Abstract
Background
Grade 3 neuroendocrine neoplasms (NENs) of gastroenteropancreatic (GEP) origin with Ki-67 indices <55% do not respond well to platinum-based chemotherapy. The combination of capecitabine and temozolomide (CAPTEM) has shown favorable responses in grade 1-2 NENs, but has rarely been studied in patients with grade 3 NENs.
Patients and methods
This open-label, single-arm phase II trial included patients with unresectable or metastatic grade 3 NENs of GEP origin with Ki-67 indices <55% enrolled between June 2017 and July 2020. Patients received oral capecitabine 750 mg/m2 twice daily on days 1 to 14 and oral temozolomide 200 mg/m2 once daily on days 10 to 14 every 4 weeks. Histologic findings were centrally reviewed after the completion of enrollment. The primary endpoint was overall response rate, and the secondary endpoints were progression-free survival (PFS), overall survival (OS), and adverse events.
Results
Of the 30 patients included in the full analysis set, 1 (3.3%) achieved complete response, 8 (26.7%) had partial responses, and 14 (46.7%) had stable disease, making the overall response rate 30.0%. At a median follow-up of 19.2 months, the median PFS was 5.9 months and the median OS was not reached. Patients with well-differentiated NENs showed significantly better median PFS (9.3 months versus 3.5 months, P = 0.005) and median OS (not reached versus 6.2 months, P = 0.004) than patients with poorly differentiated tumors. Expression of O6-methyl-guanine methyltransferase protein did not correlate with clinical outcomes. The most common grade 3-4 adverse events were thrombocytopenia (10%), anemia (6.7%), and nausea (6.7%).
Conclusions
CAPTEM was effective and well tolerated in patients with grade 3 GEP-NENs with Ki-67 indices <55%, with superior efficacy outcomes compared with the historical controls receiving platinum-based chemotherapy.
Key words: neuroendocrine tumor, capecitabine, temozolomide, MGMT
Highlights
-
•
Patients with grade 3 neuroendocrine neoplasms with a Ki-67 index <55% were treated with capecitabine and temozolomide.
-
•
Combination treatment resulted in an overall response rate of 30% and a disease control rate of 76.7%.
-
•
Median progression-free survival was 5.9 months, and median overall survival was not reached.
-
•
Treatment was well tolerated, with no new safety issues.
-
•
Capecitabine and temozolomide showed better efficacy outcomes than historical controls receiving platinum-based chemotherapy.
Introduction
Neuroendocrine neoplasms (NENs) are a heterogeneous group of tumors originating from neuroendocrine cells.1 The overall incidence of gastroenteropancreatic (GEP) NENs has been reported to be 3.53 per 100 000.2 NENs can arise from sites throughout the body, although ≥60% have been found to arise from the pancreas and the gastrointestinal tract.3 NENs are divided into well-differentiated neuroendocrine tumors (NETs), which have a relatively indolent course, and poorly differentiated neuroendocrine carcinomas (NECs), which are more aggressive.4 NENs are also classified by tumor morphology and proliferation, as represented by mitotic counts or Ki-67 proliferation indices, respectively.
The 2010 World Health Organization (WHO) classification of tumors of the digestive system has categorized NENs as grade (G) 1-2 NETs and G3 NECs, with the latter including tumors with a Ki-67 index >20 and/or a mitotic count >20/high powered field.5 Some tumors, however, were found to be well-differentiated histologically but to have a high Ki-67 index or mitotic count. Recent studies discovered that in addition to being clinicopathologically different, NETs and NECs are also molecularly distinct from each other.6 The revised 2017 WHO classification of endocrine tumors therefore subdivided G3 NECs into well-differentiated G3 NETs and poorly differentiated G3 NECs.7
Increased understanding of the heterogeneity of G3 NENs gives rise to the need for more tailored treatment strategies for these patients. Although combinations of platinum agents with etoposide have been considered standard treatment options in patients with G3 NECs,8, 9, 10 those with G3 NENs with a Ki-67 index <55% are known to respond poorly to these treatments.11 Therefore, adequate treatment choices for G3 NENs with well-differentiated morphology or low proliferative indices warrant further investigation. Retrospective studies have reported that temozolomide with or without capecitabine resulted in favorable response rates as high as 70% in patients with well- or moderately differentiated NENs.12 Although patients with Ki-67 indices of 20%-54% might benefit from the combination of capecitabine and temozolomide (CAPTEM),13,14 previous studies have included relatively few patients with G3 NENs, limiting the determination of its effectiveness.14, 15, 16 Therefore, this phase II trial was designed to prospectively evaluate the efficacy and safety of CAPTEM in patients with G3 NENs with Ki-67 indices <55%.
Methods
Study design and patients
This study was an open-label, single-arm, single-center phase II trial. Patients with histologically confirmed G3 NENs of the gastrointestinal tract or pancreatic origin were included if they (i) were aged ≥19 years with an Eastern Cooperative Oncology Group Performance Status of 0-2, (ii) had locally advanced unresectable or metastatic G3 NET or NEC, as determined by the 2017 WHO classification,7 with a Ki-67 labeling index >20% and <55%, (iii) had adequate bone marrow, renal, and hepatic functions, and (iv) had at least one measurable lesion according to RECIST version 1.1. Prior systemic therapy for the management of NENs was allowed.
The study protocol was approved by the Institutional Review Board of Asan Medical Center, Seoul, Republic of Korea (#2016-0579). This study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization guidelines for Good Clinical Practice. All participants provided written informed consent. This study was registered at ClinicalTrials.gov (NCT03079440).
Treatment
All study participants were administered oral capecitabine 750 mg/m2 twice daily on days 1-14, and oral temozolomide 200 mg/m2 once daily on days 10-14, of each 4-week cycle until disease progression or unacceptable toxicity. Absolute neutrophil counts ≥1.5 × 109/l and platelet counts ≥75 × 109/l were required for a new cycle of treatment. Capecitabine and temozolomide were generously provided by Ildong Pharmaceutical Co., Ltd., Seoul, Republic of Korea. Radiologic tumor responses were evaluated every two cycles (8 weeks). Concomitant anticancer therapy including somatostatin analogues, targeted agents, cytotoxic chemotherapy, or radionuclide therapy was not allowed.
Biomarker analysis
After the completion of the study, the pathology of enrolled patients was centrally reviewed by two academic pathologists (SMH, JHS) according to the revised WHO classification.17 O6-methyl-guanine methyltransferase (MGMT) protein expression and MGMT promotor methylation status were assessed. Formalin-fixed paraffin-embedded needle biopsy or surgically resected tissue specimens were divided into 4 μm thick sections and mounted on to silanized slides. Following heat-induced epitope retrieval by incubation with Cell Conditioning 1 buffer for 32 min in a BenchMark XT automatic immunostainer (Ventana Medical Systems, Tucson, AZ), the slides were incubated with a 1 : 40 dilution of mouse monoclonal anti-MGMT antibody (MT 3.1; Thermo Fisher Scientific, Waltham, MA), with bands detected using an OptiView DAB IHC Detection Kit (Ventana Medical Systems). Evaluation of the immunoreactivity was done by two pathologists (SMH, JHS), who were blinded to clinical information. MGMT expression was evaluated by expression scores (ranges from 0 to 300), calculated by multiplying nuclear staining intensity (negative as 0, weak as 1, moderate as 2, and strong intensity as 3) and percentage of stained cells (0 to 100). Endothelial cells were used as internal control staining. Loss of MGMT protein expression was considered negative if the MGMT expression score was ≤50.18
MGMT methylation status was assessed in 500 ng aliquots of tumor DNA by methylation-specific polymerase chain reaction (MS-PCR) using EZDNA Methylation-Lightning™ Kits (Zymo Research, Irvine, CA), according to the manufacturer's instructions, with human HCT116 non-methylated and methylated DNA standards (Zymo Research) used as negative and positive controls, respectively. The results were analyzed by comparing the bands representing PCR products of tumor samples with those of controls.
Post hoc correlative analysis with results of nuclear imaging
In this study, correlation analysis between nuclear imaging findings and efficacy outcomes was not preplanned. Considering the potential relevance of nuclear imaging in predicting the prognosis of patients with GEP-NETs,19 post hoc analysis was carried out for patients who underwent nuclear imaging (Gallium 68-DOTATOC [68Ga-DOTATOC] and/or 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) before treatment. Briefly, 68Ga-DOTATOC and 18F-FDG PET/CT images from the vertex to the upper thigh were acquired approximately 60 min after intravenous administration of 68Ga-DOTATOC 148 MBq (4 mCi) and 18F-FDG 0.14 mCi/kg, respectively (GE Discovery PET/CT 690, 690 Elite, or 710; GE Medical Systems, Milwaukee, WI). Maximum standardized uptake values (SUVmax) of the parametric PET images generated by the imaging software, as well as Krenning scores,20 were reviewed by a nuclear medicine physician (YK).
Statistical analysis
The primary endpoint was the overall response rate (ORR) assessed by RECIST 1.1. Secondary endpoints included progression-free survival (PFS), defined as the time between the initiation of study treatment and disease progression or death, whichever occurred first; overall survival (OS), defined as the time between the initiation of study treatment and death from any cause; and toxicity assessed by Common Terminology Criteria for Adverse Events, version 4.03. The disease control rate (DCR) was defined as the percentage of patients who achieved complete response (CR), partial response (PR), or stable disease (SD) by RECIST 1.1.
Based on previous literature, the response rate of NENs with Ki-67 <55% to the etoposide/cisplatin combination was considered 15%.11 Using the Fleming single-stage procedure,21 a sample size of 28 was calculated to provide 80% power to detect an improvement of ORR to 35% with CAPTEM at a one-sided significance level of 0.05. Assuming a dropout rate of 10%, a total of 31 patients were required. The full analysis set (FAS) was defined as all patients who met the inclusion criteria and received at least one dose of the study drug, whereas the per-protocol (PP) set was defined as all patients in the FAS with Ki-67 indices confirmed as <55% by central review.
Baseline characteristics and toxicities were assessed using a descriptive method. Survival outcomes were estimated using the Kaplan–Meier method and compared by log-rank tests. Univariable and multivariable analyses of factors associated with PFS and OS were carried out using the Cox regression method. All tests were two-sided, and a P value of <0.05 was considered statistically significant. All statistical analyses were carried out using R version 4.0.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
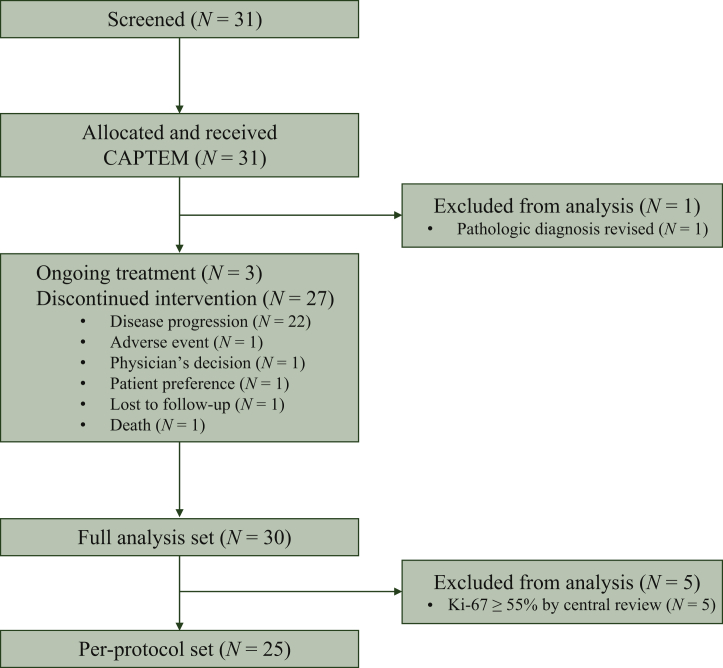
Between June 2017 and July 2020, 31 patients were screened; of these, 1 patient whose histologic diagnosis was revised other than NENs after pathologic review was excluded. The remaining 30 patients were included in the analysis (Figure 1). At data cut-off (27 December 2020), 3 of the 30 patients were still on treatment. The median follow-up duration was 19.2 months [95% confidence interval (CI), 11.9-27.3 months).
Figure 1.
CONSORT diagram.
CAPTEM, capecitabine and temozomide.
Patients
The baseline characteristics of the 30 patients in the study population are shown in Table 1. Of these 30 patients, 23 (76.7%) had well-differentiated G3 NETs, whereas 7 (23.3%) had poorly differentiated NECs. Primary tumor locations included the pancreas in 13 patients (43.3%), other non-pancreatic areas of the gastrointestinal tract in 12 (40.0%), and unknown primary with liver metastases in 5 (16.7%). At screening, all patients had documented NENs with Ki-67 indices <55%; however, post hoc central pathology review revealed that 5 patients (16.7%) had NENs with Ki-67 indices ≥55%, of whom 3 had large-cell NECs and 2 had small-cell NECs.
Table 1.
Baseline characteristics of the study population
| N = 30 | |
|---|---|
| Age, years | |
| Median (range) | 55 (27-75) |
| Sex, n (%) | |
| Male | 19 (63.3) |
| ECOG PS, n (%) | |
| 0 | 3 (10.0) |
| 1 | 27 (90.0) |
| Primary tumor location, n (%) | |
| Pancreas | 13 (43.3) |
| Stomach | 1 (3.3) |
| Small bowel | 4 (13.3) |
| Biliary tract | 4 (13.3) |
| Rectum | 3 (10.0) |
| Unknown primary with liver metastases | 5 (16.7) |
| Carcinoid symptomsb, n (%) | |
| Absent | 24 (80.0) |
| Presentb | 6 (20.0) |
| Histology by WHO 2019 classification, n (%) | |
| W/D NET G3 | 23 (76.7) |
| P/D NEC, large-cell | 2 (6.7) |
| P/D NEC, small-cell | 5 (16.7) |
| Ki-67 index, n (%) | |
| >20, <30 | 10 (33.3) |
| ≥30, <55 | 20 (66.6) |
| Ki-67 index, centrally reviewed, n (%) | |
| >20, <30 | 11 (36.7) |
| ≥30, <55 | 14 (46.7) |
| ≥55 | 5 (16.7) |
| Krenning score by 68Ga-DOTATOC PET/CT, n (%) | |
| 0-1 | 0 (0.0) |
| 2 | 2 (6.7) |
| 3-4 | 13 (43.3) |
| Unknown | 15 (50.0) |
| Disease status at enrollment, n (%) | |
| Locally advanced, inoperable | 1 (3.3) |
| Initially metastatic | 18 (60.0) |
| Recurrent after curative local treatment | 11 (36.7) |
| Number of metastatic sites, n (%) | |
| 0 | 1 (3.3) |
| 1 | 13 (43.3) |
| ≥2 | 16 (53.3) |
| Sites of metastasis, n (%) | |
| Liver | 27 (90.0) |
| Distant lymph node | 11 (36.7) |
| Peritoneum | 3 (10.0) |
| Lung | 4 (13.3) |
| Previous treatment, n (%) | |
| Surgery | 11 (36.7) |
| Radiotherapy | 1 (3.3) |
| Somatostatin analog | 5 (16.7) |
| PRRT | 0 (0.0) |
| Cytotoxic chemotherapy | 18 (60.0) |
| Previous chemotherapy regimen, n (%) | |
| Etoposide and cisplatin | 14 (46.7) |
| Cyclophosphamide, doxorubicin, and vincristine | 4 (13.3) |
| Other cytotoxic chemotherapy | 6 (20.0) |
| Everolimus | 1 (3.3) |
| Previous lines of palliative systemic treatmenta, n (%) | |
| 0 | 11 (36.7) |
| 1 | 12 (40.0) |
| ≥2 | 7 (23.3) |
| MGMT protein expression by IHC, n (%) | N = 26 |
| Loss of expression (≤50) | 14 (53.8) |
| Expressed (>50) | 12 (46.2) |
| MGMT MS-PCR, n (%) | N = 25 |
| Methylated | 24 (96.0) |
| Not methylated | 1 (4.0) |
CT, computed tomography; ECOG PS, European Cooperative Oncology Group performance status; IHC, immunohistochemistry; MGMT, O6-methyl-guanine methyltransferase; MS-PCR, methylation-specific polymerase chain reaction; P/D, poorly differentiated; PET, positron emission tomography; PRRT, peptide receptor radionuclide therapy; W/D, well differentiated; WHO, World Health Organization.
Includes somatostatin analogs, chemotherapy, and PRRT.
Includes one patient with Zollinger-Ellison syndrome and five patients with diarrhea.
Efficacy
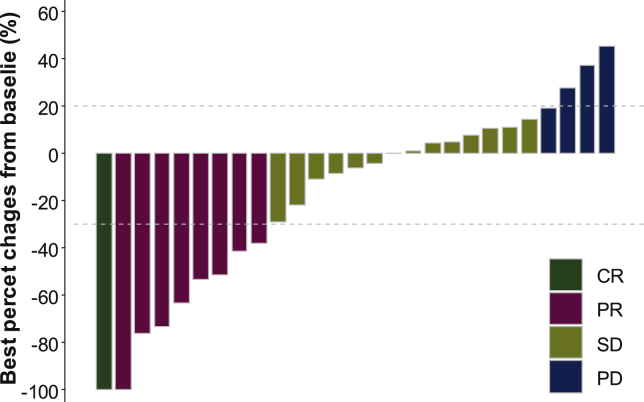
The median number of treatment cycles in the FAS was 6 (range, 1-43). The best overall response was CR in 1 patient (3.3%), PR in 8 (26.7%), SD in 14 (46.7%), and progressive disease (PD) in 4 (13.3%) (Figure 2). Two patients were not evaluated, including one who was lost to follow-up and one who stopped treatment by the patient's will before the first response evaluation. The ORR was 30.0% (9/30) and DCR was 76.7% (23/30).
Figure 2.
Waterfall plot showing the best percentage changes in the sum of target lesions from baseline.
CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
ORR (30.8% versus 29.4%, P = 1.000) and DCR (76.9% versus 76.5%, P = 1.000) did not differ significantly in patients with primary pancreatic and non-pancreatic tumors. Both ORR (14.3% versus 34.8%, P = 0.393) and DCR (42.9% versus 87.0%, P = 0.033) were lower in patients with poorly differentiated NEC than in those with well-differentiated G3 NET. Also, ORR and DCR were lower in patients with Ki-67 ≥55% per central pathology review than patients with Ki-67 <55% (for patients with Ki-67 >20% and <30% versus ≥30% and <55% versus ≥55%; ORR were 18.2% versus 50.0% versus 0%, P = 0.079, and DCR were 72.7% versus 92.9% versus 40.0%, P = 0.049) (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100119).
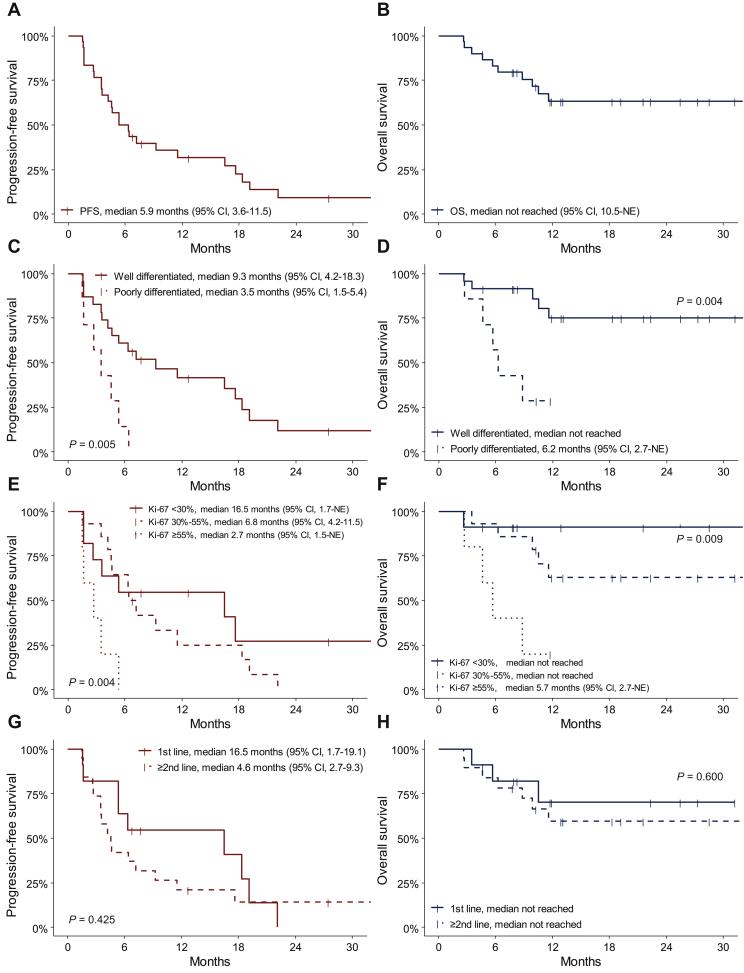
In the FAS population, the median PFS was 5.9 months (95% CI, 3.6-11.5 months; Figure 3A), and the median OS was not reached [95% CI, 10.5 months-not estimated (NE); Figure 3B]. OS rates at 6 and 12 months were 83.1% (95% CI, 64.0-92.6%) and 63.2% (95% CI, 42.1-78.4%), respectively. Patients with well-differentiated NETs showed significantly better survival outcomes than those with poorly differentiated NECs; median PFS [9.3 months (95% CI, 4.2-18.3 months) versus 3.5 months (95% CI, 1.5-5.4 months), P = 0.005; Figure 3C] and OS [not reached versus 6.2 months (95% CI, 2.7 months-NE), P = 0.004; Figure 3D]. High Ki-67 index was associated with poor PFS and OS; median PFS and OS were 2.7 months (95% CI, 1.5 months-NE) and 5.7 months (95% CI, 2.7 months-NE), respectively, in patients with Ki-67 index ≥55%; 6.8 months (95% CI, 4.2-11.5 months) and not reached, respectively, in patients with Ki-67 indices 30%-55%; and 16.5 months (95% CI, 1.7 months-NE) and not reached, respectively, in patients with Ki-67 <30% (P = 0.004 for PFS, P = 0.009 for OS) (Figure 3E and F). Median PFS was numerically longer in patients who received CAPTEM as first-line than as second- or greater-line treatment [16.5 months (95% CI, 1.7-19.1 months) versus 4.6 months (95% CI, 2.7-9.3) months], but the difference was not statistically significant (P = 0.425). Median OS was not reached in both groups (P = 0.600) (Figure 3G and H).
Figure 3.
Kaplan–Meier curves of (A) progression-free survival, (B) overall survival, (C) PFS by differentiation, (D) OS by differentiation, (E) PFS by Ki-67 index, (F) OS by Ki-67 index, (G) PFS by line of treatment, and (H) OS by line of treatment.
CI, confidence interval; NE, not estimated; OS, overall survival; PFS, progression-free survival.
In the PP analysis set of 25 patients who had Ki-67 indices <55% per central pathology review, the median PFS was 7.2 months (95% CI, 4.6-17.6 months), and the median OS was not reached (95% CI, 11.6 months-NE). ORR was 36.0% (9/25), and DCR was 84.0% (21/25).
Safety
Adverse events are summarized in Table 2. During study treatment, eight patients (26.7%) experienced grade 3-4 adverse events. The most common grade 3-4 adverse events were thrombocytopenia in three patients (10.0%), anemia in two (6.7%), and nausea in two (6.7%). Doses of CAPTEM were delayed in four patients (13.3%) and reduced in seven patients (23.3%). Most common reasons for requiring dose modification were nausea (N = 4, 13.3%), thrombocytopenia (N = 3, 10.0%), and neutropenia (N = 2, 6.7%). No treatment-related deaths occurred.
Table 2.
Adverse events
| Adverse events | Any grade | Grade 3-4 |
|---|---|---|
| Any | 28 (93.3) | 8 (26.7) |
| Hematologic, n (%) | ||
| Neutropenia | 2 (6.7) | 1 (3.3) |
| Anemia | 4 (13.3) | 2 (6.7) |
| Thrombocytopenia | 4 (13.3) | 3 (10.0) |
| Febrile neutropenia | 0 (0.0) | 0 (0.0) |
| Non-hematologic, n (%) | ||
| Anorexia | 4 (13.3) | 0 (0.0) |
| Nausea | 9 (30.0) | 2 (6.7) |
| Constipation | 7 (23.3) | 0 (0.0) |
| Diarrhea | 3 (10.0) | 0 (0.0) |
| Gastrointestinal hemorrhage | 1 (3.3) | 1 (3.3) |
| Fatigue | 6 (20.0) | 0 (0.0) |
| Fever | 3 (10.0) | 1 (3.3) |
| Hand-foot syndrome | 5 (16.7) | 0 (0.0) |
| Mucositis | 1 (3.3) | 0 (0.0) |
| Increased AST | 5 (16.7) | 1 (3.3) |
| Increased ALT | 4 (13.3) | 1 (3.3) |
| Increased ALP | 1 (3.3) | 0 (0.0) |
| Increased bilirubin | 2 (6.7) | 0 (0.0) |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Correlative analysis
MGMT protein expression was assessed by immunochemistry in 26 patients with available tumor tissue samples. Median MGMT expression score was 35 (range: 0-300). Fourteen patients (53.8%) were considered to have a loss of MGMT expression with scores of ≤50. MGMT promoter methylation results using MS-PCR were available for 25 patients. All but one patient had negative results. The one patient who was positive for MGMT promoter methylation also had a loss of MGMT expression.
Assessment of the 26 patients with MGMT immunohistochemistry results showed that neither ORR (21.4% versus 25.0%, P = 1.000) nor DCR (78.6% versus 75.0%, P = 1.000) differed significantly in patients with loss of MGMT expression and those without. Median PFS [4.1 months (95% CI, 1.7-16.5 months) versus 6.3 months (95% CI, 1.7-18.3 months), P = 0.712] and median OS (not reached in both groups, P = 0.332) also did not differ significantly in patients with loss of MGMT expression and those without.
Before study treatment, 15 and 20 patients underwent 68Ga-DOTATOC and 18F-FDG PET/CT scanning, respectively. ORR (46.2% versus 0%, P = 0.486) and DCR (84.6% versus 0%, P = 0.057) tended to be higher in patients with Krenning scores of ≥3 than in those with scores ≤2. In addition, median PFS was significantly longer [17.6 months (95% CI, 3.6-22.1 months) versus 3.1 months (95% CI, 1.7 months-NE), P = 0.031] and median OS was numerically longer [not reached versus 6.2 months (95% CI, 6.2 months-NE), P = 0.150] in patients with Krenning scores of ≥3 than in those with scores ≤2. Median SUVmax of tumors on 68Ga-DOTATOC PET/CT was higher in patients who achieved disease control (CR, PR, or SD) than in those with PD {28.9 [interquartile range (IQR), 20.9-64.5] versus 11.8 (IQR, 7.1-16.5)}, but the difference was not statistically significant (P = 0.103). Similarly, median SUVmax of the tumors on 18F-FDG PET/CT did not significantly differ in patients with disease control and PD [5.3 (IQR, 3.7-8.6) versus 11.7 (IQR, 11.6-11.8), P = 0.157]. In addition, the SUVmax of 68Ga-DOTATOC PET/CT and 18F-FDG PET/CT did not show statistically significant associations with the ORR, DCR, PFS, or OS (Supplementary Tables S1 and S2, available at https://doi.org/10.1016/j.esmoop.2021.100119).
Multivariable analysis of factors prognostic for survival
Factors associated with PFS and OS were assessed by univariable and multivariable Cox regression analyses (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2021.100119). Univariable analysis showed that poorly differentiated histology and high Ki-67 index were associated with shorter PFS and OS, and high Krenning score was associated with longer PFS. By contrast, primary tumor site (pancreatic versus non-pancreatic), MGMT protein expression, MGMT promoter methylation status, and SUVmax on 68Ga-DOTATOC and 18F-FDG PET/CT were not associated with PFS and OS.
Multivariable analysis showed that poorly differentiated histology was significantly associated with poorer PFS [hazard ratio (HR), 6.95 (95% CI, 1.45-33.35), P = 0.015] and tended to be associated with poorer OS, although not statistically significant [HR, 11.71 (95% CI, 0.64-212.65), P = 0.096].
Discussion
In this prospective phase II study evaluating the efficacy and safety of CAPTEM for patients with G3 NENs with Ki-67 <55%, the ORR was 30% including one patient who achieved CR, and the DCR was 77%. The median PFS was 5.9 months and the median OS was not reached. No new safety issues were observed.
Since the definition of G3 NETs and NECs has been revised recently, few data are available on the clinical outcomes by treatment modalities in these patients, particularly in patients with G3 NETs. Although platinum-based chemotherapy has been widely used for the management of G3 GEP-NENs, efficacy outcomes for G3 NENs with well-differentiated histology or Ki-67 <55% were limited, with an ORR of 15%-17% and a median PFS of 2.4-4 months.11,22 CAPTEM has been investigated mainly in patients with G1-2 NENs, and limited evidence was available for patients with G3 NENs with Ki-67 <55%, with previous studies being retrospective in design and having small sample sizes.13,14,23 The present study showed an ORR of 30.0% in the FAS population and 36.0% in the PP population which included only those patients with Ki-67 indices <55% per central pathology review. These outcomes were comparable to those of two prior phase II trials, which showed an ORR of 33%-45% in patients with G1-2 pancreatic NETs.24,25 In addition, the current analysis showed favorable median PFS of 5.9 months in the FAS population, and of 7.2 months in the PP population. In comparison, platinum-based therapy in the NORDIC NEC trial resulted in a median PFS of 4 months in patients with G3 NENs and Ki-67 indices <55%.11 These findings suggest that CAPTEM may provide better efficacy outcomes than platinum-based chemotherapy for patients with G3 GEP-NENs with Ki-67 <55%.
Subgroup analyses showed that tumor differentiation was associated with efficacy outcomes in patients with CAPTEM. ORR tended to be higher (34.8% versus 14.3%), and median PFS (9.3 versus 3.5 months) and OS (not reached versus 6.2 months) significantly longer, in patients with well-differentiated NETs than poorly differentiated NECs. These outcomes indicate that CAPTEM should be prioritized for patients with well-differentiated G3 NETs considering its improved outcomes in this subgroup. In contrast, in patients with poorly differentiated G3 NECs with Ki-67 <55%, platinum-based chemotherapy would be an appropriate frontline option considering their clinical outcomes were not improved with CAPTEM compared with historical data on platinum-based chemotherapy. Considering the discrepancies in the efficacy outcomes on the same chemotherapy regimen, poorly differentiated NEC and well-differentiated NET should be separately investigated in future clinical trials.
One of the potential biomarkers for temozolomide-based chemotherapy is MGMT, which has been studied well in patients with high-grade gliomas.26 In NENs, the ability of MGMT status to predict the efficacy of temozolomide-based chemotherapy is yet unclear.18,27,28 In this study, neither MGMT protein expression nor MGMT promoter methylation status was associated significantly with tumor response or survival outcomes.
Post hoc analyses of the correlation between CAPTEM efficacy and the results of 68Ga-DOTATOC and 18F-FDG PET/CT found that response rates were higher and PFS longer in patients with high (≥3) than low (≤2) Krenning scores on 68Ga-DOTATOC PET/CT, which reflect tumor expression of somatostatin receptors. This finding might be limited because it was not preplanned analysis and only a subset of patients were available for this analysis. However, considering the tumor heterogeneity of advanced NENs in terms of Ki-67 indices or somatostatin receptor expressions, nuclear imaging modalities might provide the valuable information for therapeutic decisions when added to pathologic findings.29,30
The safety profile in our study was consistent with prior studies of CAPTEM, and there were no new safety issues.16,31 The most common grade 3-4 adverse events were thrombocytopenia in 10% of these patients, and no patient discontinued the study treatment because of adverse events.
Our study has several limitations. This study was designed as a single-center, non-randomized, single-arm trial. Because of the small sample size, statistical power was insufficient to assess factors predictive of the efficacy of CAPTEM. Nevertheless, to our knowledge, this study is the first prospective trial to explore outcomes of CAPTEM in patients with G3 GEP-NENs.
In conclusion, CAPTEM was effective and well tolerated in patients with G3 GEP-NENs with Ki-67 indices <55%. In patients with well-differentiated G3 GEP-NENs with Ki-67 <55%, CAPTEM showed favorable efficacy outcomes when compared with historical data on platinum-based chemotherapy.
Acknowledgements
Ildong Pharmaceutical Co., Ltd. generously provided capecitabine and temozolomide for this study.
Funding
This work was funded in part by Ildong Pharmaceutical Co., Ltd.
Disclosure
CY has received research grants (no grant number) and honoraria from Ipsen and Ildong Pharmaceutical Co., Ltd. All other authors have declared no conflicts of interest.
Contributor Information
B.-Y. Ryoo, Email: ryooby@amc.seoul.kr.
C. Yoo, Email: yooc@amc.seoul.kr.
Supplementary data
References
- 1.Yao J.C., Hassan M., Phan A. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35 825 cases in the United States. J Clin Oncol. 2008;26(18):3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 2.Dasari A., Shen C., Halperin D. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3(10):1335–1342. doi: 10.1001/jamaoncol.2017.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oronsky B., Ma P.C., Morgensztern D., Carter C.A. Nothing but NET: a review of neuroendocrine tumors and carcinomas. Neoplasia. 2017;19(12):991–1002. doi: 10.1016/j.neo.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Eeden S., Quaedvlieg P.F., Taal B.G., Offerhaus G.J.A., Lamers C.B.H.W., van Velthuysen M.-T.F. Classification of low-grade neuroendocrine tumors of midgut and unknown origin. Hum Pathol. 2002;33(11):1126–1132. doi: 10.1053/hupa.2002.129204. [DOI] [PubMed] [Google Scholar]
- 5.Bosman F.T., World Health Organization., International Agency for Research on Cancer . 4th ed. International Agency for Research on Cancer; Lyon: 2010. WHO classification of tumours of the digestive system. [Google Scholar]
- 6.Yachida S., Vakiani E., White C.M. Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol. 2012;36(2):173–184. doi: 10.1097/PAS.0b013e3182417d36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lloyd R.V., Osamura R.Y., Klöppel G., Rosai J., editors. WHO Classification of Tumours of Endocrine Organs. IARC; Lyon, France: 2017. [Google Scholar]
- 8.Strosberg J.R., Coppola D., Klimstra D.S. The NANETS consensus guidelines for the diagnosis and management of poorly differentiated (high-grade) extrapulmonary neuroendocrine carcinomas. Pancreas. 2010;39(6):799–800. doi: 10.1097/MPA.0b013e3181ebb56f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoo C., Oh C.R., Kim S.-T. Systemic treatment of advanced gastroenteropancreatic neuroendocrine tumors in Korea: literature review and expert opinion. Cancer Res Treat. 2021;53(2):291–300. doi: 10.4143/crt.2020.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pavel M., Öberg K., Falconi M. Gastroenteropancreatic neuroendocrine neoplasms: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(7):844–860. doi: 10.1016/j.annonc.2020.03.304. [DOI] [PubMed] [Google Scholar]
- 11.Sorbye H., Welin S., Langer S.W. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol. 2013;24(1):152–160. doi: 10.1093/annonc/mds276. [DOI] [PubMed] [Google Scholar]
- 12.Strosberg J.R., Fine R.L., Choi J. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer. 2011;117(2):268–275. doi: 10.1002/cncr.25425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas K., Voros B.A., Meadows-Taylor M. Outcomes of capecitabine and temozolomide (CAPTEM) in advanced neuroendocrine neoplasms (NENs) Cancers (Basel). 2020;12(1):206. doi: 10.3390/cancers12010206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sahu A., Jefford M., Lai-Kwon J., Thai A., Hicks R.J., Michael M. CAPTEM in metastatic well-differentiated intermediate to high grade neuroendocrine tumors: a single centre experience. J Oncol. 2019;2019:9032753. doi: 10.1155/2019/9032753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fine R.L., Gulati A.P., Krantz B.A. Capecitabine and temozolomide (CAPTEM) for metastatic, well-differentiated neuroendocrine cancers: the pancreas center at Columbia University experience. Cancer Chemother Pharmacol. 2013;71(3):663–670. doi: 10.1007/s00280-012-2055-z. [DOI] [PubMed] [Google Scholar]
- 16.de Mestier L., Walter T., Evrard C. Temozolomide alone or combined with capecitabine for the treatment of advanced pancreatic neuroendocrine tumor. Neuroendocrinology. 2020;110(1-2):83–91. doi: 10.1159/000500862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagtegaal I.D., Odze R.D., Klimstra D. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76(2):182–188. doi: 10.1111/his.13975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cros J., Hentic O., Rebours V. MGMT expression predicts response to temozolomide in pancreatic neuroendocrine tumors. Endocr Relat Cancer. 2016;23(8):625–633. doi: 10.1530/ERC-16-0117. [DOI] [PubMed] [Google Scholar]
- 19.Kim Y.I., Yoo C., Oh S.J. Tumour-to-liver ratio determined by [(68)Ga]Ga-DOTA-TOC PET/CT as a prognostic factor of lanreotide efficacy for patients with well-differentiated gastroenteropancreatic-neuroendocrine tumours. EJNMMI Res. 2020;10(1):63. doi: 10.1186/s13550-020-00651-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krenning E.P., Valkema R., Kooij P.P. Scintigraphy and radionuclide therapy with [indium-111-labelled-diethyl triamine penta-acetic acid-D-Phe1]-octreotide. Ital J Gastroenterol Hepatol. 1999;31(suppl 2):S219–S223. [PubMed] [Google Scholar]
- 21.A'Hern R.P. Sample size tables for exact single-stage phase II designs. Stat Med. 2001;20(6):859–866. doi: 10.1002/sim.721. [DOI] [PubMed] [Google Scholar]
- 22.Heetfeld M., Chougnet C.N., Olsen I.H. Characteristics and treatment of patients with G3 gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer. 2015;22(4):657–664. doi: 10.1530/ERC-15-0119. [DOI] [PubMed] [Google Scholar]
- 23.Chatzellis E., Angelousi A., Daskalakis K. Activity and safety of standard and prolonged capecitabine/temozolomide administration in patients with advanced neuroendocrine neoplasms. Neuroendocrinology. 2019;109(4):333–345. doi: 10.1159/000500135. [DOI] [PubMed] [Google Scholar]
- 24.Fine R.L., Gulati A.P., Tsushima D. Prospective phase II study of capecitabine and temozolomide (CAPTEM) for progressive, moderately, and well-differentiated metastatic neuroendocrine tumors. J Clin Oncol. 2014;32(suppl 3):179. [Google Scholar]
- 25.Kunz P.L., Catalano P.J., Nimeiri H. A randomized study of temozolomide or temozolomide and capecitabine in patients with advanced pancreatic neuroendocrine tumors: a trial of the ECOG-ACRIN Cancer Research Group (E2211) J Clin Oncol. 2018;36(suppl 15):4004. doi: 10.1200/JCO.22.01013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hegi M.E., Diserens A.-C., Gorlia T. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 27.Walter T., van Brakel B., Vercherat C. O6-Methylguanine-DNA methyltransferase status in neuroendocrine tumours: prognostic relevance and association with response to alkylating agents. Br J Cancer. 2015;112(3):523–531. doi: 10.1038/bjc.2014.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cives M., Ghayouri M., Morse B. Analysis of potential response predictors to capecitabine/temozolomide in metastatic pancreatic neuroendocrine tumors. Endocr Relat Cancer. 2016;23(9):759–767. doi: 10.1530/ERC-16-0147. [DOI] [PubMed] [Google Scholar]
- 29.Frilling A., Sotiropoulos G.C., Radtke A. The impact of 68Ga-DOTATOC positron emission tomography/computed tomography on the multimodal management of patients with neuroendocrine tumors. Ann Surg. 2010;252(5):850–856. doi: 10.1097/SLA.0b013e3181fd37e8. [DOI] [PubMed] [Google Scholar]
- 30.Binderup T., Knigge U., Loft A., Federspiel B., Kjaer A. 18F-fluorodeoxyglucose positron emission tomography predicts survival of patients with neuroendocrine tumors. Clin Cancer Res. 2010;16(3):978–985. doi: 10.1158/1078-0432.CCR-09-1759. [DOI] [PubMed] [Google Scholar]
- 31.Owen D.H., Alexander A.J., Konda B. Combination therapy with capecitabine and temozolomide in patients with low and high grade neuroendocrine tumors, with an exploratory analysis of O(6)-methylguanine DNA methyltransferase as a biomarker for response. Oncotarget. 2017;8(61):104046–104056. doi: 10.18632/oncotarget.22001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.