure of the pharmacoeconomic model: A cost-utility analysis was performed through a partitioned survival model [6], incorporating 3 mutually exclusive health states: progression free (starting point), progression and death (final state), and from the perspective of the Spanish National Health System (SNS). In consideration of limitations to the available information, a time horizon of 43 months was chosen. This is 3 months longer than the maximum survival registered in the EMBRACA study, whose 40-month survival rate in the talazoparib arm is 0%. The intention behind selecting this time-frame was to maximize the observation time of remaining life, ensuring that important differences between options could be detected for both costs and intervention outcomes.
In order to evaluate talazoparib relative to standard habitual treatment (capecitabine, vinorelbine, gemcitabine or eribulin) [7,8], two scenarios were considered. Scenario 1 included patients pre-treated with anthracyclines, taxanes and hormone therapy, or who were not candidates for any of these, with the comparator being capecitabine. On the other hand, patients in scenario 2 had been pre-treated with capecitabine or were not candidates. In this case, the comparator was eribulin. Table 1 presents treatments used following relapse and the proportions employed. Selection frequencies were extrapolated for each control arm of the clinical trial pertaining to the corresponding scenario. The time in each state was estimated according to follow-up data for overall survival and progression-free survival, with both of these being obtained from the EMBRACA clinical trial [4]. Progression-free survival was considered as the time elapsed up until progression or death. In the clinical trial, progression was evaluated by an independent radiologist according to evaluation response criteria for solid tumours (RECIST).
Table 1.
Treatment lines considered and the proportion of patients in treatment.
| Lines | Talazoparib | Actual standard |
|---|---|---|
| Scenario 1: after anthracyclines/taxanes | ||
| 1st treatment | talazoparib (100%) | capecitabine (100%) |
| After relapse | capecitabine (44%) eribulin (40%) gemcitabine (10%) vinorelbine (7%) |
eribulin (70%) gemcitabine (18%) vinorelbine (12%) |
| Scenario 2: after anthracyclines/taxanes and capecitabine | ||
| 1st treatment | talazoparib (100%) | eribulin (100%) |
| After relapse | eribulin (70%) gemcitabine (18%) vinorelbine (12%) |
gemcitabine (59%) vinorelbine (41%) |
Scenario 1. Talazoparib use relative to capecitabine treatment in patients pre-treated with anthracyclines/taxanes. Scenario 2. Talazoparib use relative to treatment with eribulin in a subsequent line for patients pre-treated with anthracyclines/taxanes and capecitabine.
Effectiveness: Results pertaining to health were measured according to quality-adjusted life years (QALY). This is the time spent in each health state weighted by quality of life (utility). Adverse events included in the model pertained to the grades observed in the clinical trial which were grades 3–4. The method described by Fleurence et al. [9]was used to describe the likelihood of occurrence.
Overall survival and progression-free survival curves were modelled in order to determine the average time spent by patients in each of the aforementioned states. Kaplan-Meier curves were recreated from points obtained by digitalising the survival curves and from the aggregate data obtained from a prior publication. For this, the algorithm described by Guyot (2012) [10] was applied. Hazard ratios (HR) were calculated from Cox regression in order to compare obtained data with original data. Various parametric distributions were analysed (Gamma, Lognormal, Weibull, Gompertz, generalised Gamma, Exponential, Log-logistic, Generalised and Royston-Parmar). The final model was selected based on visual inspection and fit values according to Akaike Information Criteria (AIC) and Bayesian Information Criteria (BIC). All of these analyses were performed using the statistical program “R” in its version 3.3.2 and the Flexsurv package.
Quality of life (Utility): Utility scores were obtained from a systematic review performed by Paracha (2016) [11]. The aim of this analysis was to identify utility values relating to different breast cancer health states. These states were obtained using direct preference methods designed for health technology evaluation agencies. Utility (or lack thereof) values associated with the presence of adverse events were neither estimated nor applied.
Use of resources and costs: Overall health costs associated with each one of the concepts considered in the SNS context were estimated. Given the complexity and difficulty of collecting all potential subsequent-line treatments after disease progression, most likely treatment options were agreed upon. This was done in consideration of indications approved by the EMA, Therapeutic Positioning Reports of the Spanish Agency of Medicines and Health Products (Table 1), and the guide to an Integrated Breast Cancer Care Process distributed by the Andalusian Public Health System [8]. Official medication prices were used as indicated in the official gazetteer (March 2020) [12]. Standard patients were assumed to have a standard weight of 70 Kg and a body height of 1.7 m2. In the case of talazoparib, price approval is still pending and, therefore, the current price for olaparib (for ovarian cancer) was taken as a reference as this is an alternative with a similar therapeutic value [13]. Finally, it was assumed that all patients who had terminal cancer ceased treatment one month earlier.
In order to estimate the cost of adverse events (grade 3 or 4)we defined associated activities, established standards and, finally, identified associated resources. Cost data for diagnostic tests, appointments and procedures was extracted from the Official Bulletin of the Andalusian Government [14,15]. The recommended prices of medications used for adverse events on sale in pharmacies were extracted from the Bot-Plus database [16] (Table 2). Appointments with specialists were included in costs of adverse events and pharmaceutical treatment. In the talazoparib arm, probability values relating to the TSM arm were used to indicate the frequency of adverse events following relapse. The appendix details some of the considerations included in the estimation of direct health costs used for the estimation of the monthly cost of different adverse events, follow-up, pharmaceutical treatment and associated hospital visits (Table A1).
Table 2.
Cost and effectiveness values employed in the budgetary impact analysis and economic evaluation.
| Parameters of the modela | ||
| Concept |
Average value |
|
| Monthly cost of medication (euros) | ||
| Talazoparib | 5413.69€ | |
| Capecitabine | 162.97€ | |
| Eribulin | 2695.50€ | |
| Gemcitabine | 488.10€ | |
| Vinorelbine | 1022.66€ | |
| Cost of adverse events (euros) | ||
| Anemia | 8483.74€ | |
| Neutropenia | 427.26€ | |
| Diarrhoea | 61.53€ | |
| Nausea and vomiting | 56.58€ | |
| Constipation | 58.25€ | |
| Cost of follow-up (euros) | ||
| Follow-up | 111.33€ | |
| Utility values | ||
| Paracha (2016) | ||
| Scenario 1 |
Scenario 2 |
|
| Free | 0.66 | 0.64 |
| After relapse | 0.447 | 0.447 |
| Death | 0 | 0 |
| Ettl (2018) | ||
| Talazoparib | 0.619 (TAD: 24 months) | |
| TSM | 0.609 (TAD:6 months) | |
| Death | 0 | |
The cost of pharmaceutical treatment and adverse events includes visits to the specialist; TSM: treatment suggested by the patient’s breast cancer medical doctor. TAD: time to deterioration.
The different data used were validated by various health professionals (hospital pharmacists and drug economy experts) in order to guarantee that the assumptions made reflected the reality of SNS clinical practice.
Analysis: Analyses were conducted using Microsoft Excel 2016. In order to evaluate uncertainty in the variables included in the model, univariate sensitivity analysis was conducted as follows:
-
1)
Using utility values from the article published by Ettl [17]. Time to deterioration was evaluated in the EMBRACA trial through a quality of life questionnaire (EORTC QLQ-30). It was assumed that, from the point of deterioration, quality of life remained constant throughout the duration of remaining life.
-
2)
Given that significant differences in overall survival were not observed between arms, the same curve was used for both treatment options in scenario 1 and fo rEttl [17] and Paracha [11] utility values. This enabled evaluation of the possibility that there had not been any improvement in this variable, a fundamental element of the estimations made.
-
3)
Finally, in order to be able to recommend a price for talazoparib treatment, cost-utility thresholds of 21,000, 24,000, 25,000 and 60,000€/QALY were used for both scenarios. These thresholds have been previously established in existing literature conducted within the Spanish context [18,19].
Budgetary impact: It is estimated that in 2019, 33,307 new cases of breast cancer were diagnosed in Spain [1]. Of these, 5% are gBRCA+. Further, it is considered that 10% of diagnosed breast cancer cases are in the metastatic state and that 30% of those diagnosed with localized cancer suffer setbacks following treatment, experiencing metastatic recurrence. Following this, it is assumed that a range of 70–90% of patients are candidates for first line treatment. Of these, 50% receive mono-chemotherapy treatment (scenario 1) [20]. Following progression, 60% of these will be eligible to receive another subsequent-line treatment (scenario 2) [20]. All of this represents a total of 157–202 patients for scenario 1, whilst for scenario 2, the number of patients decreases to 94–121.
3. Results
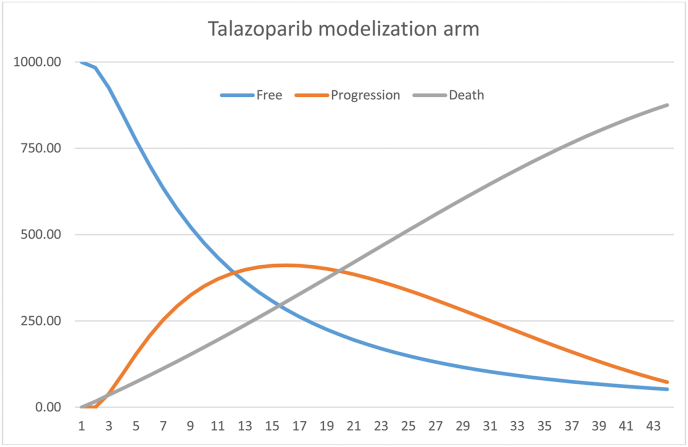
Fig. 1, Fig. 2 show the 43-month models. With regards to effectiveness, it is observed that the intervention group obtained an average value of 1.93 years of survival (1.07 QALY), relative to 1.58 years (0.84 QALY) in the standard treatment group. This represents a different of 0.35 life years and 0.23 QALY.
Fig. 1.
Modelling of the intervention group treated with talazoparib.
Fig. 2.
Modelling of the regular treatment group.
In scenario 1, the average cost of treating women with talazoparib at 43 months was 84,360.86€. Breaking down the costs, it is observed that 92.45% (77,990.29€) of this is due to pharmacological treatment. The average cost for the standard group was 26,683.90€, with pharmacological treatment representing 83.38% of that cost (22,249.10€). Next, the incremental cost-utility ratio (ICUR) was calculated at 252,420.04€/QALY. This represents an additional cost for achieving an additional QALY when changing from current treatment to talazoparib. From the sensitivity analysis performed with effectiveness values from the article published by Ettl (2018), an ICUR of 223,517.55€/QALY was obtained.
In analysing the second scenario, it is observed that the average cost of treating patients with talazoparib at 43 months was 92,515.05€, whilst the cost of the standard group was 33,195.36€. Thus, the ICUR was 259,609.36€/QALY (Table 3), whilst sensitivity analysis with effectiveness values from the article published by Ettl (2018) revealed an ICUR of 229,883.69€/QALY.
Table 3.
Incremental cost-utility of talazoparib treatment relative to current standard treatment, using a 43-month time-horizon.
| Alternative | Cost in euros (€) | Incremental cost (€) | Effectiveness (QALY) | Incremental Effectiveness (QALY) | ICURa |
|---|---|---|---|---|---|
| Scenario 1. Utility values taken from Paracha (2016) | |||||
| Talazoparib | 84,360.86 | 1.08 | |||
| Standard | 26,683.90 | 57,676.96 | 0.85 | 0.23 | 252,420.04 |
| Scenario 1. Utility values taken from Ettl (2018) | |||||
| Talazoparib | 84,360.86 | 1.09 | |||
| Standard | 26,683.90 | 57,676.96 | 0.83 | 0.26 | 223,517.55 |
| Scenario 2. Utility values taken from Paracha (2016) | |||||
| Talazoparib | 92,515.05 | 1.06 | |||
| Standard | 33,195.36 | 59,319.69 | 0.84 | 0.22 | 259,609.36 |
| Scenario 2. Utility values taken from Ettl (2018) | |||||
| Talazoparib | 92,515.05 | 1.09 | |||
| Standard | 33,195.36 | 59,319.69 | 0.83 | 0.26 | 229,883.69 |
| Scenario 1. Without overall survival benefit. Utility values taken from Paracha (2016) | |||||
| Talazoparib | 81,050.70 | 1.00 | |||
| Standard | 31,558.09 | 49,492.62 | 0.93 | 0.07 | 704,726.22 |
| Scenario 1. Without overall survival benefit. Utility values taken from Ettl (2018) | |||||
| Talazoparib | 81,050.70 | 0.99 | |||
| Standard | 31,558.09 | 49,492.62 | 0.91 | 0.08 | 646,084.56 |
ICUR: incremental cost-utility ratio; QALY: quality-adjusted life years.
Scenario 1: talazoparib relative to capecitabine as a preliminary treatment in patients pre-treated with anthracyclines/taxanes. Scenario 2: talazoparib relative to eribulin in subsequent-line treatment for patients pre-treated with anthracylcines/taxanes and capecitabine.
Cost per quality-adjusted life year (QALY) when changing from current standard treatment to talazoparib.
Through development of the model assuming that there would ultimately be no differences in overall survival, a cost of 68,238.72€ was obtained for the talazoparib arm, with a cost of 31,558.09€ for the standard arm. Resultantly, the ICUR would be 704,726.22€/QALY and 646,084.56€/QALY, for effectiveness values reported by Paracha and Ettl, respectively (Table 3).
With regards to budgetary impact, for scenario 1, differences in 3-year cost between talazoparib treatment and standard treatment ranged between 6.9 and 9 million euros. In the same way, the cost differences estimated for scenario 2 were found to be 5.2 and 6.8 million euros over 3 years.
Finally, monthly price limits were approached for talazoparib treatment according to different incremental cost-utility thresholds. In this way, the maximum price for a threshold of 21,000€/QALY was 931.33€ and 794.5€, for scenarios 1 and 2, respectively. These maximum prices were 1673.58€ and 1536.75€, respectively, for the 60,000€/QALY threshold (Table 4) (see Table 5).
Table 4.
Maximum price for talazoparib in order to fall within the cost-effective threshold range.
| Incremental cost-utility ratio threshold | |||
|---|---|---|---|
| 21,000€/QALY | 24,000€/QALY | 25,000€/QALY | 60,000€/QALY |
| Maximum Price for talazoparib (Scenario 1) | |||
| 931.33€ | 988.42 | 1007.46€ | 1673.58€ |
| Maximum Price for talazoparib (Scenario 2) | |||
| 794.5€ | 851.6€ | 870.63€ | 1536.75€ |
Scenario 1: Anthracylcines/taxanes and talazoparib adminstration relative to capecitabine administration as preliminary treatment in patients pre-treated with anthracyclines/taxanes. Scenario 2: talazoparib relative to eribulin in subsequent-line treatment for patients pre-treated with capecitabine.
Table 5.
Estimation of the budgetary impact of treatment with talazoparib relative to current standard treatment.
| Budgetary impact | ||||
|---|---|---|---|---|
| 1 year | 2 years | 3 years | 43 months | |
| Scenario 1 (n = 157) | ||||
| Talazoparib | 6,824,386.00 € | 9,095,179.49 € | 9,759,608.55 € | 9,861,106.41 € |
| Standard | 1,723,340.56 € | 2,668,125.05 € | 2,763,792.08 € | 2,764,345.96 € |
| Difference | 5,101,045.44 € | 6,427,054.44 € | 6,995,816.47 € | 7,096,760.45 € |
| Scenario 1 (n = 202) | ||||
| Talazoparib | 8,780,420.20 € | 11,702,078.07 € | 12,556,948.58 € | 12,687,538.18 € |
| Standard | 2,217,291.68 € | 3,432,874.27 € | 3,555,961.78 € | 3,556,674.42 € |
| Difference | 6,563,128.53 € | 8,269,203.80 € | 9,000,986.79 € | 9,130,863.76 € |
| Scenario 2 (n = 94) | ||||
| Talazoparib | 4,253,481.10 € | 5,821,830.34 € | 6,295,319.95 € | 6,365,316.45 € |
| Standard | 616,657.97 € | 970,211.58 € | 1,005,586.39 € | 1,005,786.86 € |
| Difference | 3,636,823.10 € | 4,851,618.75 € | 5,289,733.56 € | 5,359,529.60 € |
| Scenario 2 (n = 121) | ||||
| Talazoparib | 5,475,225.6 € | 7,494,058.20 € | 8,103,550.15 € | 8,193,652.03 € |
| Standard | 793,083.03 € | 1,247,764.87 € | 1,293,166.94 € | 1,293,409.25 € |
| Difference | 4,682,142.60 € | 6,246,293.33 € | 6,810,383.20 € | 6,900,242.78 € |
Scenario 1: talazoparib relative to capecitabine as preliminary treatment in patients pre-treated with anthracyclines/taxanes. Scenario 2: talazoparib relative to eribulin in subsequent-line treatment for patients pre-treated with anthracyclines/taxanes and capecitabine.
4. Discussion
PARP inhibitor drugs (iPARP), including olaparib, rucaparib, niraparib and talazoparib have begun to be used to treat ovarian cancer. Further, they are currently being studied and approved in new neoplastic locations, encompassing similar therapeutic breast cancer scenarios to the one studied in the present research. The most significant associated adverse events include haematological complications, which must be kept under observation and managed therapeutically [21].
As far as can be deduced from information provided by authors in the field, this is the first economic evaluation conducted in relation to iPARP in advanced/metastatic breast cancer. From the scientific literature, various economic evaluations are found in relation to iPARP treatment for recurrent platinum-sensitive ovarian cancer [[22], [23], [24]]. It serves to highlight that in the aforementioned cost-utility analysis, the effectiveness measures used were progression-free survival [[22], [23], [24]] and survival adjusted for quality of life [23]. Despite the differences encountered in relation to various items (medication, outcome and method), the three articles consulted on ovarian cancer produced incremental cost-effectiveness ratios (ICER) which ranged between 235 and 287 thousand dollars. These results are similar for other breast cancer treatments, such as eribulin for advanced breast cancer with an ICER of € 220.608/QALY. These values are similar to the ICUR described in the present article for breast cancer. Taking these results into account, the price of talazoparib should be lower than that taken as a reference to reach the cost-utility thresholds. In this sense, the National Institute for Health and Care Excellence did not recommend the use of trastuzumab for breast cancer in the National Health System due to its cost-utility ratio (£ 166,000/QALY, close to € 200,000/QALY); subsequently, the drug was added after a price negotiation.
The present work has various limitations, which should be considered when interpreting the results. Firstly, quality of life values from the study conducted by Ettl are not based on preferences. Whilst this could represent a drawback as this method is highly recommended for conducting economic evaluation studies, values were obtained from a population with similar characteristics making them appropriate. Further, quality of life values described in the study by Ettl (2018) are highly similar to those described by Hurvitz (2018) [26], which were obtained through the ABRAZO clinical trial (phase II) for talazoparib. Another potential limitation is that no reduction was applied to the effectiveness values of patients who did not present complications. This could represent a limitation given that some of these complications have a significant impact on patient quality of life, for instance inducing fatigue, within those treated with talazoparib [4]. With regards to cost, the scientific literature indicates that administration of talazoparib requires BRCA determination and this cost was not considered here. This aspect is particularly relevant as, in addition to being indicators of the effectiveness of iPARP, mutations to the BRCA gene also have implications for patients’ relatives. Thus, relatives of these patients should be invited to attend appointments relating to genetic advice [27].
Another limitation of the present work is that olaparib was not included as an alternative to talazoparib. This decision was made despite this treatment having the same indication and studies using it having similar designs and populations. The reason for this was that a previously conducted indirect comparative analysis following methodology outlined by Bucher, concluded that both drugs are equivalent. This was seen through the emergence of progression-free survival data that was statistically similar between treatment groups (HR 1.074; 95%CI 0.71–1.626) [13].
The number of patients in each health state was determined directly from underlying survival curves and this characterised the partitioned survival analysis model used. This model presents two advantages relative to the Markov model. Firstly, it avoids the need to estimate transition probabilities and, secondly, it does not invoke additional assumptions. For example, it enabled death to be modelled in all health states. However, it limits opportunities to perform sensitivity analysis [28].
The model was constructed taking the perspective of the health sector and, therefore, indirect costs were not included such as, for example, the time in working hours spent on treatment administration. Had the present study taken a social perspective, including indirect costs could have produced an increase in overall costs and it is possible that ICUR could also have further increased. On the other hand, it should be noted that there are other drugs from the same therapeutic group that are being evaluated for the same indication. However, the results of this economic evaluation cannot be extrapolated to these cases directly [29,30].
For the estimation of treatment costs in scenario 1 (capecitabine), data obtained by the pivotal study carried out by the TSM were utilised as this study lacked a reliable analysis of outcomes for each individual agent (eribulin, capecitabine, vinorelbine and gemcitabine) and relevant differences were not demonstrated [7].
It is relevant to highlight that drug choices and proportions following relapse were agreed upon by different experts. Thus, these decisions could be different depending on the health centre. However, the drug proportions used were also similar to those described in the OlympiaD [29] study which evaluated olaparib treatment for the same indication analysed in the present article.
National health systems are experiencing continuously increasing costs. Expenses related to hospital medications particularly stand out due to the arrival of new highly priced medications for haematology-oncology, chronic autoimmune conditions etc. According to the literature, the average ICER reported for medications against cancer is more than double the average ICER for non-oncology medications [31]. Inclusion of these medications in clinical practise has resulted in a notable increase in care costs for this type of patients. Thus, in this case it is recommended to carry out a downward revision of estimated medication prices in order to contribute to the sustainability of the health system. Such recommendations have already been made for olaparib treatment of ovarian cancer [22,23]. Other arguments for lowering prices include incremental cost-effectiveness ratio values, slight increases in survival of breast cancer patients, budgetary impact analysis and recommended prices according to different thresholds. In light of all of this, the delicate situation created following the COVID-19 pandemic should also be considered. This situation calls for a strict evaluation of relevant benefits for the patient and the way in which these relate to additional costs. Finally, it is recommended to conduct further studies on talazoparib given that limited evidence exists of its efficacy. Overall survival should be considered in these studies as a main variable.
The use of economic evaluations, together with the price negotiation, is a tool that helps policy makers to decide, whether to finance or not a new technology, in order to maximize the health of the population taking into account the limitation of resources. This work focuses on this scenario, initially assuming a price that would imply a modest reduction on the price of a similar drug previously financed for another indication. Maintaining this threshold, the proposed cost-effectiveness thresholds would be widely exceeded.
Novelty and impact
As far as the authors know, this paper is the first economic evaluation of iPARP in advanced/metastatic breast cancer. The intervention is not cost-effective. Additionally, talazoparib does not extend the median survival time compared to the alternatives of capecitabine, vinorelbine and eribulin and results in low values of quality-adjusted survival. Considering the adverse events, high cost and low survival improvement of talazoparib, it should be used with caution in patients with breast cancer BRCA mutation.
Funding
The present research work was carried out without funding.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2021.04.004.
Appendix A.
Cost considerations.
-
-
Pharmaceutical drug cost considerations: Three-month computed tomography scans were incorporated into follow-up costs, in addition to 15-day complete blood count (CBC) analyses. With regards to specialist visits, it was assumed that all patients receiving oral treatment had monthly specialist appointments, whilst patients who were receiving intra-venous treatment had specialist appointments for each administration. Further, one visit to the specialist was imputed for each patient at the beginning of follow-up. Finally, it was assumed that all patients diagnosed with terminal cancer, ceased treatment one month beforehand.
-
-
Adverse event cost considerations: For each adverse event, one specialist visit was recorded in addition to pharmacological treatment. In the talazoparib arm, probabilities for the TSM arm were used for the frequency of adverse events following relapse.
-
-
Appointment considerations: Appointment costs were extracted from the Official Bulletin of the Andalusian Government (BOJA) which was published in 2005 and updated in 2019 through the consumer price index (IPC).
Table A1.
Direct health costs used to estimate the monthly cost of different adverse events, follow-up, pharmacological treatment and associated hospital appointments.
| Cost/unit | Monthly cost | ||
|---|---|---|---|
| Adverse events | |||
| Anemiaa | Transfusions | 3891.17 | 8483.74 € |
| Epoetin alfa | 175.35 | ||
| Neutropeniab | Follow-up consultation (specialised care) | 54.58 | 427.26 € |
| Follow-up consultation (primary care) | 17.84 | ||
| ratiograstim (filgrastim biosimilar) | 34.12 | ||
| ratiograstim (filgrastim biosimilar) | 54.59 | ||
| Diarrhoea | Loperamide | 6.95 | 61.53 € |
| Follow-up consultation (specialised care) | 54.58 | ||
| Nausea and vomiting | metoclopramide | 2 | 56.58 € |
| Follow-up consultation (specialised care) | 54.58 | ||
| Constipation | Lactulose | 3.67 | 58.25 € |
| Follow-up consultation (specialised care) | 54.58 | ||
| Follow-up | |||
| Computerized Tomography Scan every 3 months | 138.45 | 111.33 € | |
| Haemogram | 5.3 | ||
| Follow-up consultation (specialised care) | 54.58 | ||
| Medications | |||
| Capecitabine (1,250 mg/m2 Oral) two/day. Days 1–14 every 21 days | 85.00 € | ||
| Eribulin (1.4 mg/m2 IV). Days 1–8 every 21 days | 2472.73€ | ||
| Gemcitabine (1,250mg/m2 IV). Days 1 and 8 every 21 days | 265.32 € | ||
| Vinorelbine (30 mg/m2 IV). Days 1, 8 and 15 every 21 days | 688.50 € | ||
| Talazoparib 1 mg/day | 5335.71€ | ||
| Cost of visits associated with the medications | |||
| Capecitabine (1,250mg/m2 Oral) two/day. Days 1–14 every 21 days | 77.97 € | ||
| Eribulin (1.4 mg/m2 IV). Days 1 and 8 every 21 days | 222.78 € | ||
| Gemcitabine (1,250 mg/m2 IV). Days 1 and 8 every 21 days | 222.78 € | ||
| Vinorelbine (30 mg/m2 IV). Days 1, 8 and 15 every 21 days | 334.16 € | ||
| Talazoparib 1 mg/day | 77.97 € | ||
a 2 blood transfusions and 4 administrations of epoetin alfa were considered.
b One visit to primary care services and another to specialised care services were considered, in addition to adding filgrastim growth factors 1–2 times a week.
Appendix ASupplementary data
The following is the Supplementary data to this article:
References
- 1.Asociación Española contra el cáncer. Datoscáncer de mama; 2019. https://www.aecc.es/sites/default/files/content-file/Datos-cancer-mama_2019_0.pdf Available at: [Google Scholar]
- 2.Haddad G., Saade M.C., Eid R. PARP inhibitors: a tsunami of indications in different malignancies. Pharmacogenomics. 2020;21(3):221–230. doi: 10.2217/pgs-2019-0113. [DOI] [PubMed] [Google Scholar]
- 3.Exman P., Barroso-Sousa R., Tolaney S.M. Evidence to date: talazoparib in the treatment of breast cancer. OncoTargets Ther. 2019;12:5177. doi: 10.2147/OTT.S184971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Litton J.K., Rugo H.S., Ettl J. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379(8):753–763. doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agencia española de medicamentos y productos sanitarios. Informe de Posicionamiento Terapéutico de eribulin (Halaven®) en el cáncer de mama. 2015. pp. 2–7. Available at: aemps.gob.es/medicamentosUsoHumano/informesPublicos/docs/eribulina-Halaven.pdf?x17133. [Google Scholar]
- 6.Woods B., Sideris E., Palmer S., Latimer N., Soares M. 2017;June. Nice dsu technical support document 19: partitioned survival analysis for decision modelling in health care: a critical review report by the decision support unit; pp. 1–72.http://www.nicedsu.org.uk Available from: [Google Scholar]
- 7.Cardoso F., Senkus E., Costa A. 4th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 4) Ann Oncol. 2018;29(8):1634–1657. doi: 10.1093/annonc/mdy192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Consejería de salud de la Junta de Andalucía . Consejería de Salud; 2011. Proceso Asistencial Integrado Cáncer de mama.https://www.juntadeandalucia.es/organismos/saludyfamilias/areas/calidad-investigacion-conocimiento/gestion-conocimiento/paginas/pai-cancer-mama.html Available at: [Google Scholar]
- 9.Fleurence R.L., Hollenbeak C.S. Rates and probabilities in economic modelling. Pharmacoeconomics. 2007;25(1):3–6. doi: 10.2165/00019053-200725010-00002. [DOI] [PubMed] [Google Scholar]
- 10.Guyot P., Ades A.E., Ouwens M.J.N.M. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9. doi: 10.1186/1471-2288-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paracha N., Thuresson P.O., Moreno S.G. Health state utility values in locally advanced and metastatic breast cancer by treatment line: a systematic review. Expert Rev Pharmacoecon Outcomes Res. 2016;16(5):549–559. doi: 10.1080/14737167.2016.1222907. [DOI] [PubMed] [Google Scholar]
- 12.Real Decreto-ley 8/2010, de 20 de mayo, por el que se adoptan medidas extraordinarias para la reducción del déficit público. Boletín Oficial del Estado; 2013. pp. 45070–45128.https://www.hacienda.gob.es/Documentacion/Publico/NormativaDoctrina/Tributaria/IRPF/RDL_8-2010.pdf Available at: [Google Scholar]
- 13.Camean-Castillo M., Fenix-Caballero S., Gil-Sierra M.D. 2SPD-009 Analysis of olaparib and talazoparib as possible therapeutic alternatives in advanced breast cancer and a germline BRCA mutation. Eur J Hosp Pharm. 2019;26(Suppl 1) A23 LP-A23. [Google Scholar]
- 14.ORDEN de 14 de octubre de . 2005. Por la que se fijan los precios públicos de los servicios sanitarios prestados por Centros dependientes del Sistema Sanitario Público de Andalucía.https://www.juntadeandalucia.es/boja/2005/210/28 Available at: [Google Scholar]
- 15.Orden de 1 de marzo de . 2006. Por la que se fijan los precios públicos de publicaciones editadas por esta Consejería.https://www.juntadeandalucia.es/boja/2006/56/17 Available at: [Google Scholar]
- 16.Bot plus 2. https://botplusweb.portalfarma.com Available from:
- 17.Ettl J., Quek R.G.W., Lee K.H. Quality of life with talazoparib versus physician’s choice of chemotherapy in patients with advanced breast cancer and germline BRCA1/2 mutation: patient-reported outcomes from the EMBRACA phase III trial. Ann Oncol. 2018;29(9):1939–1947. doi: 10.1093/annonc/mdy257. [DOI] [PubMed] [Google Scholar]
- 18.Vallejo-Torres L., García-Lorenzo B., Castilla I., Valcárcel Nazco C., GarcíaPérez L., Linertová R., Serrano-Aguilar P. Informes de Evaluación de Tecnologías Sanitarias; 2015. Valor Monetario de un Año de Vida Ajustado por Calidad: estimación empírica del coste de oportunidad en el Sistema Nacional de Salud. Ministerio de Sanidad, Servicios Sociales e Igualdad. Servicio de Evaluación del Servicio Canario de la Salud. [Google Scholar]
- 19.Sacristan J.A., Oliva J., Campillo-Artero C. [What is an efficient health intervention in Spain in 2020?] Gac Sanit. 2020;34(2):189–193. doi: 10.1016/j.gaceta.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Nice Talazoparib for treating BRCA 1 or 2 mutated advanced breast cancer after prior chemotherapy (ID1342) https://www.nice.org.uk/guidance/indevelopment/gid-ta10366 Available from:
- 21.Hennes E.R., Dow-Hillgartner E.N., Bergsbaken J.J. PARP-inhibitor potpourri: a comparative review of class safety, efficacy, and cost. J Oncol Pharm Pract. 2020;1078155219895066 doi: 10.1177/1078155219895066. [DOI] [PubMed] [Google Scholar]
- 22.Zhong L., Tran A.T., Tomasino T. Cost-effectiveness of niraparib and olaparib as maintenance therapy for patients with platinum-sensitive recurrent ovarian cancer. J Manag care Spec Pharm. 2018;24(12):1219–1228. doi: 10.18553/jmcp.2018.24.12.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dottino J.A., Moss H.A., Lu K.H. U.S. Food and drug administration-approved poly (ADP-Ribose) polymerase inhibitor maintenance therapy for recurrent ovarian cancer: a cost-effectiveness analysis. Obstet Gynecol. 2019;133(4):795–802. doi: 10.1097/AOG.0000000000003171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith H.J., Walters C.L., Arend R.C. PARP inhibitor maintenance therapy for patients with platinum-sensitive recurrent ovarian cancer: a cost-effectiveness analysis. Gynecol Oncol. 2015;139(1):59–62. doi: 10.1016/j.ygyno.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 26.Hurvitz S.A., Quek R.G.W., Turner N.C. Quality of life with talazoparib after platinum or multiple cytotoxic non-platinum regimens in patients with advanced breast cancer and germline BRCA1/2 mutations: patient-reported outcomes from the ABRAZO phase 2 trial. Eur J Canc. 2018;104:160–168. doi: 10.1016/j.ejca.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Jerez Y., Marquez-Rodas I., Aparicio I. Poly (ADP-ribose) polymerase inhibition in patients with breast cancer and BRCA 1 and 2 mutations. Drugs. 2020;80(2):131–146. doi: 10.1007/s40265-019-01235-5. [DOI] [PubMed] [Google Scholar]
- 28.Williams C., Lewsey J.D., Mackay D.F. Estimation of survival probabilities for use in cost-effectiveness analyses: a comparison of a multi-state modeling survival analysis approach with partitioned survival and Markov decision-analytic modeling. Media Decis. 2017;37(4):427–439. doi: 10.1177/0272989X16670617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robson M., Ruddy K.J., Im S.A. Patient-reported outcomes in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer receiving olaparib versus chemotherapy in the OlympiAD trial. Eur J Canc. 2019;120:20–30. doi: 10.1016/j.ejca.2019.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diéras V., Han H.S., Kaufman B. Veliparib with carboplatin and paclitaxel in BRCA-mutated advanced breast cancer (BROCADE3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(10):1269–1282. doi: 10.1016/S1470-2045(20)30447-2. [DOI] [PubMed] [Google Scholar]
- 31.Bae Y.H.J., Mullins C.D. Do value thresholds for oncology drugs differ from nononcology drugs? J Manag care Spec Pharm. 2014;20(11):1086–1092. doi: 10.18553/jmcp.2014.20.11.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.