Abstract
Development of bactericides and fungicides in terms of isolation and identification of substances is an important area of research. Presently, under the concept of integrated pest management, all possible plant pest and disease control methods are integrated to minimize the excessive use of synthetic chemicals and also the incidence of disease.
The potential of various essential oils against Gram-positive and Gram-negative bacteria and fungi is being actively investigated in various laboratories across the world. Rice the most important crop, suffers from huge yield losses due to blast and blight diseases. Most of the labs have focussed to use transgenic approaches, the use of environmentally friendly natural products, as disease control strategies. In this context, we propose to evaluate the antimicrobial properties of essential oils and their ability to control diseases of rice. Seven Essential oils from seven different plants were selected for the study. The antimicrobial activity was assessed in terms of their antibacterial activity towards non-pathogenic bacteria and pathogenic drug resistant bacteria by means of their ability to sensitize the drug resistant bacteria in plasmid curing and, ß-lactamase inhibition and as antifungal agents. In conclusion, out of the seven essential oils used, lemongrass, palm rosa and eucalyptus were found to be good antimicrobial agents.
Keywords: Plant essential oils, Anti microbial activity, Natural products, Gram + bacteria, Gram, Bacterial, Rice diseases
Plant essential oils, Anti microbial activity, Natural products, Gram + bacteria, Gram - Bacterial, Rice diseases.
1. Introduction
Plants produce a large and diverse array of organic compounds that appear to have no direct function in growth and development, and these are named as secondary metabolites. They differ from primary metabolites of plants such as chlorophyll, amino acids, nucleotides, simple carbohydrates etc. in having a restricted distribution in the plant kingdom. Plant secondary metabolites can be divided mainly into three chemically distinct groups: Terpenes, Phenolic compounds and Nitrogen-containing secondary products, such as alkaloids, which are primarily biosynthesized from amino acids.
The biological properties displayed by plants have been attributed to their ability to synthesize such compounds [1]. The ability of these compounds to form complexes with certain enzymes or directly inhibit enzymes, toxic effects on membrane structure and integrity, quenching of free radicals, stimulation of natural killer cells in the humans, modulation of steroid concentrations etc. could be among important mechanisms underlying their biological activity. However, quite often, exact mechanisms in particular cases remain to be clarified [2, 3, 4].
One of the important biological properties of these essential oils is their antimicrobial nature, and in many cases this activity was found to be due to the presence of active monoterpene constituents [5, 6]. Another property of the plant products that is generating considerable interest is their ability to control plant diseases. Till now chemical control remains the main measure to reduce the incidence of plant diseases. Two serious problems, development of resistance by plant pathogenic fungi & bacteria and the presence of high level toxic residues in agricultural products, hamper against the effective use of the chemical fungicides and bactericides in controlling plant pathogenic microbes. Hence the exploitation of natural substances such as essential oils, safer to consumers and the environment, for the control of plant diseases is presently looked upon. Reports by Tahir et al., [7] and Letessier et al., [8] showed that the application of 0.05% hyssops oil can reduce the rust infection of broad bean caused by Uromyces viciae-fabae, while application of 0.05% hyssops oil, post inoculation, reduced the infection of powdery mildew of barley seedlings caused by Blumeria graminis f. sp. Hordei.
Many plant essential oils were tested for their antimicrobial activities. The essential oils of Artemisia afra, Pteronia incana and Rosmarinus officinalis were found to display antimicrobial activity against 41 microbial strains, which includes food spoilage and common human/plant pathogenic bacterial and yeast strains. The essential oil of hyssop was found to inhibit plant pathogenic fungi like Pyrenophora avenae and Pyricularia oryzae, in in vitro conditions. Further it was found to inhibit the germination of conidia and uredospores of Botrytis fabae and Uromyces viciae-fabae respectively (Letessier et al., 2001). The essential oil of Melaleuca alternifolia (Tea tree oil), which is well characterized and found to contain approximately a hundred terpenes and their related alcohols was found to possess antibacterial, antifungal, antiviral and anti-inflammatory properties in vitro [9].
The essential oils used in the present study were known for their antimicrobial activity. The essential oil of Cymbopogon citratus (lemon grass) was shown to have appreciable activity against some Gram + bacteria [10], and the essential oil of C. nardus (citronella) was shown to have broad spectrum fungistatic activity against Aspergillus, Chaetomium, Myrothecium, Pencillium and Trichoderma spp. [11, 12]. Further, there is no agreement among various groups of scientists on the preferential ability of plant essential oils to inhibit Gram + and Gram - bacteria. For these reasons there is a need to study the antimicrobial properties of plant essential oils comprehensively. The main objectives of the present study are to explore the antibacterial activity of essential oils and their components against Gram- & Gram- non-pathogenic bacteria, identification of major components of selected essential oils by gas chromatography, to determine the efficacy of essential oils and their major components against human pathogenic drug resistant bacteria, to study the effect of essential oils and their major components on curing of drug resistance plasmid pBR 322 in bacteria (E. coli HB101); and the activity of β-lactamase in drug resistant bacteria.
2. Materials & methods
2.1. Bacterial cultures
The following Gram - and Gram + non-pathogenic cultures were used for testing the antibacterial activity of the essential oils and their major components.
2.1.1. Gram negative bacteria (non-pathogenic)
E. coli NCIM – 2089; E. coli HB 101/pBR 322 – ampr, tetr; Salmonella typhimurium NCIM – 2501; Proteus vulgaris NCIM – 2027; Pseudomonas aeruginosa NCIM – 2036.
2.1.2. Gram positive bacteria (non-pathogenic)
Staphylococcus aureus NCIM – 2079; Streptococcus faecalis NCIM – 2080; Bacillus subtilis NCIM – 2063; Pure cultures of the above bacteria were obtained from National Chemical Laboratory (NCL), Pune, except for E. coli HB 101 from University of Hyderabad.
Plant Pathogenic Bacteria: Xanthomonas oryzae and X. malvacearum were also used to study the antibacterial activity of essential oils and their major components. X. Malvacearum was obtained from NCL, Pune, while X. oryzae was isolated from blight infected leaves collected from Directorate of Rice Research (DRR), Hyderabad.
All the bacterial cultures, except plant pathogenic bacteria X. oryzae and X. alvacearum, were maintained in nutrient broth at 37 °C. Xanthomonas oryzae and X. malvacearum were maintained on Sucrose peptone medium at 28 °C.
2.2. Fungal cultures
The following fungal cultures were used to assess the antifungal activity of the essential oils and their major components.
Aspergillus niger, Fusarium oxysporum, Fusarium udum, Magnaporthe grisea
Magnoporthe grisea was isolated from infected blast leaves collected from DRR, Hyderabad. Other three fungal cultures were obtained from the Dept. of Botany, Osmania University, Hyderabad.
The fungal cultures were maintained on PDA slants. In case of M. grisea the incubation was for 7 days at 28 °C. The cultures were sub-cultured three times before using for antifungal assays.
2.3. Plant essential oils
Seven essential oils were selected for the present study. These oils were extracted by steam distillation from Cymbopogon flexuosus (lemon grass-LG), C. martini (palm rosa-PR), Eucalyptus citridora (EC), Tagetus minuta (TM), Pelargonium sp (geranium- GE), C. winterianus (citronella-CI) and Mentha arvensis (MA). These essential oils contain terpenoids and their derivatives. All the essential oils used in the present study were kindly supplied by Central Institute for Medicinal and Aromatic Plants, Boduppal, Hyderabad.
2.3.1. Major components of essential oils
Citral, citronellal and geraniol were obtained from M/s Sigma Chemicals.
The concentration of essential oil/the components provided by manufacturers was determined by Semenova et al., [13] and then the final concentration was brought to 10 mg/ml and then used for the experiments.
2.4. Determination of minimum bactericidal concentration of the essential oils and their major components using non-pathogenic bacteria
Non-pathogenic Gram + and Gram - bacterial strains and plant pathogenic bacteria were used for measuring minimum bactericidal concentration (MBC) of the test compounds by serial dilution method [14, 15]. The test compounds include the essential oils and major components.
Test compounds in 1% Tween 20 solution were added in to the nutrient broth and thoroughly shaken. Two-fold serial dilution of the test compound was carried out in the nutrient broth/sucrose peptone broth. To each test tube 104–105 cells/ml of actively growing bacterial culture in log phase was inoculated. The culture tubes were incubated at 37 °C for 24h and at 28 °C for 48h in case of plant pathogenic bacteria. After the incubation, a loop full of these treated cultures was streaked on the nutrient agar/sucrose peptone agar plates. The plates were incubated and were checked for the growth of bacteria, and the Minimum Bactericidal Concentration (MBC) of each test compound was determined. The MBC was expressed in μl/ml (v/v).
Control: Control cultures (in absence of test compounds) in presence of 1% Tween 20 solution were maintained to check for growth of the organism. MBC of commonly used antibiotics viz ciprofloxacin, gentamicin, ampicillin, streptomycin and chloramphenicol was determined to compare the effectiveness of test compounds.
2.5. Determination of time course of lethal action of test compounds
Initially, the MBC of the test compounds and ampicillin was determined. Then the bacteria (E. coli, X. oryzae & Staphylococcus) were treated with test compounds at MBC values. At regular time intervals, aliquots of treated culture were drawn, diluted appropriately and plated on nutrient agar/sucrose peptone agar for colony development. The number of colonies was counted, and the number of viable cells was estimated.
2.6. Effect of test compounds (essential oils and their major components) on drug resistant pathogenic bacteria
The antibacterial activity of the essential oils and their major components on drug resistant pathogenic bacteria was tested by filter paper disc method [16, 17]. Actively growing log phase cultures were mixed in soft agar (nutrient broth with 1% agar) and plated. Two different dilutions of the essential oils and their major components were used for testing the antibacterial efficacy. The essential oils and their major components were loaded onto to filter paper discs prepared from Whatman No. 1 filter paper. The discs were then placed on the agar medium containing the culture, and incubated for 24 h at 37 °C. The diameter of zone of growth inhibition was recorded. The same procedure was followed to screen the antibacterial activity of essential oils and their major components against non-pathogenic bacteria initially.
2.7. Gas chromatography analysis of essential oils
The five essential oils viz., lemon grass, palm rosa, eucalyptus, geranium, citronella were chromatographed using a Varian star CP 3800 gas chromatograph equipped with a ZB wax capillary column (30 m length x 0.25 mm i.d. x 0.25 μdf), FID, ECD and PFPD with auto sampler. The carrier gas was helium, by a rate of 1 ml/min. The make up gas was helium and make up flow was 25 ml/min. Air flow was kept at 300 ml/min. The FID temperature was 250 °C (range-12), and the injector temperature was 225 °C (split ratio-100:5). Column temperature was initially kept for 2 min at 70 °C, and was then gradually increased at a rate of 5 °C/min to 275 °C and held for 12min. Total run time was 55min [18].
2.8. Effect of essential oils and their components on curing of drug resistant (pBR 322 in E. coli HB101)
Curing experiment (or elimination of plasmid) was carried out according to the method described by Kresge et al., [19]. Briefly, the drug resistant E. coli HB101 containing pBR 322 Ampr & Tetr was treated by at sub-inhibitory concentration of the test compounds, and plasmid was extracted and electrophoresed using agarose.
2.9. Effect of essential oils and their components on β - lactamase enzyme activity
Inhibition of β-lactamase in bacterial cultures was studied as per the procedure of Bush and Bradfold [20]. Whatman No. 1 filter paper strips (7 × 4mm) were soaked in 1% starch solution, dried and resoaked in ampicillin solution for 10′ and spread smoothly in a petridish. E. coli HB101 treated with the test compounds at sub-inhibitory concentrations for overnight were streaked on nutrient agar plate, and incubated for 24 h for colony development. Colonies were transferred to the surface of starch- ampicillin paper strips and were spread over an area of 2–3 mm, then incubated at 37 °C for 30′ to facilitate the production of enzyme stained with Gram's iodine solution for 30 s and checked for the presence/absence of the zone of decolorization around the culture streak.
2.10. Test for development of resistance of bacteria against test compounds
Three essential oils viz., lemon grass, palm rosa and eucalyptus were tested against E. coli by exposing the bacterium to sub-MIC (1/2 of MIC) of each extract for 18 h at 37 °C, for 3 passages and then the culture was streaked on nutrient agar plates containing MIC of the extract and then incubated for 24 h at 37 °C. In this manner, the culture was tested 10 times making a total of 30 passages and assessed for the development of resistance to the given extract.
2.11. Antifungal activity of essential oils and their major components
The essential oils and major components were used for testing the antifungal ability. Initially, the compounds were screened at various concentrations by disc diffusion assay. After screening the test samples for antifungal activity, they were tested in terms of inhibition of biomass production by the fungus.
NOTE: All the experiments were conducted in triplicates and average values were presented in the results. As the data obtained was huge, SD was not included. However an SD of ±15% of average value was obtained.
3. Results
3.1. Screening of plant essential oils for anti bacterial activity against pathogenic bacteria
Seven plant essential oils were screened for their anti bacterial activity using paper disc method. All the seven test essential oils showed antibacterial activity against at least few bacteria tested. The essential oils of lemon grass, palm rosa and eucalyptus showed a very good antibacterial activity against all the tested bacteria. The essential oils of geranium and citronella showed good antibacterial activity against all the bacteria tested at high concentration. The essential oils of tagetus and mentha are active against only few bacteria and both showed very low zones of inhibition (Table 1).
Table 1.
Antibacterial activity of essential oils determined by disc diffusion assay.
| Essential Oil → |
LG |
PR |
Ec |
Ge |
Cit |
Ta |
Me |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacterial Strain ↓ | 4 μl | 8 μl | 4 μl | 8 μl | 4 μl | 8 μl | 4 μl | 8 μl | 4 μl | 8 μl | 4 μl | 8 μl | 4 μl | 8 μl |
| E.coli | 11 | 22 | 9 | 17 | 8 | 18 | 7 | 14 | 7 | 11 | 5 | 5 | NI | NI |
| E.coli HB 101 | 12 | 24 | 8 | 16 | 8 | 16 | 6 | 13 | 6 | 10 | 4 | 6 | NI | NI |
| S.typhimurium | 9 | 19 | 11 | 17 | 6 | 11 | 7 | 13 | 6 | 11 | NI | 8 | 4 | 6 |
| P.vulgaris | 7 | 18 | 6 | 12 | 5 | 12 | 5 | 12 | 5 | 8 | 5 | 9 | 4 | 6 |
| P.aeruginosa | 7 | 16 | 6 | 11 | 7 | 11 | 8 | 10 | 4 | 6 | NI | NI | NI | 6 |
| S.aureus | 11 | 24 | 10 | 21 | 7 | 17 | 10 | 19 | 6 | 9 | 6 | 10 | NI | 7 |
| S.faecalis | 9 | 22 | 8 | 18 | 8 | 19 | 9 | 14 | 5 | 11 | 4 | 3 | NI | NI |
| B.subtilis | 6 | 11 | 5 | 12 | 6 | 11 | NI | 6 | NI | 5 | NI | NI | NI | NI |
| X.oryzae | 14 | 22 | 10 | 18 | 9 | 18 | 7 | 15 | 8 | 11 | 6 | 9 | NI | 5 |
| X.malvacearum | 18 | 24 | 10 | 14 | 12 | 18 | 7 | 15 | 6 | 10 | 7 | 10 | NI | 6 |
Note:
1. The above values represent diameter of the zone of inhibition (in mm) at 4μ and 8μl concentration of essential oil/disc (Average value of triplicate experiment).
2. NI – No Zone of inhibition.
3. Lg-lemon grass oil; Pr-palm rosa oil; Ec-eucalyptus oil; Ge-geranium oil; Cit-citronella oil; Tatagetus oil; Me-mentha oil.
3.2. MBC of plant essential oils against non- pathogenic bacteria
Among the Gram - bacteria, highest activity was recorded against E. coli, E. coli HB101 and Proteus vulgaris. Among the Gram + bacteria both S. aureus and S. faecalis were inhibited at 0.98 μl/ml concentration (Table 2).
Table 2.
Determination of Minimum Bactericidal Concentration of Essential oils.
| Essential Oil → |
LG | PR | Ec | Ge | Cit | Ta | Me |
|---|---|---|---|---|---|---|---|
| Bacterial Strain ↓ | |||||||
| E.coli | 0.98 | 31.25 | 15.625 | 31.25 | 62.5 | 250 | NA |
| E.coli HB 101 | 0.98 | 31.25 | 15.625 | 31.25 | 62.5 | 250 | NA |
| S.typhimurium | 1.9 | 15.625 | 31.25 | 125 | 125 | 250 | 250 |
| P.vulgaris | 0.98 | 3.9 | 31.25 | 31.25 | 62.5 | 125 | 250 |
| P.aeruginosa | 1.9 | 7.8 | 15.625 | 31.25 | 125 | 250 | 500 |
| S.aureus | 0.98 | 3.9 | 15.625 | 15.625 | 62.5 | 31.25 | 250 |
| S.faecalis | 0.98 | 3.9 | 15.625 | 31.25 | 62.5 | 125 | NA |
| B.subtilis | 7.8 | 3.9 | 15.625 | 250 | 125 | 250 | NA |
| X.oryzae | 1.9 | 1.9 | 7.8 | 7.8 | 31.25 | 62.5 | 62.5 |
| X.malvacearum | 19 | 1.9 | 15.625 | 15.625 | 31.25 | 125 | 125 |
Note:
1. MBC values were represented in μl of essential oil/ml of the liquid medium.
2. NA- No Activity.
3. The values represent average of three experiments.
4. Lg-lemon grass oil; Pr-palm rosa oil; Ec-eucalyptus oil; Ge-geranium oil; Cit-citronella oil; Tatagetus oil; Me-mentha oil.
All the tested Gram + bacteria were inhibited by Palm rosa oil at a minimum concentration of 3.9 μl/ml concentration. Among the Gram - bacteria Proteus vulgaris and Pseudomonas aureus were more sensitive than others (Table 2).
The essential oil of eucalyptus inhibited all Gram + bacteria at a minimum concentration of 15.625 μl/ml, Gram - bacteria, drug sensitive E. coli, drug resistant E. coli HB101 and P. aeruginosa were inhibited at a minimum concentration of 15.625 μl/ml (Table 2).
The essential oil of geranium inhibited S. aureus and S. Faecalis at a minimum concentration of 15.625 μl/ml and 31.25 μl/ml respectively. However B. subtilis was not very sensitive (Table 2).
The essential oil of citronella displayed only moderate activity against both Gram + and Gram - bacteria. The essential oil of tagetus showed marginal bacterial activity. The essential oil of mentha showed very poor antibacterial activity. The minimum bactericidal concentration of plant essential oils was also checked against two plant pathogenic bacteria viz. X. oryzae and X. malvacearum. All the plant essential oils showed antibacterial activity against both the bacteria tested. Both X. oryzae and X. malvacearum showed a very high sensitivity towards lemon grass and palm rosa essential oils. Essential oils of eucalyptus and geranium also showed a very high antibacterial activity against both X. oryzae and X. malvacearum. Essential oil of citronella inhibited both the organisms at a minimum concentration of 31.25 μl/ml. Essential oils of tagetus and menthe were effective against X. oryzae and X.malvacearum (Table 2).
3.3. Antibacterial activity of major components of essential oils against non-pathogenic bacteria
The antibacterial activity results of major components by disc diffusion method are presented in Table 3 MBCs were presented in Table 4. Among the components tested citral, a major constituent of lemon grass showed the highest activity followed by geraniol and citronellal.
Table 3.
Antibacterial activity of the major components determined by disc diffusion assay.
| Essential Oil → |
Citral |
Geraniol |
Citronellal |
|||
|---|---|---|---|---|---|---|
| Bacterial Strain ↓ | 4 μl | 8 μl | 4 μl | 8 μl | 4 μl | 8 μl |
| E.coli | 9 | 15 | 6 | 11 | 5 | 8 |
| E.coli HB 101 | 8 | 16 | 6 | 12 | 6 | 8 |
| S.typhimurium | 10 | 17 | 9 | 14 | 8 | 10 |
| P.vulgaris | 7 | 12 | 5 | 8 | 5 | 7 |
| P.aeruginosa | 7 | 13 | 5 | 7 | 5 | 6 |
| S.aureus | 10 | 16 | 9 | 14 | 8 | 11 |
| S.faecalis | 8 | 14 | 9 | 12 | 8 | 10 |
| B.subtilis | 5 | 10 | NI | 7 | NI | 8 |
| X.oryzae | 12 | 20 | 10 | 16 | 8 | 10 |
| X.malvacearum | 14 | 22 | 11 | 17 | 7 | 12 |
Note:
1. The above values represent diameter of the zone of inhibition (in mm) at 4μl and 8μl concentration of major component/disc (Average value of triplicate experiment).
2. NI – No Zone of inhibition.
Table 4.
Determination of minimum bactericidal concentration (MBC) of major components.
| Essential Oil → |
Citral | Geraniol | Citronellal |
|---|---|---|---|
| Bacterial Strain ↓ | |||
| E.coli | 3.9 | 31.25 | 62.5 |
| E.coli HB 101 | 3.9 | 31.25 | 62.5 |
| S.typhimurium | 7.8 | 62.5 | 62.5 |
| P.vulgaris | 3.9 | 31.25 | 125 |
| P.aeruginosa | 7.8 | 31.25 | 125 |
| S.aureus | 3.9 | 15.625 | 31.25 |
| S.faecalis | 3.9 | 31.25 | 62.5 |
| B.subtilis | 15.625 | 125 | 125 |
| X.oryzae | 1.9 | 7.8 | 15.625 |
| X.malvacearum | 3.9 | 15.625 | 31.25 |
Note:
1. MBC (Minimum Bactericidal Concentration) values were represented in μl of major component/ml of the liquid medium.
2. The values represent average of three experiments.
Citral, a major constituent of lemon grass oil showed very high bactericidal activity.
Geraniol, showed bactericidal activity against the Gram + bacteria Staphylococcus aureus, Streptococcus faecalis and Bacillus subtilis (Table 3).
The Gram + bacteria S. aureus, S. faecalis and B. Subtilis was inhibited by Citronellal at a minimum concentration of 31.25 μl/ml, 62.5 μl/ml and 125 μl/ml respectively. Among Gram - bacteria, drug sensitive E. coli, drug resistant E. coli HB101, S. typhimurium, P. vulgaris and P. aeruginosa were inhibited (Table 4).
All the three components showed good bactericidal activity against both X. oryzae and X. malvacearum (Table 4). The results suggest that citral shows the highest antibacterial activity followed by geraniol and citronellal.
3.4. Comparison of antibacterial activity of essential oils their major components and antibiotics against non-pathogenic bacteria and plant pathogenic bacteria
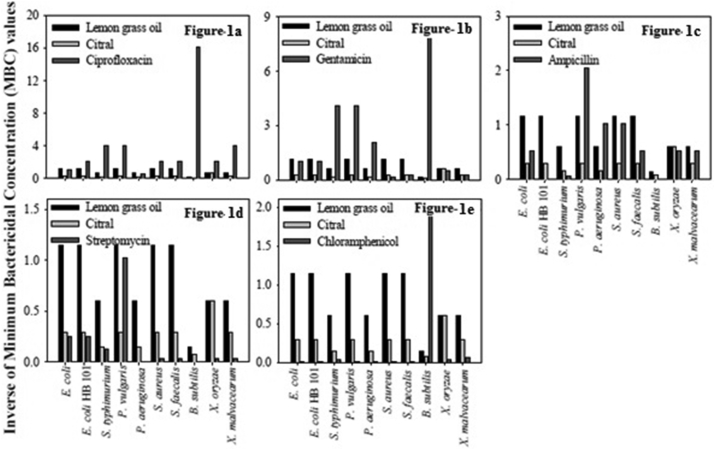
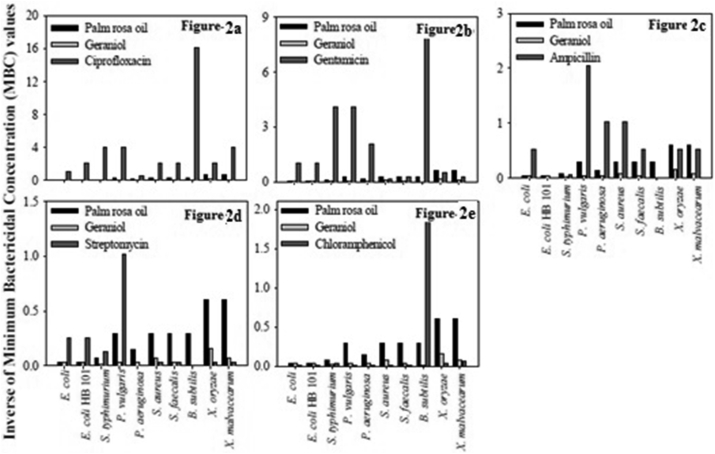
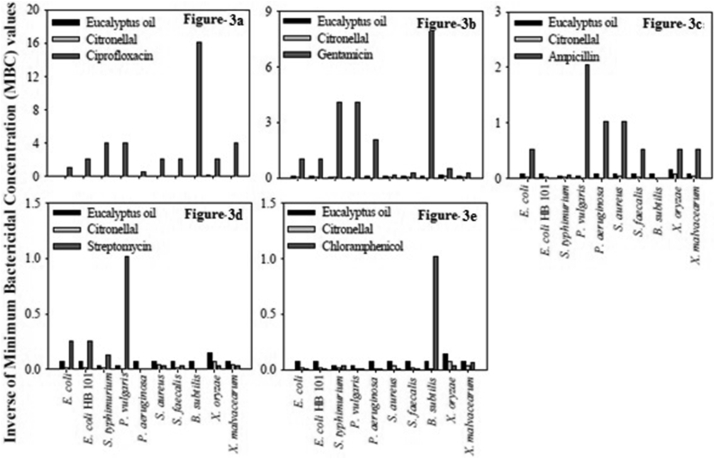
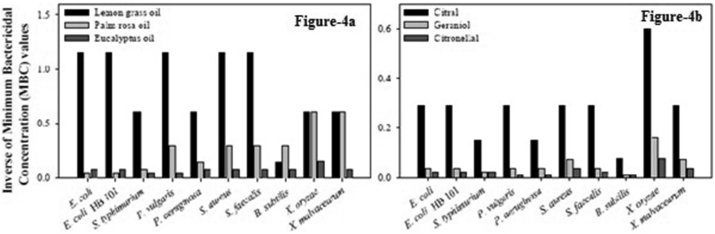
For comparison purpose, the minimum bactericidal concentrations in μg/ml (w/v) of some commonly used antibiotics viz., ciprofloxacin, gentamicin, ampicillin, streptomycin, chloramphenicol was determined and the results are presented in Table 5. The antibacterial activity of the antibiotics, the three essential oils viz. lemon grass, palm rosa, eucalyptus and their major components viz. citral, geraniol and citronellal was compared and illustrated in Figures 1, 2, and 3. From the results it is clear that among the essential oils under investigation the best oils in terms of antibacterial activity are lemon grass, palm rosa and eucalyptus oils (Figure 4a and b).
Table 5.
Determination of MBC of Common Antibiotics Against the Gram- Positive and Gram-Negative Bacteria (including X. oryzae and X. malvacearum).
| Essential Oil → |
Ciprofloxacin | Gentamicin | Ampicillin | Streptomycin | Chloramphenicol |
|---|---|---|---|---|---|
| Bacterial Strain ↓ | |||||
| E.coli | 0.98 | 0.98 | 1.9 | 3.9 | 250 |
| E.coli HB 101 | 0.49 | 0.98 | NA | 3.9 | 250 |
| S.typhimurium | 0.245 | 0.245 | 15.625 | 7.8 | 31.25 |
| P.vulgaris | 0.245 | 0.245 | 0.49 | 0.98 | 125 |
| P.aeruginosa | 1.9 | 0.49 | 0.98 | 250 | 125 |
| S.aureus | 0.49 | 7.8 | 0.98 | 31.25 | 250 |
| S.faecalis | 0.49 | 3.9 | 1.9 | 31.25 | 125 |
| B.subtilis | 0.062 | 0.122 | 125 | 125 | 0.98 |
| X.oryzae | 0.49 | 1.9 | 1.9 | 31.25 | 31.25 |
| X.malvacearum | 0.245 | 3.9 | 1.9 | 62.5 | 15.625 |
Note:
1. The antibacterial activity of the antibiotics was in the order of Ciprofloxacin > Gentamycin > Ampicillin > Streptomycin > Chloramphenicol.
2. MBC (Minimum Bactericidal Values) were represented in μg of antibiotic/ml of the liquid medium.
3. NA- No Activity.
4. The values represent average of three experiments.
Figure 1.
Comparison of MBCs of lemon grass oil and its major component citral. a to e: Comparison of minimum bactericidal concentrations of lemon grass oil and its major component citral, with antibiotics viz- ciprofloxacin, gentamicin, ampicillin, streptomycin and chloramphenicol respectively, against various Gram + and Gram- bacteria. The inverses of MBC values are represented in the figures to have a better presentation.
Figure 2.
Comparison of MBCs of Palm Rosa oil and its major component Geraniol. a to e: Comparison of minimum bactericidal concentration values of palm rosa oil and its major component geraniol, with antibiotics viz- ciprofloxacin, gentamicin, ampicillin, streptomycin and chloramphenicol respectively against various Gram + & Gram –ve bacteria. The inverses of MBC values are represented in the figures to have a better presentation.
Figure 3.
Comparison of MBCs of Eucalyptus oil and its major component Citronellal. a to e: Comparison of MBC values of Eucalyptus oil and its major component citronellal, with antibiotics viz., ciprofloxacin, gentamicin, ampicillin, streptomycin and chloramphenicol respectively, against various Gram + and Gram –ve bacteria. The inverses of MBC values are represented in the figures to have a better presentation.
Figure 4.
Comparison of minimum bactericidal concentration values of essential oils and their major components against various Gram + and Gram –ve bacteria. a: Comparison of minimum bactericidal concentration values of essential oils lemon grass, palm rosa, eucalyptus against various Gram + and Gram –ve bacteria. The inverses of MBC values are represented in the figures to have a better presentation. b: Comparison of minimum bactericidal concentration (MBC) values of major components citral, geraniol and citronellal against various Gram + and Gram –ve bacteria. The inverses of MBC values are represented in the figures to have a better presentation.
3.5. Antibacterial activity of essential oils and their major components on drug resistant pathogenic bacteria
Lemon grass essential oil showed best antibacterial activity against all the ten drug resistant pathogenic bacteria tested. The zone of inhibition against all the five E. coli isolates at 4 μl concentration was between 9-11 mm, while at 8 μl the inhibition was 21mm against EC-2 and EC-5. Against EC-1, EC-3 and EC-4 isolates the zone of inhibition was 16 mm, 18 mm and 17 mm respectively. Against Staphylococcus aureus isolates, lemon grass oil at 4μl showed 8–11 mm zones of inhibition and at 8 μl showed 21–22 mm against SA-1, SA-3 and SA-4 isolates, while against SA-2 the zone of inhibition was 17 mm. Lemon grass essential oil against drug resistant Pseudomonas sp. isolate showed a zone of inhibition of 7 mm and 12 mm at 4 μl per disc and 8 μl per disc concentrations respectively. The palm rosa oil also showed good antibacterial activity against all the ten pathogenic antibacterial isolates (Table 6).
Table 6.
Antibacterial activity of essential oils determined by disc diffusion method against drug resistant pathogenic bacteria.
| Essential Oil → |
LG |
PR |
Ec |
Ge |
Cit |
Ta |
Me |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacterial Strain ↓ | 4 μl | 8 μl | 4 μl | 8 μl | 4 μl | 8 μl | 4 μl | 8 μl | 4 μl | 8 μl | 4 μl | 8 μl | 4 μl | 8 μl |
| EC-1 | 9 | 16 | 8 | 16 | 8 | 17 | 7 | 13 | 6 | 11 | 4 | 7 | NI | NI |
| EC-2 | 11 | 21 | 9 | 17 | 7 | 15 | 6 | 10 | 5 | 11 | 4 | 6 | NI | NI |
| EC-3 | 9 | 18 | 8 | 15 | 9 | 18 | 6 | 10 | 6 | 12 | NI | 6 | NI | NI |
| EC-4 | 9 | 17 | 7 | 15 | 6 | 14 | 6 | 15 | NI | NI | 5 | 7 | NI | NI |
| EC-5 | 10 | 21 | 8 | 16 | 9 | 17 | 7 | 14 | 5 | 9 | NI | 5 | NI | NI |
| Pseudomonas sp. | 7 | 12 | 8 | 11 | 6 | 11 | 4 | 9 | 4 | 8 | 5 | 8 | 4 | 6 |
| SA-1 | 11 | 22 | 10 | 18 | 8 | 19 | 8 | 15 | 5 | 8 | NI | NI | 4 | 8 |
| SA-2 | 8 | 17 | 9 | 18 | 8 | 16 | 7 | 15 | 4 | 9 | NI | NI | NI | 7 |
| SA-3 | 11 | 22 | 10 | 18 | 7 | 15 | 8 | 17 | 5 | 11 | NI | 5 | NI | 6 |
| SA-4 | 10 | 21 | 8 | 15 | 7 | 19 | 7 | 15 | 4 | 10 | NI | NI | 4 | 7 |
Note:
1. The above values represent diameter of the zone of inhibition (in mm) at 4 μl and 8 μl concentration of essential oil/disc (Average value of triplicate experiment).
2. N = No Zone of inhibition.
The oils geranium and citronella showed moderate antibacterial activity against all the ten drug resistant pathogenic bacterial isolates. The geranium oil showed good bactericidal activity against S. aureus isolates, recording more than a diameter of 15mm zone of inhibition. The essential oils of tagetus and mentha showed a very poor anti bacterial activity against the ten isolates (Table 6). The major component citral showed good antibacterial activity against all the ten isolate.s The active component geraniol also showed good antibacterial activity. The active component citronellal showed moderate activity (Table 7).
Table 7.
Antibacterial activity of major components determined by disc diffusion method against drug resistant pathogenic bacteria.
| Essential Oil → |
Citral |
Geraniol |
Citronellal |
|||
|---|---|---|---|---|---|---|
| Bacterial Strain ↓ | 4 μl | 8 μl | 4 μl | 8 μl | 4 μl | 8 μl |
| EC-1 | 8 | 17 | 6 | 14 | 5 | 11 |
| EC-2 | 9 | 16 | 7 | 13 | 6 | 9 |
| EC-3 | 7 | 14 | 5 | 10 | 8 | 11 |
| EC-4 | 7 | 15 | 7 | 11 | 5 | 8 |
| EC-5 | 9 | 18 | 6 | 14 | 5 | 11 |
| Pseudomonas sp. | 6 | 14 | 4 | 8 | NI | 8 |
| SA-1 | 10 | 18 | 7 | 16 | 6 | 12 |
| SA-2 | 9 | 16 | 7 | 16 | 6 | 13 |
| SA-3 | 10 | 18 | 6 | 14 | 6 | 13 |
| SA-4 | 9 | 17 | 6 | 15 | 5 | 14 |
Note:
1. The above values represent diameter of the zone of inhibition (in mm) at 4 μl and 8 μl concentration of major component/disc (Average value of triplicate experiment).
2. N = No Zone of inhibition.
3.6. Time course of lethal action of essential oils, major components on bacteria
The essential oils and their major components at MBC values were used to determine the time taken for the lethal action against E. coli, X. oryzae and S. aureus. To compare the efficacy of these test compounds with those of known antibiotics like ampicillin, the time course of lethal action of ampicillin against E. coli, X. oryzae and S. aureus was also studied.
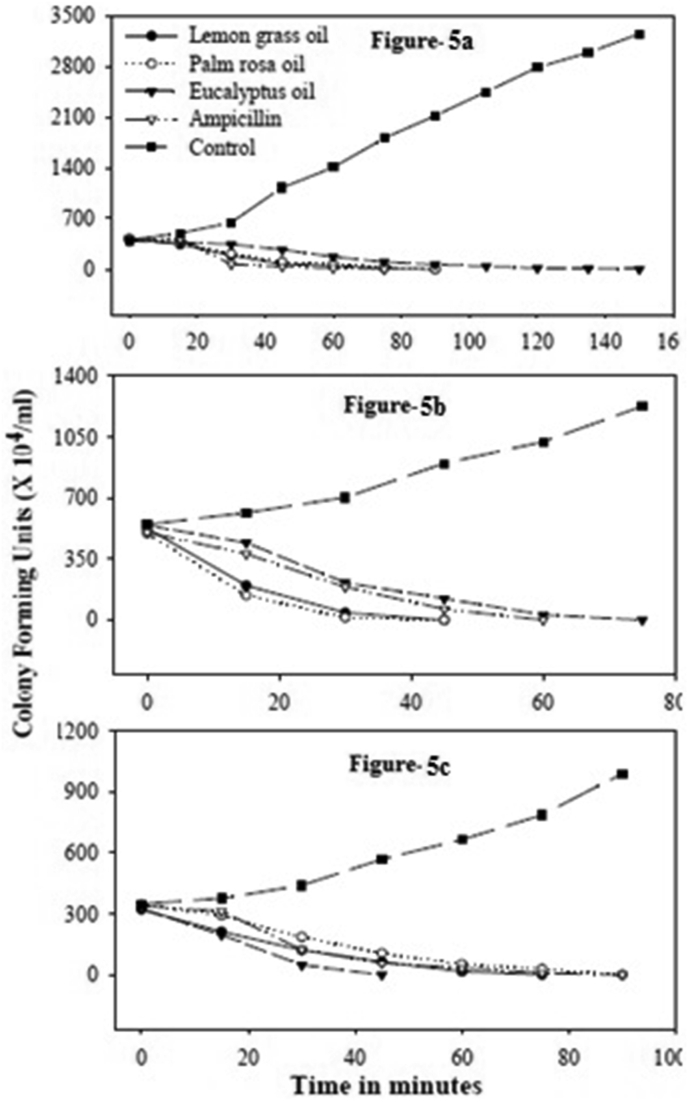
The essential oil of lemon grass was very active. 100 % inhibition of E. Coli was observed at 90 min and for 75 min for X. Oryzae. Against the Gram + S. aureus, lemon grass oil took 45 min for 100 % inhibition.
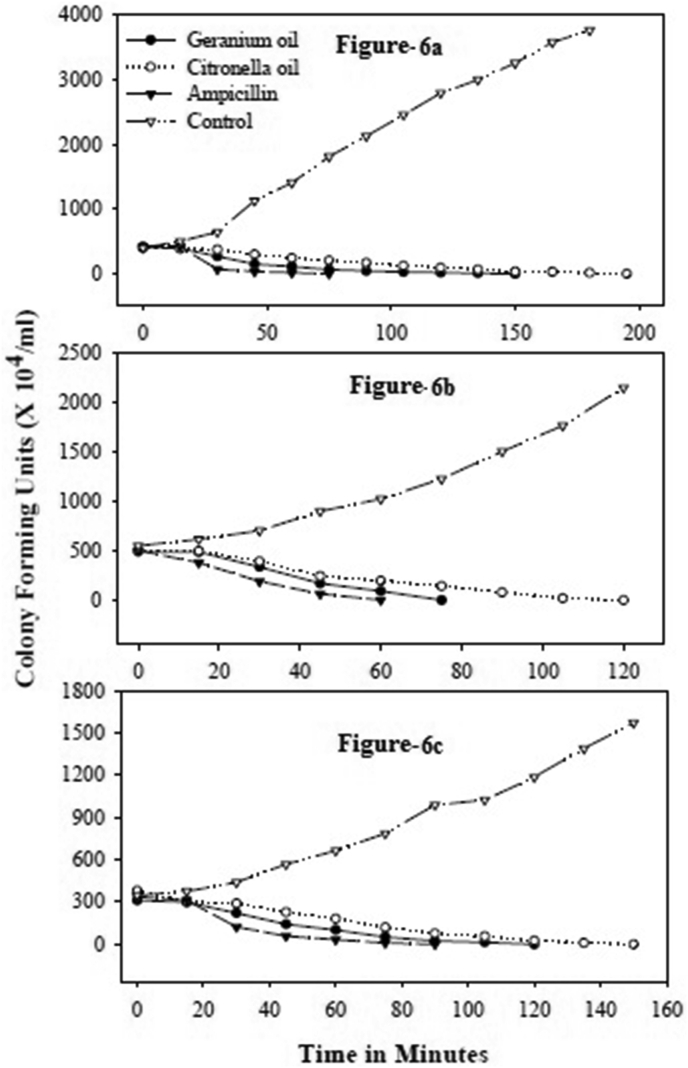
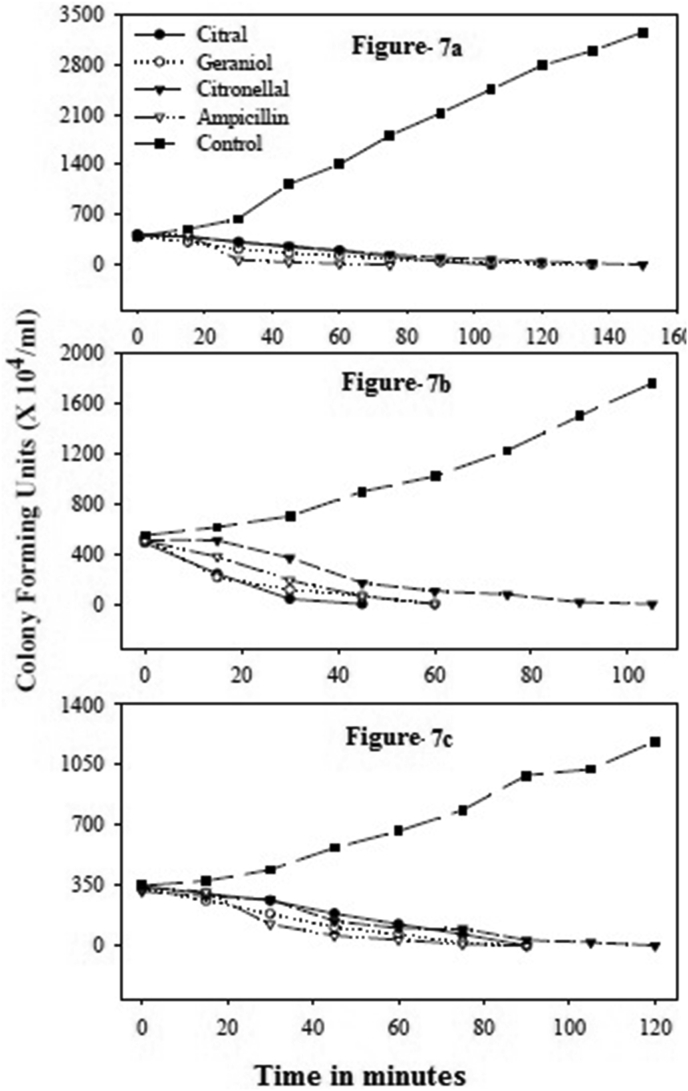
Palm rosa oil took 90 min for 100% inhibition of E. coli and X. oryzae, while it took 45 min for 100 % inhibition of S. aureus (Figure 5a–c). In both the above cases the lethal activity was observed from 15 min. The eucalyptus oil took 150 min for 100% inhibition of E. coli. In presence of eucalyptus oil there was no drastic reduction in colony forming units (CFU) of E. coli for the first 30 min. The essential oil of geranium took 150 min for 100% inhibition of E. Coli, 120 min for X. oryzae and 75 min for S. aureus. The essential oil of citronella took 195 min for E. Coli, 150 min and 120 min for X. Oryzae and S. aureus respectively for 100% inhibition (Figure 6a–c). Among the major components citral showed a very fast activity. It showed 100% inhibition of E. coli, X. oryzae and S. aureus in 105 min, 90 min and 45 min respectively (Figure 7a–c).
Figure 5.
Time course of lethal action of lemon grass, palm rosa and eucalyptus. a–c: Time course of lethal action of essential oils lemon grass, palm rosa and eucalyptus against E. coli (a), S. aureus (b) and X. oryzae (c).
Figure 6.
Time course of lethal action of essential oils geranium and citronella. a–c: Time course of lethal action of essential oils geranium and citronella against E.coli (a), S. aureus (b) and X. oryzae (c).
Figure 7.
Time course of lethal action of citral, geraniol and citronellal. a–c: Time course of lethal action of major components citral, geraniol and citronellal against E. coli (a), S. aureus (b) and X. oryzae (c).
3.7. Effect of essential oils and their major components in curing pBR322 (ampr, tetr) in E. coli HB101
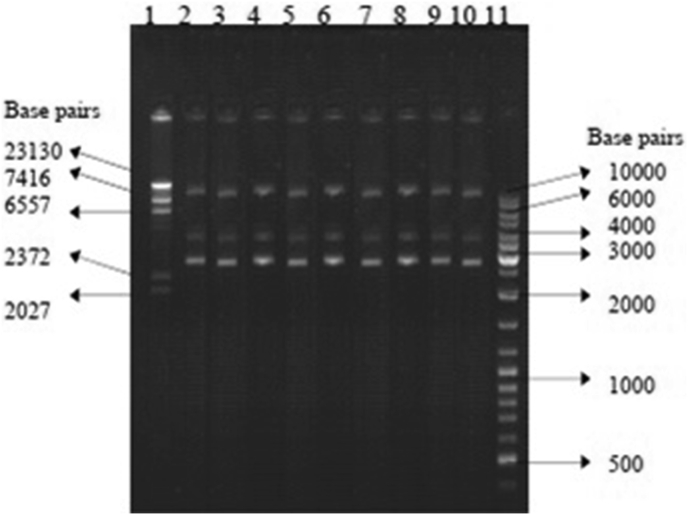
To determine the ability to eliminate or cure the plasmid pBR322 from the bacterium E. coli HB101, the essential oils of lemon grass, palm rosa, eucalyptus, geranium and citronella which showed good antibacterial activity were selected at their sub-lethal concentrations (half the MIC). Similarly, the three major components present in these oils were also tested for their curing abilities. All the essential oils tested and their major components did not eliminate or cure the plasmid (Figure 8) (which confers ampicillin and tetracycline drug resistant phenotype) from the host bacterium E. coli HB101 (Table 8).
Figure 8.
Ability of essential oils & their major components in curing pBR322. Lane 1: DNA/Hind III Digest (Bangalore Genei), Lane 2: pBR 322 from untreated E. coli HB101 (Control), Lane 3: pBR322 from lemon grass oil treated E. coli HB101, Lane 4: pBR322 from palm rosa oil treated E. coli HB101, Lane 5: pBR322 from eucalyptus oil treated E. coli HB101, Lane 6: pBR322 from geranium oil treated E. coli HB101, Lane 7: pBR322 from citronella oil treated E. coli HB101, Lane 8: pBR322 from citral treated E. coli HB101, Lane 9: pBR322 from geraniol treated E. coli HB101, Lane 10: pBR322 from citronellal treated E. coli HB101, Lane 11: 1 kb DNA ladder Mix (MBI Fermentas).
Table 8.
Curing Activity of Essential Oils and Their Major Components on pBR 322 (ampr, tetr) in E. coli HB101.
| Essential Oil | Curing Activity | Remarks |
|---|---|---|
| Lemon Grass | Not Observed | Plasmid not eliminated |
| Palm Rosa | Not Observed | Plasmid not eliminated |
| Eucalyptus | Not Observed | Plasmid not eliminated |
| Geranium | Not Observed | Plasmid not eliminated |
| Citronella |
Not Observed |
Plasmid not eliminated |
|
Major Components | ||
| Citral | Not Observed | Plasmid not eliminated |
| Geraniol | Not Observed | Plasmid not eliminated |
| Citronellal | Not Observed | Plasmid not eliminated |
| Control | Observed | Plasmid Present |
Note:
1. Sub – inhibitory concentrations of the test compounds were used to test the curing ability. The culture was treated for 24 h.
2. Curing of plasmid was not observed with the test compounds.
3. Control/untreated culture showed the presence of pBR 322 plasmid.
3.8. Effect of essential oils and their major components on β -lactamase activity in E. coli HB101
The ability of the essential oils lemon grass, palm rosa, eucalyptus, geranium, citronella and their major components to inhibit the β-lactamase activity of E. coli HB101 at sub-lethal concentration was studied. None of the test compounds could inhibit the β-lactamase activity (Table 9).
Table 9.
Effect of Essential Oils and Their Major Components on ß-lactamase activity in E. coli HB101.
| Essential Oil | Enzyme Inhibition | Remarks |
|---|---|---|
| Lemon Grass | Not Observed | No inhibition of β-lactamase |
| Palm Rosa | Not Observed | No inhibition of β-lactamase |
| Eucalyptus | Not Observed | No inhibition of β-lactamase |
| Geranium | Not Observed | No inhibition of β-lactamase |
| Citronella |
Not Observed |
No inhibition of β-lactamase |
|
Major Components | ||
| Citral | Not Observed | No inhibition of β-lactamase |
| Geraniol | Not Observed | No inhibition of β-lactamase |
| Citronellal | Not Observed | No inhibition of β-lactamase |
Note:
1. Sub-inhibitory concentrations of the test compounds were used to testing the inhibition of enzyme activity. The culture was treated for 24 h.
2. Approximately 100 colonies of each treated culture were tested for enzyme activity.
3. None of the test compounds could inhibit β-lactamase activity.
3.9. Test for development of resistance of bacteria against essential oils
Five essential oils viz. lemon grass, palm rosa, eucalyptus, geranium and citronella (which showed high antibacterial activity) were tested against E. coli, S. aureus and X. oryzae to study whether these bacteria develop resistance against the essential oils after prolonged exposure. Interestingly none of the three bacterial cultures developed resistance to these essential oils up to thirty passages in the presence of the oils.
3.10. Antifungal activity of essential oils and their major components
-
a)
Antifungal activity of the seven plant essential oils and their major components against A. niger, F. udum, F. oxysporum and M. grisea by the disc diffusion method as described by Elgayyar et al., [16].
Five of the seven essential oils viz. lemon grass, palm rosa, eucalyptus, geranium and citronella showed antifungal activity against all the four fungi tested (Table 10). Further, it was observed that essential oils of lemon grass and eucalyptus showed delayed sporulation started at the end of 7th and 6th day respectively in A. Niger, compared to control where, sporulation started by the end of the fourth day. The two essential oils of tagetus and mentha did not inhibit A. niger, F. udum and F. oxysporum. However, these two essential oils showed antifungal activity against M. Grisea Among all these, lemon grass oil was the most potent one in terms of inhibiting the growth of fungi around the disc loaded with this oil (Table 10; Figure 9). All the three major components viz., citral, citronellal and geraniol were found to be effective in inhibiting the growth of all the four fungi. Citral was found to be the most potent of all the three major components tested (Table 11).
-
b)
Effect of Essential Oils and Major components on Biomass production by Fungi
Table 10.
Antifungal activity of essential oils determined by disc diffusion assay.
| Essential Oil → |
LG |
PR |
Ec |
Ge |
Cit |
Ta |
Me |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacterial Strain ↓ | 4 μl | 8 μl | 4 μl | 8 μl | 4 μl | 8 μl | 4 μl | 8 μl | 4 μl | 8 μl | 4 μl | 8 μl | 4 μl | 8 μl |
| A.niger | 18 | 28 | 12 | 18 | 12 | 22 | NI | 8 | 6 | 12 | NI | NI | NI | NI |
| F.udum | 13 | 18 | 16 | 19 | 14 | 20 | NI | 8 | 6 | 8 | NI | NI | NI | NI |
| F.oxysporum | 15 | 18 | 14 | 16 | 14 | 18 | NI | 8 | NI | 8 | NI | NI | NI | NI |
| M.grisea | 26 | 32 | 18 | 26 | 22 | 30 | 7 | 16 | 8 | 14 | 6 | 12 | 6 | 14 |
Note:
1. The above values represent diameter of the zone of inhibition (in mm) at 4 μl and 8 μl concentration of essential oil/disc (Average value of triplicate experiment).
2. N = No Zone of inhibition.
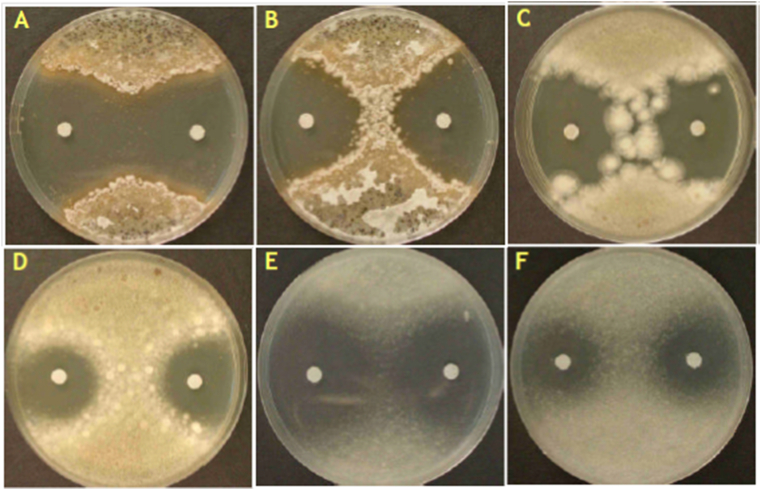
Figure 9.
Zone of inhibition of M. Grisea by various essential oils. (A) zone of inhibition of M. grisea around the paper disks loaded with 4 μl and 8 μl of lemon grass essential oil, (B) zone of inhibition of M. grisea around the paper disks loaded with 4 μl and 8 μl of citral, (C) zone of inhibition of A. niger around the paper disks loaded with 4 μl and 8 μl of lemon grass essential oil, (D) zone of inhibition of A. niger around the paper disks loaded with 4 μl and 8 μl of citral, (E) zone of inhibition of F. udum around the paper disks loaded with 4 μl and 8 μl of lemon grass essential oil, and (F) Represents the zone of inhibition of F. Udum around the paper disks loaded with 4 μl and 8 μl of citral.
Table 11.
Antifungal activity of major components determined by disc diffusion assay.
| Essential Oil → |
Citral |
Geraniol |
Citronellal |
|||
|---|---|---|---|---|---|---|
| Bacterial Strain ↓ | 4 μl | 8 μl | 4 μl | 8 μl | 4 μl | 8 μl |
| A.niger | 14 | 22 | 7 | 12 | 6 | 13 |
| F.udum | 14 | 18 | 5 | 9 | 6 | 12 |
| F.oxysporum | 12 | 16 | 6 | 10 | 5 | 10 |
| M.grisea | 20 | 30 | 12 | 19 | 10 | 17 |
Note:
1. The above values represent diameter of the zone of inhibition (in mm) at 4 μl and 8 μl concentration of major component/disc (Average value of triplicate experiment).
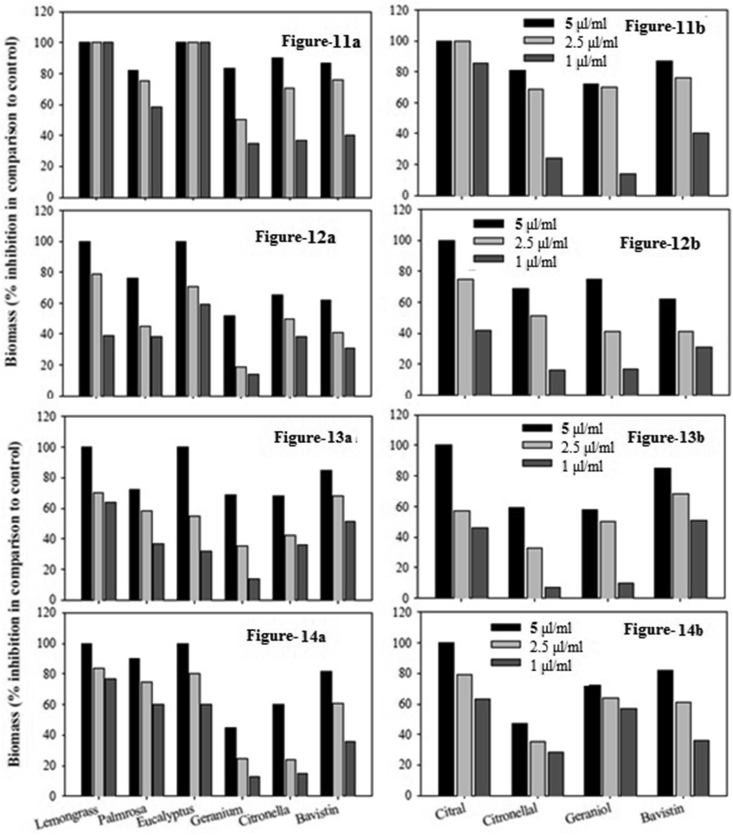
The essential oils and major components for tested for their ability on the production of biomass by fungi. For this purpose the five essential oils, which showed good antifungal activity (in disc diffusion assay) viz., lemon grass, palm rosa, eucalyptus, geranium, citronella and their major components citral, geraniol and citronellal were selected. The results are illustrated in Figure 10a–h. Biomass inhibition in the presence of bavistin, an anti fungal compound was also measured for comparison purpose.
Figure 10.
Biomass inhibition of fungi by essential oils. Biomass inhibition of Aspergillus niger (a), Fusarium udum (b), Fusarium oxysporum (c) and Magnoporthe grisea (d) in presence of essential oils lemon grass, palm rosa, eucalyptus, geranium and citronella. Biomass inhibition of A. niger (e), F. udum (f), F. oxysporum (g) and M. grisea (h) in presence of essential oil major components citral, geraniol and citronellal.
The essential oils of lemon grass and eucalyptus showed significant reduction in biomass production of all the four fungi tested viz. A. niger, F. udum, F. oxysporum and M. grisea. The amount of biomass produced in milligrams was measured after 72 h for A. niger, F. udum, F. oxysporum and after 7 days for M. Grisea. Citral is the most effective among the major components tested, followed by citronellal and geraniol (Figure 10b and f).
Lemongrass oil and eucalyptus oil along with palm rosa showed better inhibition than bavistin. Citronella and geranium showed comparable activity to that of bavistin. The major components citral showed best activity followed by geraniol and citronellal. Similar sort of results could be observed against F. oxysporum (Figure 10c and g).
Against M. grisea all the compounds tested showed good biomass inhibition. In case of this organism the values were recorded after 7 days of incubation. Lemon grass, eucalyptus and Palm rosa oils showed better activity than bavistin. Geraniol showed better inhibitory activity than citronellal (Figure 10d and h).
3.11. Gas-chromatography analysis
The GC analysis of the essential oils was carried. Citral, citronellal, and geraniol (from Sigma chemicals) were used as standards. Citral was found to be the major component in lemon grass, and was found to be present up to 81.84%. Geraniol is present at a concentration of 0.28%. In palm rosa oil 63.79% of geraniol and 3.72 % of citral was detected. In eucalyptus 76.8% of citronellal and 0.3% of geraniol was detected. In geranium oil 22.38% of geraniol and 2.47% of citronellal was detected. In citronella oil 29.2% of geraniol and 34.1% of citronellal was detected (Table 12).
Table 12.
Gas Chromatography Analysis of Essential oils.
| Major Component → |
Citral (%) | Geraniol (%) | Citronellal (%) |
|---|---|---|---|
| Essential Oil ↓ | |||
| Lemon grass | 81.84 | ND | ND |
| Palm rosa | 3.72 | 63.79 | ND |
| Eucalyptus | ND | 0.3 | 76.8 |
| Geranium | ND | 22.38 | 2.47 |
| Citronella | ND | 29.2 | 34.1 |
Note:
1. The values in the table indicate the percentage of major components present in the respective essential oils.
2. ND – Not detected/present in negligible quantity.
4. Discussion
One among the defense mechanisms, against infection and predation has been the chemical defenses developed by these organisms for survival. It is well-known that usage of antibiotics, fungicides, and pesticides excessively and in indiscriminate fashion has resulted in ill effects on soil health, environmental pollution, development of resistant microbes and pests [21, 22]. Therefore, alternatives to these compounds are needed which resulted in screening of many plants for potential biological activity [23, 24].
In the present study, we have studied the antimicrobial properties of seven essential oils, and their major components. Initially we have screened the antimicrobial activity of the test essential oils and their major components using paper disc assays. However, using the size of inhibition zone to indicate relative antimicrobial activity of essential oil and its constituents is not adequate. The zone of inhibition may be altered by the solubility and rate of diffusion of the test compounds in agar medium. Moreover, evaporation of the essential oils and their constituents can affect the doses applied to paper discs and thus the results. Hence we have determined the relative antimicrobial activity of the test compounds using liquid culture assay by determining their minimum inhibitory concentrations. Based on paper disc assay the most effective antibacterial essential oil is lemongrass followed by palmrosa, eucalyptus and geranium. Our liquid culture assays also specify the same.
Boukhatem et al., [10], studied the antibacterial activity of the lemongrass essential oil from Cymbopogon citratus and reported its potent activity against Gram + organisms compared to Gram - organisms. Our present results with lemongrass oil isolated from Cymbopogon flexousus show that this particular essential oil is equally effective against both Gram + and Gram - bacteria and shows no preferential activity towards a particular Gram reaction (Table 2).
The results of minimum bactericidal concentration (MBC) values of palm rosa essential oil show that it has a good antibacterial activity. However, the MBC results of palm rosa essential oil it is not very clear whether there is any preferential activity towards Gram reaction or not, though it appears that in general it is more active against Gram + bacteria.
The results of essential oil of eucalyptus show that it was active against all Gram + bacteria and against Gram - bacteria such as E. coli, E. coli HB101 and Pseudomonas aeruginosa. Our studies showed that the essential oil of geranium inhibits both E. coli and P. Vulgaris which was is in agreement with the results of Dorman and Deans [25]. From this study, the essential oil with the widest spectrum of activity was found to be lemon grass followed by palm rosa, eucalyptus, geranium and citronella, tagetus and mentha (Table 2).
The antibacterial activity of major components present in lemon grass, palm rosa, eucalyptus, geranium and citronella were also determined. Jansen et al., [26] have reported that changes in the composition of an essential oil due to many factors influence its antimicrobial activity. Keeping these things in view, we have selected the five essential oils that were showing good antibacterial activity for GC analysis.
Our GC analysis indicated that Citral content in the lemon grass essential oil (C. flexuosus) and geraniol in Palm rosa [27]. Similarly eucalyptus was known to have citronellal as its major component [27], and our GC analysis indicates that eucalyptus contains Citronellal as its major component and Geraniol (0.3%). Based on the literature available the Java type of citronella oil, which we are using in the present studies should contain 25–45% of geraniol and 25–55% of citronellal. Our GC analysis indicates that citronella oil contain 29.2% of geraniol and 34.1% of citronellal similar to [28] report.
Among the three major components, citral showed the best antibacterial activity. The results from the disc diffusion assay and liquid culture assay indicate that citral could be the main antibacterial component of the lemon grass essential oil. The antibacterial activity of palm rosa oil was found to be greater than that of geraniol. In contrast to this, essential oil of citronella, showed less antibacterial activity than geraniol. These two observations suggest two things which needs to be tested further thoroughly 1) In palm rosa in addition to geraniol some other components might be acting synergistically against the bacteria, and 2) In citronella oil geraniol is present up to 29%, and it could be that this is the only component which is showing antibacterial activity, while the rest of the components are either not showing any antibacterial activity or show only weak antibacterial activity.
Two methods were trialed to determine the antimicrobial activity of essential oils and their major components. The disc diffusion assay and serial dilution assay were used to determine the minimum inhibitory concentrations of the test compounds. Both the assays showed that lemon grass oil has a broad spectrum of inhibitory activity. From the results it could be observed that the bactericidal activity of the essential oil was higher than their major components suggesting the role of other components of oils in antimicrobial activity. This was in contrast to the results of [29].
Next we observed that lemon grass oil and palm rosa essential oil showed higher bactericidal activity than streptomycin and chloramphenicol, and comparable activity with ampicillin and gentamicin. Similarly the major component of lemon grass essential oil, citral showed higher bactericidal activity than ampicillin, streptomycin, and chloramphenicol against some bacteria. The antibacterial activity of palm rosa was higher than streptomycin against P. aeruginosa, X. oryzae and X. malvaceraum.
The major component of palm rosa i.e. Geraniol showed higher antibacterial activity than chloramphenicol against all the bacteria tested, except against B. subtilis. The essential oil of eucalyptus showed higher antibacterial activity compared to chloramphenicol. From the above studies it can be clearly understood that among the various essential oils tested for antibacterial activity lemon grass oil, followed by its major component citral, palm rosa oil, eucalyptus oil showed highest bactericidal activity in that order. Studies on the time course of lethal action were carried out on E. coli, S. aureus and X. oryzae. The results indicate that the lethal rate by lemon grass oil, palm rosa oil and citral was comparable with ampicillin against S. aureus. Against E. coli. Against X. oryzae, eucalyptus oil was very quick in its action though its antibacterial activity was less compared to lemon grass, palm rosa and ampicillin. One interesting observation from these studies is that, in general the essential oils are very quick in their action against the Gram + bacteria, S. aureus than Gram - bacteria. This could be due to the higher tolerance to lipophilic compounds shown by the outer membrane of Gram - bacteria as suggested by Heipieper et al., [30] and Sikkema et al., [31]. This might be playing a role in slower action of essential oils against Gram - bacteria in terms of time taken for total inhibition.
After establishing the antibacterial activity and time course of lethal action of the essential oils and the major components, their activity against the drug resistant pathogenic bacteria isolated from human patients was studied. As expected lemon grass oil was found to be the most potent against all the pathogenic drug resistant bacteria tested. However the studies clearly demonstrate antibacterial properties of some of the test compounds and suggest their potential use as chemotherapeutic agents, disinfectants or food preservants.
As it was reported that some phytochemicals have the ability to alter or inhibit the activity of several enzymes, the effect of the test compounds on the ß lactamase was studied. In our studies it was observed that despite the high bactericidal action against drug resistant pathogenic and non-pathogenic bacteria, none of the test compounds could neither eliminate the plasmid (Table 8) nor inhibit ß lactamase enzyme (Table 9). We feel that through studies, which are out of scope for present work, on plasmid elimination using properly formulated test compounds needs to be undertaken before coming to any strong conclusion about their ability to cure plasmids.
Though the results on sensitization of bacteria were not encouraging, the studies on development of resistance by E. coli to the essential oils were encouraging. The bacterium was not found to develop any resistance against the test compounds even after 30 passages in presence of sub lethal concentrations of the test compounds. These developments and the associated increase in fungal infections of animals and plants intensified the search for new, safer, and more efficacious agents to combat serious fungal infections. The crop losses due to fungal infections are enormous which led to indiscriminate use of synthetic fungicides. Moreover most of the studies on essential oils were done on their antibacterial activity. This prompted us to study the efficacy of the test compounds for their antifungal activity. For our studies we have selected plant pathogenic fungi as the losses due to fungi is seen more in agriculture. One interesting thing here is that from among the compounds tested, lemon grass oil and citral were the best antibacterial and antifungal agents. Palm rosa which was next to lemon grass in antibacterial activity showed lesser anti fungal activity than eucalyptus oil. Geranium essential oil could not show any zone of inhibition at 4μl/disc concentration against all the tested fungi, except M. grisea. Tagetus and mentha oils showed very poor anti fungal activity.
In conclusion, the essential oils of lemongrass, palm rosa and eucalyptus were found to be good antimicrobial agents. Next, we planned to study the ability of these essential oils in controlling the plant diseases caused by bacteria and fungi. Till now most of the work on the antimicrobial effects of essential oils has been performed on human pathogens, spoilage microorganisms, and dermatophytes, with very little research on phytopathogenic microorganism, both in vitro and in vivo. Hence we further examined the effect of these compounds against phytopathogens in vivo. The in vitro results show that these compounds are active against X. oryzae and M. grisea and hence we studied the ability of these compounds to control blight and blast diseases of rice.
Declarations
Author contribution statement
M. Naga Parameswari: Performed the experiments.
P. Shravan Kumar: Analyzed and interpreted the data.
J. Naveena Lavanya Latha: Conceived and designed the experiments; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the commercial, public, or not-for-profit sectors.
Data availability statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are grateful to the support provided by Osmania University, Hyderabad, DRR, Hyderabad and YVNR Govt. Degree College, Kaikaluru, Vikrama Simhapuri University, Nellore, A.P. for carrying out the present research.
Footnotes
Antimicrobial activity of essential oils.
References
- 1.Górniak I., Bartoszewski R., Króliczewski J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochemistry Rev. 2019;18:241–272. [Google Scholar]
- 2.Valko M., Morris H., Cronin M.T. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 3.Jomova K., Valko M. Advances in metal-induced oxidative stress and human disease Toxicol. 2011;283:65–87. doi: 10.1016/j.tox.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Yahia Y., Benabderrahim M.A., Tlili N., Bagues M., Nagaz K. Bioactive compounds, antioxidant and antimicrobial activities of extracts from different plant parts of two Ziziphus Mill. species. PloS One. 2020;15(5) doi: 10.1371/journal.pone.0232599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duarte A.E., de Menezes I.R., Bezerra Morais Braga M.F. Antimicrobial activity and modulatory effect of essential oil from the leaf of rhaphiodon echinus (Nees & Mart) Schauer on some antimicrobial drugs. Molecules. 2016;21(6):743. doi: 10.3390/molecules21060743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knobloch K., Pauli A., Iberl B., Weis N., Weigand H. Antibacterial activity and antifungal properties of essential oil components. J. Essent. Oil Res. 1988;1:119–128. [Google Scholar]
- 7.Tahir Mohd, Khushtar Mohammad, Fahad Mohd, Azizur Rahman Md. Phytochemistry and pharmacological profile of traditionally used medicinal plant Hyssop (Hyssopus officinalis L.) J. Appl. Pharmaceut. Sci. 2018;8(7):132–140. [Google Scholar]
- 8.Letessier M.P., Svoboda K.P., Walters D.R. Antifungal activity of the essential oil of Hyssop (Hyssopus officinalis) J. Phytopathol. 2001;149:673–678. [Google Scholar]
- 9.Szweda Piotr, Kot Barbara. 2018. Bee Products and Essential Oils as Alternative Agents for Treatment of Infections Caused by S. Aureus/Book Chapter in Frontiers in Staphylococcus aureus; pp. 203–224. [Google Scholar]
- 10.Boukhatem M.N., Ferhat M.A., Kameli A., Saidi F., Kebir H.T. Lemon grass (Cymbopogon citratus) essential oil as a potent anti-inflammatory and antifungal drugs. Libyan J. Med. 2014;9:25431. doi: 10.3402/ljm.v9.25431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khosravi A.R., Minooeianhaghighi M.H., Shokri H., Emami S.A., Alavi S.M., Asili J. The potential inhibitory effect of cuminum cyminum, ziziphora clinopodioides and nigella sativa essential oils on the growth of Aspergillus fumigatus and Aspergillus flavus. Braz. J. Microbiol. 2011;42(1):216–224. doi: 10.1590/S1517-838220110001000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delespaul Q., de Billerbeck V.G., Roques C.G., Michel G., Marquier-Vinuales C., Bessiere J.M. The antifungal activity of essential oils as determined by different screening methods. J. Essent. Oil Res. 2000;12:256–266. [Google Scholar]
- 13.Semenova E., Presnyakova V., Goncharov D., Goncharov M., Presnyakova E., Presnyakov S., Moiseeva I., Kolesnikova S. Spectrophotometric method for quantitative measuring essential oil in aromatic water and distillate with rose smell. J. Phy. 2017 Conference Series 784. [Google Scholar]
- 14.Stokes E.J. Clinical Bacteriology. Edward Arnold Ltd.; London: 208: 1975. [Google Scholar]
- 15.Pavitra Vani K., Bhagya Lakshmi O. Antibacterial Activity of black pepper (Piper nigrum Linn.) with special reference to its mode of action on bacteria. Indian J. Nat. Prod. Reseour. 2010;1(2):213–215. 2010. [Google Scholar]
- 16.Elgayyar M., Draughon F.A., Golden D.A., Mount J.R. Antimicrobial activity of essential oils from plants against selected pathogenic and saprophytic microorganisms. J. Food Protect. 2001;64(7):1019–1024. doi: 10.4315/0362-028x-64.7.1019. [DOI] [PubMed] [Google Scholar]
- 17.Kumara Swamy M., Akhtar Mohd Sayeed, Uma Rani S. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: an updated review. Evidence-Based Compl. Alternat. Med. 2016;2016:21. doi: 10.1155/2016/3012462. Article ID 3012462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan S., Chang J., Zong Y., Hu G., Jia J. GC-MS analysis of the composition of the essential oil from Dendranthema indicum Var. Aromaticum using three extraction methods and two columns. Molecules. 2018;23(3):576. doi: 10.3390/molecules23030576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kresge N.R., Simoni D., Hill R.L. The molecular genetics of bacteriophage: the work of Norton Zinder. J. Biol. Chem. 2011;286(25):4–5. doi: 10.1074/jbc.O111.000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bush K., Bradford P.A. β-Lactams and β-lactamase inhibitors: an overview. Cold Spring Harb. Perspect. Med. 2016;6:a025247. doi: 10.1101/cshperspect.a025247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roy N.K., Dureja P. New eco-friendly pesticides for integrated pest management. Pestic. World. 1998;3:16–21. [Google Scholar]
- 22.Fortunati E., Mazzaglia A., Balestra G.M. Sustainable control strategies for plant protection and food packaging sectors by natural substances and novel nanotechnological approaches. J. Sci. Food Agric. 2019;99(3):986–1000. doi: 10.1002/jsfa.9341. [DOI] [PubMed] [Google Scholar]
- 23.Recio M.C., Rios J.L. A review of some antimicrobial compounds isolated from medicinal plants reported in the literature 1978-1988. Phytopathol. Res. 1989;3:117–124. [Google Scholar]
- 24.Rios J.L., Recio M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005;100:80–84. doi: 10.1016/j.jep.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 25.Deans S.G., Ritchie G. Antibacterial properties of plant essential oils. Int. J. Food Microbiol. 1987;5:165–180. [Google Scholar]
- 26.M Jansen A., Scheffer J.J.C., Svendsen A.B. Antimicrobial activity of essential oils: a 1976-1986 literature review. Aspects of the test methods. Planta Med. 1987;5:395–398. doi: 10.1055/s-2006-962755. [DOI] [PubMed] [Google Scholar]
- 27.Gildemeister E., Hoffmann F., Schimmel . Wiley; New York: 1913. The Volatile Oils. [Google Scholar]
- 28.Formacek V., Kubeczka K.H. Einsatzmöglichkeiten der 13C-NMR Spektrobei der Analyse ätherischer Öle. In: Kubeczka K.-H., editor. Vorkommen und Analytik ätherischer Öle. Thieme Verlag; Stuttgart: 1979. p. 130. [Google Scholar]
- 29.Grace O., Onawunmi Evaluation of the antimicrobial activity of citral. Lett. Appl. Microbiol. 2008;9(3):105–108. [Google Scholar]
- 30.Heipieper H., Martínez-Lavanchy Paula. 2010. Toxicity of Hydrocarbons to Microorg. [Google Scholar]
- 31.Sikkema J., de Bont J.A.M., Poolman B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 1995;59:201–222. doi: 10.1128/mr.59.2.201-222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.