Abstract
Macrophages are important for the first line of defense against microbial pathogens. Integrin CD11b, which is encoded by Itgam, is expressed on the surface of macrophages and has been implicated in adhesion, migration, and cell-mediated cytotoxicity. However, the functional impact of CD11b on the inflammatory responses of macrophages upon microbial infection remains unclear. Here, we show that CD11b deficiency resulted in increased susceptibility to sepsis induced by methicillin-resistant Staphylococcus aureus (MRSA) infection by enhancing the pro-inflammatory activities of macrophages. Upon infection with MRSA, the mortality of Itgam knockout mice was significantly higher than that of control mice, which is associated with increased production of TNF-α and IL-6. In response to MRSA, both bone marrow-derived macrophages and peritoneal macrophages lacking CD11b produced elevated amounts of pro-inflammatory cytokines and nitric oxide. Moreover, CD11b deficiency upregulated IL-4-induced expression of anti-inflammatory mediators such as IL-10 and arginase-1, and an immunomodulatory function of macrophages to restrain T cell activation. Biochemical and confocal microscopy data revealed that CD11b deficiency augmented the activation of NF-κB signaling and phosphorylation of Akt, which promotes the functional activation of macrophages with pro-inflammatory and immunoregulatory phenotypes, respectively. Overall, our experimental evidence suggests that CD11b is a critical modulator of macrophages in response to microbial infection.
Keywords: CD11b, Methicillin-resistant Staphylococcus aureus, Sepsis, Inflammation, Macrophages
INTRODUCTION
Sepsis is a life-threatening infectious disease and a leading cause of death in hospitals (1). Upon pathogen infection, the host immune system elicits an appropriate inflammatory response to eliminate the invading microbe, followed by active resolution of the inflammation to maintain tissue homeostasis (2). Otherwise, hyperactivation and/or sustained activation of neutrophils and macrophages, upregulation of pro-inflammatory cytokines such as TNF-α, and the enhanced tissue necrosis contribute to the pathogenesis of sepsis (3). Immunocompromised patients are at a higher risk of sepsis partially due to an impaired capability of clearing pathogens, leading to the continued activation of inflammatory cells (4,5). Antibiotics are currently used to treat sepsis (6); however, antimicrobial resistance has become a major global healthcare problem (1). The increasing prevalence of methicillin-resistant Staphylococcus aureus (MRSA), for which multiple antibiotic resistances are conferred by mutations in a penicillin-binding protein, has been one of the serious threats (7). Indeed, MRSA bacteremia is closely associated with a poor outcome because antibiotic resistances can lead to sustained systemic inflammatory responses against the accumulated bacteria (8).
Macrophages have a pivotal role in the innate immune responses to remove pathogenic microbes and in tissue homeostasis. Pattern recognition receptors including TLRs sense invading pathogens and trigger inflammation by producing soluble factors that include IL-1β, TNF-α, and IL-6 and recruiting other immune cells to the inflamed tissue (9,10). In addition, macrophages modulate adaptive immune responses by presenting foreign antigens in the context of MHC molecules supplemented with costimulators and inhibitory ligands such as CD80 and PD-L1, respectively (11). After clearing the infection, macrophages responsible for anti-inflammation and tissue remodeling are essential to maintain tissue homeostasis (12). The secretion of anti-inflammatory cytokines such as IL-10 and TGF-β and the production of immunomodulatory proteins including arginase-1 are involved in this later stage of infection. Tissue-resident macrophage populations are also responsible for peripheral tolerance by producing anti-inflammatory cytokines such as IL-10 and/or by inducing regulatory T cells (13). In this regard, there are two functional classes of macrophages, classically activated macrophages with a pro-inflammatory phenotype and alternatively activated macrophages with an immune regulatory phenotype (14). The septic symptom can be derived from the attenuated function of the regulatory macrophages and the hyperactivation activation of pro-inflammatory macrophages against pathogens (15,16). Thus, a balance between functionally distinct macrophages in the context of heterogeneous populations is critical for many inflammatory disorders, including infectious diseases, autoimmune diseases, and sepsis. However, it remains unclear how macrophages decide their fates to differentiate into the two distinct populations, which attribute to the pathogenesis of inflammatory diseases.
The integrin protein CD11b is encoded by the ITGAM gene and expressed on the surface of many innate immune cells that include macrophages, granulocytes, NK cells, subsets of dendritic cells (DCs), and B cells (17). CD11b is important for the adhesion, migration, and phagocytosis of macrophages as a subunit of the integrin αMβ2/Mac-1/CR3. Besides its role as an integrin, CD11b is reported to modulate several other biological functions in macrophages, DCs, and B cells (18-20). Single nucleotide polymorphisms in ITGAM are highly associated with susceptibility to autoimmune diseases such as arthritis, nephritis, and systemic lupus erythematosus (SLE) (21,22), suggesting a crucial role for CD11b in immune tolerance. Using KO animal approaches, Han and colleagues (18,23) reported that Itgam −/− mice are more susceptible to sepsis induced by endotoxin shock and Escherichia coli infection and colitis in dextran sodium sulfate-treated mice. These reports suggested that CD11b negatively regulates pro-inflammatory responses of macrophages but also promotes anti-inflammatory function by IL-10 production. In contrast, other study claimed that CD11b facilitates pro-inflammatory responses of macrophages as inhibition of CD11b using a blocking antibody, small molecule inhibitor or genetic ablation protected mice against septic death in LPS- and polymicrobial-induced inflammation models (24). Similarly, Schmid et al. (25) reported that pharmacological activation of CD11b promoted polarization of pro-inflammatory macrophage whereas CD11b deficiency led to immunosuppressive macrophages. Moreover, Ling and colleagues (19) suggested that CD11b positively cooperates with TLR as an LPS co-receptor to elicit optimal inflammatory responses in DCs. At the same time, another group proposed that the expression of CD11b confers DCs with a regulatory phenotype in a collagen-induced arthritis model (26). These studies revealed that CD11b has a pivotal role in the balance between pro-inflammatory and immune regulatory responses, although the conflicting results remain to be clarified in terms of immune populations and disease conditions.
To delineate the role of CD11b in activation and functional polarization of macrophages against pathogenic microbes, in this study, we infected Itgam−/− mouse with the antibiotic-resistant bacteria MRSA. We found that the CD11b deficiency conferred a higher susceptible to bacteremia and sepsis induced by MRSA infection. In line with previous studies using LPS or other infection models (18,23), we observed increased activation of the pro-inflammatory macrophages upon MRSA infection in vivo and in vitro. Intriguingly, the CD11b deficiency also enhanced functional activities of macrophages induced by IL-4 treatment, suggesting a modulatory role of CD11 in macrophages with both the pro-inflammatory and regulatory phenotypes against pathogen infection. Our experimental evidence sheds new light on the regulatory role of CD11b in macrophages and suggests that CD11b is required for the fine-tuning of immune responses to microbial infection.
MATERIALS AND METHODS
Bacteria
The MRSA MW2 strain was kindly provided by Bok Leul Lee (Pusan National University, Busan, Korea) and cultured at 37°C in Columbia broth (BD Bioscience, Franklin Lakes, NJ, USA) containing 2% NaCl in a shaking incubator (27). Bacteria cultured overnight were diluted in the Columbia broth at 1:100 and grown to an OD600 of 0.8 for the in vitro and in vivo infection experiments. To measure bacterial colony forming units (CFUs), samples were serially diluted in PBS and plated on the Columbia broth plates containing 1.5% agar, followed by the enumeration of the CFUs.
Mice and MRSA infection
C57BL/6J mice were purchased from DBL (Eumseong-gun, Korea), and Itgam−/− mice were obtained from Jackson Laboratories (Bar Harbor, ME, USA). OT-II mice were provided by Mark Boothby (Vanderbilt University, Nashville, TN, USA). The mice were housed in specific pathogen-free conditions and used under experimental protocols (Hallym 2017-14) approved by the Institutional Animal Care and Use Committee of Hallym University. The Itgam−/− mice were backcrossed with C57BL/6J, and the direct analyses of each type of control mice (Itgam+/+ and Itgam+/−) were compared to the knockout mice. Eight- to twelve-week-old male mice were injected intraperitoneally with MRSA (8×108 CFU/mouse) and monitored over 7 days. Peripheral blood was collected from the tail vein 8 h after MRSA infection and treated with 5 mM EDTA. The peritoneum was infused with ice-cold PBS containing 2% FBS and the peritoneal lavage was used for determining the bacterial titer and for the flow analysis.
Macrophage culture
Bone marrow-derived macrophages (BMDMs) were prepared as described elsewhere (28). Briefly, bone marrow cells were isolated from the femurs and tibias and resuspended in complete RPMI1640 medium supplemented with 10% FBS. Cells were cultured for 4 days in the presence of 5 ng/ml GM-CSF or 20 ng/ml M-CSF at 37°C in a 5% CO2 atmosphere, and adherent cells were grown in fresh media containing GM-CSF or M-CSF for an additional 3 days. Prevalence of F4/80hi cells in the cultured BMDMs was more than 90% (data not shown). Peritoneal macrophages were isolated from mice 4 days after pretreatment with 3% thioglycollate. BMDMs and peritoneal macrophages were allowed to adhere for at least 2 h in well plates and cultured in antibiotic-free RPMI1640 containing 10% FBS with 5 multiplicities of infection (MOI) of MRSA for 8 h. Alternatively activated macrophages were treated with 10 ng/ml IL-4 for 18 h.
Migration assay
The migration of macrophages was measured using a Boyden chamber transwell plate with a 5 μm porous membrane. Peritoneal macrophages were loaded onto the upper chamber of the transwells in complete RPMI1640 medium. Four hours after incubation with or without 200 ng/ml LPS in the lower chamber at 37°C, the cells were removed from the upper side of membranes and the migratory cells in the lower side of the membranes were stained with crystal violet. The number of migratory cells was counted from random microscopic fields for each sample.
Phagocytosis assay
BMDMs or peritoneal macrophages were treated with 200 ng/ml LPS for 8 h and incubated with 5 MOI of MRSA in an antibiotic-free medium in 12-well plates. Half an hour after incubation with MRSA at 37°C, the cells were washed in PBS to remove the free bacteria and incubated in a new medium for an additional 1.5 h. The cells were lysed in 0.2% Triton X-100 in PBS and then diluted in PBS to measure live bacterial titers.
Measurement of cytokines and nitric oxide (NO)
The protein levels of IL-12p70, TNF-α, IFN-γ, MCP-1 and IL-6 were determined using the Cytokine Bead Array Mouse Inflammation Kit (BD Bioscience) and LEGENDplex Multiplex Assay kit (BioLegend, San Diego, CA, USA) following the manufacturer's instructions. IL-10 levels were measured by the Cytokine Beads Array Mouse IL-10 Enhanced Sensitivity Flex Set (BD Bioscience). The NO assay was performed as described (29). Briefly, macrophages were treated with 5 MOI of MRSA for 8 h, and the culture medium was collected to measure the amount of nitrite, a stable metabolite of NO. One hundred microliters of the culture medium were incubated with equal amount of Griess reagent (Promega, Madison, WI, USA) at room temperature for 10 min, and the absorbance was measured at 540 nm using a microplate reader. The quantity of nitrite was determined from a standard curve of sodium nitrate.
Coculture with OT-II T cells
Spleen cells were isolated from class II MHC-restricted TCR transgenic OT-II mice, and CD4 T cells were purified using anti-CD4 microbeads and MACS cell separation reagents (Miltenyi Biotec, Seoul, Korea). OT-II T cells were covalently fluorescein-labeled using the CellTrace Violet Cell Proliferation Kit (ThermoFisher, Waltham, MA, USA) and cultured with BMDMs in the presence of 1 μg/ml OVA323–339 peptides for 4 days. For intracellular cytokine staining, the cells were restimulated with 50 ng/ml PMA and 1 μg/ml ionomycin for 6 h in the presence of the Golgi-stop (BD Bioscience) followed by surface FACS staining. After fixed with 4% paraformaldehyde and permeabilized with 1% saponin, the cells were stained with antigen presenting cell (APC)-conjugated anti-IFN-γ and analyzed by flow cytometry.
Flow cytometry
Immunofluorescent staining of peritoneal cells and cultured macrophage was conducted as described (20). Fluorescein-conjugated anti-CD11b (M1/70), anti-F4/80 (T45-2342), anti-CD19 (ID3), anti-TCRβ (H57-0597), anti-CD11c (N418), anti-CD86 (GL1), anti-I-A/I-E (M5/114.15.2), anti-38 (90/CD38), and anti-CD278 (TY25) antibodies were purchased from BD Bioscience or eBioscience (San Diego, CA, USA). Data were acquired using the BD FACSCanto-II with the FACSDiva software (BD Bioscience) and analyzed using FlowJo (Tree Star, Ashland, OR, USA). Macrophages were defined as CD11b+ F4/80+ TCRβ− B220− cells in a viable forward scatter and side scatter gate.
Western blotting and quantitative real-time RT-PCR
Cells were lysed in 20 mM HEPES (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, protease inhibitors and phosphatase inhibitors (GenDEPOT, Hanam, Korea) and used for SDS-PAGE. Immunoblotting was performed using the indicated antibodies, followed by the appropriate HRP-conjugated secondary antibodies. All primary antibodies were purchased from Cell Signaling Technologies except for NF-κB p65 (Santa Cruz Biotechnology, Dallas, TX, USA) and β-actin (Sigma-Aldrich, St. Louis, MO, USA). Total RNA was extracted using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA), and cDNA was synthesized by M-MLV reverse transcriptase (Promega) with random nonamers (Genotech, Daejeon, Korea). Using the primer pairs tabulated in Table 1, quantitative PCR was performed with a SYBR PCR mix (Toyobo, Osaka, Japan) and the CFX Real-Time PCR detection system (Bio-Rad, Hercules, CA, USA). Data were normalized to the levels of Actb expression in each sample and quantified using the comparative 2−ΔΔCT method (20).
Table 1. Primer sequences used in the quantitative RT-PCR analysis.
| Gene name | Sequence (5' to 3') |
|---|---|
| Tnf | GATCGGTCCCCAAAGGGATG |
| CACTTGGTGGTTTGCTACGAC | |
| Il6 | GTTCTCTGGGAAATCGTGGA |
| TGTACTCCAGGTAGCTATGG | |
| Il1b | GGATGAGGACATGAGCACCT |
| GGAGCCTGTAGTGCAGTTGT | |
| Il12b | CACGGCAGCAGAATAAATA |
| CTTGAGGGAGAAGTAGGAATG | |
| Nos2 | TCCTGGACATTACGACCCCT |
| CTCTGAGGGCTGACACAAGG | |
| Il10 | CCCATTCCTCGTCACGATCTC |
| TCAGACTGGTTTGGGATAGGTTT | |
| Arg1 | GGGACCTGGCCTTTGTTGAT |
| GCTTCCAACTGCCAGACTGT | |
| Ym1 | GAAGGAGCCACTGAGGTCTG |
| TGAGCCACTGAGCCTTCAAC | |
| Fizz1 | AGTGCCCTGTGTTTCAGAGA |
| TGTGGAAGTTCACGCTCCAG | |
| Irf4 | CTTTGAGGAATTGGTCGAGAGG |
| GAGAGCCATAAGGTGCTGTCA | |
| Actb | GGCACCACACCTTCTACAATG |
| GGGGTGTTGAAGGTCTCAAAC |
Confocal microscopy
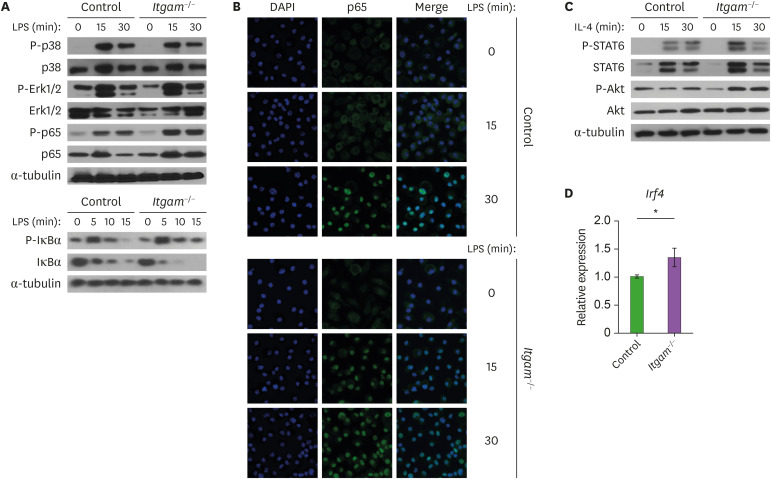
Indirect immunofluorescent staining was performed as described (30). BMDMs were placed onto coverslips and treated with 200 nM LPS for the indicated times. After washing with ice-cold PBS, the cells were fixed with 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, and stained using anti-NF-κB p65 antibody (Santa Cruz Biotechnology) followed by secondary antibody conjugated with Alexa Fluoro 488 (ThermoFisher). After stained with DAPI (Sigma-Aldrich), the cells were scanned using a Zeizz LSM710 laser scanning confocal device attached to an Axiovert 100 microscope (Carl Zeiss, Oberkochen, Germany).
Statistics
All of the experiments were performed in duplicate or triplicate, and the results of more than 3 independent experiments are presented as the mean±SEM. Differences between the samples were analyzed using an unpaired, 2-tailed nonparametric t-test of significance (Instat; GraphPad Inc., San Diego, CA, USA). The Kaplan-Meier log rank test was used to analyze mouse mortality data using R (The R Foundation for Statistical Computing, Vienna, Austria). In the figures, * and ** denote p-values less than 0.05 and 0.01, respectively.
RESULTS
CD11b deficiency confers increased susceptibility to sepsis induced by MRSA infection
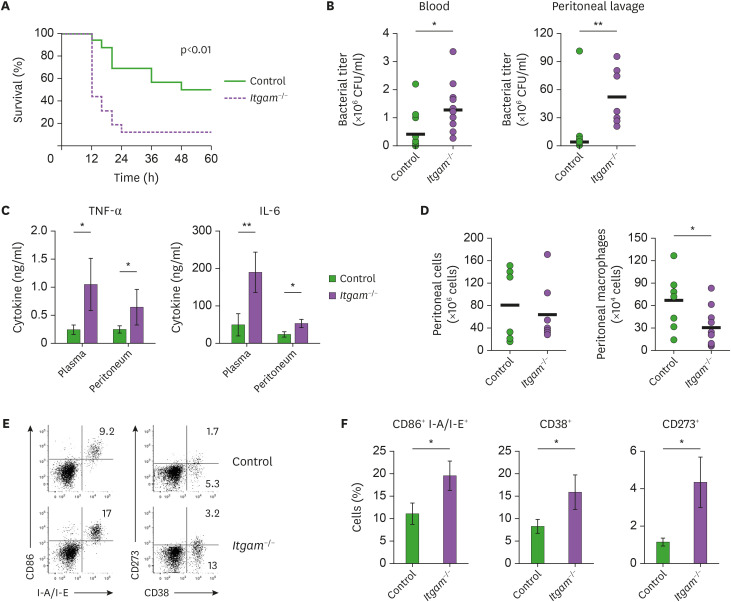
CD11b has an important role in immunity as an integrin, but there have been conflicting reports on its function regulating inflammatory responses against microbial (18,24,31,32). To define the impact of CD11b on the pathogen-driven immune responses, we infected mice lacking CD11b (Itgam−/−) and control mice with MRSA. Intraperitoneal inoculation of MRSA with 3×108 CFU resulted in septic death in 50% of the control C57BL/6 mice (Fig. 1A). The mortality of the Itgam−/− mice upon MRSA infection was substantially higher (87%) than that of the control mice, and all the sepsis mice were dead within 24 h post-infection (Fig. 1A). The MRSA titers in the blood circulation and peritoneum of the Itgam−/− mice were significantly higher than in those of the control mice (Fig. 1B), suggesting that CD11b deficiency might impede the ability to clear the invading bacterial pathogen. On the other hand, CD11b-deficient mice produced elevated amounts of pro-inflammatory cytokines such as TNF-α and IL-6 in the plasma and peritoneum upon MRSA infection (Fig. 1C and Supplementary Fig. 1), which was correlated with the higher susceptibility to septic death in the Itgam−/− mice.
Figure 1.
CD11b-deficient mice are more susceptible to sepsis induced by MRSA infection. (A) Mice were inoculated intraperitoneally with MRSA, and the mortality was monitored: n=16 Itgam−/− vs. 16 control mice. (B) Bacterial titers in the peripheral blood and peritoneum were determined 8 h after infection: bar indicates the mean values of CFUs. (C) Amounts of TNF-α and IL-6 in the plasma and peritoneal lavage were measured 8 h after the infection, and shown as mean (±SEM) of cytokine levels. (D) Viable cells in the peritoneal lavage were counted 8 h after infection and analyzed by flow cytometry: bar indicates the mean numbers of total peritoneal leukocytes as CD45+ and macrophages as TCRβ− B220− F4/80+ cells, respectively. (E) Representative FACS profiles of peritoneal macrophages with frequencies of indicated subsets in the viable TCRβ− B220− F4/80+ gates. (F) Shown are mean (±SEM) prevalence of the indicated subsets obtained from 2 independent experiments.
*p<0.05; **p<0.01.
CD11b is reported to be involved in the integrin-mediated cellular processes including migration and recruitment of innate immune cells including macrophages to the inflamed tissues (17). Accordingly, there were fewer Ly-6Chi Ly-6G- monocytes and Gr1lo F4/80hi macrophages cells in the peritoneum of the Itgam−/− mice upon MRSA infection, while the total numbers of peritoneal leukocytes were comparable (Fig. 1D and Supplementary Fig. 2). In contrast, prevalence of Gr1hi F4/80int granulocytes was higher in the peritoneum of Itgam−/− mice (Supplementary Fig. 2), in agreement with other studies in which CD11b deficiency promoted neutrophil infiltration into the lung upon pulmonary infection (33,34). The surface expression of pro-inflammatory activation markers such as CD86 and class II MHC (I-A/I-E) was higher on the peritoneal macrophages of the Itgam−/− mice than on those of the control mice (Fig. 1E and F). Glycoprotein CD38 is selectively expressed on the classically activated macrophages, whereas expression of CD273 is largely confined in the alternatively activated macrophages (35). We observed predominant induction of CD38+ macrophages in the MRSA-inoculated peritoneum, and the prevalence of the CD38+ macrophages was significantly higher in the Itgam−/− mice (Fig. 1E and F). Intriguingly, the surface expression of CD273 on peritoneal macrophages was also markedly upregulated by CD11b deficiency (Fig. 1E and F). Collectively, the data suggest a regulatory role of CD11b in the inflammatory responses of macrophages and that CD11b-deficiency causes exacerbated inflammatory responses upon MRSA infection.
Impaired migration, but enhanced bacterial killing ability of CD11b-deficient macrophages
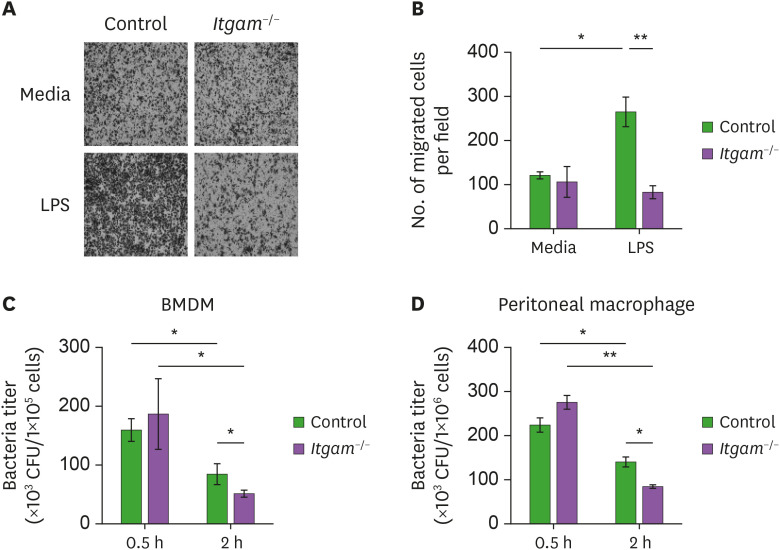
MRSA infection experiments showed that CD11b deficiency resulted in decreased macrophages and higher bacterial burden in the in the peritoneum of the Itgam−/− mice upon MRSA infection (Fig. 1). To determine the requirement of CD11b in the recruitment of macrophages, we performed an ex vivo migration assay using a Boyden-chamber well plate with peritoneal macrophages. While basal migration was comparable, LPS-induced migration of CD11b-deficient BMDMs was substantially impaired (Fig. 2A and B). This result was consistent with the attenuated recruitment of macrophages in the inflamed peritoneum of the Itgam−/− mice in vivo. Macrophages are the primary phagocytes for invading microorganisms and eliminate the pathogens by producing cytotoxic molecules such as NO, ROS, and lysosomal hydrolases (10). As shown in Fig. 2C and D, uptake of bacteria by the BMDMs and peritoneal macrophages 0.5 h after incubation with MRSA was not affected by CD11b deficiency. Live bacteria in both CD11b-deficient and control macrophages were significantly declined by additional 1.5 h incubation, which indicated an increment of the bacteria killing activity. Of note, CD11b deficiency did not impair the bacteria killing activity of the activated macrophages against MRSA, although previous studies reported a requirement of CD11b in a phagocytic function against other bacterial pathogens (36,37). Instead, we observed an enhancement of the bactericidal activity (Fig. 2C and D), implying a distinct regulation of CD11b in the phagocytic pathway to ingest and destroy the pathogen MRSA. Overall, these ex vivo experimental results indicate that the increased MRSA burden in the Itgam−/− mice was likely due to impaired migration rather than an altered phagocytic function of macrophages.
Figure 2.
Impaired migration but normal in bacteria killing ability of CD11b-deficient macrophages. (A) Peritoneal macrophages were isolated from Itgam−/− or control mice 4 days after thioglycollate treatment, and migration assay was performed using a Boyden chamber transwell plate. Shown are representative pictures (100×) of macrophages stained with crystal violet on the bottom side of the membranes 4 h after the cells were incubated in the upper insert wells in the presence or absence of LPS. (B) Shown are mean (±SEM) numbers of macrophages on the bottom side of the membranes obtained from 3 experiments. (C, D) BMDMs and peritoneal macrophages were activated with LPS for 8 h and treated with MRSA for 0.5 h. After washed to remove cell-free bacteria, some macrophages were lysed in 0.2% Triton X-100 (denoted by “0.5 h”) and the others were incubated in a new antibiotics-free medium for additional 1.5 h (denoted by “2 h”). Live bacteria inside the macrophages were determined as a CFU.
Increased pro-inflammatory activities of CD11b-deficient macrophages
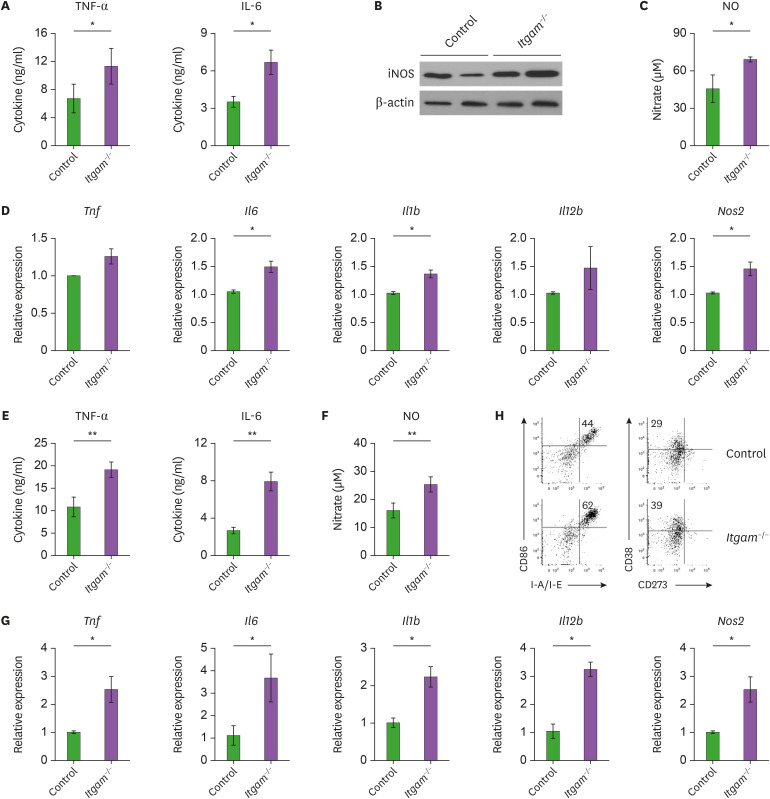
CD11b deficiency resulted in increased production of IL-6 and TNF-α after MRSA infection, and a higher susceptibility to sepsis (Fig. 1). To explore whether CD11b is associated with functional activation of macrophages, we measured the secretion of pro-inflammatory mediators. In response to MRSA treatment in vitro, BMDMs lacking CD11b produced higher amounts of pro-inflammatory cytokines such as TNF-α and IL-6 than control BMDMs (Fig. 3A). Enzyme NO synthesis 2 (inducible nitric oxide synthase [iNOS]) and the resulting production of NO were also significantly increased in the Itgam−/− macrophages (Fig. 3B and C). According to the increased amounts of pro-inflammatory cytokines and NO, we detected the elevated expression of mRNAs encoding TNF-α, IL-6, IL-1β, and iNOS in the CD11b-deficient macrophages (Fig. 3D), suggesting a regulatory effect of CD11b at the transcriptional level. Ling et al. (19) reported that CD11b deficiency upregulated LPS-induced expression of IL-6 and TNF-α in thioglycolate-elicited peritoneal macrophages but not in resident peritoneal macrophages or BMDMs. However, our data show that CD11b deficiency also promotes functional activation of BMDMs with pro-inflammatory phenotypes such as the production of TNF-α and NO. Moreover, similar results were obtained with peritoneal macrophages elicited with thioglycollate (Fig. 3E-G). These data could explain why CD11b-deficient mice exhibited exaggerated inflammatory responses against the MRSA infection. In addition to the secretion of pro-inflammatory mediators, flow cytometry analysis revealed an increased induction of CD86, class II MHC, and CD38 on the surface of CD11b-deficient BMDMs compared with that of the control BMDMs (Fig. 3H). In line with the elevated pro-inflammatory gene expression (Fig. 3D and G), these results imply that CD11b functions as a negative modulator in the activation and function of pro-inflammatory macrophages upon MRSA infection.
Figure 3.
CD11b-defieicnt macrophages produce increased amounts of pro-inflammatory mediators. (A) BMDMs were treated with MRSA for 8 h, and cytokine levels in the culture supernatants were measured using the Cytokine Bead Array. (B) iNOS expression was assessed by immunoblotting and intensity of iNOS signal in each band was normalized by β-actin. Shown is the representative of 3 independent experiments. (C) NO production was measured as nitrate levels using the Griess reagent. (D) Expression of mRNA of the indicated cytokines was analyzed by quantitative real-time RT-PCR. Concentration of each mRNA was normalized to Actb, and depicted as the relative expression levels to those of the control macrophages. Data are presented the mean (±SEM) from 2 independent experiments with 3 biological replicates. (E-G) Peritoneal macrophages were treated with MRSA for 8 h, and cytokine levels (E), NO production (F) and mRNA expression (G) were determined as described above. (H) BMDMs were treated with MRSA for 8 h, and analyzed by flow cytometry. Shown are representative FACS profiles with frequencies of indicated subsets in the viable TCRβ− B220− F4/80+ gates.
Given that MRSA is a gram-positive bacterium, BMDMs derived from Itgam−/− or control mice were activated with TLR ligands that include lipoteichoic acid (LTA), peptidoglycan (PGN) and bacterial DNA containing immunostimulatory CpG motifs (CpG-DNA), as well as LPS. Consistently, BMDMs lacking CD11b produced significantly higher amounts of TNF-α and IL-6 in response to LTA, PGN, and CpG-DNA (Supplementary Fig. 3), which are major constituents of gram-positive bacteria and recognized by TLR2, TLR2/4 and TLR9, respectively (9). Thus, we speculated that CD11b could restrain pro-inflammatory activation of macrophages in response to various microbial stimuli.
CD11b deficiency enhances functional activation of alternatively activated macrophages
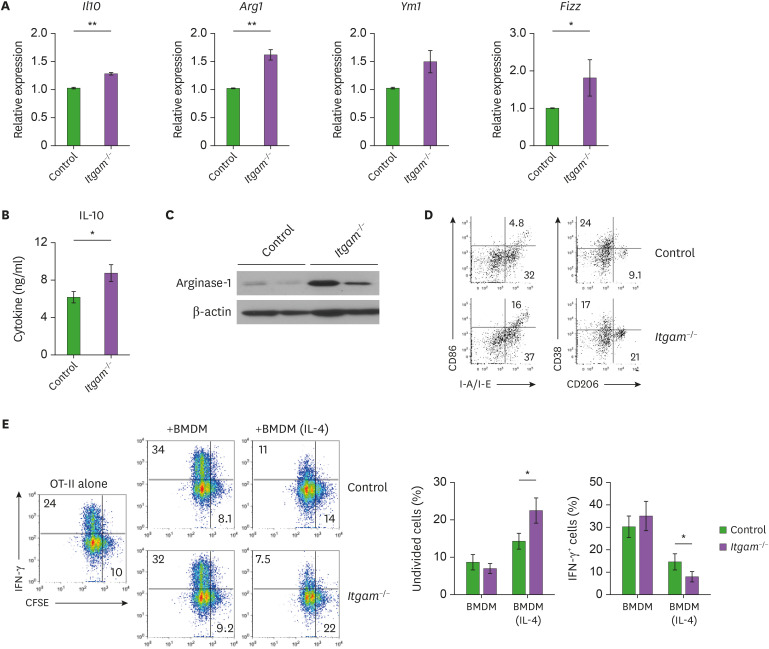
The alternatively activated macrophages produce IL-10 and arginase-1, which exert anti-inflammatory functions by transducing immunomodulatory signals by its cognate receptor or depleting arginine as a source of NO, respectively (14). In addition, immunomodulatory macrophages are also involved in tissue remodeling during wound healing and tumor growth by expressing several enzyme genes, including Arg1, Ym1, and Fizz (38). To determine whether increased production of pro-inflammatory molecules in CD11b-deficient macrophages was due to the attenuated function of the alternatively activated macrophages, we activated BMDMs with the type 2 cytokine IL-4. The IL-4-induced expression of the alternatively activated macrophage markers, including Il10, Arg1 and Fizz was significantly higher in the CD11b-deficient BMDMs than in the control macrophages (Fig. 4A). We confirmed that the protein amounts of IL-10 and arginase-1 were increased in the IL-4-activated BMDMs by CD11b deficiency (Fig. 4B and C), suggesting that the CD11b deficiency led to enhanced functional activation of alternatively activated macrophages. Consistently, flow cytometry analysis showed a higher prevalence of the mannose receptor CD206, a marker for alternatively activated macrophages (Fig. 4D). In line with the higher prevalence of CD273+ macrophages along with the increased CD38+ cells in the inflamed peritoneum (Fig. 1E and F), these results imply a regulatory role of CD11b in the acquisition of phenotypes associated with the alternatively activated macrophages.
Figure 4.
Enhanced functional activation of CD11b-deficient macrophages with an immune regulatory phenotype. Peritoneal macrophages were treated with IL-4 for 18 h, and quantitative real-time RT-PCR was performed as in Fig. 3. Shown is the relative expression of the indicated genes obtained from 2 independent experiments, each involving 3 mice per group. (B) Amount of IL-10 in the culture supernatants was measured using the Cytokine Bead Array. (C) Arginase-1 expression was assessed by immunoblotting and intensity of arginase-1 signal in each band was normalized by β-actin. (D) Representative FACS profiles of surface markers including CD86, class II MHC (I-A/I-E), CD38 and CD206 with frequencies of indicated subsets in the viable TCRβ− B220− F4/80+ gates are shown. (E) OT-II T cells were labeled with CellTraceTM Violet, and cocultured with BMDMs in the presence of OVA323–339 peptide. Four days after the coculture, cell division and IFN-γ expression of the T cells were analyzed by flow cytometry. Representative FACS profiles with frequencies of undivided cells and IFN-γ+ cells in the CD4 T cell gates and mean (±SEM) prevalence of the indicated subsets are shown.
To further determine the functional impact of CD11b on macrophages, we activated class II MHC-restricted TCR-transgenic OT-II T cells with an antigenic peptide in the presence of BMDMs as APCs and measured the T cell expansion and cytokine production of the activated T cells. While coculturing with unactivated BMDMs did not affects the antigen-induced cell division of OT-II T cells, the BMDMs markedly upregulated the antigen-induced expression of interferon-γ as shown by the CFSE dilution and intracellular cytokine staining in Fig. 4E. On the other hand, OT-II T cells that were cocultured with IL-4-activated BMDMs divided with a lower efficiency than the T cells cocultured with unactivated BMDMs and expressed a less amount of interferon-γ (Fig. 4E). Intriguingly, coculturing with IL-4-activated BMDMs lacking CD11b resulted in further attenuated cell division and lesser interferon-γ expression of activated OT-II T cells (Fig. 4E), suggesting that CD11b deficiency augments the ability of immunomodulatory macrophages to restrain the inflammatory T cell activation. Therefore, we might exclude the possibility that the exaggerated inflammatory responses of CD11b-deficient macrophages were attributed to an elevated immunomodulatory function of the alternatively activated macrophages.
CD11b promotes functional activation of macrophages by regulating NF-κB and Akt signaling pathways
To address the molecular mechanisms by which CD11b regulates the activation and function of macrophages, we investigated impact of CD11b-deficiency on signal transduction pathways. The TLR4 pathway in the pro-inflammatory macrophages that is induced by LPS stimulation is quite well characterized. TLR4 triggering initiates the MAPKs, including p38 and Erk, that activates transcription factors associated with the expression of pro-inflammatory cytokines (28). LPS-induced phosphorylation of p38 and Erk in BMDMs was not affected by the CD11b deficiency (Fig. 5A). The transcription factor NF-κB regulates the expression of a wide array of pro-inflammatory genes that include Il1b, Il6, Il12b, and Tnf (39). When CD11b-deficient BMDMs were treated with LPS, we observed an enhanced NF-κB activation as shown by the increased phosphorylation of NF-κB p65 and IκBα, along with the subsequent degradation of IκBα (Fig. 5A). Confocal microscopy analysis also showed accelerated nuclear localization of NF-κB p65 in the CD11b-deficiecnt macrophages in response to LPS (Fig. 5B). These results indicate that integrin CD11b could restrain the activation of pro-inflammatory macrophages through the modulation of NF-κB signaling.
Figure 5.
Increased activation of NF-κB signaling and phosphorylation of Akt in CD11b-deficient macrophages. (A) BMDMs were activated with LPS for the indicated times, and analyzed by Western blotting. Representative results from 2 or 3 independent experiments are shown. Immunoblotting of α-tubulin and unphosphorylated proteins was used as a loading control. (B) Accelerated nuclear induction of NF-κB p65 in CD11b-deficient macrophages. BMDMs were treated with LPS for the indicated times, and stained using anti-p65, followed by Alexa Fluor 488-conjugated secondary antibody. Shown are representative confocal images obtained from 3 independent experiments. DAPI (blue) was used to stain nucleus. (C) BMDMs were treated with IL-4, and phosphorylation of STAT6 and Akt was analyzed as in (A). (D) mRNA expression of Irf4 was measured quantitative real-time RT-PCR 8 h after IL-4 treatment. Data are presented the mean (±SEM) from 2 independent experiments with 3 biological replicates.
STAT6 is an essential signaling mediator and transcription factor in IL-4-induced activation of many immune cells and involved in the transcriptional programming of alternatively the activated macrophages (14). When BMDMs were activated with IL-4, we did not observe any apparent difference in the phosphorylation of STAT6 between the control and Itgam−/− macrophages (Fig. 5C). Hyperactive PI3K signaling was reported to suppress activation of the pro-inflammatory macrophages, which instead leads to preferential polarization toward the alternatively activated macrophages, partially via Akt (40,41). While IL-4 treatment did not increase the basal phosphorylation of Akt in the control BMDMs compared to untreated cells, it did enhance the phosphorylation of Akt in BMDM lacking CD11b (Fig. 5C). Furthermore, expression of interferon regulatory factor 4 (IRF4) that is reported to have a role in the IL-4-induced metabolic reprogramming to support the alternatively activated macrophages (42) was higher in CD11b-deficient BMDMs than in the control BMDMs (Fig. 5D). This result implies that the enhanced production of anti-inflammatory mediators and the immunomodulatory function of CD11b-deficient macrophages might be due to the increased ability of Akt to promote functional activation of alternatively activated macrophages. Overall, several lines of our experimental data suggested a regulatory role of CD11b in the activation of macrophages, and that CD11b modulates the activation of macrophage with both proinflammatory and immunomodulatory phenotypes by regulating the NF-κB and Akt signaling pathways.
DISCUSSION
Deregulated inflammatory responses against pathogens is a key feature of sepsis (3). Sepsis could occur when macrophages with pro-inflammatory phenotypes are hyperactivated in response to invading microbes and/or when the regulatory functions of counterbalanced macrophages are attenuated. S. aureus bacteremia is the most common serious infection worldwide, leading to sepsis (43). In this study, we investigated the role of CD11b in the activation and functional polarization of macrophages during MRSA infection. When peritonitis was induced with MRSA inoculation, CD11b deficiency resulted in an increased susceptibility to bacteremia and sepsis, which was associated with increased pro-inflammatory cytokines and hyperactivation of macrophages with pro-inflammatory phenotypes despite the attenuated migratory potential of the macrophages. In vitro studies using BMDMs and peritoneal macrophages revealed that CD11b deficiency not only led to increased secretion of pro-inflammatory mediators such TNF-α and NO but also enhanced production of anti-inflammatory proteins such as IL-10 and arginase-1 in macrophages treated with MRSA and IL-4, respectively. Therefore, our study demonstrated that CD11b has a regulatory role in both pro-inflammatory macrophages and immunomodulatory macrophages during pathogenic microbial infection.
As an integrin, CD11b is essential for many innate immune cells such as macrophages to influx into inflamed tissues and to clear invading pathogens. However, there have been conflicting reports in the impact of CD11b on the functional polarization of macrophages. The Han et al. (18) investigated the mechanism by which CD11b suppresses inflammatory responses of macrophages and revealed a cross-regulation of CD11b with TLR4 to protect undesired inflammation against microbial pathogens including E. coli. They also claimed that the increased colitis severity of Itgam−/− mice was partially due to the reduced IL-10 production of CD11b-deficient macrophages and indicated a positive regulatory role in the anti-inflammatory response of macrophages (23). In contrast, Zhou et al. (24) provide a mechanistic insight in which CD11b facilitates pro-inflammatory responses of macrophages by upregulating HMGB1 release in LPS shock or polymicrobial sepsis. Similarly, Schmid et al. identified CD11b as a pivotal regulator of anti-tumor immunity in which CD11b promotes polarization of the pro-inflammatory macrophage and suppresses tumor growth (25).
Similar to the previous experiment using E. coli, Itgam−/− mice were more sensitive to sepsis induced by MRSA infection, which was associated with enhanced functional activation of pro-inflammatory macrophages. Intriguingly, we observed higher bacterial burden in CD11b-deficient mice infected with MRSA, which is gram-positive and known to be primarily sequestered by macrophages during infection (44). This result was quite different from the Han et al.'s report (18) where they claimed that larger amount of TNF-α could promote clearance of the intracellular pathogen Listeria monocytogenes. On the contrary, the expression of immunomodulatory proteins including IL-10 and arginase-1 during MRSA infection was not affected by CD11b deficiency in vivo and in vitro (data not shown). These data imply that the increased sensitivity to sepsis upon MRSA infection might be largely due to the negative regulatory role of CD11b in pro-inflammatory macrophages. However, we could not rule out the possible that upregulation of the alternatively activated immunoregulatory macrophages might contribute to the higher susceptibility to MRSA infection. Indeed, macrophages expressing CD273+ and CD206+, phenotypic markers for the alternatively activated macrophages were increased in the inflamed peritoneum of Itgam−/− mice along with pro-inflammatory CD38+ macrophages (Fig. 1 and data not shown).
Indeed, CD11b-deficiency resulted in increased production of IL-10 and arginase-1 from macrophages activated with IL-4 (Fig. 4). Furthermore, CD11b-deficient macrophages showed an enhanced activity to suppress expansion and IFN-γ expression of antigen-stimulated T cells, implying a negative regulatory role of CD11b for macrophages to restrain an adaptive immune response. Consistently, it was reported that CD11b deficiency resulted in elevated expression of Arg1 and Ym1, markers for immunomodulatory macrophages in IL-4-activated macrophages, which contribute to improved insulin resistance in a high-fat diet model (45). In tumor transplantation experiments using LLC and B16 cancer cells, CD11b deficiency promoted tumor growth in a macrophage-dependent manner, whereas the constitutive activation of CD11b suppressed it (25). Intriguingly, it was suggested that increased IL-6 secretion from macrophages lacking CD11b led to autocrine induction of STAT-3-dependent genes including Arg1, Pdfgb and Vegfa, which in turn promoted the enhancement of immune suppressive and pro-tumorigenic activities. Overall, combined with these studies, our data revealed a potential regulatory role of CD11b in the functional polarization of the alternatively activated macrophages with an immunomodulatory phenotype.
Functional polarization of macrophages toward pro-inflammatory and immunomodulatory activities requires the engagement of a set of signaling pathways and transcriptional regulatory networks. Pattern recognition receptors such as TLRs sense microbial pathogen upon infection and induce the expression of inflammatory genes including TNF-α, IL-6, IL-12, and iNOS, by triggering MAPK and NF-κB signaling pathways (28). A cross-regulation of TLR signaling with integrin CD11b in macrophages was proposed by Han et al. (18): TLR4 ligation induces an “inside-out” activation of CD11b, which in turn promotes the degradation of MyD88 and TRIF, essential adaptor molecules downstream from the TLRs. In contrast, the concept of TLR4-coreceptor was raised by Zheng et al. (45) in which CD11b cooperates with TLR4 signaling to elicit optimal inflammatory responses of DCs by facilitating TLR4 endocytosis. Our biochemical and confocal analyses showed that CD11b deficiency accelerated NF-κB signaling in macrophages treated with TLR4 ligand, supporting the cross-regulation of CD11b in TLR signaling. Of note, we did not observe any significant changes in the MAPK signaling as shown by the phosphorylation of p38 and Erk1/2. Considering both MAPK and NF-κB signaling pathways as downstream from TLRs, it is unlikely that CD11b controls the stability of MyD88 and TRIF in a feedback loop in TLR signaling. However, it remains to be elucidated how CD11b selectively modulates NF-κB-dependent activation of pro-inflammatory macrophages.
Polarization toward the regulatory macrophages could be achieved by treatment with IL-4, IL-10, and immune complexes (14). While phosphorylation of STAT6 was not affected, phosphorylation of Akt was upregulated by CD11b deficiency when macrophages were activated with IL-4 (Fig. 5C). Hyperactivation of PI3K signaling driven by Pten loss leads to preferential activation of immunoregulatory macrophages that produce arginase-1 (46). It was also shown that TSC1 deficiency exacerbates the functional polarization of pro-inflammatory macrophages through the suppression of Akt (41). Thus, the enhanced sensitivity to IL-4-induced activation of Akt in CD11b-deficient macrophages could contribute to the alternatively activated macrophages with immunomodulatory activity. Furthermore, we observed increased transcriptional induction of IRF4, which depends on the regulation of PI3K-Akt signaling and facilitates metabolic reprogramming to support functional polarization of the regulatory macrophages (42). While CD11b deficiency accelerated TLR-triggered phosphorylation of Akt, pharmacological activation of CD11b was reported to protect mice from polymicrobial sepsis and attenuate SLE of MRL/lpr mice through the upregulation of Akt signaling (32). Overall, our experimental evidence highlights the regulatory roles of CD11b in the functional activation of macrophages upon microbial infection and suggests that CD11b modulates NF-κB and Akt signaling pathways during polarization of macrophages toward the pro-inflammatory and immune regulatory phenotypes, respectively.
ACKNOWLEDGEMENTS
This research was supported by Korean Mouse Phenotyping Project (NRF-2014M3A9D5A01073841) funded by the Ministry of Science and ICT through the National Research Foundation, Basic Science Research Program (NRF-2018R1D1A1B07048260; NRF-2019R1A6A1A11036849) funded by the Ministry of Education through the National Research Foundation, and the Hallym University Specialization Fund (HRF-S-12).
Abbreviations
- APC
antigen presenting cell
- BMDM
bone marrow-derived macrophage
- CFU
colony forming unit
- DC
dendritic cell
- iNOS
inducible nitric oxide synthase
- IRF4
interferon regulatory factor 4
- LTA
lipoteichoic acid
- MOI
multiplicity of infection
- MRSA
methicillin-resistant Staphylococcus aureus
- NO
nitric oxide
- PGN
peptidoglycan
- SLE
systemic lupus erythematosus
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Lee K.
- Data curation: Sim H, Pak S, Lee K.
- Investigation: Sim H, Jeong D, Kim HI, Pak S, Thapa B, Lee K.
- Project administration: Lee K.
- Resources: Kwon HJ.
- Supervision: Lee K.
- Writing - original draft: Sim H, Lee K.
SUPPLEMENTARY MATERIALS
CD11b-deficient mice produced elevated amounts of pro-inflammatory cytokines upon MRSA infection. Mice were inoculated intraperitoneally with MRSA as in Fig. 1 and cytokine levels were determined by the Cytokine Beads Array (BioLegend) 8 h after the infection. Shown are mean (±SEM) amounts of the indicated cytokines in the plasma (A) and peritoneal lavage (B).
CD11b deficiency attenuated infiltration of monoctyes/macrophages into the inflamed peritoneum. Cells were obtained from the peritoneal lavage 8 h after MRSA infection and analyzed by flow cytometry. (A) Representative FACS profiles of peritoneal innate immune cells with frequencies of indicated subsets in the viable TCRβ− B220− gates. (B) Shown are mean (±SEM) percentage of the indicated subsets among the viable CD45.2+ cells: n = 5 Itgam−/− vs. 5 control mice.
CD11b deficiency enhanced production of TNF-α and IL-6 in response to TLR stimuli. BMDMs were cultured in the presence of M-CSF and treated with LPS, LTA, PGN and bacterial CpG-DNA for 8 h. Cytokine levels in the culture supernatants were measured using the Cytokine Bead Array.
References
- 1.Inglis TJ, Urosevic N. Where sepsis and antimicrobial resistance countermeasures converge. Front Public Health. 2017;5:6. doi: 10.3389/fpubh.2017.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stearns-Kurosawa DJ, Osuchowski MF, Valentine C, Kurosawa S, Remick DG. The pathogenesis of sepsis. Annu Rev Pathol. 2011;6:19–48. doi: 10.1146/annurev-pathol-011110-130327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalil AC, Syed A, Rupp ME, Chambers H, Vargas L, Maskin A, Miles CD, Langnas A, Florescu DF. Is bacteremic sepsis associated with higher mortality in transplant recipients than in nontransplant patients? A matched case-control propensity-adjusted study. Clin Infect Dis. 2015;60:216–222. doi: 10.1093/cid/ciu789. [DOI] [PubMed] [Google Scholar]
- 5.Williams MD, Braun LA, Cooper LM, Johnston J, Weiss RV, Qualy RL, Linde-Zwirble W. Hospitalized cancer patients with severe sepsis: analysis of incidence, mortality, and associated costs of care. Crit Care. 2004;8:R291–R298. doi: 10.1186/cc2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bochud PY, Bonten M, Marchetti O, Calandra T. Antimicrobial therapy for patients with severe sepsis and septic shock: an evidence-based review. Crit Care Med. 2004;32:S495–S512. doi: 10.1097/01.ccm.0000143118.41100.14. [DOI] [PubMed] [Google Scholar]
- 7.Gordon RJ, Lowy FD. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2008;46(Suppl 5):S350–S359. doi: 10.1086/533591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eseonu KC, Middleton SD, Eseonu CC. A retrospective study of risk factors for poor outcomes in methicillin-resistant Staphylococcus aureus (MRSA) infection in surgical patients. J Orthop Surg. 2011;6:25. doi: 10.1186/1749-799X-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 10.Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014;5:491. doi: 10.3389/fimmu.2014.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clatza A, Bonifaz LC, Vignali DA, Moreno J. CD40-induced aggregation of MHC class II and CD80 on the cell surface leads to an early enhancement in antigen presentation. J Immunol. 2003;171:6478–6487. doi: 10.4049/jimmunol.171.12.6478. [DOI] [PubMed] [Google Scholar]
- 12.Novak ML, Koh TJ. Phenotypic transitions of macrophages orchestrate tissue repair. Am J Pathol. 2013;183:1352–1363. doi: 10.1016/j.ajpath.2013.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porta C, Rimoldi M, Raes G, Brys L, Ghezzi P, Di Liberto D, Dieli F, Ghisletti S, Natoli G, De Baetselier P, et al. Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor κB. Proc Natl Acad Sci U S A. 2009;106:14978–14983. doi: 10.1073/pnas.0809784106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hotchkiss RS, Coopersmith CM, McDunn JE, Ferguson TA. The sepsis seesaw: tilting toward immunosuppression. Nat Med. 2009;15:496–497. doi: 10.1038/nm0509-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abram CL, Lowell CA. The ins and outs of leukocyte integrin signaling. Annu Rev Immunol. 2009;27:339–362. doi: 10.1146/annurev.immunol.021908.132554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han C, Jin J, Xu S, Liu H, Li N, Cao X. Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting degradation of MyD88 and TRIF via Cbl-b. Nat Immunol. 2010;11:734–742. doi: 10.1038/ni.1908. [DOI] [PubMed] [Google Scholar]
- 19.Ling GS, Bennett J, Woollard KJ, Szajna M, Fossati-Jimack L, Taylor PR, Scott D, Franzoso G, Cook HT, Botto M. Integrin CD11b positively regulates TLR4-induced signalling pathways in dendritic cells but not in macrophages. Nat Commun. 2014;5:3039. doi: 10.1038/ncomms4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park S, Sim H, Kim HI, Jeong D, Wu G, Cho SY, Lee YS, Kwon HJ, Lee K. CD11b regulates antibody class switching via induction of AID. Mol Immunol. 2017;87:47–59. doi: 10.1016/j.molimm.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Chung SA, Taylor KE, Graham RR, Nititham J, Lee AT, Ortmann WA, Jacob CO, Alarcón-Riquelme ME, Tsao BP, Harley JB, et al. Differential genetic associations for systemic lupus erythematosus based on anti-dsDNA autoantibody production. PLoS Genet. 2011;7:e1001323. doi: 10.1371/journal.pgen.1001323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramos PS, Criswell LA, Moser KL, Comeau ME, Williams AH, Pajewski NM, Chung SA, Graham RR, Zidovetzki R, Kelly JA, et al. A comprehensive analysis of shared loci between systemic lupus erythematosus (SLE) and sixteen autoimmune diseases reveals limited genetic overlap. PLoS Genet. 2011;7:e1002406. doi: 10.1371/journal.pgen.1002406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu X, Han C, Jin J, Qin K, Zhang H, Li T, Li N, Cao X. Integrin CD11b attenuates colitis by strengthening Src-Akt pathway to polarize anti-inflammatory IL-10 expression. Sci Rep. 2016;6:26252. doi: 10.1038/srep26252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou H, Li Y, Gui H, Zhao H, Wu M, Li G, Li Y, Bai Z, Yin Z, Redmond HP, et al. Antagonism of integrin CD11b affords protection against endotoxin shock and polymicrobial sepsis via attenuation of HMGB1 nucleocytoplasmic translocation and extracellular release. J Immunol. 2018;200:1771–1780. doi: 10.4049/jimmunol.1701285. [DOI] [PubMed] [Google Scholar]
- 25.Schmid MC, Khan SQ, Kaneda MM, Pathria P, Shepard R, Louis TL, Anand S, Woo G, Leem C, Faridi MH, et al. Integrin CD11b activation drives anti-tumor innate immunity. Nat Commun. 2018;9:5379. doi: 10.1038/s41467-018-07387-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stevanin M, Busso N, Chobaz V, Pigni M, Ghassem-Zadeh S, Zhang L, Acha-Orbea H, Ehirchiou D. CD11b regulates the Treg/Th17 balance in murine arthritis via IL-6. Eur J Immunol. 2017;47:637–645. doi: 10.1002/eji.201646565. [DOI] [PubMed] [Google Scholar]
- 27.Kurokawa K, Kim MS, Ichikawa R, Ryu KH, Dohmae N, Nakayama H, Lee BL. Environment-mediated accumulation of diacyl lipoproteins over their triacyl counterparts in Staphylococcus aureus . J Bacteriol. 2012;194:3299–3306. doi: 10.1128/JB.00314-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGuire VA, Gray A, Monk CE, Santos SG, Lee K, Aubareda A, Crowe J, Ronkina N, Schwermann J, Batty IH, et al. Cross talk between the Akt and p38α pathways in macrophages downstream of Toll-like receptor signaling. Mol Cell Biol. 2013;33:4152–4165. doi: 10.1128/MCB.01691-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weichhart T, Costantino G, Poglitsch M, Rosner M, Zeyda M, Stuhlmeier KM, Kolbe T, Stulnig TM, Hörl WH, Hengstschläger M, et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29:565–577. doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 30.Lee KW, Lee Y, Kim DS, Kwon HJ. Direct role of NF-κB activation in Toll-like receptor-triggered HLA-DRA expression. Eur J Immunol. 2006;36:1254–1266. doi: 10.1002/eji.200535577. [DOI] [PubMed] [Google Scholar]
- 31.Kirby AC, Raynes JG, Kaye PM. CD11b regulates recruitment of alveolar macrophages but not pulmonary dendritic cells after pneumococcal challenge. J Infect Dis. 2006;193:205–213. doi: 10.1086/498874. [DOI] [PubMed] [Google Scholar]
- 32.Faridi MH, Khan SQ, Zhao W, Lee HW, Altintas MM, Zhang K, Kumar V, Armstrong AR, Carmona-Rivera C, Dorschner JM, et al. CD11b activation suppresses TLR-dependent inflammation and autoimmunity in systemic lupus erythematosus. J Clin Invest. 2017;127:1271–1283. doi: 10.1172/JCI88442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rijneveld AW, de Vos AF, Florquin S, Verbeek JS, van der Poll T. CD11b limits bacterial outgrowth and dissemination during murine pneumococcal pneumonia. J Infect Dis. 2005;191:1755–1760. doi: 10.1086/429633. [DOI] [PubMed] [Google Scholar]
- 34.Teschner D, Cholaszczyńska A, Ries F, Beckert H, Theobald M, Grabbe S, Radsak M, Bros M. Cd11b regulates fungal outgrowth but not neutrophil recruitment in a mouse model of invasive pulmonary aspergillosis. Front Immunol. 2019;10:123. doi: 10.3389/fimmu.2019.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jablonski KA, Amici SA, Webb LM, Ruiz-Rosado JD, Popovich PG, Partida-Sanchez S, Guerau-de-Arellano M. Novel markers to delineate murine M1 and M2 macrophages. PLoS One. 2015;10:e0145342. doi: 10.1371/journal.pone.0145342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drevets DA, Leenen PJ, Campbell PA. Complement receptor type 3 (CD11b/CD18) involvement is essential for killing of Listeria monocytogenes by mouse macrophages. J Immunol. 1993;151:5431–5439. [PubMed] [Google Scholar]
- 37.Hu C, Mayadas-Norton T, Tanaka K, Chan J, Salgame P. Mycobacterium tuberculosis infection in complement receptor 3-deficient mice. J Immunol. 2000;165:2596–2602. doi: 10.4049/jimmunol.165.5.2596. [DOI] [PubMed] [Google Scholar]
- 38.Thapa B, Lee K. Metabolic influence on macrophage polarization and pathogenesis. BMB Rep. 2019;52:360–372. doi: 10.5483/BMBRep.2019.52.6.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baker RG, Hayden MS, Ghosh S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011;13:11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arranz A, Doxaki C, Vergadi E, Martinez de la Torre Y, Vaporidi K, Lagoudaki ED, Ieronymaki E, Androulidaki A, Venihaki M, Margioris AN, et al. Akt1 and Akt2 protein kinases differentially contribute to macrophage polarization. Proc Natl Acad Sci U S A. 2012;109:9517–9522. doi: 10.1073/pnas.1119038109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Byles V, Covarrubias AJ, Ben-Sahra I, Lamming DW, Sabatini DM, Manning BD, Horng T. The TSC-mTOR pathway regulates macrophage polarization. Nat Commun. 2013;4:2834. doi: 10.1038/ncomms3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang SC, Smith AM, Everts B, Colonna M, Pearce EL, Schilling JD, Pearce EJ. Metabolic reprogramming mediated by the mTORC2-IRF4 signaling axis is essential for macrophage alternative activation. Immunity. 2016;45:817–830. doi: 10.1016/j.immuni.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thwaites GE, Edgeworth JD, Gkrania-Klotsas E, Kirby A, Tilley R, Török ME, Walker S, Wertheim HF, Wilson P, Llewelyn MJ, et al. Clinical management of Staphylococcus aureus bacteraemia. Lancet Infect Dis. 2011;11:208–222. doi: 10.1016/S1473-3099(10)70285-1. [DOI] [PubMed] [Google Scholar]
- 44.Jorch SK, Surewaard BG, Hossain M, Peiseler M, Deppermann C, Deng J, Bogoslowski A, van der Wal F, Omri A, Hickey MJ, et al. Peritoneal GATA6+ macrophages function as a portal for Staphylococcus aureus dissemination. J Clin Invest. 2019;129:4643–4656. doi: 10.1172/JCI127286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng C, Yang Q, Xu C, Shou P, Cao J, Jiang M, Chen Q, Cao G, Han Y, Li F, et al. CD11b regulates obesity-induced insulin resistance via limiting alternative activation and proliferation of adipose tissue macrophages. Proc Natl Acad Sci U S A. 2015;112:E7239–E7248. doi: 10.1073/pnas.1500396113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sahin E, Haubenwallner S, Kuttke M, Kollmann I, Halfmann A, Dohnal AM, Chen L, Cheng P, Hoesel B, Einwallner E, et al. Macrophage PTEN regulates expression and secretion of arginase I modulating innate and adaptive immune responses. J Immunol. 2014;193:1717–1727. doi: 10.4049/jimmunol.1302167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CD11b-deficient mice produced elevated amounts of pro-inflammatory cytokines upon MRSA infection. Mice were inoculated intraperitoneally with MRSA as in Fig. 1 and cytokine levels were determined by the Cytokine Beads Array (BioLegend) 8 h after the infection. Shown are mean (±SEM) amounts of the indicated cytokines in the plasma (A) and peritoneal lavage (B).
CD11b deficiency attenuated infiltration of monoctyes/macrophages into the inflamed peritoneum. Cells were obtained from the peritoneal lavage 8 h after MRSA infection and analyzed by flow cytometry. (A) Representative FACS profiles of peritoneal innate immune cells with frequencies of indicated subsets in the viable TCRβ− B220− gates. (B) Shown are mean (±SEM) percentage of the indicated subsets among the viable CD45.2+ cells: n = 5 Itgam−/− vs. 5 control mice.
CD11b deficiency enhanced production of TNF-α and IL-6 in response to TLR stimuli. BMDMs were cultured in the presence of M-CSF and treated with LPS, LTA, PGN and bacterial CpG-DNA for 8 h. Cytokine levels in the culture supernatants were measured using the Cytokine Bead Array.