Abstract
Patients with severe coronavirus disease 2019 (COVID-19) demonstrate dysregulated immune responses including exacerbated neutrophil functions. Massive neutrophil infiltrations accompanying neutrophil extracellular trap (NET) formations are also observed in patients with severe COVID-19. However, the mechanism underlying severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-induced NET formation has not yet been elucidated. Here we show that 2 viral proteins encoded by SARS-CoV-2, the nucleocapsid protein and the whole spike protein, induce NET formation from neutrophils. NET formation was ROS-independent and was completely inhibited by the spleen tyrosine kinase inhibition. The inhibition of p38 MAPK, protein kinase C, and JNK signaling pathways also inhibited viral protein-induced NET formation. Our findings demonstrate one method by which SARS-CoV-2 evades innate immunity and provide a potential target for therapeutics to treat patients with severe COVID-19.
Keywords: Severe acute respiratory syndrome coronavirus 2, Neutrophils, Neutrophil extracellular traps, Viral protein, C-type lectin receptor, Spleen tyrosine kinase
INTRODUCTION
Patients with severe coronavirus disease 2019 (COVID-19) demonstrate excessive infiltration of immune cells into the lung parenchyma and compromised immune cell function (1,2,3). Dysregulated immune cell function leads to widespread inflammation in the lung with diffuse alveolar damage, finally resulting in acute respiratory distress syndrome (ARDS) in patients with severe COVID-19 (1-3). Recent studies suggest that neutrophils are involved in the pathogenesis of severe COVID-19. Excessive infiltration of neutrophils is found in lung parenchyma in patients with severe COVID-19 (4,5). The components of neutrophil extracellular traps (NETs), web-like structures composed of DNA, histones, and antimicrobial peptides, are also found in the serum of patients with severe COVID-19 (6). Moreover, an increased presence of neutrophil precursors and mature neutrophils has been reported in patients with severe COVID-19 (7). Since exacerbated NET formation induces host tissue injury (8) and thrombosis (9), NETs are considered to play a critical role in the pathogenesis of ARDS in patients with severe COVID-19 (4,6,10). However, it is still unclear how neutrophils generate NETs in response to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. In the current study, we found that the nucleocapsid (N) proteins and spike (S) proteins of SARS-CoV-2 induce NET formation through a spleen tyrosine kinase (syk)-dependent pathway.
MATERIALS AND METHODS
Neutrophil isolation and materials
Human blood experiments were approved by the Institutional Research Board of Kyungpook National University (KNU-2020-0029). Neutrophils were isolated using a density gradient followed by dextran sedimentation, as previously described (11). Materials used in the current study are summarized in Supplementary Table 1.
Quantification of NETs formation and ROS generation
Neutrophils (2×105 cells) were stimulated with SARS-CoV-2 viral proteins for 2 h in RPMI-1640 (Gibco, Grand Island, NY, USA) supplemented with 5% FBS (HyClone, Logan, UT, USA). Recombinant viral proteins used in this study include: N protein, 40588-V08B (Sino Biological, Beijing, China); S protein, 40589-V08B1 (Sino Biological); S1 subunit protein, 40591-V08H (Sino Biological); and S2 subunit protein, 40590-V08B (Sino Biological). Extracellular NET formation was measured using Sytox Green (Thermo Fisher Scientific Inc., Waltham, MA, USA) and intracellular ROS were measured using a fluorescent probe, 2′7′-dichlorodihydrofluorescein diacetate (DCF-DA; Thermo Fisher Scientific Inc.) as previously described (12). Fluorescence was measured using Spectramax M2/e fluorescence microplate reader (Molecular Devices, San Jose, CA, USA). For inhibition of viral protein-induced NET formation, neutrophils were pre-treated with following inhibitors: an antagonist for TLR 2 (MMG11, 10 μM; Tocris, Bristol, UK), an inhibitor for TLR4 (TAK242, 10 μM; Tocris), a syk inhibitor (R406, 1 μM; Cayman, Ann Arbor, MI, USA), an anti-angiotensin converting enzyme (ACE) 2 Ab (10 μg/ml; MyBioSource, San Diego, CA, USA), a NADPH oxidase inhibitor (diphenyleneiodonium chloride [DPI], 10 μM; Santa Cruz, Dallas, TX, USA), a Ca2+ chelator (BAPTA-AM, 100 μM; Tocris), an autophagy sequestration inhibitor (3-Methyladenine [3-MA], 10 μM; Sigma-Aldrich, St. Louis, MO, USA), autophagosome degradation inhibitors (chloroquine, 10 μM; Abcam, Cambridge, UK; leupeptin, 20 μM; Sigma-Aldrich), an inhibitor of autophagosome-lysosome fusion (bafilomycin, 10 μM; Santa Cruz), an ERK MAPK inhibitor (PD90859, 10 μM; Tocris), a PI3K inhibitor (wortmannin, 1 μM; Tocris), an inhibitor of p38 MAPK (SB203580, 10 μM; Tocris), a protein kinase C (PKC) inhibitor (GF109203, 5 μM; Sigma-Aldrich), a JNK inhibitor (SP600125, 10 μM; Sigma-Aldrich), and a protein arginine deiminase 4 (PAD4) inhibitor (Cl-amide, 10 μM; Cayman).
Immunofluorescence
For immunofluorescence microscopy analysis, neutrophils were stimulated with viral proteins for 2 h, fixed with 4% para-formaldehyde (Biosesang, Seongnam, Korea), permeabilized with 0.1% Triton X, and blocked with 5% donkey serum (Sigma-Aldrich) in PBS. Cells were stained with primary Abs against myeloperoxidase (5 μg/ml; Abcam) and citrullinated histone 3 (5 μg/ml; Abcam), stained with secondary Abs, and counterstained with DAPI. Cells were visualized by immunofluorescence microscopy (Olympus IX83; Olympus, Tokyo, Japan).
Migration of neutrophil chemotaxis
Migration of neutrophils against viral proteins of SARS-CoV-2 was determined using a μ-slide chamber (ibidi, Fitchburg, WI, USA) according to the manufacturer's instruction.
Statistical analysis
Data are presented as means±SEM. Comparisons between 2 groups were performed with 2-tailed Student's t-tests (parametric). Statistical data were analyzed by Graphpad Prism 7.0e (Graphpad Software, San Diego, CA, USA).
RESULTS AND DISCUSSION
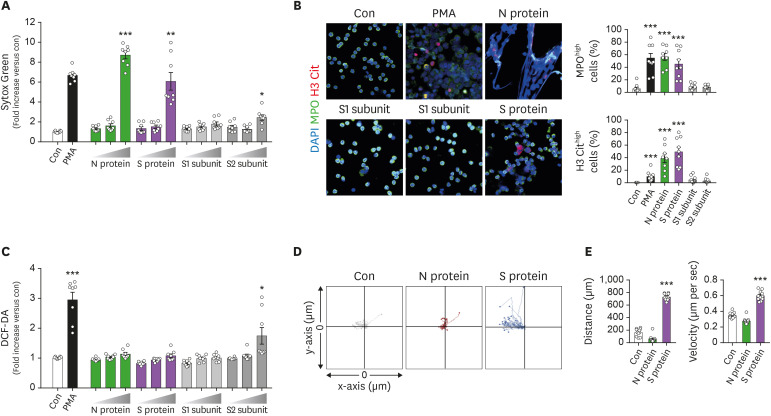
To examine whether viral proteins induced NETs formation from neutrophils, neutrophils isolated from healthy subjects were exposed to either N protein, S proteins, or subunits (S1 and S2) of SARS-CoV-2 for 2 h and NET formation was examined. N and S protein significantly enhanced NET formation (Fig. 1A). Immunofluorescence microscopic analysis showed the presence of viral protein-induced NETs (Fig. 1B). We further examined the effects of viral proteins from SARS-CoV-2 on effector functions of neutrophils. Viral proteins did not significantly affect the ROS generation in neutrophils excepting S2 protein, which slightly enhanced ROS production (Fig. 1C). Neutrophils showed significantly increased migration toward S proteins, but not N proteins (Fig. 1D). These results suggest that viral proteins of SARS-CoV-2 directly affect effector functions of neutrophils, especially ROS-independent NET formation.
Figure 1. N and S proteins of SARS-CoV-2 induce NET formation from neutrophils. (A-C) Neutrophils were incubated with various concentrations (1, 10, and 100 nM) of the N protein, whole S protein, S1 subunits of the S protein, or S2 subunits of the S protein for 2 h. (A) NET formation in response to viral proteins was determined by Sytox Green staining. (B) Representative immunofluorescence images of NET formation in response to viral proteins of SARS-CoV-2. Representative images of more than 5 experiments are shown (scale bar, 10 μm). (C) ROS generation in response to viral proteins was determined by DCF-DA staining. (D, E) The effects of viral proteins on chemotaxis of neutrophils. One side of chamber was coated with either N, S, S1, or S2 protein and chemotaxis of neutrophils toward viral proteins was examined. (D) Neutrophil migration tracking analysis. The distances traveled by neutrophils were tracked for 45 min. Representative tracking results of thirty cells per each group are shown (n=3 per group). (E) Relative mean distance and relative mean velocity of neutrophils migrating toward viral proteins. Data are expressed as means±SEMs.
Con, control; MPO, myeloperoxidase; H3Cit, citrullinated histone 3.
*p<0.05; **p<0.01; ***p<0.001 vs. Con.
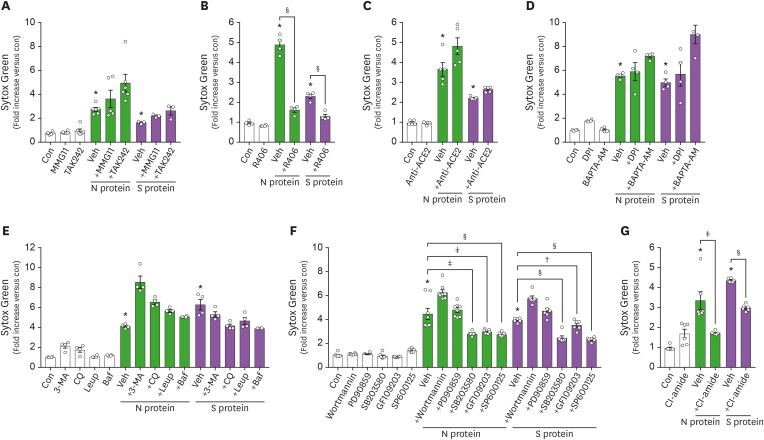
Innate immune cells are thought to recognize SARS-CoV-2 through pattern recognition receptors (3), and TLR2 has been reported to mediate the innate immune response of peripheral blood mononuclear cells against S proteins of SARS-CoV (13). Therefore, we examined whether TLRs could mediate the recognition of viral proteins by neutrophils. The pharmacological inhibition of neither TLR2 nor TLR4 affected viral protein-induced NET formation (Fig. 2A). However, the inhibition of syk, an intracellular signaling molecule of downstream of C-type lectin receptors (CLRs), significantly inhibited viral protein-induced NET formation (Fig. 2B). We also examined whether ACE2, which has been reported to facilitate the entry of SARS-CoV-2 into cells (14), might also play a role in viral protein-induced NET formation, but treatment with an anti-ACE2 Ab had no effect (Fig. 2C).
Figure 2. Neutrophils utilize a CLR-dependent pathway in viral protein-induced NET formation. (A-G) The effects of various inhibitors on viral protein-induced NET formation. Neutrophils were pre-treated with indicated inhibitors for 1 h, and further stimulated with viral proteins (100 nM) for 1 h. Veh; MMG11, a TLR2 inhibitor, 10 μM; TAK242, a TLR4 inhibitor, 10 μM; R406, a syk inhibitor, 1 μM; an anti-ACE2 Ab, 10 μg/ml; DPI, a NADPH oxidase inhibitor, 10 μM; BAPTA-AM, Ca2+ chelator, 100 μM; 3-MA, an autophagy sequestration inhibitor, 10 μM; CQ, an autophagosome degradation inhibitor, 10 μM; Leup, an autophagosome degradation inhibitor, 20 μM; Baf, an inhibitor for autophagosome-lysosome fusion, 10 μM; wortmannin, an inhibitor for PI3K, 10 μM; PD90859, an inhibitor for ERK, 10 μM; SB203580, an inhibitor for p38 MAPK, 10 μM; GF109203, a PKC inhibitor, 10 μM; SP600125, a JNK inhibitor, 10 μM; Cl-amide, a PAD4 inhibitor, 10 μM. Data are expressed as means±SEM.
Con, control; Veh, vehicle; 3-MA, 3-Methyladenine; Leup, leupeptin; CQ, chloroquine; Baf, bafilomycin.
*p<0.001 vs. Con; †p<0.05; ‡p<0.01; §p<0.001 vs. Veh.
We further identified the mechanism underlying viral protein-induced NET formation. Neither the treatment with DPI, an inhibitor for NADPH oxidase, nor BAPTA-AM, a Ca2+ chelator, affected viral protein-induced NET formation (Fig. 2D). Pharmacological inhibition of autophagic pathways, a major pathway underlying ROS-independent NET formation (11), also had no effect (Fig. 2E). Neither did inhibition of ERK MAPK nor PI3K affect viral protein-induced NET formation (Fig. 2F). Interestingly, the inhibition of p38 MAPK, PKC, and JNK significantly inhibited viral protein-induced NET formation (Fig. 2F). Moreover, the inhibition of PAD4, a key enzyme for chromatin decondensation during NETs formation, significantly inhibited viral protein-induced NET formation (Fig. 2G).
The key findings of this study are that 1) N and S proteins of SARS-CoV-2 directly induced NET formation from neutrophils, 2) viral proteins of SARS-CoV-2 induce NET formation via a syk-dependent pathway, and 3) p38 MAPK, JNK, PKC, and PAD4 mediate viral protein-induced NET formation.
Although it is still questionable whether SARS-CoV-2-induced NET formation is protective or detrimental to patients, recent studies have emphasized the detrimental effects of excessive NET formation in the pathogenesis of COVID-19. NETs are known to be associated with alveolar damage in various infectious diseases, such as influenza virus infection (15) and bacterial infection (16). Neutrophilia and extensive infiltration of neutrophils in the lung parenchyma were found in severe COVD-19 patients (4,5,17,18), and lung parenchymal autopsy samples revealed degenerated neutrophils representing NETs-released neutrophils (5). We found that viral proteins of SARS-CoV-2 are able to drive NETs formation from neutrophils directly. Recently, a correlation between the viral products of SARS-CoV-2 and poor outcomes has been reported (19), and increased loads of viral products, including viral proteins, were found in COVID-19 patients (19,20,21). Therefore, the increased load of viral proteins shed from replicating SARS-CoV-2 might drive excessive or dysregulated NET formation in patients with COVD-19.
We also found that viral proteins of SARS-CoV-2 induce NETs formation through a syk-dependent pathway. Immune cells exert immune responses against fungus and viruses through CLR recognition and syk-mediated intracellular signaling (22). Immune cells induce syk-mediated immune response against fungus and viruses through CLRs recognition (23). CLRs are binding receptors for SARS-CoV that are expressed in alveolar type II cells, endothelial cells, and dendritic cell (24,25). Neutrophils are equipped with syk-associated CLRs such as Dectin-1, Mincle, C-type lectin domain family (CLEC) 2, and CLEC5A (25), and utilize them for recognition of diverse viruses such as the dengue virus, Japanese encephalitis virus, and human immunodeficiency virus (26). Interestingly, we found that whole S protein induce NET formation from neutrophils whereas each subunit of S protein did not affect neutrophil function (Fig. 1A). Recent study suggested that immune cells might interact with S protein of SARS-CoV-2 through CLRs such as DC-SIGN, CLEC4, CLEC5A, and CLEC10A based on in silico study (27). The structure of trimeric S protein complex composes 22 glycosylated regions and SARS-CoV-2 utilize them for evading immune responses (28,29). Therefore, we suggest that the structural recognition of viral proteins by neutrophils might be critical for NETs formation. Supporting this, syk, major intracellular signaling molecule of CLR pathway, mediated NET formation in neutrophils stimulated with S protein (Fig. 2B). Dectin-1 and syk signaling is involved in immune response of neutrophils against fungal infection (30) and syk induces the activation of PKC- δ in various immune cells (31,32). In accordance with these findings, the inhibition of PKC, p38 MAPK, and JNK signaling pathways reduced viral protein-induced NET formation (Fig. 2B and F), suggesting the involvement of CLRs such as dectin-1 in viral protein-induced NET formation.
In summary, our results suggest an important insight into how neutrophils respond to SARS-CoV-2 infection. Neutrophils might recognize liberated viral proteins during SARS-CoV-2 infection through a syk-dependent pathway, leading to excessive NET formation and subsequent tissue damage. Therefore, either the inhibition of syk-dependent viral recognition or NET formation could represent possible candidates for therapeutics for severe COVD-19 patients.
ACKNOWLEDGEMENTS
This study was supported by 2019R1A2C1087814 and 2020R1A4A2002691 from the National Research Foundation of Korea (NRF).
Abbreviations
- ACE
anti-angiotensin converting enzyme
- ARDS
acute respiratory distress syndrome
- CLEC
C-type lectin domain family
- CLR
C-type lectin receptor
- COVID-19
coronavirus disease 2019
- DCF-DA
2′7′-dichlorodihydrofluorescein diacetate
- DPI
diphenyleneiodonium chloride
- N
nucleocapsid
- NET
neutrophil extracellular trap
- PAD4
protein arginine deiminase 4
- PKC
protein kinase C
- S
spike
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- syk
spleen tyrosine kinase
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Hong CW.
- Data curation: Bae JS.
- Funding acquisition: Jin HK, Bae JS, Hong CW.
- Investigation: Youn YJ, Lee YB, Kim SH.
- Supervision: Jin HK, Bae JS, Hong CW.
- Writing - original draft: Youn YJ, Hong CW.
- Writing - review & editing: Hong CW.
SUPPLEMENTARY MATERIAL
Materials used in the study
References
- 1.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vabret N, Britton GJ, Gruber C, Hegde S, Kim J, Kuksin M, Levantovsky R, Malle L, Moreira A, Park MD, et al. Immunology of COVID-19: current state of the science. Immunity. 2020;52:910–941. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes BJ, Adrover JM, Baxter-Stoltzfus A, Borczuk A, Cools-Lartigue J, Crawford JM, Daßler-Plenker J, Guerci P, Huynh C, Knight JS, et al. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J Exp Med. 2020;217:e20200652. doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fox SE, Akmatbekov A, Harbert JL, Li G, Quincy Brown J, Vander Heide RS. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8:681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA, Blair C, Weber A, Barnes BJ, Egeblad M, et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5:e138999. doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulte-Schrepping J, Reusch N, Paclik D, Baßler K, Schlickeiser S, Zhang B, Krämer B, Krammer T, Brumhard S, Bonaguro L, et al. Severe COVID-19 is marked by a dysregulated myeloid cell compartment. Cell. 2020;182:1419–1440.e23. doi: 10.1016/j.cell.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Németh T, Sperandio M, Mócsai A. Neutrophils as emerging therapeutic targets. Nat Rev Drug Discov. 2020;19:253–275. doi: 10.1038/s41573-019-0054-z. [DOI] [PubMed] [Google Scholar]
- 9.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Jr, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skendros P, Mitsios A, Chrysanthopoulou A, Mastellos DC, Metallidis S, Rafailidis P, Ntinopoulou M, Sertaridou E, Tsironidou V, Tsigalou C, et al. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J Clin Invest. 2020;130:6151–6157. doi: 10.1172/JCI141374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park SY, Shrestha S, Youn YJ, Kim JK, Kim SY, Kim HJ, Park SH, Ahn WG, Kim S, Lee MG, et al. Autophagy primes neutrophils for neutrophil extracellular trap formation during sepsis. Am J Respir Crit Care Med. 2017;196:577–589. doi: 10.1164/rccm.201603-0596OC. [DOI] [PubMed] [Google Scholar]
- 12.Shrestha S, Noh JM, Kim SY, Ham HY, Kim YJ, Yun YJ, Kim MJ, Kwon MS, Song DK, Hong CW. Angiotensin converting enzyme inhibitors and angiotensin II receptor antagonist attenuate tumor growth via polarization of neutrophils toward an antitumor phenotype. OncoImmunology. 2015;5:e1067744. doi: 10.1080/2162402X.2015.1067744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dosch SF, Mahajan SD, Collins AR. SARS coronavirus spike protein-induced innate immune response occurs via activation of the NF-kappaB pathway in human monocyte macrophages in vitro. Virus Res. 2009;142:19–27. doi: 10.1016/j.virusres.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narasaraju T, Yang E, Samy RP, Ng HH, Poh WP, Liew AA, Phoon MC, van Rooijen N, Chow VT. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol. 2011;179:199–210. doi: 10.1016/j.ajpath.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Douda DN, Jackson R, Grasemann H, Palaniyar N. Innate immune collectin surfactant protein D simultaneously binds both neutrophil extracellular traps and carbohydrate ligands and promotes bacterial trapping. J Immunol. 2011;187:1856–1865. doi: 10.4049/jimmunol.1004201. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Liu Y, Xiang P, Pu L, Xiong H, Li C, Zhang M, Tan J, Xu Y, Song R, et al. Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J Transl Med. 2020;18:206. doi: 10.1186/s12967-020-02374-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng Z, Diao B, Wang R, Wang G, Wang C, Tan Y, Liu L, Wang C, Liu Y, Liu Y, et al. The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) directly decimates human spleens and lymph nodes. medRxiv; 2020. [DOI] [Google Scholar]
- 21.Okba NMA, Müller MA, Li W, Wang C, GeurtsvanKessel CH, Corman VM, Lamers MM, Sikkema RS, de Bruin E, Chandler FD, et al. Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease patients. Emerg Infect Dis. 2020;26:1478–1488. doi: 10.3201/eid2607.200841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoving JC, Wilson GJ, Brown GD. Signalling C-type lectin receptors, microbial recognition and immunity. Cell Microbiol. 2014;16:185–194. doi: 10.1111/cmi.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mócsai A, Ruland J, Tybulewicz VL. The syk tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol. 2010;10:387–402. doi: 10.1038/nri2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gramberg T, Hofmann H, Möller P, Lalor PF, Marzi A, Geier M, Krumbiegel M, Winkler T, Kirchhoff F, Adams DH, et al. LSECtin interacts with filovirus glycoproteins and the spike protein of SARS coronavirus. Virology. 2005;340:224–236. doi: 10.1016/j.virol.2005.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeffers SA, Tusell SM, Gillim-Ross L, Hemmila EM, Achenbach JE, Babcock GJ, Thomas WD, Jr, Thackray LB, Young MD, Mason RJ, et al. CD209L (L-SIGN) is a receptor for severe acute respiratory syndrome coronavirus. Proc Natl Acad Sci U S A. 2004;101:15748–15753. doi: 10.1073/pnas.0403812101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas CJ, Schroder K. Pattern recognition receptor function in neutrophils. Trends Immunol. 2013;34:317–328. doi: 10.1016/j.it.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 27.Gollapalli P, S SB, Rimac H, Patil P, Nalilu SK, Kandagalla S, Shetty P. Pathway enrichment analysis of virus-host interactome and prioritization of novel compounds targeting the spike glycoprotein receptor binding domain-human angiotensin-converting enzyme 2 interface to combat SARS-CoV-2. J Biomol Struct Dyn. 2020 doi: 10.1080/07391102.2020.1841681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watanabe Y, Allen JD, Wrapp D, McLellan JS, Crispin M. Site-specific glycan analysis of the SARS-CoV-2 spike. Science. 2020;369:330–333. doi: 10.1126/science.abb9983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grant OC, Montgomery D, Ito K, Woods RJ. Analysis of the SARS-CoV-2 spike protein glycan shield reveals implications for immune recognition. Sci Rep. 2020;10:14991. doi: 10.1038/s41598-020-71748-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X, Utomo A, Cullere X, Choi MM, Milner DA, Jr, Venkatesh D, Yun SH, Mayadas TN. The β-glucan receptor Dectin-1 activates the integrin Mac-1 in neutrophils via Vav protein signaling to promote Candida albicans clearance. Cell Host Microbe. 2011;10:603–615. doi: 10.1016/j.chom.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strasser D, Neumann K, Bergmann H, Marakalala MJ, Guler R, Rojowska A, Hopfner KP, Brombacher F, Urlaub H, Baier G, et al. Syk kinase-coupled C-type lectin receptors engage protein kinase C-δ to elicit Card9 adaptor-mediated innate immunity. Immunity. 2012;36:32–42. doi: 10.1016/j.immuni.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacinto E, Werlen G, Karin M. Cooperation between syk and Rac1 leads to synergistic JNK activation in T lymphocytes. Immunity. 1998;8:31–41. doi: 10.1016/s1074-7613(00)80456-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Materials used in the study