Abstract
Objective
To evaluate the use of Gallium-68 prostate-specific membrane antigen positron emission tomography/computed tomography (68Ga-PSMA PET/CT), compared with conventional CT abdomen/pelvis (CTAP) and whole body single photon emission CT bone scan (BS), for detection of local or distant metastasis following biochemical failure/recurrence in post-prostatectomy patients.
Methods
We conducted a review of our prospectively maintained, institutional database to identify 384 patients with post-prostatectomy biochemical failure/recurrence who underwent PSMA PET/CT, CTAP and BS from February 2015 to August 2017 in Nepean Hospital, tertiary referral centre. The results of the three imaging modalities were analysed for their ability to detect local recurrence and distant metastases. PSMA PET/CT and CTAP imaging were separately performed on the same day and the BS was performed within several days (mostly in 24 h). Difference in detection rates was determined between the modalities and the Chi square test was used to determine significance.
Results
A total of 384 patients were identified with a median prostate-specific antigen (PSA) of 0.465 ng/mL (interquartile range =0.19–2.00 ng/mL). Overall, PSMA PET/CT was positive for 245 (63.8%) patients whereas CTAP and BS were positive in 174 patients (45.3%). A total of 98 patients (25.5%) had local or distant metastasis detected on PSMA only, while 20 patients (5.2%) had recurrences detected on CTAP but not on PSMA PET/CT.
Conclusion
The use of PSMA PET/CT has a higher detection rate of predicted local or distant metastasis compared to CTAP and BS in the staging of patients with biochemical recurrences after radical prostatectomy.
Keywords: Prostatic neoplasms, Positron-emission tomography, Prostate-specific antigen, Neoplasm staging, Bone scan
1. Introduction
Biochemical recurrence post-prostatectomy is seen in approximately 20%–30% of patients, which may be due to local recurrence and/or distant metastasis. Accurate re-staging of the patients is crucial in delivering appropriate treatment in this group [1].
Conventional imaging techniques such as Tc-99m bone scan (BS) [2] and computed tomography of the abdomen and pelvis (CTAP) lack diagnostic yield to investigate biochemical recurrence [2,3]. At a prostate-specific antigen (PSA) level of <7 ng/mL post prostatectomy, the probability of a positive BS is 5% and 11%–14% for CTAP [4]. This low diagnostic yield has led to further investigation of imaging modalities such as fludeoxyglucose positron-emission tomography (FDG-PET) and 11-choline positron emission tomography (PET) showing some earlier promising results [5,6]. However, CTAP and BS are still utilised in the setting of PSA recurrence or persistence because choline PET is not readily available and its diagnostic yield has been shown to be lower than more recently developed radiolabels which also have difficulty differentiating lesions from background physiological activity in certain areas such as around the bladder.
Prostatic-specific membrane antigen (PSMA) is expressed on the transmembrane domain of prostate cells and has 100–1000 times greater expression on prostate cancer cells [7,8]. 68-Gallium labelled ligand used in PSMA PET/computed tomography (CT) has a high affinity to this antigen and multiple studies have reported that PSMA PET/CT has a sensitivity and specificity of approximately 86% in staging prostate cancer. However not all prostate cancer express PSMA and could lead to false negative in around 5%–15% [9]. The false positive rate was reported as low as 4% in one retrospective study looking into PSMA positivity corelated with histopathology results of template lymph node dissections [10]. The PSMA false negative rate largely depends on patients’ risks such as PSA value and PSA kinetics. Early studies have shown promise in identifying PCa recurrences in postoperative setting [[11], [12], [13]]. As with any new technology, cost has been an inhibiting factor both in implementation of the modality and access for patients. Determining the location of recurrence and burden of recurrent disease management would give clinicians further information to direct management of recurrent disease in a form of salvage radiotherapy, targeted hypofractionated radiotherapy or enrolment to trial for early systemic treatment.
The aim of our study is to evaluate whether PSMA PET/CT improves the identification of local and distant lesions compared with the current modalities of CTAP and BS.
2. Patients and methods
We analysed our prospectively maintained, institutional database for patients who underwent a PSMA PET/CT, CTAP and BS for PSA recurrence or persistent PSA elevation following radical prostatectomy between February 2015 and August 2017. Institutional board approval was granted from Nepean Blue Mountains Local Health District Human Research Ethics Committee (Study No. 17-63a), with a total of 394 patients identified from our single institution. Patients without BS [7] or PSA [3] were excluded from the study. Overall, 384 patients were included for our analysis. Local recurrences were defined as an PSMA avid lesion on PSMA PET/CT scan or enhancing soft tissue lesion on CT scan in the prostatic bed. Any other recurrences were defined as distant recurrences. Both PSMA PET/CT scan and CTAP were separately performed on the same day with the BS performed within 72 h of the other imaging modalities.
2.1. PSMA PET/CT scan
Intravenous injection of 68Ga-PSMA (150–300 MBq) was performed at around 50–70 min before imaging. Oral contrast was also given to improve the delineation of small bowel. Whole body PET imaging was performed with a Philips GEMINI TOF PET/CT scanner (Phillips, Amsterdam, Netherlands). All images were obtained with an acquisition time of 2 min/bed positions with 50% overlap. Images were reconstructed with the built-in iterative algorithm. Low dose CT images were acquired for attenuation correction and anatomical localisation with a 64 slice helical scanner using 120 keV, 60 mAs and field of view (FOV) of 600 mm. The low dose CT images were reconstructed from the vertex to the mid-femoral bone with 4 mm thickness.
2.2. Bone scan
Bone scans were acquired within 72 h of the CTAP and PSMA PET/CT scan with a gamma camera (Philips Bright View XCT, Amsterdam, Netherlands). A thousand MBq of Tc-99m HDP (hydromethane diphosphate) was injected intravenously and whole-body planar imaging acquired 2–3 h post injection with a concurrent selected regional SPECT/low dose CT. Any localised uptake of Tc-99m HDP not explained by benign aetiology such as arthritis was interpreted likely to be bony metastases. The scan was considered to be negative if there was only physiological skeletal uptake of the Tc-99m HDP.
2.3. CT abdomen and pelvis scan
CTAP scans were obtained with a 64 slice helical scanner from the dome of the diaphragm to the proximal femur using 120 keV, 300 mAs and FOV of 350 mm. Each patient received IV contrast unless contraindicated. The CT images were reconstructed with 2 mm thickness.
2.4. Imaging interpretation
Two experienced dual nuclear medicine and radiology trained radiologists reported the scans on dedicated work stations. They were not blinded and had access to patient history, PSA levels and previous imaging. When there is an ambiguity in the finding, the scan was co-read by an independent radiologist with similar level of experience. They reported both PSMA PET/CT scan and CTAP concurrently.
Abnormal sites of increased Tc-99m HDP on BS, not explained by physiological uptake or benign aetiology were considered as metastases. 68Ga-PSMA uptake in organs such as salivary glands, small bowel, liver and spleen were considered physiological. Increased PSMA uptakes in sites elsewhere relative to the surrounding adjacent tissue were considered as PSMA avid metastases. PSMA avidity in the prostatic bed was viewed as local recurrence.
On CT a soft tissue lesion of 1 cm or larger in the prostatic bed or demonstrating contrast enhancement was considered suspicious for local recurrence and reported as such. A lymph node with 1 cm in short axis dimension or those that were smaller, but demonstrated contrast enhancement with irregular margins, were also considered pathological. Lytic and sclerotic bony lesions with ill-defined margins were classified as bony metastases.
2.5. Statistical analysis
Data were analysed using IBM SPSS Statistics for Windows, version 23.0 (IBM Corp., Armonk, NY, USA). Fisher's exact test was used to compare the positivity rates between different imaging modalities.
3. Results
Three hundred and eighty four patients were identified for analysis. The median age was 69.5 years with a median PSA of 0.465 (interquartile range [IQR] 0.19–2.00) ng/mL. Further patient demographics are listed in Table 1.
Table 1.
Patient demographics, postprostatectomy PSA and Gleason score.
| Characteristics | Value |
|---|---|
| Patients, n | 384 |
| Age, median (IQR), year | 69.5 (64–74) |
| PSA level, median (IQR), ng/mL | 0.465 (0.19–2.00) |
| PSA level, n (%) | |
| <0.2 ng/mL | 96 (25) |
| 0.2–0.49 ng/mL | 100 (26) |
| 0.5–0.99 ng/mL | 53 (14) |
| 1–1.99 ng/mL | 39 (10) |
| ≥2 ng/mL | 96 (25) |
| Gleason score at radical prostatectomy, n (%) | |
| 6 | 15 (4) |
| 7 | 212 (55) |
| 8 | 57 (15) |
| 9 | 97 (25) |
| 10 | 2 (0.5) |
| Unknown | 1 (0.25) |
PSA, prostate-specific antigen; IQR, interquartile range.
3.1. PSMA PET scan versus CTAP and BS combined
For distant recurrence detection, 206 patients (53.6%) had a positive PSMA PET/CT scan compared to 114 patients (29.7%) for conventional imaging (CTAP and BS); p<0.0001 (Table 2).
Table 2.
PMSA PET/CT scan versus CT abdomen pelvis and BS.
| Positive | Negative | |
|---|---|---|
| Bone metastases, n (%) | ||
| PSMA PET/CT | 70 (18.2) | 314 (81.8) |
| BS | 51 (13.2) | 333 (86.8) |
| p-Value | 0.074 | |
| Local recurrence, n (%) | ||
| PSMA PET/CT | 77 (20.1) | 307 (79.9) |
| CTAP | 70 (18.2) | 314 (81.8) |
| p-Value | 0.52 | |
| Distant metastases, n (%) | ||
| PSMA PET/CT | 206 (53.6) | 178 (46.4) |
| CTAP and BS | 114 (29.7) | 270 (70.3) |
| p-Value | <0.001 | |
| Radiological positive lesions (local and distant disease), n (%) | ||
| PSMA | 245 (63.8) | 139 (36.2) |
| CTAP and BS | 174 (45.3) | 210 (54.7) |
| p-Value | <0.001 | |
BS, bone scan; CT, computed tomography; CTAP, CT abdomen/pelvis; PET/CT, positron emission tomography/computed tomography; PSMA, prostate-specific membrane antigen.
Overall, for both local and distant recurrences, 245 patients (63.8%) had a positive PSMA PET/CT scan as opposed to 174 patients (45.3%) for conventional imaging (CTAP and BS); p<0.0001 (Table 2). A total of 98 cases (25.5% of entire cohort) had a positive PSMA PET/CT when CTAP and BS failed to detect any lesions. In comparison, CTAP and BS only detected 20 cases when PSMA scan was negative (5.2%).
In terms of oligometastatic disease, 143 patients (37.2%) were found to have oligometastatic disease with equal or less than three sites compared to 106 (27.6%) patients in CT and bone scan group (Table 3). Out of these 106 patients in CT/BS group, 65 patients also had oligometastatic disease, 22 patients had widespread metastatic disease and 19 patients had no suspicion of disease on PSMA PET/CT scan.
Table 3.
PMSA PET/CT Scan versus CT abdomen pelvis and bone scan in finding oligometastatic disease.
| Oligometastatic disease ≤3 lesions | Widespread disease >4 lesions | |
|---|---|---|
| PSMA PET/CT, n (%) | 143 (37.2) | 63 (16.4) |
| CT and BS, n (%) | 102 (26.6) | 15 (3.9) |
BS, bone scan; CT, computed tomography; PET/CT, positron emission tomography/computed tomography; PSMA, prostate-specific membrane antigen.
3.2. PSMA PET versus BS for detection of bony metastasis
A total of 70 patients (18.2%) had positive PSMA PET/CT scans compared with 51 patients (13.2%) with positive BS. This was not statistically significant (p=0.074). A total of 26 (6.7%) cases had a positive PSMA PET/CT in the setting of a negative BS. A total of seven (1.8%) cases had a positive BS in the setting of a negative PSMA PET/CT scan. The concordance of both scans was 91.4% for detection of bone lesions (Table 2).
PSMA PET/CT for detection of local, nodal and bony recurrences stratified by PSA level.
Increasing PSA level was associated with increased PSMA detection rate. Even with relatively low PSA <0.2 ng/mL, 43% of patients had PSMA scan detected lesions. This value steadily increased to 92% with PSA >2.0 ng/mL. Overall, 64% had a positive PSMA scan (Table 4). PSMA detected more distant recurrences compared with local recurrences. Around 70%–80% of detected lesions were distant recurrences (Table 5).
Table 4.
Detection rates of PSMA PET following radical prostatectomy based on PSA banding.
| PSA level, ng/mL | Sample size, n | Positive patients, n | Detection rate (%) |
|---|---|---|---|
| <0.2 | 96 | 41 | 43 |
| <0.2–0.5 | 100 | 47 | 47 |
| < 0.5–1.0 | 53 | 36 | 68 |
| <1.0–2.0 | 39 | 33 | 85 |
| >2.0 | 96 | 88 | 92 |
| Total | 384 | 245 | 64 |
PET, positron emission tomography; PSA, prostate-specific antigen; PSMA, prostate-specific membrane antigen.
Table 5.
Regions of detection PSMA PET following radical prostatectomy based on PSA banding.
| PSA level, ng/mL (n) | Bone, n (%) | Nodal, n (%) | Local, n (%) |
|---|---|---|---|
| <0.2 ng/mL (41) | 10 (24) | 26 (63) | 12 (29) |
| 0.2–0.5 ng/mL (48) | 13 (27) | 26 (54) | 15 (31) |
| <0.5–1.0 ng/mL (36) | 7 (19) | 28 (78) | 7 (19) |
| <1.0–2.0 ng/mL (33) | 13 (39) | 22 (67) | 8 (24) |
| >2.0 ng/mL (92) | 27 (31) | 61 (69) | 35 (39) |
| Total | 70 (28.6) | 163 (66.5) | 77 (31.4) |
PET, positron emission tomography; PSA, prostate-specific antigen; PSMA, prostate-specific membrane antigen.
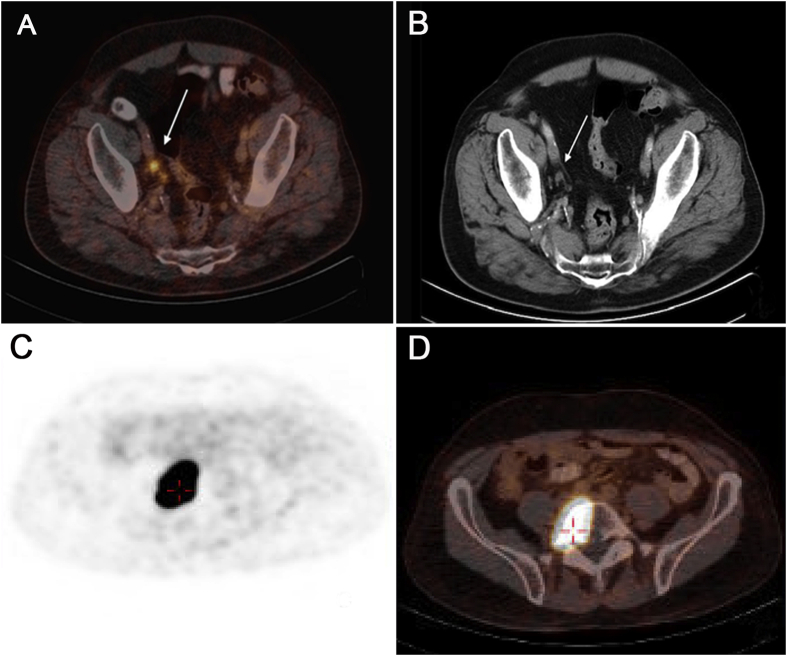
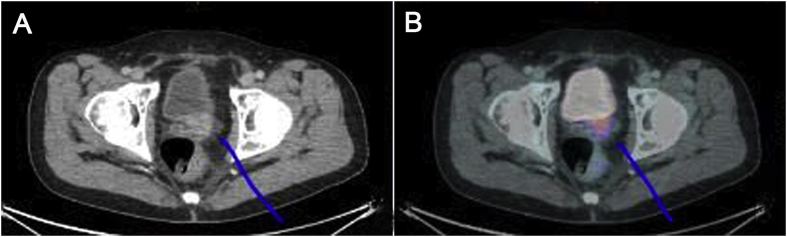
Fig. 1 illustrates a patient with concordant lesions on PSMA PET/CT scan and CTAP/BS, whereas Fig. 2 illustrates images of a patient with discordant lesions only picked up by PSMA PET/CT.
Figure 1.
The results of PET/CT and CT scan. Both PSMA PET/CT scan (A) and CT abdomen and pelvis (B) showed positive right pelvic lymph nodes; Both PSMA PET/CT scan (C) and bone scan (D) showed positive lesion on vertebral body. CT, computed tomography; PET/CT, positron emission tomography/computed tomography; PSMA, prostatespecific membrane antigen.
Figure 2.
The results of CT and PET/CT scan. (A) CT scan showing a possible lesion in the previous prostatectomy site; (B) PSMA PET/CT scan showing avid lesion at prostatic bed. CT, computed tomography; PET/CT, positron emission tomography/computed tomography; PSMA, prostatespecific membrane antigen.
4. Discussion
PSA failure post-radical prostatectomy creates a diagnostic and therapeutic dilemma for urologists and oncologists worldwide. Conventional CTAP and BS fail to show the site of recurrence in a significant proportion of patients. PSMA PET/CT provides a new diagnostic tool to investigate such a dilemma with improved detection rates at low PSA levels. However, due to lack of availability of 68Ga-PSMA tracer and compounded further by the fact that these studies incur significant costs for patients, patients may be inadequately investigated with current imaging. Accurate staging is important for guiding appropriate follow-up, choosing appropriate adjuvant/salvage therapies and avoiding unnecessary interventions. In our analysis, we showed that PSMA PET/CT identifies twice as more distant metastases than conventional re-staging scans. Accurate staging of patient's disease will allow more tailored treatment for patients with biochemical recurrence.
To our knowledge, our study is the first study reviewing a large number of post-prostatectomy patients evaluated with both PSMA PET/CT scans in comparison with CTAP and BS. Pyka et al. [14] comparing PSMA PET/CT and BS, found 22% higher sensitivity (95.8%–100% versus 83.3%) for detecting bone lesions for 49 biochemical recurrence patients with significantly higher mean PSA of 20.9 ng/mL (range: 0.3–490.0 ng/mL). Our results are similar to this study, however most of Pyka's patients had a time gap in two studies up to 3 months compared with several days in our study. Our study detected bone metastasis at 18% in PSMA PET/CT and at 13% in BS but this was not statistically significant. We attribute this finding to our much lower mean PSA population (2.0 ng/mL). Maurer et al. [10] looked into sensitivity and specificity of PSMA PET/CT scan compared with morphological CT or MRI scan in the staging of 130 preoperative intermediate to high risk prostate cancer patients. Comparison to histological diagnosis of lymph nodes was made in this study. They found that PSMA PET/CT had higher sensitivity and specificity at 65.9% and 98.9% respectively, whereas low dose morphological CT scan had 43.9% and 85.4% sensitivity and specificity. Although their population was a preoperative group of patients, our study showed similar outcomes in terms of detection rate but clinical application of our results helps to choose further treatment for post radical prostatectomy patients with persistent or recurrent raised PSA levels.
Our findings prove our hypothesis that PSMA PET/CT scans detects more lesions than traditional imaging techniques. The implication of our study is that PSMA PET/CT detects more suspicious lesions allowing more accurate staging of patients which may guide more appropriate treatments for the patient. This may help patients avoid unnecessary local salvage radiotherapy or patients may be eligible for combined chemo-hormonal therapy if PSMA PET/CT scan detects earlier metastasis. Furthermore, this modality may also detect solitary metastasis for potential oligometastatic treatment or locoregional treatment. This group of patients may benefit from salvage radiotherapy or trials for early salvage treatment including chemotherapy and novel hormone agents. Also, if PSMA PET/CT is negative for distant metastases, it is more likely that the patients will receive benefits from salvage radiotherapy for local recurrences.
There are several limitations to our study. The first is the lack of a histopathological gold standard to correlate with the positive lesions on the imaging study modalities or to follow up to validate our findings. Our finding would have been validated better with biopsy of local or distant recurrences or follow-up scans. However, positive PSMA PET/CT scan in preoperative setting has proven to have very high specificity to almost 100% [10]. In addition, Afshar-Oromieh et al. [11] reported the lesion based analysis of sensitivity, specificity, NPV and PPV values of 76.6, 100, 91.4% and 100% on histopathology correlated lesions shown on PSMA PET/CT on post-prostatectomy group. There is an ongoing randomised trial comparing PMSA PET/CT to conventional CTAP and BS in preoperative setting to address this question [15]. Mena et al. [16] showed that 60% of patients with negative conventional imaging had positive PSMA PET/CT scan in this clinical setting with mean PSA of 4.4 ng/mL.
Secondly, our radiologists reported PSMA PET/CT scan and CTAP at the same time and were thus not blinded to the results of the other imaging modality. This likely resulted in our higher local recurrence detection rate by CTAP (19.8%) given that CTAP is not used for local recurrence detection at all. This lack of blinding may have masked a significant difference between PSMA PET/CT and CTAP for the detection of local recurrence. Our study showed that PSMA PET/CT has a higher detection rate of local recurrence (20.8%) compared with the historical rate of 3.4% for CTAP in a cohort with much higher PSA values than in our study [3]. This also applies to our higher detection rate (13.2%) in BS for detection of metastasis since it was interpreted in conjunction to PSMA scan. Also, this is evident in overall high positive rate of CT and bone scan of 45.3%. Furthermore, dedicated CT chest was not performed and it is not possible to draw any conclusion regarding the comparison of two methods in the thorax.
Lastly, our cross-sectional study has not assessed the long-term outcomes of these patients after PSMA PET/CT to determine if PSMA PET/CT changed management decisions or long-term outcomes for our patients [17]. It would have been useful to monitor treatment response of salvage radiotherapy as PSMA PET/CT can change radiation planning [18] and negative PSMA PET/CT has shown to have higher response rate to radiotherapy [19]. Future studies are required to investigate histopathological correlation of PSMA lesions and how PSMA affects management decisions and long-term patient outcomes.
5. Conclusion
This study provides evidence that PSMA PET/CT has a higher detection rate of lesions when compared with CTAP and BS in patients with biochemical recurrence post-prostatectomy. PSMA and BS concordance was higher in the detection of bone metastases than the concordance of PSMA and CTAP for detection of local or distant disease. Further studies are needed to correlate PSMA PET/CT finding with histopathology and how these results lead to a change in clinical outcome of this group of patients.
Author contributions
Study concept and design: Chris Rothe, Yuigi Yuminaga, Jonathan Kam.
Data acquisition: Mohan Arianayagam, Chuong Bui, Bertram Canagasingham, Richard Ferguson, Mohamed Khadra, Raymond Ko, Celi Varol, Diep Nguyen.
Data analysis: Jonathan Kam, Chris Rothe, Kieran Beattie.
Drafting of manuscript: Yuigi Yuminaga, Jonathan Kam, Ken Le.
Critical revision of the manuscript: Matthew Winter, Jonathan Kam, Ken Le.
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of Second Military Medical University.
References
- 1.Han M., Partin A.W., Pound C.R., Epstein J.I., Walsh P.C. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol Clin. 2001;28:555–565. doi: 10.1016/s0094-0143(05)70163-4. [DOI] [PubMed] [Google Scholar]
- 2.Kramer S., Gorich J., Gottfried H.W., Riska P., Aschoff A.J., Rilinger N. Sensitivity of computed tomography in detecting local recurrence of prostatic carcinoma following radical prostatectomy. Br J Radiol. 1997;70:995–999. doi: 10.1259/bjr.70.838.9404201. [DOI] [PubMed] [Google Scholar]
- 3.Kane C.J., Amling C.L., Johnstone P.A.S., Pak N., Lance R.S., Thrasher J.B. Limited value of bone scintigraphy and computed tomography in assessing biochemical failure after radical prostatectomy. Urology. 2003;61:607–611. doi: 10.1016/s0090-4295(02)02411-1. [DOI] [PubMed] [Google Scholar]
- 4.Mottet N., Bellmunt J., Bolla M., Briers E., Cumberbatch M.G., De Santis M. EAU-ESTRO-SIOG guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71:618–629. doi: 10.1016/j.eururo.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Seltzer M.A., Barbaric Z., Belldegrun A., Naitoh J., Dorey F., Phelps M.E. Comparison of helical computerized tomography, positron emission tomography and monoclonal antibody scans for evaluation of lymph node metastases in patients with prostate specific antigen relapse after treatment for localized prostate cancer. J Urol. 1999;162:1322–1328. [PubMed] [Google Scholar]
- 6.Taneja S.S. Imaging in the diagnosis and management of prostate cancer. Rev Urol. 2004;6:101–113. [PMC free article] [PubMed] [Google Scholar]
- 7.Eder M., Schafer M., Bauder-Wust U., Hull W.E., Wangler C., Mier W. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjugate Chem. 2012;23:688–697. doi: 10.1021/bc200279b. [DOI] [PubMed] [Google Scholar]
- 8.Afshar-Oromieh A., Malcher A., Eder M., Eisenhut M., Linhart H.G., Hadaschik B.A. PET imaging with a [68Ga] gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imag. 2013;40:486–495. doi: 10.1007/s00259-012-2298-2. [DOI] [PubMed] [Google Scholar]
- 9.Eapen R.S., Nzenza T.C., Murphy D.G., Hofman M.S., Cooperberg M., Lawrentschuk N. PSMA PET applications in the prostate cancer journey: from diagnosis to theranostics. World J Urol. 2019;37:1255–1268. doi: 10.1007/s00345-018-2524-z. [DOI] [PubMed] [Google Scholar]
- 10.Maurer T., Gschwend J.E., Rauscher I., Souvatzoglou M., Haller B., Weirich G. Diagnostic efficacy of (68)gallium-PSMA positron emission tomography compared to conventional imaging for lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. J Urol. 2016;195:1436–1443. doi: 10.1016/j.juro.2015.12.025. [DOI] [PubMed] [Google Scholar]
- 11.Afshar-Oromieh A., Avtzi E., Giesel F.L., Holland-Letz T., Linhart H.G., Eder M. The diagnostic value of PET/CT imaging with the 68Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imag. 2015;42:197–209. doi: 10.1007/s00259-014-2949-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Afshar-Oromieh A., Haberkorn U., Eder M., Eisenhut M., Zechmann C.M. [68Ga]Gallium-labelled PSMA ligand as superior PET tracer for the diagnosis of prostate cancer: comparison with 18F-FECH. Eur J Nucl Med Mol Imag. 2012;39:1085–1086. doi: 10.1007/s00259-012-2069-0. [DOI] [PubMed] [Google Scholar]
- 13.Meredith G., Wong D., Yaxley J., Coughlin G., Thompson L., Kua B. The use of 68Ga-PSMA PET CT in men with biochemical recurrence after definitive treatment of acinar prostate cancer. BJU Int. 2016;118:49–55. doi: 10.1111/bju.13616. [DOI] [PubMed] [Google Scholar]
- 14.Pyka T., Okamoto S., Dahlbender M., Tauber R., Retz M., Heck M. Comparison of bone scintigraphy and 68Ga-PSMA PET for skeletal staging in prostate cancer. Eur J Nucl Med Mol Imag. 2016;43:2114–2121. doi: 10.1007/s00259-016-3435-0. [DOI] [PubMed] [Google Scholar]
- 15.Hofman M.S., Murphy D.G., Williams S.G., Nzenza T., Herschtal A., Lourenco R.A. A prospective randomized multicentre study of the impact of gallium-68 prostate-specific membrane antigen (PSMA) PET/CT imaging for staging high-risk prostate cancer prior to curative-intent surgery or radiotherapy (proPSMA study): clinical trial protocol. BJU Int. 2018;122:783–793. doi: 10.1111/bju.14374. [DOI] [PubMed] [Google Scholar]
- 16.Mena E., Lindenberg M.L., Shih J.H., Adler S., Harmon S., Bergvall E. Clinical impact of PSMA-based (18)F-DCFBC PET/CT imaging in patients with biochemically recurrent prostate cancer after primary local therapy. Eur J Nucl Med Mol Imag. 2018;45:4–11. doi: 10.1007/s00259-017-3818-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy D.G., Hofman M., Lawrentschuk N., Maurer T. Bringing clarity or confusion? The role of prostate-specific membrane antigen positron-emission/computed tomography for primary staging in prostate cancer. BJU Int. 2017;119:194–195. doi: 10.1111/bju.13600. [DOI] [PubMed] [Google Scholar]
- 18.Calais J., Czernin J., Cao M., Kishan A.U., Hegde J.V., Shaverdian N. 68Ga-PSMA-11 PET/CT mapping of prostate cancer biochemical recurrence after radical prostatectomy in 270 patients with a PSA level of less than 1.0 ng/mL: impact on salvage radiotherapy planning. J Nucl Med. 2018;59:230–237. doi: 10.2967/jnumed.117.201749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emmett L., van Leeuwen P.J., Nandurkar R., Scheltema M.J., Cusick T., Hruby G. Treatment outcomes from 68Ga-PSMA PET/CT-informed salvage radiation treatment in men with rising PSA after radical prostatectomy: prognostic value of a negative PSMA PET. J Nucl Med. 2017;58:1972–1976. doi: 10.2967/jnumed.117.196683. [DOI] [PubMed] [Google Scholar]