Abstract
Extracellular vesicles (EVs) are vesicle-like substances released by eukaryotic cells. Based on their origin and size, EVs are mainly divided into exosomes, microvesicles and apoptotic bodies, and they are secreted by eukaryotic cells under physiological and pathological conditions. EVs are enriched with nucleic acids, proteins and other factors. EVs can regulate the function of adjacent and distant cells, and they are even involved in the pathogenesis of diseases. They contain proteins associated with the pathogenesis of neurodegenerative diseases (NDs), such as the α-synuclein (α-syn) and tau proteins, which suggest potential roles for EVs as biomarkers and carriers of drugs and other therapeutic molecules that can cross the blood–brain barrier to treat NDs. In this review, we summarized the function of EVs in the pathogenesis of different NDs and related advances in EVs as diagnostic biomarkers and treatments for diseases.
Keywords: Biomarker, Exosomes, Extracellular vesicles, Neurodegenerative diseases
Neurodegenerative diseases (NDs) mainly include Alzheimer's disease (AD), Parkinson's disease (PD), amyotrophic lateral sclerosis (ALS), and Huntington's disease (HD), which are very serious diseases. AD and PD are also very common in elderly people. However, because the early clinical manifestations of these diseases are not typical, NDs may easily be confused with many other diseases. Because of the lack of effective biological markers, early diagnosis of NDs is quite difficult. Furthermore, although much research has been carried out in the past few decades, and the pathogenesis of NDs has been more recognized, there are still no drugs or measurements that can cure or delay the development of diseases in clinical practice, which brings profound psychological pressure and economic burden to patients and families. Many recent studies have reported that extracellular vesicles (EVs) may be involved in the pathogenesis of NDs1,2 and have potential as diagnostic biomarkers3 and therapeutic measures.4,5 This paper reviewed the research progress into the relationship between EVs and NDs.
Introduction of EVs
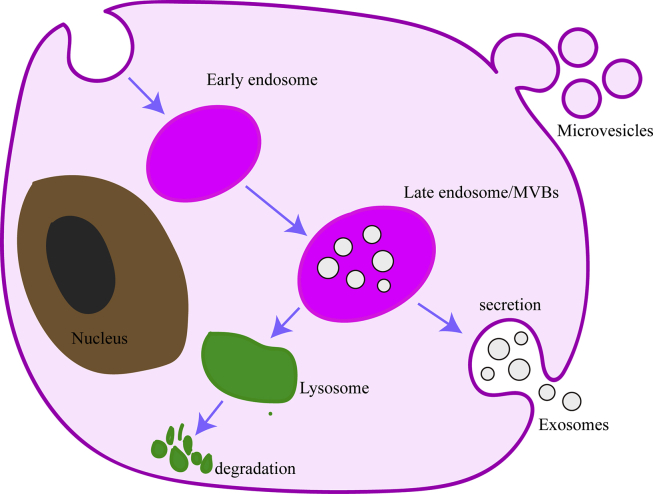
EVs arise either from late endosomes, which are typically referred to as exosomes (50–150 nm in diameter), or they bud directly from the plasma membrane (PM), in which case they are referred to as microvesicles (100–1000 nm in diameter) (Fig. 1).6,7 In addition to these two main members of the EV family, other extracellular structures have been described, such as apoptotic bodies (100–5000).7 So far, the most studied type is the exosomes.
Figure 1.
The release process of microvesicles and exosomes. Invagination of the plasma membrane form early endosome, followed by budding of payload into the endosomal membrane to form multivesicular endosomes (MVBs). MVBs can fuse with lysosome for degradation of their contents or fuse with the plasma membrane to release their intraluminal vesicles, then called exosomes. Microvesicles bud directly from the plasma membrane.
The secretion of exosomes is complicated because the vesicle structure secreted by cells directly enters the external environment. The International Society for Extracellular Vesicles (ISEV) clearly assist in EV definitions, isolation methods, but a lack of standards remains widespread.8, 9, 10 EVs in the size range of exosomes are not able to effectively separated from due to overlapping physical properties and biometrics of the two7,11; although many articles use the term “exosomes” referring to EVs preparations isolated by physical biological processes, they are likely referring to a mixture of the exosomes and non-exosome-like small EVs.7
EVs are rich in nucleic acids nucleic acids, proteins, and lipids.11 To date, according to the EXOCARTA database (www.exocarta.org), 41,860 proteins, 3408 mRNAs, 2838 miRNAs, and 1116 lipids have been isolated from exosomes of different cell origins.
The important role for EVs in intercellular communication occurs via the transfer of proteins, lipids and nucleic acids, which has been confirmed in numerous studies.12, 13, 14, 15 EVs can also be isolated from various body fluids, such as the cerebrospinal fluid, blood, urine, and saliva.8, 9, 10,16 Exosomes are very stable under different conditions and can protect “bio-goods (such as proteins or miRNAs)” from degradation and denaturation in the extracellular environment.17 Increasing evidence has shown that they can function as biomarkers for NDs and that they are more reliable than biological fluids, such as pure cerebrospinal fluid, blood, or urine.18 Finally, increasing evidence shows that EVs can also cross the blood–brain barrier,19, 20, 21 and their characteristic low immunogenicity lays a theoretical foundation for them to function as biomarkers for diseases and “drug” delivery vehicles for the treatment of NDs.
Roles of EVs in the central nervous system
In the central nervous system (CNS), EVs are secreted by many cells, such as dendritic cells, neurons, oligodendrocytes, and astrocytes.22 Emerging evidence indicates that EVs are key players in the intercellular communication that underlies physiological processes, such as synaptic plasticity and the maintenance of myelination.23,24 Furthermore, upon injury to the CNS, EVs may propagate inflammation across the blood–brain barrier and beyond, and they also appear to mediate neuroprotection and modulate regenerative processes.23 For example, Kramer-Albers and colleagues25 show that functional EVs can be transmitted bidirectionally between oligodendrocytes and neurons; furthermore, the results indicate that oligodendrocyte-derived exosomes mediate neuroprotective functions. Moreover, the cargo delivered by exosomes includes a number of enzymes with metabolic functions, including catalase and superoxide dismutase-1, which can help neurons resist oxidative stress. Consistently, neurons exposed to oxidative stress or other harmful conditions survived better if they had been treated with oligodendroglial exosomes, suggesting that exosomes are protective and can increase the neuronal stress tolerance.25,26 Xu et al27 reported that exosomes transfer neuronal miR-132 from neurons to endothelial cells (ECs) to regulate the integrity of brain vasculature. Perez-Gonzalez et al28 demonstrated that EVs containing CysC protect cultured cells from starvation-induced death. Unfortunately, EVs also have somewhat neurotoxic effects, and several studies have shown that EVs can promote the spread of proteins associated with the pathogenesis of NDs in the CNS.29 Microglia-derived EVs carry bioactive lipids, including endocannabinoids, which acutely impact the neuronal firing rate.30, 31, 32
EVs and occurrence of NDs
EVs and occurrence of AD
Senile plaques and neurofibrillary tangles (NFTs) are two specific neuropathological features of AD. Senile plaques are mainly composed of deposited amyloid β-protein (Aβ), while NFTs are mainly composed of hyperphosphorylated tau protein. According to current views, the accumulation of Aβ and tau proteins is toxic to neurons, but exosomes can mediate the diffusion of these toxic proteins among AD neurons.17 Yakama et al33 confirmed that exosomes can release AD-related proteins and peptides, namely, amyloid precursor protein (APP), APP C-terminal fragment, or APP intracellular domain, to the outside of cells. In addition, other research groups have found exosomal marker proteins in amyloid plaques deposited in mouse brain tissue and the brains of postmortem AD patients, indicating that exosomes have transported Aβ to plaques during the pathogenesis of AD.34 Furthermore, Zheng35 observed in an AD model that plasma exosomes aggregate around Aβ, and these findings provide a possible interpretation for the extracellular amyloid deposition in the brain of AD patients. It is reported that the neurotoxicity of exosomes may be due to exosome lipids promoting the formation of soluble Aβ out of extracellular insoluble Aβ. Aβ is internalized by microglia and then sorted into exosomes, in which it exists as the toxic form of Aβ.35 In addition to the release and aggregation of Aβ, exosomes are also associated with tau in the pathogenesis of AD. Studies have found that microglia can diffuse tau by releasing tau in exosomes, and microglia can significantly reduce the synthesis of exosomes, resulting in significantly reduced abnormal aggregation of tau proteins in vitro and in vivo. These studies indicate that exosomes contribute to the spread of tau protein.36 The neurotoxic effects of exosomes on AD patients are related not only to pathological proteins but also probably to the fact that exosomes can impair the neuronal function during pathological progression of AD by other means; for example, AD patient exosome contents induce neuronal apoptosis in AD models. In 2012, Wang37 performed a series of experiments and demonstrated that Aβ induces the secretion of exosomes containing ceramide and proapoptotic response factor-4 (PAR-4) through the neutral sphingomyelinase 2 (nSMase2)-dependent pathway, leading to the apoptosis of astrocytes; Eitan38 found that EVs may also cause dysregulation of neuronal Ca2+ and impair mitochondrial function, leading to neurons’ vulnerability to excitotoxicity and further damage. All of the above data illustrate the neurotoxicity of exosomes. On the other hand, EVs also have neuroprotective effects in AD. Yuyama39 added a mixture of exosomes and Aβ to primary cortical cells, which then significantly inhibited oligomerization and the resultant toxicity, revealing the ability of exosomes to capture Aβ and promote its clearance from microglia. Correspondingly, there is also in vivo evidence that exosomes derived from mouse neuroblastoma N2a cells or human cerebrospinal fluid can eliminate the neurotoxicity caused by Aβ derived from the brains of patients with AD,33 but they believe that this protective effect was due to the exosome proteins; the proteins can interfere with the assembly of toxic Aβ rather hydrolyze the Aβ protein.33 Finally, cystatin C, a protein thought to be neuroprotective against AD, has been shown to secrete mouse primary neurons out of the cell through the exosomes40; immunoproteomics analysis has revealed the presence of at least 9 different cystatin C glycosylation forms in exosomes. In addition, the overexpression of familial AD-associated presenilin gene mutations can result in decreased levels of all cystatin C forms (natural and glycosylated) and exosomal APP metabolites.40 In conclusion, despite all of this evidence, the pathogenic or protective effects of exosomes have not been totally confirmed in AD patients, and whether the dual role of exosomes is ubiquitous in all exosomes and to what extent can they promote or prevent the clearance of Aβ peptides remains controversial. To date, there is no marker information on exosomes derived from different neuronal cell types; further research is needed to solve these problems step by step.
EVs and occurrence of PD
Due to the central role of α-synuclein (α-syn) in sporadic PD, the potential association of α-syn with exosomes has begun to attract attention. However, the pathogenesis of PD is related not only to α-syn but also to the DJ-1 protein and leucine-rich repeat kinase 2 (LRRK2) protein; researchers have also identified and studied them. A number of studies have shown that neuron-derived exosomes transfer α-syn between neurons and nonneuronal cells (such as astrocytes and microglia), thereby contributing to the diffusion of PD. The deposition of α-syn in glial cells induces inflammation, which can be further transmitted to other glial cells and neurons.41,42 Tofaris43 suggested that when the lysosomal function is inhibited, the amount of exosome-released α-syn increases, and the exosomes promote the uptake of α-syn by recipient cells; then, these exosome-associated α-syn oligomers are more likely to be absorbed by recipient cells than free α-syn, which results in toxicity. The above evidence indicate that the exosomes promote the transmission and aggregation of α-syn, but the exact trigger mechanism for the incorporation of α-syn into exosomes or for its release remain unclear. Further, Chang et al44 once reported that α-syn was capable of inducing secretion of exosomes from microglia, which are rich in major histocompatibility complex (MHC II), tumor necrosis factor (TNF), and proinflammatory cytokines, and the exosomes induced neuronal apoptosis in a TNF-dependent manner. Whether the exosomes have neuroprotective effects has not yet been confirmed. Because the role of exosomes in PD-related brain inflammation has not been well known, further research in this area should be performed.
EVs and occurrence of HD
HD is a neurodegenerative disease that mainly affects motor function. It is an autosomal dominant hereditary disease caused by expanded repeats of the CAG base sequence in the IT15 gene. The repeated CAG sequence occurs in a gene that encodes a polyglutamine sequence (PolyQ); the number of repeats is less than 35 in healthy individuals and more than 37 in patients. Its pathogenic gene (IT15) encodes a protein with a relative molecular weight of 350,000Da, consisting of 3144 amino acids, and is named mutant Huntingtin (mHtt). The main pathological change observed in HD is the appearance of inclusion bodies and aggregates formed by mHtt in the neurons of the central nervous system. Zhang45 used human embryonic kidney 293T cells as donor cells for EVs in an experiment and found that the released EVs contained polyglutamine (polyQ) and RNA with CAG repeats, indicating that EVs have the potential to transmit the repeated RNA of a toxically amplified trinucleotide from one cell to another. These EVs may be used as biomarkers for disease states and responses to treatment, but more research is needed to confirm this.
EVs and occurrence of ALS
ALS is a fatal ND characterized by progressive muscle paralysis that is caused by motor neuron degeneration. ALS is accompanied by the accumulation of pathological protein superoxide dismutase-1 (SOD1), TAR DNA binding protein-43 (TDP- 43), and sarcoma fusion transporter (FUS), which interferes with neuronal function and ultimately leads to cell death. The pathogenesis of ALS mainly includes abnormal aggregation of proteins, mitochondrial dysfunction, glutamate excitotoxicity, oxidative stress, and neuroinflammation.46 Recent studies have shown that peripheral blood mononuclear cells also play an important role in the pathogenesis of ALS.47 Therefore, some researchers48 stimulated CD14++ monocytes with exosomes isolated from the serum of ALS patients and interestingly found that the mononuclear cells showed an obvious inflammatory reaction; further, this change was not observed in the control group. This indicates that exosomes may play a role in promoting the ALS inflammatory response; many studies have also confirmed that exosomes can transport proteins associated with the pathogenesis of ALS,49 but fluid tissues have been researched more than solid tissues. Therefore, Silverman2 once isolated the exosomes from the frozen brain tissue of ALS mice and found that CNS-derived EVs contained pathogenic ALS proteins; further, the astrocytes and neurons, instead of the microglia, were the main source of EVs, which confirmed that exosomes are involved in the transmission of toxic proteins during ALS progression. It is possible that the exosomes from different sources have different effects on the pathogenesis of ALS, but further development of techniques for identifying exosomes from different sources are needed to address this issue.
EVs and diagnosis of NDs (Table .1)
Table 1.
The biomarkers in NDs.
| Diseases | Source | Biomarker | Reference |
|---|---|---|---|
| AD | plasma exosomes | P-T181-tau, P–S396-tau, Aβ1-42 | 50,51 |
| plasma exosomes | NRGN, REST | 51 | |
| plasma exosomes | IRS-1, P-IRS-1 | 52 | |
| plasma exosomes | cathepsin D, LAMP-1, ubiquitinylated proteins, heat-shock protein 70 | 53 | |
| plasma exosomes | neurexin 2α, GluA4-containing glutamate receptor, neuroligin 1, neuronal pentraxin 2 | 54 | |
| CSF exosomes | miR-193b | 55 | |
| plasma exosomes | miR-23b-3p, miR-24-3p, miR-29b-3p, miR-125b-5p, miR-138-5p, miR-139-5p, miR-141-3p, miR-150-5p, miR-152-3p, miR-185-5p, miR-338-3p, miR-342-3p, miR-342-5p, miR-548at-5p, miR-659-5p, miR-3065-5p, miR-3613-3p, miR-3916, miR-4772-3p, miR-5001-3p | 56 | |
| PD | CSF exosomes | α-synuclein | 58 |
| plasma exosomes | α-synuclein | 62 | |
| plasma exosomes | tau | 62 | |
| salivary EVs | α-synuclein | 64 | |
| urine exosomes | DJ-1 | 65 | |
| urine exosomes | Ser(P)-1292 LRRK2 | 66 | |
| urine exosomes | ratio of phosphorylated Ser-1292 LRRK2 to total LRRK2 | 67 | |
| CSF exosomes | miR-153, miR-409-3p, miR-10a-5p, let-7g-3p | 68 | |
| serum microvesicles. | miR-19b, miR-24 miR-195 | 69 | |
| ALS | serum exosomes | miR-27a-3p | 74 |
EVs and diagnosis of AD
Exosomes have the potential to be diagnostic markers for AD. Fiandacaf50 measured the levels of total tau protein, two versions of phosphorylated tau protein (P-T181-tau and P–S396-tau), and Aβ1-42 in neuron-derived exosomes isolated from the blood of AD patients; the research showed that the expression levels of P–S396-tau, P-T181-tau, and Aβ1-42 were significantly higher 1–10 years before the diagnosis of AD, and the blood exosomal level of Aβ1-42 continued to increase from the preclinical stage to the diagnosis of AD. These results suggest that the above three proteins have the potential to be AD biomarkers; it is also speculated that exosome-derived Aβ 1–42 may be the biomarkers for monitoring disease progression. Later, Winston51 performed a similar study and confirmed that during patient progress from mild cognitive impairment (MCI) to dementia, the levels of the above three markers increased; they also found that Neurogranin (NRGN) and neuron-restricted silencing factor (REST) were elevated, so these five indicators can be used to accurately predict the transformation of MCI to AD dementia; other studies have suggested that neuronal-derived AD pathogenesis-associated exosomes may carry proteins that have the potential to become AD biomarkers, such as phosphorylated insulin receptor 1 protein,52 cysteine D (cathepsin D),53 and synaptic protein (Synaptic protein).54
In addition to proteins, miRNAs have been shown to be biomarkers in several studies. In 2014, Liu et al55 showed that miR-193b may bind to the 3′-untranslated region of APP and inhibit the expression of APP-related mRNA and protein, suggesting that miR-193b may be involved in the progression of NDs. Compared with MCI and the control group, in AD patients, the expression levels of miR-193b in the exosomes of cerebrospinal fluid and blood were lower, demonstrating that exosome miR-193b is a potentially unique biomarker of AD; thereafter, Lugli et al56 used Illumina deep sequencing and differential centrifugation to separate the plasma exosomes and identify the miRNAs expressed by the exosomes. There were 20 miRNAs that were significantly changed in the AD group (miR-23b-3p, miR-24-3p, miR-29b-3p, miR-125b-5p, miR-138-5p, miR-139-5p, miR-141-3p, miR-150-5p, miR-152-3p, miR-185-5p, miR-338- 3p, miR-342-3p, miR-342-5p, miR-548at-5p, miR-659-5p, miR-3065-5p, miR-3613-3p, miR-3916, miR-4772-3p, and miR-5001-3p); these miRNAs are expected to be diagnostic markers for AD. However, there are conflicting reports about these miRNAs, which is probably due to different separation and identification techniques. Research on different cell sources and different uses may require tissue-specific exosome markers, and further studies are needed in the future.
EVs and diagnosis of PD
Wang57 suggested that the level of leucine-rich repeat kinase 2 (LRRK2) protein in CSF exosomes is not related to the diagnosis of PD; Stuendl58 measured the α-syn content in the CSF exosomes of PD patients. The results showed that the α-syn content in exosomes from PD patients was lower than that in the control group, suggesting that α-syn be used as a biomarker for PD patients. Blood can be easily obtained than CSF, so blood is also being studied by many people. Because peripheral cells, especially red blood cells and platelets, can produce abundant a-syn,59 they cannot be directly used as diagnostic markers.60 Therefore, some people have studied α-syn in the exosomes from plasma patients with PD.61 The comparison of dozens of PD patients without GABI gene mutations with a control group revealed that the level of α-syn in plasma exosomes was significantly higher in the PD group than in the control group, and the ratio of plasma exosome α-syn to plasma total α-syn was found to be inversely related to the severity of the disease. Later, Shi collected a few hundred samples and found that the α-syn content in plasma exosomes from PD patients was significantly higher than that in the control group,62 which confirms that the plasma exosome α-syn is likely to be a useful marker for later PD diagnosis; then, Shi applied a similar method to detect the exogenous tau content of CNS-derived plasma exosomes. Surprisingly, tau in plasma exosomes of PD patients was also found to have the potential to be a biomarker.63 In general, CSF, plasma exosome α-syn, and plasma exogenous tau protein may become clinically significant biomarkers.
Because saliva collection is simple and noninvasive, in 2018, Cao64 measured the content of α-syn in EVs from the saliva collected from dozens of patients with PD and a control group. The results showed that the content of oligomeric α-syn and the ratio of oligomeric α-syn to total α-syn can be used as diagnostic markers for PD. However, this study has some limitations, such as a small sample size. There are few studies on salivary exosomes as diagnostic markers for PD, and the diagnosis of PD by Parkinson's disease-associated salivary exosomes remains to be further studied.
Urine is another example of an accessible biological fluid. One study65 reported the detection of PD-related proteins, DJ-1 and LRRK2, in urine exosomes, of which DJ-1 showed significant sex differences. The level of DJ-I in urine exosomes was significantly higher in male PD patients than in the control group. This study showed that DJ-1 could be used as a marker for the diagnosis of male PD patients, but LRRK2 does not show a difference between the two groups. However, in another study, Fraser66 reported sex differences in phosphorylated LRRK2 (Ser(P)-1292 LRRK2) levels in urinary exosomes, which shows that in patients with idiopathic PD, the level of Ser(P)-1292 LRRK2 in male exosomes is higher than that in female exosomes, and the level of Ser(P)-1292 LRRK2 was positively correlated with the severity of the disease. In the same year, these authors focused on familial PD and found that in LRRK2 mutation carriers, the ratio of Ser(P)-1292 LRRK2 to total LRRK2 was related to the risk of PD. The higher the ratio was, the higher the risk of PD. In the future, more research is expected to focus on whether lowering the ratio can reduce the incidence risk of PD.67 In 2017, Wang57 showed that Ser(P)-1292 LRRK2 in urine exosomes may have a diagnostic effect on male PD. In general, the levels of DJ-1 and Ser(P)-1292 LRRK2 in urine exosomes have the potential to diagnose PD, and Ser(P)-1292 LRRK2 may also have the ability to monitor the progression and prognosis of the disease.
In studies of miRNAs in exosomes from PD patients, CSF exosomal miRNAs have been identified, and the results reveal that miR-1 and miR-19b-3p are significantly downregulated, while miR-153, miR-409-3p, and miR-10a-5p are upregulated significantly. These 6 miRNAs are expected to serve as diagnostic markers for PD.68 Another study collected plasma from 109 PD patients and 40 normal controls and identified 24 miRNAs (miR-24, mi R-30a-3p, mi R-30e-3p, miR-195, miR-223, miR -324-3p, miR-331-5p, miR-338-3p, miR-505, miR-626, miR-15b, miR-162-3p, miR-19a, miR-19b, miR-29a, miR-29c, miR-30c, miR-148b, miR-181a, miR-185, miR-221, miR-339-5p, miR-450b-3p, and miR-1294); the results show that miR-24 and miR-195 are expressed at higher levels than the control group, while miR-19b expression is low. It is thought that miR-24, miR-195, and miR-19b have potential as diagnostic markers for PD.69 This evidence demonstrates the potential value of cerebrospinal fluid and plasma exosome miRNAs in the diagnosis and assessment of PD. Among the miRNAs, miR-153 has been shown to bind to the 3′-untranslated region of α-syn and downregulate its mRNA and protein levels, thus participating in the pathogenesis of PD70; in addition, it is also confirmed that the pathogenic role of miR-7 in PD has a similar effect as that caused by miR-153,71 but the above potential markers do not overlap, and large-sample control studies are needed in the future to verify the roles and diagnostic utilities of the above miRNAs.
EVs and diagnosis of HD
Studies have shown that in all types of blood cells, the platelets contain the highest concentration of mHtt,72 so Denis73 collected samples from 59 HD patients and 54 control groups; however, no HTT was detected in platelet-derived EVs, and the correlation analysis also failed to reveal any association between the number of platelet-derived EVs and patients' age, CAG repeats, total exercise score of unified Huntington's disease rating scale, total function score, or disease burden score. Therefore, platelet-derived EVs from HD patients are not valuable biomarkers for HD. However, there are few studies on exosomes as HD biomarkers, so more research is needed to find better circulating biomarkers in the blood.
EVs and diagnosis of ALS
There are studies using exosomes as biomarkers. Xu74 measured the expression of miR-27a-3p in serum exosomes from 10 ALS patients and healthy subjects and found that miR-27a-3p is downregulated in patients with ALS; therefore, miR-27a-3p is thought to be a biomarker for ALS. In addition to miRNAs, Otake75 recently suggested that exosome mRNAs and genes may also be biomarkers of diseases. In general, there are few studies on ALS biomarkers. This may be due to the small number of ALS cases in general. In the future, large-sample multiple-center studies are needed to verify the above results and transition into clinical application as soon as possible.
EVs and treatment of NDs
EVs and treatment of AD
Exosomes are also attractive candidates as therapeutic delivery vehicles for NDs. In 2011, Alvarez20 applied exogenous small interfering RNA (siRNA) to Lamp2b protein (an exosome membrane protein fused to a neuron-specific rabies virus glycoprotein)-expressible dendritic cell-derived exosomes by electroporation. Intravenously injected exosomes targeted the specific transmission of siRNA to neurons, microglia, and oligodendrocytes in the brain, and BACE1 gene knockout was observed; moreover, both BACE1 mRNA (60%) and protein (62%) were downregulated. Recently, Li76 revealed a new method of loading therapeutic substances into exosomes, which was mediated by fusing the exosomal membrane protein CD9 with an RNA-binding protein with high affinity for miR-155. The fused CD9-RNA enriched miR-155 in the exosomes, and the encapsulated miR-155 was efficiently delivered to recipient cells, where it recognized endogenous targets. This method can also be designed to enable exosome enrichment with CRISPR/dCas9. These studies have laid the foundation for the use of exosomes to treat NDs in the clinic.
Wang et al found that exosomes carrying curcumin inhibited tau phosphorylation, holding great potential for improving targeted drug delivery and the recovery of neuronal function in AD therapy.21
EVs and treatment of PD
In terms of treatment, Hall et al77 integrated a therapeutic PD protein catalase into exosomes. When activated macrophages were treated with these exosomes, the levels of reactive oxygen species in treated macrophages decreased, which was confirmed later in C57BL/6 mice. Other research groups also loaded glial cell-derived neurotrophic factor (GDNF) into exosomes and introduced them to a 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP)-induced monkey PD model; the results confirm that exosome-mediated GDNF delivery has a strong neuroprotective effect.78 All of these findings indicate that exosomes are good carriers for delivering drugs to the CNS, but more research is needed in the future on the delivery of therapeutic drugs such as siRNAs and miRNAs and on the development of instruments or methods for engineering exosomes.
EVs and treatment of HD
Lee79 injected exosomes from adipose-derived stem cells (ASCs) into R6/2 mice and observed reduced mHtt aggregates in R6/2 mouse neurons. These exosomes also appear to improve abnormal apoptotic protein levels and reduce mitochondrial dysfunction and apoptosis in an in vitro HD model, but this study did not clarify which substances in the exosomes provided these protective effects. Didiot80 coincubated Cy3-labeled hydrophobically modified small interfering RNA (Cy3-h siRNA) with exosomes to produce Cy3-h siRNA-carrying exosomes and then injected the exosomes into the unilateral corpus striatum of mice. It is exciting to observe the silencing of bilateral target mRNA. The widespread distribution and efficacy of siRNA-carrying exosomes is expected to advance the development of therapeutic approaches for the treatment of HD and other NDs. Subsequently, human exosomal miRNAs have been explored in research. Lee81 transfected a miR-124 expression vector into HEK293 cells to generate a cell line stably expressing miR-124 and confirmed the miR-124 expression by qPCR. Then, the HEK293 cells overexpressing miR-124 were cultured in exosome-free Dulbecco's modified Eagle's medium, and the exosomes inside were isolated from the culture medium. The EXO-124 exosomes were injected into the bilateral corpus striatum of 6-week-old R6/2-type transgenic HD mice. The results showed that the expression of target gene was reduced. Although this treatment did not reduce the motor symptoms of mice, it showed the feasibility of using exosomes to deliver miRNA to the brain for the treatment of degenerative diseases. Current studies have provided evidence for the delivery of exosome-based siRNAs and miRNAs to the brain. To further validate the possibility of exosomes as a neurodegenerative drug carrier, more research is needed.
EVs and treatment of ALS
Studies have also applied exosomes to the treatment of ALS. Lee82 found that exosomes released from ADSCs could reduce the accumulation of mutant SOD1 in G93A neurons. This is further validated by Bonafede,83 who found that exosomes secreted by mouse ADSCs (0.2 μg/ml) could protect NSC-34 cells from oxidative damage and increase cell viability, thereby protecting the cells. These findings indicate that exosomes released by ADSCs have therapeutic potential against ALS, but further research is needed on whether these methods can be used for clinical treatment.
Summary and outlook
The field of EVs research is an emerging and rapidly developing field, and in recent years, it has received increasing attention from researchers. Over the past 10 years, EVs have evolved from the initial characterization as the “trash can” of cells into a key player in many biological processes in the physiological and pathological environment of the body. EVs play an important role in the pathogenesis, diagnosis, and treatment of NDs, but research on EVs still requires a great deal of technical development. First, the extraction process of EVs must be simple, fast, and inexpensive. Preanalytical procedures should be used to maintain the structural and molecular integrity of EVs; second, gold standards for instrumentation and analysis of exosome-associated biomarkers are urgently needed. Moreover, proteomics combined with other histological screenings, such as lipidomics, metabolomics, or transcriptomics, may quickly identify useful candidate biomarkers for validation in a wide range of clinical studies. Standard operating procedures for the collection, storage, processing, and analysis of EVs are still urgently needed. Although EVs have been shown to have the ability to spread diseases, it is believed that techniques related to EVs can still be used to benefit patients.
Funding
Shandong Nature Fund (ZR2019MH065).
Conflict of Interests
The authors declare no conflict of interests.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Grey M., Dunning C.J., Gaspar R. Acceleration of alpha-synuclein aggregation by exosomes. J Biol Chem. 2015;290(5):2969–2982. doi: 10.1074/jbc.M114.585703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silverman J.M., Christy D., Shyu C.C. CNS-derived extracellular vesicles from superoxide dismutase 1 (SOD1)(G93A) ALS mice originate from astrocytes and neurons and carry misfolded SOD1. J Biol Chem. 2019;294(10):3744–3759. doi: 10.1074/jbc.RA118.004825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.You Y., Ikezu T. Emerging roles of extracellular vesicles in neurodegenerative disorders. Neurobiol Dis. 2019;130:e104512. doi: 10.1016/j.nbd.2019.104512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rufino-Ramos D., Albuquerque P.R., Carmona V., Perfeito R., Nobre R.J., Pereira de Almeida L. Extracellular vesicles: novel promising delivery systems for therapy of brain diseases. J Control Release. 2017;262:247–258. doi: 10.1016/j.jconrel.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Liew L.C., Katsuda T., Gailhouste L., Nakagama H., Ochiya T. Mesenchymal stem cell-derived extracellular vesicles: a glimmer of hope in treating Alzheimer's disease. Int Immunol. 2017;29(1):11–19. doi: 10.1093/intimm/dxx002. [DOI] [PubMed] [Google Scholar]
- 6.Szatanek R., Baj-Krzyworzeka M., Zimoch J., Lekka M., Siedlar M., Baran J. The methods of choice for extracellular vesicles (EVs) characterization. Int J Mol Sci. 2017;18(6):e1153. doi: 10.3390/ijms18061153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathieu M., Martin-Jaular L., Lavieu G., Thery C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 8.Das S., Extracellular RNACC, Ansel K.M. The extracellular RNA communication consortium: establishing foundational knowledge and technologies for extracellular RNA research. Cell. 2019;177(2):231–242. doi: 10.1016/j.cell.2019.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srinivasan S., Yeri A., Cheah P.S. Small RNA sequencing across diverse biofluids identifies optimal methods for exRNA isolation. Cell. 2019;177(2):446–462. doi: 10.1016/j.cell.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thery C., Witwer K.W., Aikawa E. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):e1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Niel G., D'Angelo G., Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 12.Chaput N., Thery C. Exosomes: immune properties and potential clinical implementations. Semin Immunopathol. 2011;33(5):419–440. doi: 10.1007/s00281-010-0233-9. [DOI] [PubMed] [Google Scholar]
- 13.Bobrie A., Colombo M., Raposo G., Thery C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12(12):1659–1668. doi: 10.1111/j.1600-0854.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- 14.Thery C., Ostrowski M., Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9(8):581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 15.Ratajczak M.Z., Kucia M., Jadczyk T. Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies? Leukemia. 2012;26(6):1166–1173. doi: 10.1038/leu.2011.389. [DOI] [PubMed] [Google Scholar]
- 16.Colombo M., Raposo G., Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 17.Iranifar E., Seresht B.M., Momeni F. Exosomes and microRNAs: new potential therapeutic candidates in Alzheimer disease therapy. J Cell Physiol. 2019;234(3):2296–2305. doi: 10.1002/jcp.27214. [DOI] [PubMed] [Google Scholar]
- 18.Wu X., Zheng T., Zhang B. Exosomes in Parkinson's disease. Neurosci Bull. 2017;33(3):331–338. doi: 10.1007/s12264-016-0092-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skog J., Wurdinger T., van Rijn S. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alvarez-Erviti L., Seow Y., Yin H., Betts C., Lakhal S., Wood M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 21.Wang H., Sui H., Zheng Y. Curcumin-primed exosomes potently ameliorate cognitive function in AD mice by inhibiting hyperphosphorylation of the Tau protein through the AKT/GSK-3beta pathway. Nanoscale. 2019;11(15):7481–7496. doi: 10.1039/c9nr01255a. [DOI] [PubMed] [Google Scholar]
- 22.Mrowczynski O.D., Zacharia B.E., Connor J.R. Exosomes and their implications in central nervous system tumor biology. Prog Neurobiol. 2019;172:71–83. doi: 10.1016/j.pneurobio.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Holm M.M., Kaiser J., Schwab M.E. Extracellular vesicles: multimodal envoys in neural maintenance and repair. Trends Neurosci. 2018;41(6):360–372. doi: 10.1016/j.tins.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Prada I., Gabrielli M., Turola E. Glia-to-neuron transfer of miRNAs via extracellular vesicles: a new mechanism underlying inflammation-induced synaptic alterations. Acta Neuropathol. 2018;135(4):529–550. doi: 10.1007/s00401-017-1803-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fruhbeis C., Frohlich D., Kuo W.P. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 2013;11(7) doi: 10.1371/journal.pbio.1001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frohlich D., Kuo W.P., Fruhbeis C. Multifaceted effects of oligodendroglial exosomes on neurons: impact on neuronal firing rate, signal transduction and gene regulation. Philos Trans R Soc Lond B Biol Sci. 2014;369(1652) doi: 10.1098/rstb.2013.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu B., Zhang Y., Du X.F. Neurons secrete miR-132-containing exosomes to regulate brain vascular integrity. Cell Res. 2017;27(7):882–897. doi: 10.1038/cr.2017.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perez-Gonzalez R., Sahoo S., Gauthier S.A. Neuroprotection mediated by cystatin C-loaded extracellular vesicles. Sci Rep. 2019;9(1):e11104. doi: 10.1038/s41598-019-47524-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trotta T., Panaro M.A., Cianciulli A., Mori G., Di Benedetto A., Porro C. Microglia-derived extracellular vesicles in Alzheimer's Disease: a double-edged sword. Biochem Pharmacol. 2018;148:184–192. doi: 10.1016/j.bcp.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 30.Antonucci F., Turola E., Riganti L. Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism. EMBO J. 2012;31(5):1231–1240. doi: 10.1038/emboj.2011.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gabrielli M., Battista N., Riganti L. Active endocannabinoids are secreted on extracellular membrane vesicles. EMBO Rep. 2015;16(2):213–220. doi: 10.15252/embr.201439668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riganti L., Antonucci F., Gabrielli M. Sphingosine-1-Phosphate (S1P) impacts presynaptic functions by regulating synapsin I localization in the presynaptic compartment. J Neurosci. 2016;36(16):4624–4634. doi: 10.1523/JNEUROSCI.3588-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuyama K., Igarashi Y. Exosomes as carriers of Alzheimer's amyloid-ss. Front Neurosci. 2017;11:e229. doi: 10.3389/fnins.2017.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quek C., Hill A.F. The role of extracellular vesicles in neurodegenerative diseases. Biochem Biophys Res Commun. 2017;483(4):1178–1186. doi: 10.1016/j.bbrc.2016.09.090. [DOI] [PubMed] [Google Scholar]
- 35.Zheng T., Pu J., Chen Y. Plasma exosomes spread and cluster Around beta-amyloid plaques in an animal model of Alzheimer's disease. Front Aging Neurosci. 2017;9:e12. doi: 10.3389/fnagi.2017.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asai H., Ikezu S., Tsunoda S. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat Neurosci. 2015;18(11):1584–1593. doi: 10.1038/nn.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang G., Dinkins M., He Q. Astrocytes secrete exosomes enriched with proapoptotic ceramide and prostate apoptosis response 4 (PAR-4): potential mechanism of apoptosis induction in Alzheimer disease (AD) J Biol Chem. 2012;287(25):21384–21395. doi: 10.1074/jbc.M112.340513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eitan E., Hutchison E.R., Marosi K. Extracellular vesicle-associated abeta mediates trans-neuronal bioenergetic and Ca(2+)-handling deficits in Alzheimer's disease models. NPJ Aging Mech Dis. 2016;2 doi: 10.1038/npjamd.2016.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuyama K., Sun H., Usuki S. A potential function for neuronal exosomes: sequestering intracerebral amyloid-beta peptide. FEBS Lett. 2015;589(1):84–88. doi: 10.1016/j.febslet.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 40.Ghidoni R., Paterlini A., Albertini V. Cystatin C is released in association with exosomes: a new tool of neuronal communication which is unbalanced in Alzheimer's disease. Neurobiol Aging. 2011;32(8):1435–1442. doi: 10.1016/j.neurobiolaging.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chistiakov D.A., Chistiakov A.A. alpha-Synuclein-carrying extracellular vesicles in Parkinson's disease: deadly transmitters. Acta Neurol Belg. 2017;117(1):43–51. doi: 10.1007/s13760-016-0679-1. [DOI] [PubMed] [Google Scholar]
- 42.Soria F.N., Pampliega O., Bourdenx M., Meissner W.G., Bezard E., Dehay B. Exosomes, an unmasked culprit in neurodegenerative diseases. Front Neurosci. 2017;11:e26. doi: 10.3389/fnins.2017.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tofaris G.K. A critical assessment of exosomes in the pathogenesis and stratification of Parkinson's disease. J Parkinson's Dis. 2017;7(4):569–576. doi: 10.3233/JPD-171176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang C., Lang H., Geng N., Wang J., Li N., Wang X. Exosomes of BV-2 cells induced by alpha-synuclein: important mediator of neurodegeneration in PD. Neurosci Lett. 2013;548:190–195. doi: 10.1016/j.neulet.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 45.Zhang X., Abels E.R., Redzic J.S., Margulis J., Finkbeiner S., Breakefield X.O. Potential transfer of polyglutamine and CAG-repeat RNA in extracellular vesicles in Huntington's disease: background and evaluation in cell culture. Cell Mol Neurobiol. 2016;36(3):459–470. doi: 10.1007/s10571-016-0350-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bonafede R., Mariotti R. ALS pathogenesis and therapeutic approaches: the role of mesenchymal stem cells and extracellular vesicles. Front Cell Neurosci. 2017;11:e80. doi: 10.3389/fncel.2017.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fyfe I. Motor neuron disease: proinflammatory monocytes might contribute to ALS progression. Nat Rev Neurol. 2017;13(7):e385. doi: 10.1038/nrneurol.2017.74. [DOI] [PubMed] [Google Scholar]
- 48.Zondler L., Feiler M.S., Freischmidt A. Impaired activation of ALS monocytes by exosomes. Immunol Cell Biol. 2017;95(2):207–214. doi: 10.1038/icb.2016.89. [DOI] [PubMed] [Google Scholar]
- 49.Sproviero D., La Salvia S., Giannini M. Pathological proteins are transported by extracellular vesicles of sporadic amyotrophic lateral sclerosis patients. Front Neurosci. 2018;12:e487. doi: 10.3389/fnins.2018.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fiandaca M.S., Kapogiannis D., Mapstone M. Identification of preclinical Alzheimer's disease by a profile of pathogenic proteins in neurally derived blood exosomes: a case-control study. Alzheimers Dement. 2015;11(6):600–607 e601. doi: 10.1016/j.jalz.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Winston C.N., Goetzl E.J., Akers J.C. Prediction of conversion from mild cognitive impairment to dementia with neuronally derived blood exosome protein profile. Alzheimers Dement. 2016;3:63–72. doi: 10.1016/j.dadm.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kapogiannis D., Boxer A., Schwartz J.B. Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer's disease. FASEB J. 2015;29(2):589–596. doi: 10.1096/fj.14-262048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goetzl E.J., Boxer A., Schwartz J.B. Altered lysosomal proteins in neural-derived plasma exosomes in preclinical Alzheimer disease. Neurology. 2015;85(1):40–47. doi: 10.1212/WNL.0000000000001702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goetzl E.J., Abner E.L., Jicha G.A., Kapogiannis D., Schwartz J.B. Declining levels of functionally specialized synaptic proteins in plasma neuronal exosomes with progression of Alzheimer's disease. FASEB J. 2018;32(2):888–893. doi: 10.1096/fj.201700731R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu C.G., Song J., Zhang Y.Q., Wang P.C. MicroRNA-193b is a regulator of amyloid precursor protein in the blood and cerebrospinal fluid derived exosomal microRNA-193b is a biomarker of Alzheimer's disease. Mol Med Rep. 2014;10(5):2395–2400. doi: 10.3892/mmr.2014.2484. [DOI] [PubMed] [Google Scholar]
- 56.Lugli G., Cohen A.M., Bennett D.A. Plasma exosomal miRNAs in persons with and without alzheimer disease: altered expression and prospects for biomarkers. PLoS One. 2015;10(10) doi: 10.1371/journal.pone.0139233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang S., Liu Z., Ye T. Elevated LRRK2 autophosphorylation in brain-derived and peripheral exosomes in LRRK2 mutation carriers. Acta Neuropathol Commun. 2017;6(1):e86. doi: 10.1186/s40478-017-0492-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stuendl A., Kunadt M., Kruse N. Induction of alpha-synuclein aggregate formation by CSF exosomes from patients with Parkinson's disease and dementia with Lewy bodies. Brain. 2016;139(Pt 2):481–494. doi: 10.1093/brain/awv346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barbour R., Kling K., Anderson J.P. Red blood cells are the major source of alpha-synuclein in blood. Neurodegener Dis. 2008;5(2):55–59. doi: 10.1159/000112832. [DOI] [PubMed] [Google Scholar]
- 60.Shi M., Zabetian C.P., Hancock A.M. Significance and confounders of peripheral DJ-1 and alpha-synuclein in Parkinson's disease. Neurosci Lett. 2010;480(1):78–82. doi: 10.1016/j.neulet.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cerri S., Ghezzi C., Sampieri M. The exosomal/total alpha-synuclein ratio in plasma is associated with glucocerebrosidase activity and correlates with measures of disease severity in PD patients. Front Cell Neurosci. 2018;12:e125. doi: 10.3389/fncel.2018.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shi M., Liu C., Cook T.J. Plasma exosomal alpha-synuclein is likely CNS-derived and increased in Parkinson's disease. Acta Neuropathol. 2014;128(5):639–650. doi: 10.1007/s00401-014-1314-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shi M., Kovac A., Korff A. CNS tau efflux via exosomes is likely increased in Parkinson's disease but not in Alzheimer's disease. Alzheimers Dement. 2016;12(11):1125–1131. doi: 10.1016/j.jalz.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cao Z., Wu Y., Liu G. alpha-Synuclein in salivary extracellular vesicles as a potential biomarker of Parkinson's disease. Neurosci Lett. 2019;696:114–120. doi: 10.1016/j.neulet.2018.12.030. [DOI] [PubMed] [Google Scholar]
- 65.Ho D.H., Yi S., Seo H., Son I., Seol W. Increased DJ-1 in urine exosome of Korean males with Parkinson's disease. BioMed Res Int. 2014;2014 doi: 10.1155/2014/704678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fraser K.B., Rawlins A.B., Clark R.G. Ser(P)-1292 LRRK2 in urinary exosomes is elevated in idiopathic Parkinson's disease. Mov Disord. 2016;31(10):1543–1550. doi: 10.1002/mds.26686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fraser K.B., Moehle M.S., Alcalay R.N., West A.B., Consortium L.C. Urinary LRRK2 phosphorylation predicts parkinsonian phenotypes in G2019S LRRK2 carriers. Neurology. 2016;86(11):994–999. doi: 10.1212/WNL.0000000000002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gui Y., Liu H., Zhang L., Lv W., Hu X. Altered microRNA profiles in cerebrospinal fluid exosome in Parkinson disease and Alzheimer disease. Oncotarget. 2015;6(35):37043–37053. doi: 10.18632/oncotarget.6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cao X.Y., Lu J.M., Zhao Z.Q. MicroRNA biomarkers of Parkinson's disease in serum exosome-like microvesicles. Neurosci Lett. 2017;644:94–99. doi: 10.1016/j.neulet.2017.02.045. [DOI] [PubMed] [Google Scholar]
- 70.Doxakis E. Post-transcriptional regulation of alpha-synuclein expression by mir-7 and mir-153. J Biol Chem. 2010;285(17):12726–12734. doi: 10.1074/jbc.M109.086827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Junn E., Lee K.W., Jeong B.S., Chan T.W., Im J.Y., Mouradian M.M. Repression of alpha-synuclein expression and toxicity by microRNA-7. Proc Natl Acad Sci U S A. 2009;106(31):13052–13057. doi: 10.1073/pnas.0906277106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Denis H.L., Lamontagne-Proulx J., St-Amour I. Platelet abnormalities in Huntington's disease. J Neurol Neurosurg Psychiatry. 2019;90(3):272–283. doi: 10.1136/jnnp-2018-318854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Denis H.L., Lamontagne-Proulx J., St-Amour I. Platelet-derived extracellular vesicles in Huntington's disease. J Neurol. 2018;265(11):2704–2712. doi: 10.1007/s00415-018-9022-5. [DOI] [PubMed] [Google Scholar]
- 74.Xu Q., Zhao Y., Zhou X., Luan J., Cui Y., Han J. Comparison of the extraction and determination of serum exosome and miRNA in serum and the detection of miR-27a-3p in serum exosome of ALS patients. Intractable Rare Dis Res. 2018;7(1):13–18. doi: 10.5582/irdr.2017.01091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Otake K., Kamiguchi H., Hirozane Y. Identification of biomarkers for amyotrophic lateral sclerosis by comprehensive analysis of exosomal mRNAs in human cerebrospinal fluid. BMC Med Genomics. 2019;12(1):e7. doi: 10.1186/s12920-019-0473-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li Z., Zhou X., Wei M. In Vitro and in vivo RNA inhibition by CD9-HuR functionalized exosomes encapsulated with miRNA or CRISPR/dCas9. Nano Lett. 2019;19(1):19–28. doi: 10.1021/acs.nanolett.8b02689. [DOI] [PubMed] [Google Scholar]
- 77.Hall J., Prabhakar S., Balaj L., Lai C.P., Cerione R.A., Breakefield X.O. Delivery of therapeutic proteins via extracellular vesicles: review and potential treatments for Parkinson's disease, glioma, and schwannoma. Cell Mol Neurobiol. 2016;36(3):417–427. doi: 10.1007/s10571-015-0309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Garbayo E., Ansorena E., Lana H. Brain delivery of microencapsulated GDNF induces functional and structural recovery in parkinsonian monkeys. Biomaterials. 2016;110:11–23. doi: 10.1016/j.biomaterials.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 79.Lee M., Liu T., Im W., Kim M. Exosomes from adipose-derived stem cells ameliorate phenotype of Huntington's disease in vitro model. Eur J Neurosci. 2016;44(4):2114–2119. doi: 10.1111/ejn.13275. [DOI] [PubMed] [Google Scholar]
- 80.Didiot M.C., Hall L.M., Coles A.H. Exosome-mediated delivery of hydrophobically modified siRNA for Huntingtin mRNA silencing. Mol Ther. 2016;24(10):1836–1847. doi: 10.1038/mt.2016.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee S.T., Im W., Ban J.J. Exosome-based delivery of miR-124 in a Huntington's disease model. J Mov Disord. 2017;10(1):45–52. doi: 10.14802/jmd.16054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee M., Ban J.J., Kim K.Y. Adipose-derived stem cell exosomes alleviate pathology of amyotrophic lateral sclerosis in vitro. Biochem Biophys Res Commun. 2016;479(3):434–439. doi: 10.1016/j.bbrc.2016.09.069. [DOI] [PubMed] [Google Scholar]
- 83.Bonafede R., Scambi I., Peroni D. Exosome derived from murine adipose-derived stromal cells: neuroprotective effect on in vitro model of amyotrophic lateral sclerosis. Exp Cell Res. 2016;340(1):150–158. doi: 10.1016/j.yexcr.2015.12.009. [DOI] [PubMed] [Google Scholar]