Introduction
Nevoid melanoma (NeM) is deemed to be one of the most difficult melanomas to diagnose as it mimics a benign nevus clinically and histologically.1 There is no evidence to suppose at this time that it represents any more than a morphologic subtype without any special prognostic significance.2 Nevertheless, as NeM can be confused clinically and even pathologically for a nevus, there may be a delay in diagnosis, which can lead to a worse prognosis.3,4 In vivo reflectance confocal microscopy (RCM) is a noninvasive, high-resolution skin-imaging device, which has become an important tool in the diagnosis of malignant melanoma (MM),5 but it has some pitfalls.6 Few data on the use of RCM in NeM cases have been published. Herein, we describe RCM findings in 4 NeM cases and their clinical and dermoscopic features.
Case series
Case 1
An 82-year-old man was referred for evaluation of a pigmented lesion on the upper portion of the back. On cutaneous examination, a well-demarcated light-to-dark brown, 12-mm lesion was observed. Dermoscopy revealed a slightly asymmetric lesion with a typical reticular pattern in the periphery and a hyperpigmented blotch in the center, with a small blue-gray area (Fig 1). RCM was performed, revealing dendritic and round cells in a pagetoid spread, disrupting the honeycomb pattern in the epidermal spinous-granular layers (Fig 2) and nonedged papillae with many atypical refractile cells in the epidermal ridges. Dense nests were observed in most areas of the papillary dermis. Suspecting a melanoma developing in a nevus, the lesion was excised. Histopathology revealed a 0.69 mm-thick NeM. On scanning magnification, the lesion appeared with both junctional and dermal medium-large nests. At a higher magnification, these nests were composed of moderately discohesive atypical cells, and a prominent pagetoid spread was observed.
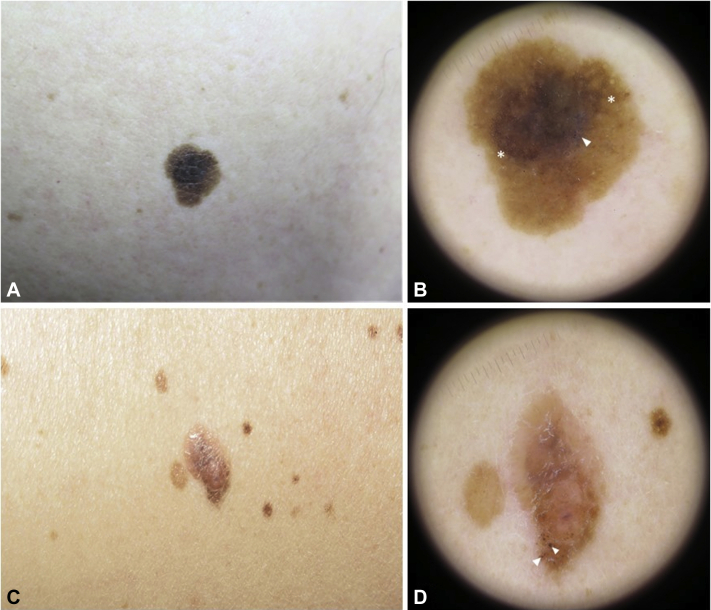
Fig 1.
Cases 1 and 2. A, Clinical picture of case 1. B, Dermoscopy image of case 1 showing a typical reticular pattern at the periphery (asterisks) and a small blue-gray area (arrowhead) next to a hyperpigmented blotch. C, Clinical picture of case 2. D, Dermoscopy image of case 2, revealing an asymmetric lesion with atypical irregular globules (arrowheads).
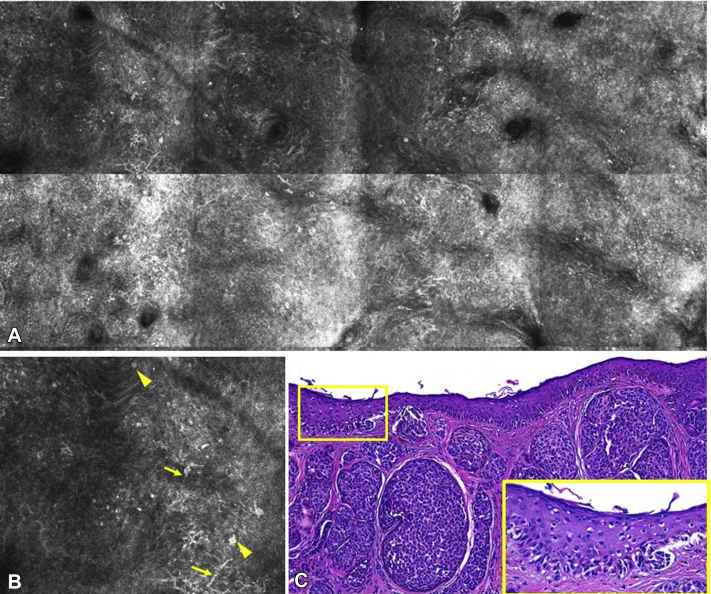
Fig 2.
Cytological atypia and pagetoid spread in the epidermis in case 1. A, Reflectance confocal microcopy mosaic (2 × 1 mm) revealing atypical cells disrupting the honeycomb pattern of the epidermal spinous-granular layers. B, Detail of the dendritic (yellow arrows) and round atypical cells (yellow arrowheads). C, Histopathology image of case 1 showing large nests of atypical melanocytes and prominent pagetoid spread (yellow square). (Hematoxylin-eosin stain; original magnification: C, ×100).
Case 2
A 53-year-old man with more than 50 nevi was referred for evaluation. On general examination a brownish-pink, well-demarcated, soft and slightly raised, 16-mm lesion was identified on the patient's right flank. Dermoscopy revealed an asymmetric lesion with typical homogenous-reticular pattern areas and atypical irregular globules (Fig 1). The RCM showed nonedged papillae and severe atypical junctional thickenings at the dermo-epidermal junction. Moderate numbers of dendritic cells in the upper layers of the epidermis were also observed. As observed in the previous case, typical dense nests were also seen in the papillary dermis. Histopathology confirmed the diagnosis of a 1.17 mm-thick NeM.
Case 3
A 69-year-old man was referred for a periodic skin examination. A suspicious pigmented lesion was noticed on his back. Clinically, the lesion was a 7-mm, homogenous, light-brown, flat lesion. Dermoscopically, the lesion was asymmetric and characterized by a prevalent globular pattern with pigmented globules varying in size and color (Fig 3). The RCM revealed the presence of large, compact nests filling up the interpapillary spaces while distorting and enlarging them. On close examination, the nests were irregular in shape and size with slightly irregular reflectance (Fig 4). Integrating all the findings, a diagnosis of melanoma was made, and the lesion was excised. Histopathology revealed a 0.67 mm-thick NeM. Unlike the previous cases, no pagetoid spread or junctional component was observed.
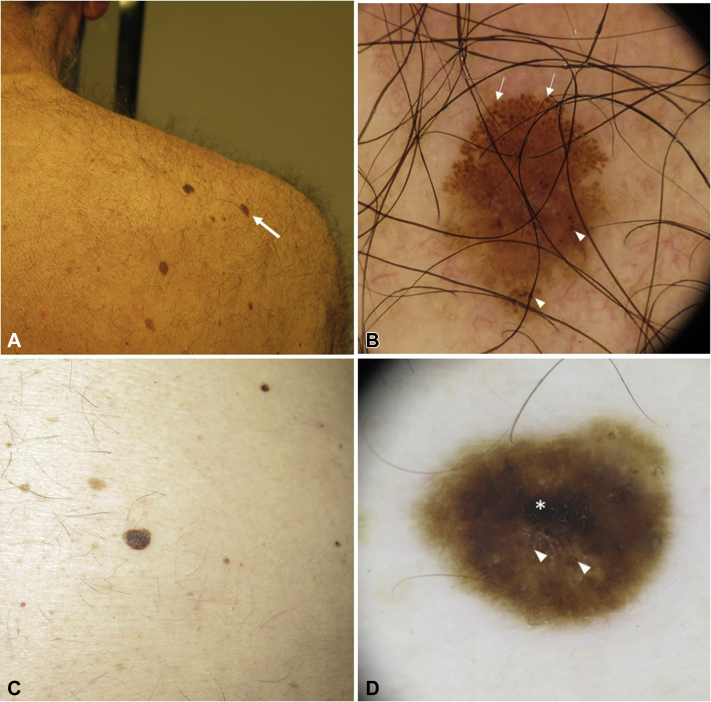
Fig 3.
Cases 3 and 4. A, Clinical picture of case 3 (arrow). B, Dermoscopy image of case 3 showing an asymmetric lesion with large globules in the top border (arrows) and irregular globules (arrowheads). C, Clinical picture of case 4. D, Dermoscopy image of Case 4 with a hyperpigmented blotch (asterisk) and irregularly distributed atypical globules (arrowheads).
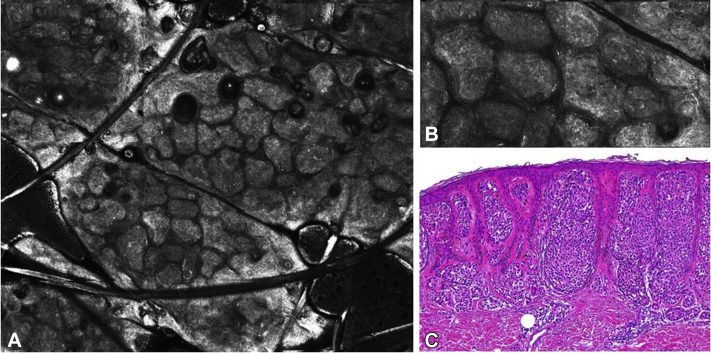
Fig 4.
Atypical nests in case 3. A, Reflectance confocal microcopy mosaic (3 × 3 mm) of case 3 showing the nest pattern in the papillary dermis. B, Detail of the clustered large nests with slightly irregular reflectance. C, Histopathology image of case 3 revealing large melanocyte nests in the dermal papillae, flattening the dermo-epidermal junction (Hematoxylin-eosin stain; original magnification: C, ×100).
Case 4
A 79-year-old man presented to the clinic with actinic keratosis in the face. On examination, a brownish, well-demarcated, flat, 6-mm lesion was identified on the patient's abdomen. Dermoscopic examination of the brown macule revealed a symmetric lesion with a homogeneous-reticular pattern but with a hyperpigmented blotch and irregularly distributed atypical globules (Fig 3). The RCM showed nests irregular in shape and size with slightly irregular reflectance and cytologic atypia. Histopathologic examination revealed a 0.48 mm-thick NeM. No pagetoid spread or junctional component was observed histologically or by RCM.
Discussion
NeMs are a rare morphological variant of MM, representing <1% of all melanomas.3 The significance lies in the stage at the time of the diagnosis, with a mean Breslow thickness of >2 mm in most series.3,4 Clinically, a NeM is described as a brown nodule or papule with or without a papilomatous surface located on the trunk or the proximal limbs; macular variants have been described.7 Dermoscopically, most NeMs have a nevus-like pattern, but with irregular globules/dots and atypical vascular structures.8 Irregular globules were a constant feature in our 4 cases, suggesting possible melanoma. NeMs are classically classified into 3 histologic subtypes: large-nest NeM, papillomatous NeM and diffuse/nodular NeM. All of our 4 cases showed the large-nest pattern. Regardless of the subtype, the histologic diagnosis of NeMs is challenging. Cytological and architectural changes are bland and can be missed, and the lesion can simulate depth on maduration.2, 3, 4,9 The RCM findings can also be subtle and may be overlooked, requiring the integration of clinical, Dermoscopy, and RCM features. In a retrospective study evaluating 710 lesions with RCM but without clinical data, the NeMs constituted a common pitfall and resulted in 4 of the 13 false-negative MMs.10 The presence of atypical cells in the suprabasal layer of the epidermis and the dermo-epidermal junction were instrumental in establishing correct diagnosis in first 2 cases, which correlated with the histologic image of pagetoid ascent and atypical melanocytic nests. However, not all NeMs exhibit a pagetoid spread or a junctional component on histologic examination; most of them have these features at the periphery of the lesion.9 This lack of epidermal and junctional component can make the diagnosis more challenging by RCM, as we can see in the other 2 cases, in which preserved structures were observed in the epidermis along with edged papillae or typical mesh pattern at the dermo-epidermal junction. These cases showed large dermal nests of irregular size with nonhomogeneous reflectance filling up the large intrapapillary nests of the NeMs. Similar findings were published in a recent case report.11 Although with bland changes, our cases presented malignant-suggestive features either in the epidermis and/or at the dermo-epidermal junction, as in cases 1 and 2, or in the papillary dermis' nests, as in cases 3 and 4. The difference between the nests of the first 2 cases and those of the latter 2 could be due to the depth at which they were located, since they were histologically similar.
In conclusion, while NeMs may be difficult to differentiate from naevi, RCM may provide clues to help identify their malignancy. The melanoma-suggestive findings by RCM can be subtle in NeMs, so our case series highlights that the correct diagnosis of these lesions relies on integrating clinical data, dermoscopic features, and RCM findings.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
References
- 1.Cabrera R., Recule F. Unusual clinical presentations of malignant melanoma: a review of clinical and histologic features with special emphasis on dermatoscopic findings. Am J Clin Dermatol. 2018;19(suppl 1):15–23. doi: 10.1007/s40257-018-0373-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diwan A.H., Lazar A.J. Nevoid melanoma. Clin Lab Med. 2011;31(2):243–253. doi: 10.1016/j.cll.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Zembowicz A., McCusker M., Chiarelli C. Morphological analysis of nevoid melanoma: a study of 20 cases with a review of the literature. Am J Dermatopathol. 2001;23(3):167–175. doi: 10.1097/00000372-200106000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Schmoeckel C., Castro C.E., Braun-Falco O. Nevoid malignant melanoma. Arch Dermatol Res. 1985;277(5):362–369. doi: 10.1007/BF00509234. [DOI] [PubMed] [Google Scholar]
- 5.Pezzini C., Kaleci S., Chester J., Farnetani F., Longo C., Pellacani G. Reflectance confocal microscopy diagnostic accuracy for malignant melanoma in different clinical settings: systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2020;34(10):2268–2279. doi: 10.1111/jdv.16248. [DOI] [PubMed] [Google Scholar]
- 6.Waddell A., Star P., Guitera P. Advances in the use of reflectance confocal microscopy in melanoma. Melanoma Manag. 2018;5(1):MMT04. doi: 10.2217/mmt-2018-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kossard S., Wilkinson B. Small cell (naevoid) melanoma: a clinicopathologic study of 131 cases. Australas J Dermatol. 1997;38(suppl 1):S54–S58. doi: 10.1111/j.1440-0960.1997.tb01011.x. [DOI] [PubMed] [Google Scholar]
- 8.Longo C., Piana S., Marghoob A. Morphological features of naevoid melanoma: results of a multicentre study of the International Dermoscopy Society. Br J Dermatol. 2015;172(4):961–967. doi: 10.1111/bjd.13524. [DOI] [PubMed] [Google Scholar]
- 9.Yélamos O., Busam K.J., Lee C. Morphologic clues and utility of fluorescence in situ hybridization for the diagnosis of nevoid melanoma. J Cutan Pathol. 2015;42(11):796–806. doi: 10.1111/cup.12627. [DOI] [PubMed] [Google Scholar]
- 10.Guitera P., Menzies S.W., Longo C., Cesinaro A.M., Scolyer R.A., Pellacani G. In vivo confocal microscopy for diagnosis of melanoma and basal cell carcinoma using a two-step method: analysis of 710 consecutive clinically equivocal cases. J Invest Dermatol. 2012;132(10):2386–2394. doi: 10.1038/jid.2012.172. [DOI] [PubMed] [Google Scholar]
- 11.Jain M., Marghoob A.A. Integrating clinical, dermoscopy, and reflectance confocal microscopy findings into correctly identifying a nevoid melanoma. JAAD Case Rep. 2017;3(6):505–508. doi: 10.1016/j.jdcr.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]