Abstract
Lev/MSNs/n-HA/PU has been proved to be a novel scaffold material to treat bone defect caused by chronic osteomyelitis. We have previously identified that this material can effectively treat chronic osteomyelitis caused by Staphylococcus aureusin vivo. However, the potential mechanisms of antibacterial and osteogenic induction properties remain unclear. Thus, for osteogenesis property, immunohistochemistry, PCR, and Western blot were performed to detect the expression of osteogenic markers. Furthermore, flow cytometry and TUNEL were applied to analyze MC3T3-E1 proliferation and apoptosis. For antibacterial property, the material was co-cultivated with bacteria, bacterial colony forming units was counted and the release time of the effective levofloxacin was assayed by agar disc-diffusion test. Moreover, scanning electron microscope was applied to observe adhesion of bacteria. In terms of osteogenic induction, we found BMSCs adherently grew more prominently on Lev/MSNs/n-HA/PU. Lev/MSNs/n-HA/PU also enhanced the expression of osteogenic markers including OCN and COL1α1, as well as effectively promoted the transition from G1 phase to G2 phase. Furthermore, Lev/MSNs/n-HA/PU could reduce apoptosis of MC3T3-E1. Besides, both Lev/MSNs/n-HA/PU and n-HA/PU materials could inhibit bacterial colonies, while Lev/MSNs/n-HA/PU possessed a stronger antibacterial activities, and lower bacterial adhesion than n-HA/PU. These results illustrated that Lev/MSNs/n-HA/PU composite scaffold possess favorable compatibility in vitro, which induce osteogenic differentiation of MSCs, promote proliferation and differentiation of MC3T3-E1, and inhibit apoptosis. Moreover, clear in vitro antibacterial effect of Lev/MSNs/n-HA/PU was also observed. In summary, this study replenishes the potential of Lev/MSNs/n-HA/PU composite scaffold possess dual functions of anti-infection and enhanced osteogenesis for future clinical application.
Keywords: Antibacterial, Composite scaffold, Levofloxacin, Nanobiomaterial, Osteogenesis
Introduction
Chronic osteomyelitis remains a catastrophic and challenging problem in clinics.1,2 Treatment of chronic osteomyelitis not only requires radical debridement that would resulting in large bone defects, but also demands high-dose, prolonged intravenous antibiotic therapy, which brings much severe systemic adverse effects but may not able to maintain minimum inhibitory concentration.3 Therefore, management of bone defects and implementation of appropriate antibiotic therapy are crucial issues to deal with the refractory osteomyelitis.
Although antibiotic-loaded polymethylmethacrylate (PMMA) is the gold standard biomaterial against osteomyelitism,4, 5, 6, 7 it bears serious shortcomings including limited and irregular antibiotic release, incompatibility with many types of antibiotics, demand a second surgery to remove and lack of bone regeneration or conduction.8,9 Thus, design and construction of alternative, biodegradable materials to deliver antibiotics locally and promote osteogenesis have been given high priority. Extensive biodegradable and resorbable materials have been explored preclinically or used clinically for local antibiotic delivery, and the only apparent merit of these materials over PMMA is the potential to reduce the number of surgeries to date. Similarly, we also designed a novel biodegradable antibiotic delivery system, aimed to achieve the synergistic effect of antibacterial and osteogenesis in the treatment of osteomyelitis. Nano-hydroxyapatite (n-HA) is considered to be the one of the most promising material for the treatment of bone defects as its similar structure to normal bone. Such structure makes good mechanical properties and biocompatibility of n-HA.10,11 Polyurethane (PU) is widely used in bone tissue engineering due to its good biodegradability, biocompatibility, and osteo-inductive properties.12 Moreover, mesoporous silica nanoparticles (MSNs) are currently widely used in bio-nanotechnology and nanomedicine to encapsulate biological small molecules, chemical drugs, DNA, or siRNAs.13, 14, 15, 16 In addition, Levofloxacin can be effectively encapsulated in MSNs as its small molecular weight, turn out to be ideal choice for local releasing.17,18 We combined Levofloxacin loaded MSNs with porous n-HA/PU composite scaffold and developed a novel biodegradable antibiotic material—Lev/MSNs/n-HA/PU.
Our previous in vivo studies have demonstrated that Lev/MSNs/n-HA/PU composite scaffold can effectively inhibit the process of chronic osteomyelitis soon after implantation, provided mechanical support for bone repair, and the degradation of the biomaterial contributed to new bone formation,19 which indicated that this novel synthetic scaffold may provide a solution for the treatment of chronic osteomyelitis. In this research, our aimed to further explore the osteogenic activity and antibacterial ability of this novel material in vitro.
Materials and methods
Fabrication of composite scaffold
Both n-HA/PU and Lev/MSNs/n-HA/PU materials were synthesized by Sichuan University. Briefly, the n-HA/PU composite porous scaffold were successfully manufactured using the situ foaming method.20 To further synthesize Lev/MSNs/n-HA/PU, n-HA/PU was immersed in the solution containing 0.5wt% chitosan (CS) and 2% (w/w) acetic acid for 20 min, next in the Lev/MSNs suspension for 30 min, then dried in a vacuum oven at 40 °C.19
For agar disc-diffusion assay, uniform piece (1 mm × 6 mm × 5.5 mm) of different scaffold materials were prepared. For bacterial colony forming unit counting experiments and MTT assay experiments, material extracts were prepared in a way that the materials (10 mm × 6 mm × 6 mm) were immersed in 5 ml phosphate-buffered saline (PBS). PBS was changed on daily basis and the extracts were collected.
All materials were sterilized by the gamma Co-60 ray irradiation.
Cell isolation and culture, microbial strains
All research involving animals was approved by The First Affiliated Hospital of Chongqing Medical University. Bone marrow mesenchymal stem cells (BMSCs) were isolated from bones of Sprague Dawley rats limbs and cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% FBS and PS (100 units/ml penicillin, 100 μg/ml streptomycin) at 37 °C in humidified atmosphere of 5% CO2. In the control group, BMSCs were inoculated into a 24-well culture plate and were adjusted to a density of 105 cells/ml in co-cultured group. The cell droplets were added dropwise to the material until they were no longer absorbed. After 4 h of incubation at 37 °C, 0.5 ml fresh medium was gently added alongside the wall of the plate, and the osteogenic induction medium was replaced every 2 days.
The murine pre-osteoblastic MC3T3-E1 cell was purchased from ATCC (Manassas, VA, USA) and cultured in α-MEM with 10% FBS and PS (100 units/ml penicillin, 100 μg/ml streptomycin) at 37 °C in a 5% CO2 incubator.
The bacterial strains Escherichia coli (E. coli) ATCC25922 and Staphylococcus aureus (S. aureus) ATCC25923 were obtained from ATCC. Unless otherwise specified, all reagents were purchased from Thermo Fisher Scientific (Waltham, MA, USA).
Antimicrobial activity in vitro
The activity of this novel material against E. coli ATCC25922 and S. aureus ATCC25923 in vitro was determined according to disc diffusion method and colony forming unit (CFU) enumeration method.
E. coli ATCC 25922 and S. aureus ATCC 25923 stock cultures were sub-cultured into fresh Luria Broth (LB) medium at 37 °C with shaking. To obtain working cultures, a loopful of culture was inoculated into LB and incubated overnight at 37 °C. After 24 h of incubation, the cultures were then diluted in medium to adjust the cell density to approximately 1.0 × 105 CFU/mL, which was determined at 600 nm using the UV–visible spectrophotometer.
In disc diffusion method, 200 μL of bacterial suspension (1.0 × 105 CFU/ml) was first seeded on LB broth agar plates, and then uniform pieces (1 mm thick, 6 mm length and 5.5 mm width) of different scaffold materials were prepared and subsequently placed on E. coli and S. aureus culture plates. The plates were incubated for 24 h at 37 °C. The relative antibacterial effect was found by measuring the zone of inhibition (ZOI) around each specimen with a digital caliper. Counting the number of colony formation was also used for determining antibacterial activity. Previous bacterial suspension (1.0 × 105 CFU/ml) was diluted 1:100 in fresh LB broth, followed by addition of Lev@MSNs/n-HA/PU and n-HA/PU, and the tubes were then incubated with 220 rpm agitation in an orbital shaker at 37 °C and for 24 h. After the overnight co-culture with samples, co-culture suspension was collected and diluted 10000-fold, 100 μL bacterial suspensions were uniformly spread onto a LB agar plate and incubated under 37 °C for 24 h. The bacterial growth on the plate was visualized directly.
Quantitative real-time PCR
After 14 days of incubation, total RNA from osteoblasts grew on different scaffold materials was isolated using the TRIzoI reagent and reversely transcribed into cDNA as per the manufacturer's protocol in the treatment group (Lev@MSNs/n-HA/PU composite scaffold) and the control group (n-HA/PU composite scaffold and negative control group). The expression of osteogenesis-related genes, including Osteocalcin (OCN) and type I collagen gene (COL1α1), were measured with a quantitative real-time polymerase chain reaction (qRT-PCR) detection system and analyzed by using the gene-specific primers (Table S1).
Western blot
After the osteoblasts were seeded onto scaffold for 14 days, total cell protein was extracted by RIPA cell lysis reagent and subjected to Western blot. The protein concentration was determined with a BCA Protein Assay kit (Thermo scientific, Rockford, Illinois, USA) according to the manufacturer's instructions. The β-actin was used as an internal standard. Briefly, protein samples were mixed in a 5 × loading buffer and loaded into SDS-PAGE, and transferred electrophoretically to polyvinylidene difluoride (PVDF) membranes. The PVDF membrane was washed in TBST and then blocked with 5% skim milk blocking solution for 2 h at room temperature. Subsequently, the membranes were incubated with COL-I antibody (Cell signaling Technology, 1:1000) or β-actin antibody (Cell signaling Technology, 1:1000) at 4 °C overnight, and followed by incubation with secondary antibody labeled with horseradish peroxidase-labeled (Beyotime, Jiangsu, China) at room temperature for 2 h. Enhanced chemiluminescence (ECL) was captured by a Millipore ECL system (Millipore, USA).
Immunohistochemistry analysis
Immunohistochemistry were performed for ALP, OCN, and OPN proteins detection as previously study described. After co-cultured for 14 days, cells were digested in 0.5% trypsin and seeded on slides at 2 × 104 cells/ml. After the cell's adherent, osteogenic induction medium was added to continue cultivation for 3 days. After fixation cells in 4% paraformaldehyde for 10–20 min, slides were incubated with goat serum to block non-specific binding, following incubation with primary antibody (ALP, 1:100; OCN, 1:50; OPN, 1:100) overnight at 4 °C. Subsequently, HRP conjugated secondary antibody (dilution ratio 1:50) was applied to the slides for 30 min at room temperature. The negative control replaced the primary antibody with PBS.
MTT analysis
The MTT assay was performed to evaluate cell proliferation activity within different materials. Briefly, after 2, 4 and 7 days of co-culture, MC3T3-E1 cells were digested and seeded in 96-well cell culture plates, and adjusted to 1.0 × 105 cells/ml. After 24 h, the culture medium was removed and MTT solution was added and incubated for 4 h. After the cultivation, supernatant was discarded, and 150 μl dimethyl sulfoxide (DMSO) were added and shaken for 10min. The absorbance was measured at 490 nm. Measurements were repeated at least 3 times in each group were performed at each time point.
SEM analysis
MC3T3-E1 cells were seeded on different scaffold materials (1 mm thick and 7 mm diameter) with 4.0 × 104 cells and incubated in complete medium at 37 °C for 7 days, then the medium was removed and the scaffold materials were rinsed twice with PBS and processed for SEM analysis (SEM, JSM-7500F, JEOL, USA).
Flow cytometry analysis
MC3T3-E1 cells and materials were co-cultured for 14 days, and the cells were digested and fixed, following resuspend in 0.5 mL of propidium iodide and incubated at 37 °C for 30 min. Red fluorescence was detected at a wavelength of 488 nm using a flow cytometer (BD Biosciences, San Jose, CA, USA). Simultaneously, light scattering was detected.
TUNEL assay
After 14 days of co-culture, cells were digested and seeded on slice for 24 h. TUNEL imaging assays were performed according to the manufacturer's protocol (Roche, Stockholm, Sweden). Briefly, cells were fixed with 4% paraformaldehyde for 20 min, then incubated in TUNEL reaction mixture (50 μL per well) for 60 min at 37 °C, following incubation with 50 μL convert-POD at 37 °C for 30 min. Labeled cells could be directly detected by bright-field microscopy after 3,3′-diaminobenzidine (DAB) exposure followed by hematoxylin QS counterstaining.
Statistical analysis
All statistical analyses were performed using SPSS software (Version 21, IBM, USA). The data are expressed as the mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was performed to analyze inter- and intragroup differences, and Student's t-test was used to compare between any two groups. P < 0.05 was considered as statistically significant.
Results
In vitro biocompatibility
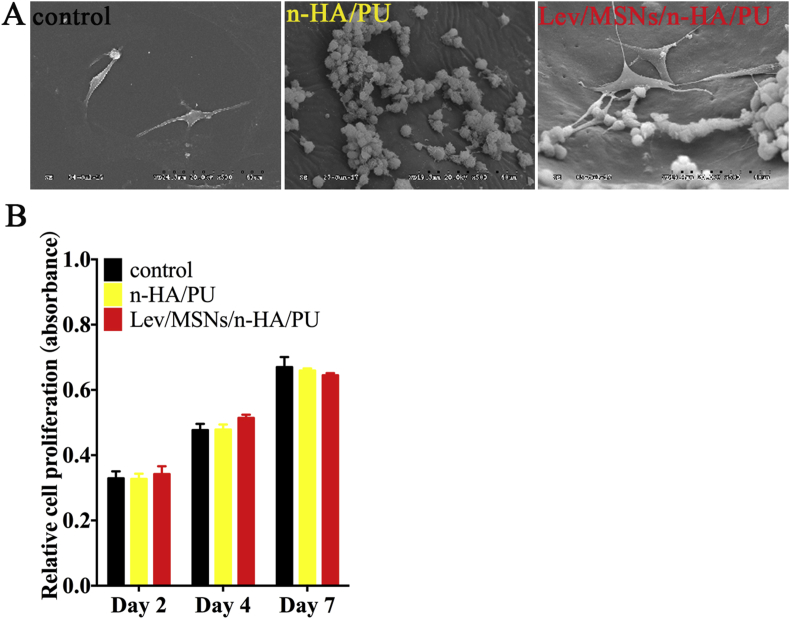
For explore the biocompatibility of Lev/MSNs/n-HA/PU materials in vitro, BMSCs were seeded at glass slides, n-HA/PU and Lev/MSNs/n-HA/PU materials. After 7 days of culture, electron microscopy scan was used to examine the adhesion and the proliferative activity of BMSCs. The results showed that BMSCs could effectively grow on n-HA/PU and Lev/MSNs/n-HA/PU materials. In particular, the prosthetic foot and pseudopodia in Lev/MSNs/n-HA/PU material groups were exhibited more extension, compared with that in the n-HA/PU group (Fig. 1A).
Figure 1.
Effect of novel Lev/MSNs/n-HA/PU composite scaffold materials on biocompatibility and proliferation of BMSCs. (A) SEM micrographs of the BMSCs adhesion in different materials for 7 days. (B) MTT assay for proliferation of BMSCs cultured with different materials at 2, 4 and 7 days. Each experiment was repeated three times. “*” means P < 0.05.
We further explored whether the material will release some cytotoxic substances that may affect cell proliferation activity. In vitro, we co-cultured BMSCs with Lev/MSNs/n-HA/PU materials, n-HA/PU materials, and negative control. After 2, 4, and 7 days, the effect of material on cell proliferation activity was detected by MTT assay. The experimental results showed a significant time-dependent increase of cell proliferation activity at each time point. And BMSCs on the Lev/MSNs/n-HA/PU material showed similar cell proliferation activity as the control and the n-HA/PU materials groups. Interestingly, the cell viability in the Lev/MSNs/n-HA/PU material group after 7 days of culture showed a tendency of decrease compared to the control group and the n-HA/PU material group (Fig. 1B).
In vitro osteogenic performances
Differentiation property
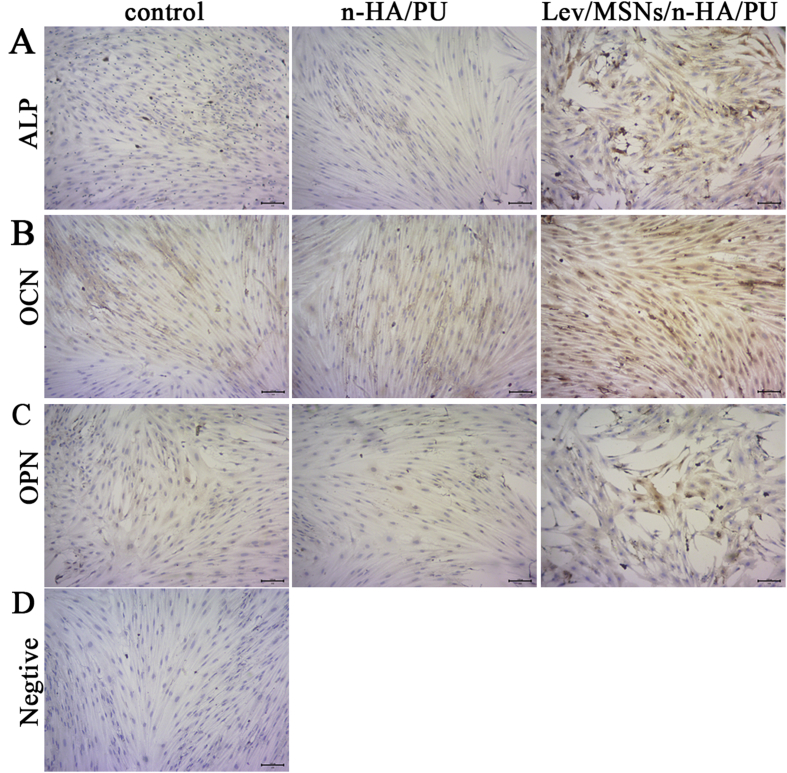
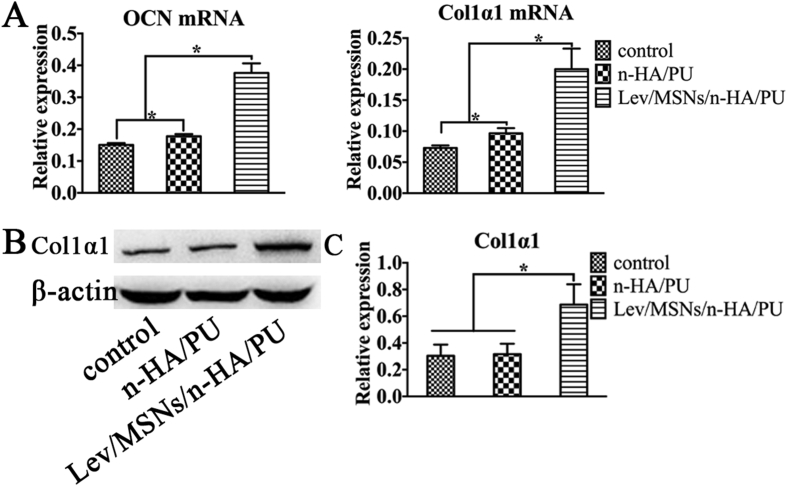
Our previous studies found that Lev/MSNs/n-HA/PU can effectively repair bone defects in a rabbit model of chronic osteomyelitis and promote local new bone formation. To further investigate the osteogenic induction of Lev/MSNs/n-HA/PU materials in vitro, we isolated primary rat BMSCs and co-cultured with the material. After 14 days, immunohistochemistry was used to determine the expression of osteogenic differentiation-related molecules. We found that the expression of ALP, OCN, and OPN increased more significantly in the Lev/MSNs/n-HA/PU material group compared with that in the negative control and n-HA/PU groups during osteogenic differentiation process (Fig. 2). Similarly, the expression of CON and COL1α1, the late osteogenic differentiation markers, were analyzed by qPCR and Western blot. The results showed that the mRNA expression of OCN and COL1α1 in n-HA/PU material group was higher than that in the control group, while the expression of COL1α1 protein was not statistically significant different. Significantly, mRNA transcription of OCN and COL1α1 and expression of COL1α1 protein were elevated in the Lev/MSNs/n-HA/PU material group compared to the control and n-HA/PU material groups (Fig. 3). These results indicate that Lev/MSNs/n-HA/PU material can significantly induce osteogenic differentiation of BMSCs in vitro.
Figure 2.
Immunohistochemistry assay of osteogenic markers for composite scaffold materials. BMSCs were cultured on different scaffold materials for 7 days and next on slides for 3 days. They were subjected to immunohistochemistry assay of the expression of ALP (A), OCN (B) and OPN (C). The negative control was added with PBS instead of primary antibody (D). Black bar represents 100 μm.
Figure 3.
Q-PCR and Western-Blot assay of osteogenic markers for composite scaffold materials. BMSCs were cultured on different scaffold materials for 14 days. RNA was isolated and subjected to qPCR analysis of the expression of OCN and COL1α1 (A) in osteoblast cells. The expression of COL1α1 was detected by Western-Blot (B, C). “*” means P < 0.05.
Osteogenic capability
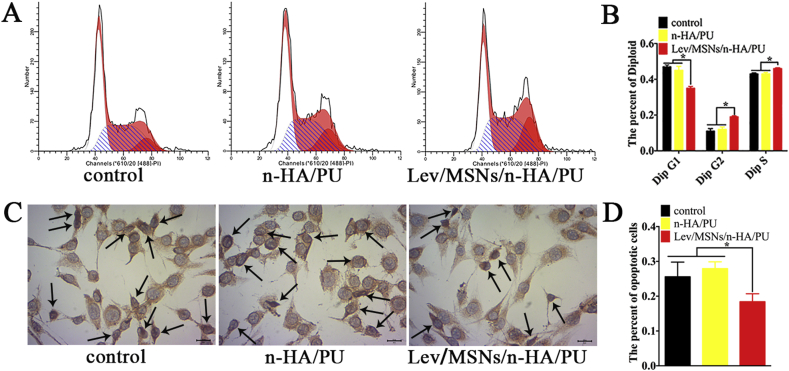
To elucidate the mechanism by which Lev/MSNs/n-HA/PU material promotes osteogenic differentiation, we also co-cultured pre-osteoblast MC3T3-E1 in vitro with the scaffold material. After 14 days of co-culture, the split cycle of pre-osteoclast MC3T3 E1 was analyzed by flow cytometry. Overall, we found that most of the cells were in the G1 and S phases of the cell cycle. However, cells co-cultured with Lev/MSNs/n-HA/PU material were more in S and G2 phases than the control and n-HA/PU material groups (Figs. 4A and B).
Figure 4.
Effect of novel Lev/MSNs/n-HA/PU composite scaffold materials on proliferation and apoptosis of MC3T3-E1. (A) MC3T3-E1 was cultured on different scaffold materials for 14 days and digested for assay of flow cytometry. (B) The percentage of cell proliferation cycles treated with different materials. (C) TUNEL assay for evaluation of apoptosis of MC3T3-E1 co-cultured with different materials for 14 days. (D) The percentage of TUNEL positive cell treated with different materials, and black arrows represent positive cells. Each experiment was repeated three times. “*” means P < 0.05.
The effects of Lev/MSNs/n-HA/PU materials on apoptosis of pre-osteoblast MC3T3-E1 were further examined. We found no significant difference in the apoptosis of the MC3T3-E1 pre-osteoblasts between the control group and the n-HA/PU material by TUNEL assay. However, Lev/MSNs/n-HA/PU materials can significantly reduce MC3T3-E1 apoptosis (Figs. 4C and D).
In vitro antibacterial property
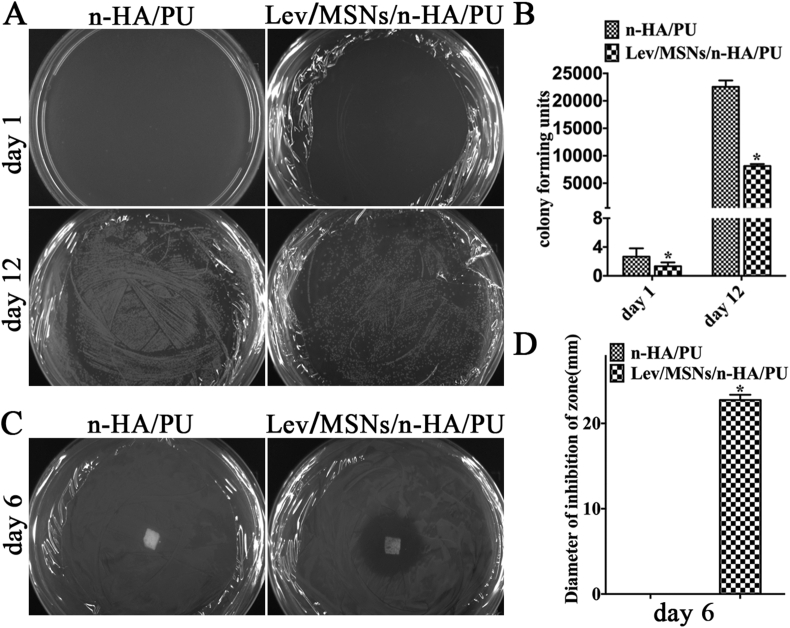
Our previous experiments found that Lev/MSNs/n-HA/PU scaffold materials can significantly treat S. aureus-induced chronic osteomyelitis in rabbit tibia. To further confirm the antibacterial property of Lev/MSNs/n-HA/PU scaffold materials, we used bacterial agar plate to examine the effect of Lev/MSNs/n-HA/PU scaffold materials on bacterial growth. Bacteria and materials were co-cultured and spread evenly on LB agar plates overnight. The results showed that Lev/MSNs/n-HA/PU material can significantly inhibit the growth of both E. coli and S. aureus, there was no E. coli CFU, and only two S. aureus colony forming units (CFU) per 100 μL of culture. While there were 8129 E. coli CFUs and 22552 S aureus CFUs per 100 μL of culture in the n-HA/PU material group, which indicated that Lev/MSNs/n-HA/PU material had significantly inhibited growth of bacterial colony forming units (CFU–B) (Fig. 5A and B).
Figure 5.
The antibacterial effect of novel Lev/MSNs/n-HA/PU composite scaffold materials. (A) Bacterial colony formation after treatment with material extracts at 1 st and 12 th day, and (B) Number of bacterial colony units. (C) The zone of inhibition (ZOI) around n-HA/PU and Lev/MSNs/n-HA/PU scaffold materials. (D) The diameter of ZOI around the materials. Each experiment was repeated three times. Representative results are shown. “*” means P < 0.05 compared with n-HA/PU group.
Lev/MSNs/n-HA/PU scaffold materials also lead to formation of larger size of ZOI. Based on the analysis of the ZOI, there was an obvious ZOI around both Lev/MSNs/n-HA/PU materials after co-cultured with S. aureus and E. coli for 24 h, while the value of ZOI in the n-HA/PU material group was no longer visible, which indicated that Lev/MSNs/n-HA/PU materials were able to inhibit bacterial growth (Fig. 5C and D). These results could be due to the porous structure of n-HA and PU degradability in Lev/MSNs/n-HA/PU materials, as well as the controlled release of MSN-based system.
Antibacterial adhesion performance
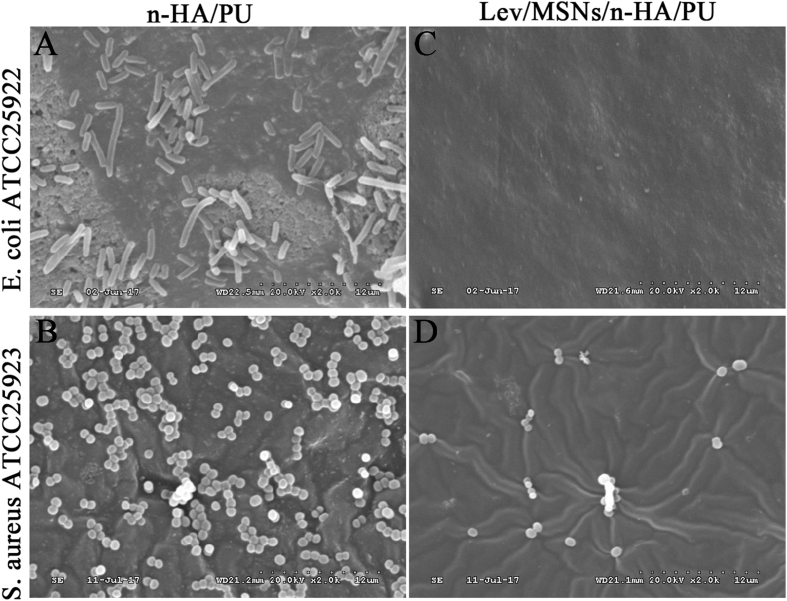
We also tested in vitro the adhesion of E. coli and S. Aureus on Lev/MSNs/n-HA/PU scaffold. The SEM results showed that a large amount of both E. coli and S. aureus could adhere to n-HA/PU materials, while only a small fraction of S. aureus without E. coli adhered to Lev/MSNs/n-HA/PU materials (Fig. 6), which indicated that Lev/MSNs was able to inhibit microbial adhesion or viability of S. aureus and E. coli.
Figure 6.
The adhesive effect of novel Lev/MSNs/n-HA/PU composite scaffold materials on E. coli and S. aureus. Scanning electron microscope (SEM) was used to detect the adhesion of Escherichia coli (upper) and Staphylococcus aureus (lower) to the composite scaffold materials n-HA/PU (A-B), and the adhesion to novel composite scaffold materials Lev/MSNs/n-HA/PU (C-D). Representative results are shown.
Discussion
Management of bone defects caused by chronic osteomyelitis remains a century-long, troublesome problem. In clinical practice, we have experienced periods of systemic application of large amounts of antibiotics and currently antibiotics loaded PMMA beads or spacers are widely used. Notably, PMMA remains deficient due to non-degradation that requires surgical removal, which would leads to large bone defects or local bacterial biofilms formation which can provide a substratum for bacterial colonization, causing recurrence of secondary infections.21, 22, 23 Especially the amount of antibiotic released decreases over time and eventually becomes ineffective.23 These disadvantages of antibiotics loaded PMMA have led to the pursuit of alternative, degradable delivery devices, and the development of novel material with multiple biological functions (such as osteogenesis, antibiosis) has been more powerful development and has led to the most important technological advancements in recent.24, 25, 26, 27
Hydroxyapatite (HA) resembles the main inorganic component of biological apatite (mineralized tissues) and has high biocompatibility, which in combination with its non-toxicity and, most importantly, osteoconductive properties makes it an asset for biomaterial engineering.28, 29, 30 HA materials have been widely used as a component of hybrid biomaterials. Facing chronic osteomyelitis, one major concern in the field of orthopaedic, recent developments of materials engineering are focused on delivery antibiotics directly into the affected bone. HA-based materials are frequently chosen as the system matrix for antibacterial drugs. Porous structures of HA could be used as temporary scaffold for newly formed osseous tissue.31,32 Moreover, it has further uses in polymer bone composite materials, not only as a bioactive material but also as a provider of desirable mechanical properties.29,30 Similarly, MSNs also gained wide popularity over the recent years, which has uniform and tunable pore size, easy independent functionalization of the surface, internal and external pores and the gating mechanism of the pore opening, making it a versatile and promising carrier.33 A mesoporous silica nanoparticles/hydroxyapatite (MSNs/HA) hybrid drug carrier, which not only enhanced the biodegradability of silica, but also has high drug loading efficiency.32 MSNs composed into Nano-hydroxyapatite material (MSNs/n-HA) structure contains mesopores and during the initial drug loading period, levofloxacin penetrates the pores of the MSNs where it coats the surfaces and its porous network. The levofloxacin contained in these porosities will be released gradually. Hence, the process of polymeric matrix composites containing drug carrying particulate matter can be thought to proceed in progressive dissolution process.
Previously, our research group developed a novel biodegradable antibiotic material—Lev/MSNs/n-HA/PU composite scaffold, which were manufactured by loading levofloxacin on nano-mesoporous silica and then compounding with n-HA/PU.19,34 We expect this novel scaffold materials to have large-scale loading of antibiotics with sustained release, biocompatibility, degradation and bone-inducing performance.34 Our previous studies have confirmed that the composite scaffold could be used to treat chronic osteomyelitis caused by S. aureus in rabbits.19 Apparently, the composite scaffold are not cytotoxic in animals, and there is degradation around the composite scaffold, as well as a large amount of new bone formation and defect healing. To further explore the osteogenesis-induction and antibacterial of this material, we further explored its in-vitro potential osteoinductive and antibacterial properties. As a result, we found that this novel scaffold materials can inhibit bacterial growth and bacterial adhesion in vitro effectively, but also induce osteogenic differentiation of BMSCs, promote proliferation and differentiation of MC3T3-E1 and inhibit apoptosis progress.
Repair and regeneration processes in chronic osteomyelitis can be enhanced by using synthetic scaffold materials, which promoting osteoblast proliferation and differentiation.35,36 Bone regeneration requires biocompatibility to mimic the environment of the extracellular matrix of natural bone tissue, osteoblasts, and signaling molecules for tissue differentiation and vascularization. Renaissance of bone tissue needs scaffold having biocompatibility that simulates the extracellular matrix (ECM) niche of natural bone, osteogenic cells as well as signaling molecules aimed at specific tissue differentiation along with vascularization of host bed.37,38 New bone development at the site of malfunctioning areas needs numerous series of processes like adhesion of osteogenic cells, as well as its survival and proliferation.
The differentiated process from osteo-progenitor cells or undifferentiated stem cells into osteoblast is integrated between the scaffold and host bone.36 Besides, it has been verified that nano-ceramics having smaller pores environment could stimulate osteochondral formation followed by osteogenesis as well as vascularization.39, 40, 41 The dissolution products of scaffold materials are found to up-regulate genes controlling osteogenesis.42 Hence, many approaches have been made for application of nanotechnology in regenerative medicine, nanofiber scaffolding, and modification of nano-topography of scaffold. Similarly, our results illustrated that Lev/MSNs/n-HA/PU, as a novel nano-hydroxyapatite composite scaffold, could induce expression of specific markers of osteogenic differentiation. Unsatisfactorily, the tangible substances of dissolution that induce osteogenic differentiation in our study was not further investigated.
Further, Moorthi et al in his study reported that bioglass at nano scale induces the expression levels of cyclins C and E to stimulate the osteoblasts to go in cell cycle activation phase.43 Furthermore, it might decrease growth cycle passing G1 and S phases following fast entrance addicted to G2 phase by the improved dissolution of silicon and calcium forming the nanoceramics, leading to heightened osteoblast proliferation.44 In our study, we found Lev/MSNs/n-HA/PU could effectively enhance the transition from G1 phase to S phase of cells, rapidly arrest at G2 phase and promote proliferation and differentiation. Simultaneously, it will reduce apoptosis of osteogenic cells. These might be the dual role of mesoporous silica and nano-hydroxyapatite, which provides a relatively better patching environment for cell proliferation and differentiation. Mechanistically, it has been found that calcium silicate (CS, CaSiO3) coating over inert substrates leading to release of SiO3 ions addicted to the biological fluid resulting in its hydrolysis to HSiO3- ions along with Ca2+ ions initiating numerous cellular actions and endorses improved cell attachment, proliferation and differentiation.45 Exactly, Our composite scaffold also contain MSNs and is capable of releasing it stably, which may be related to its ability to significantly induce osteogenic differentiation, and additionally n-HA possess properties similar to structure of natural bone matrix, which can further induce cells to attachment, subsequently proliferation and differentiation.
In this study, we identified that Lev/MSNs/n-HA/PU composite scaffold materials could induce osteogenic differentiation of BMSCs, promote proliferation and differentiation of MC3T3-E1, and inhibit apoptosis, which also have stronger antibacterial properties in vitro compared with n-HA/PU, it can effectively inhibit bacterial growth and bacterial adhesion in vitro. In summary, the results of this study replenish the potential of Lev/MSNs/n-HA/PU materials with dual functions of anti-infection and enhanced osteogenesis for future clinical applications.
Funding
This research was funded by the National High Technology Research and Development Program of China (863 Program, grant number 2013AA032203).
Authors contribution
Conceptualization, Zhiping Kuang and Wei Huang; methodology, Zhiping Kuang, Guangming Dai, Ruijie Wan, Dongli Zhang, Chen Zhao, Cheng Chen, Jidong Li, Hongchen Gu,; software, Guangming Dai, Ruijie Wan, Dongli Zhang, Chen Zhao, Cheng Chen,; validation, Zhiping Kuang, Jidong Li, Hongchen Gu, Wei Huang; formal analysis, Dongli Zhang, Chen Zhao, Cheng Chen; investigation, Zhiping Kuang, Ruijie Wan, Dongli Zhang, Chen Zhao, Cheng Chen; resources, Jidong Li and Hongchen Gu; data curation, Zhiping Kuang, Guangming Dai, Ruijie Wan, Dongli Zhang, Chen Zhao, Cheng Chen; writing—original draft preparation, Zhiping Kuang, Guangming Dai, Ruijie Wan, Dongli Zhang, Chen Zhao, Cheng Chen; writing—review and editing, Jidong Li, Hongchen Gu, Wei Huang; visualization, Guangming Dai, Ruijie Wan, Dongli Zhang, Chen Zhao, Cheng Chen; supervision, Jidong Li, Hongchen Gu, Wei huang; project administration, Zhiping Kuang; funding acquisition, Wei Huang.
Conflict of Interests
The authors declare no conflict of interest.
Acknowledgements
We are very grateful to the Institute of Life Sciences of Chongqing Medical University for providing support for our experiments and thanks to Dr. Wang Qi for providing technical support for our experiments.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gendis.2019.09.014.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Walter G., Kemmerer M., Kappler C., Hoffmann R. Treatment algorithms for chronic osteomyelitis. Dtsch Arzteblatt Int. 2012;109(14):e257. doi: 10.3238/arztebl.2012.0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jorge L.S., Chueire A.G., Rossit A.R.B. Osteomyelitis: a current challenge. Braz J Infect Dis. 2010;14(3):310–315. [PubMed] [Google Scholar]
- 3.Hatzenbuehler J., Pulling T.J. Diagnosis and management of osteomyelitis. Am Fam Physician. 2011;84(9):e1027. [PubMed] [Google Scholar]
- 4.Klemm K. Gentamicin-PMMA-beads in treating bone and soft tissue infections (author's transl) Zentralblatt fur Chir. 1979;104(14):934–942. [PubMed] [Google Scholar]
- 5.Klemm K. The use of antibiotic-containing bead chains in the treatment of chronic bone infections. Clin Microbiol Infect. 2000;7(1):28–31. doi: 10.1046/j.1469-0691.2001.00186.x. [DOI] [PubMed] [Google Scholar]
- 6.Elmarsafi T., Steinberg J.S., Kim P.J., Attinger C.E., Evans K.K. Viability of permanent PMMA spacer with combined free fasciocutaneous tissue transfer for failed charcot reconstruction: a 38 month prospective case report. Int J Surg Case Rep. 2017;41:174–179. doi: 10.1016/j.ijscr.2017.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mills D.K., Jammalamadaka U., Tappa K., Weisman J. Studies on the cytocompatibility, mechanical and antimicrobial properties of 3D printed poly (methyl methacrylate) beads. Bioact Mater. 2018;3(2):157–166. doi: 10.1016/j.bioactmat.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walenkamp G., Vree T. Gentamicin-PMMA beads. Pharmacokinetic and nephrotoxicological study. Clin Orthop Relat Res. 1986;(205):171–183. [PubMed] [Google Scholar]
- 9.Lalidou F., Kolios G., Drosos G. Bone infections and bone graft substitutes for local antibiotic therapy. Surg Technol Int. 2014;24:353–362. [PubMed] [Google Scholar]
- 10.Ferraz M., Mateus A., Sousa J., Monteiro F. Nanohydroxyapatite microspheres as delivery system for antibiotics: release kinetics, antimicrobial activity, and interaction with osteoblasts. J Biomed Mater Res A. 2007;81(4):994–1004. doi: 10.1002/jbm.a.31151. [DOI] [PubMed] [Google Scholar]
- 11.Jiang J.L., Li Y.F., Fang T.L. Vancomycin-loaded nano-hydroxyapatite pellets to treat MRSA-induced chronic osteomyelitis with bone defect in rabbits. Inflamm Res. 2012;61(3):207–215. doi: 10.1007/s00011-011-0402-x. [DOI] [PubMed] [Google Scholar]
- 12.Gorna K., Gogolewski S. Preparation, degradation, and calcification of biodegradable polyurethane foams for bone graft substitutes. J Biomed Mater Res Part A: Off J Soc Biomater – Jpn Soc Biomater – Aust Soc Biomater – Korean Soc Biomater. 2003;67(3):813–827. doi: 10.1002/jbm.a.10148. [DOI] [PubMed] [Google Scholar]
- 13.Li X., Zhang J., Gu H. Study on the adsorption mechanism of DNA with mesoporous silica nanoparticles in aqueous solution. Langmuir. 2012;28(5):2827–2834. doi: 10.1021/la204443j. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y., Gu H. Core–shell-type magnetic mesoporous silica nanocomposites for bioimaging and therapeutic agent delivery. Adv Mater. 2015;27(3):576–585. doi: 10.1002/adma.201401124. [DOI] [PubMed] [Google Scholar]
- 15.Khatoon S., Han H.S., Lee M. Zwitterionic mesoporous nanoparticles with a bioresponsive gatekeeper for cancer therapy. Acta Biomater. 2016;40:282–292. doi: 10.1016/j.actbio.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Xiao X., Liu Y., Guo M. pH-triggered sustained release of arsenic trioxide by polyacrylic acid capped mesoporous silica nanoparticles for solid tumor treatment in vitro and in vivo. J Biomater Appl. 2016;31(1):23–35. doi: 10.1177/0885328216637211. [DOI] [PubMed] [Google Scholar]
- 17.Popa C., Mircheva M., Krämer B.K., Berghofen A., Krüger B. Ceftolozane-tazobactam versus levofloxacin in urinary tract infection. The Lancet. 2015;386(10000):1241–1242. doi: 10.1016/S0140-6736(15)00262-7. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y., Ding X., Chen Y. Antibiotic-loaded, silver core-embedded mesoporous silica nanovehicles as a synergistic antibacterial agent for the treatment of drug-resistant infections. Biomaterials. 2016;101:207–216. doi: 10.1016/j.biomaterials.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Wang Q., Chen C., Liu W. Levofloxacin loaded mesoporous silica microspheres/nano-hydroxyapatite/polyurethane composite scaffold for the treatment of chronic osteomyelitis with bone defects. Sci Rep. 2017;7:e41808. doi: 10.1038/srep41808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li L., Zuo Y., Zou Q. Hierarchical structure and mechanical improvement of an n-HA/GCO–PU composite scaffold for bone regeneration. ACS Appl Mater Interfaces. 2015;7(40):22618–22629. doi: 10.1021/acsami.5b07327. [DOI] [PubMed] [Google Scholar]
- 21.Baker A., Greenham L. Release of gentamicin from acrylic bone cement. Elution and diffusion studies. J Bone Joint Surg Am Vol. 1988;70(10):1551–1557. [PubMed] [Google Scholar]
- 22.Khoury A.E., Lam K., Ellis B., Costerton J.W. Prevention and control of bacterial infections associated with medical devices. Am Soc Artif Intern Organs J. 1992;38(3):M174–M178. doi: 10.1097/00002480-199207000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Simpson P., Dall G., Breusch S., Heisel C. In vitro elution and mechanical properties of antibiotic-loaded SmartSet HV and Palacos R acrylic bone cements. Der Orthopäde. 2005;34(12):1255–1262. doi: 10.1007/s00132-005-0861-2. [DOI] [PubMed] [Google Scholar]
- 24.Gong M., Huang C., Huang Y. Core-sheath micro/nano fiber membrane with antibacterial and osteogenic dual functions as biomimetic artificial periosteum for bone regeneration applications. Nanomed Nanotechnol Biol Med. 2019;17:124–136. doi: 10.1016/j.nano.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Zhao Q., Yi L., Hu A., Jiang L., Hong L., Dong J. Antibacterial and osteogenic activity of a multifunctional microporous coating codoped with Mg, Cu and F on titanium. J Mater Chem B. 2019;7(14):2284–2299. doi: 10.1039/c8tb03377c. [DOI] [PubMed] [Google Scholar]
- 26.Zhao C., Zeng Z., Qazvini N.T. Thermoresponsive citrate-based graphene oxide scaffold enhances bone regeneration from BMP9-stimulated adipose-derived mesenchymal stem cells. ACS Biomater Sci Eng. 2018;4(8):2943–2955. doi: 10.1021/acsbiomaterials.8b00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi R., Gong M., Chi C. Nano twin-fiber membrane with osteogenic and antibacterial dual functions as artificial periosteum for long bone repairing. J Biomed Nanotechnol. 2019;15(2):272–287. doi: 10.1166/jbn.2019.2687. [DOI] [PubMed] [Google Scholar]
- 28.Marković S., Veselinović L., Lukić M.J. Synthetical bone-like and biological hydroxyapatites: a comparative study of crystal structure and morphology. Biomed Mater. 2011;6(4):e045005. doi: 10.1088/1748-6041/6/4/045005. [DOI] [PubMed] [Google Scholar]
- 29.Ridi F., Meazzini I., Castroflorio B., Bonini M., Berti D., Baglioni P. Functional calcium phosphate composites in nanomedicine. Adv Colloid Interface Sci. 2017;244:281–295. doi: 10.1016/j.cis.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Wang C., Wang Y., Meng H. Research progress regarding nanohydroxyapatite and its composite biomaterials in bone defect repair. Int J Polym Mater Polym Biomater. 2016;65(12):601–610. [Google Scholar]
- 31.Elkassas D., Arafa A. The innovative applications of therapeutic nanostructures in dentistry. Nanomed Nanotechnol Biol Med. 2017;13(4):1543–1562. doi: 10.1016/j.nano.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 32.Szurkowska K., Laskus A., Kolmas J. Hydroxyapatite-based materials for potential use in bone tissue infections. Hydroxyapatite: Adv Compos Nanomater – Biomed Appl Technol Facets. 2018;109 [Google Scholar]
- 33.Narayan R., Nayak U., Raichur A., Garg S. Mesoporous silica nanoparticles: a comprehensive review on synthesis and recent advances. Pharmaceutics. 2018;10(3):e118. doi: 10.3390/pharmaceutics10030118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei J., Wang Y., Jiang J. Development of an antibacterial bone graft by immobilization of levofloxacin hydrochloride-loaded mesoporous silica microspheres on a porous scaffold surface. J Biomed Nanotechnol. 2019;15(5):1097–1105. doi: 10.1166/jbn.2019.2743. [DOI] [PubMed] [Google Scholar]
- 35.Arvidson K., Abdallah B., Applegate L. Bone regeneration and stem cells. J Cell Mol Med. 2011;15(4):718–746. doi: 10.1111/j.1582-4934.2010.01224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiara G., Letizia F., Lorenzo F. Nanostructured biomaterials for tissue engineered bone tissue reconstruction. Int J Mol Sci. 2012;13(1):737–757. doi: 10.3390/ijms13010737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allo B.A., Costa D.O., Dixon S.J., Mequanint K., Rizkalla A.S. Bioactive and biodegradable nanocomposites and hybrid biomaterials for bone regeneration. J Funct Biomater. 2012;3(2):432–463. doi: 10.3390/jfb3020432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amini A.R., Laurencin C.T., Nukavarapu S.P. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng. 2012;40(5):363–408. doi: 10.1615/critrevbiomedeng.v40.i5.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeimaran E., Pourshahrestani S., Djordjevic I., Pingguan-Murphy B., Kadri N.A., Towler M.R. Bioactive glass reinforced elastomer composites for skeletal regeneration: a review. Mater Sci Eng C. 2015;53:175–188. doi: 10.1016/j.msec.2015.04.035. [DOI] [PubMed] [Google Scholar]
- 40.Martin R.A., Yue S., Hanna J.V. Characterizing the hierarchical structures of bioactive sol–gel silicate glass and hybrid scaffold for bone regeneration. Philos Trans R Soc A Math Phys Eng Sci. 2012;370(1963):1422–1443. doi: 10.1098/rsta.2011.0308. [DOI] [PubMed] [Google Scholar]
- 41.Hong Z., Reis R.L., Mano J.F. Preparation and in vitro characterization of novel bioactive glass ceramic nanoparticles. J Biomed Mater Res Part A: Off J Soc Biomater – Jpn Soc Biomater – Aust Soc Biomater – Korean Soc Biomater. 2009;88(2):304–313. doi: 10.1002/jbm.a.31848. [DOI] [PubMed] [Google Scholar]
- 42.Gerhardt L.-C., Boccaccini A.R. Bioactive glass and glass-ceramic scaffold for bone tissue engineering. Materials. 2010;3(7):3867–3910. doi: 10.3390/ma3073867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moorthi A., Parihar P., Saravanan S., Vairamani M., Selvamurugan N. Effects of silica and calcium levels in nanobioglass ceramic particles on osteoblast proliferation. Mater Sci Eng C. 2014;43:458–464. doi: 10.1016/j.msec.2014.07.040. [DOI] [PubMed] [Google Scholar]
- 44.Sun J.Y., Yang Y.S., Zhong J., Greenspan D.C. The effect of the ionic products of Bioglass® dissolution on human osteoblasts growth cycle in vitro. J Tissue Eng Regenerat Med. 2007;1(4):281–286. doi: 10.1002/term.34. [DOI] [PubMed] [Google Scholar]
- 45.Wang X., Zhou Y., Xia L. Fabrication of nano-structured calcium silicate coatings with enhanced stability, bioactivity and osteogenic and angiogenic activity. Colloids Surfaces B Biointerfaces. 2015;126:358–366. doi: 10.1016/j.colsurfb.2014.11.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.