Abstract
There have been significant improvements in surgical techniques and implant designs of elbow arthroplasty over the last five decades. These advances have resulted in improved outcomes and expansion of indications for total elow arthroplasty (TEA). As the proportion of TEAs being performed for inflammatory arthritis has been decreasing in recent years, TEAs are being performed more commonly for the management of acute distal humerus fractures in the elderly, post-traumatic sequelae, and primary osteoarthritis. Appropriate patient selection and meticulous attention to surgical technique including the surgical approach, implant positioning and fixation will result in acceptable outcomes. Future advances in the design, instrumentation, and surgical technique will allow for further improvement in outcomes as the indications for TEA continue to expand.
Keywords: Elbow, Arthroplasty, Replacement, Unlinked, Linked, Convertible
1. Background
Total elbow arthroplaty (TEA) was first utilized in the 1970s for the management of patients with rheumatoid elbow arthritis.1, 2, 3, 4 These early implants were fully constrained hinged prostheses utilizing a single axis pin that only allowed flexion and extension of the elbow. The increased constraint of these implants compared to the “loose hinge” of the native elbow led to high rates of early loosening due to stresses at the implant-cement-bone interface.5 Thus, the linkage design was changed from a fully constrained prosthesis to a “sloppy” hinge semi-constrained prosthesis allowing for some varus-valgus and internal-external rotation laxity during elbow motion (Fig. 1).6 Currently available total elbow arthroplasty implants are categorized as linked, unlinked, and convertible implants based on the presence, absence, or the option of having a mechanical link between the ulnar and humeral components.
Fig. 1.
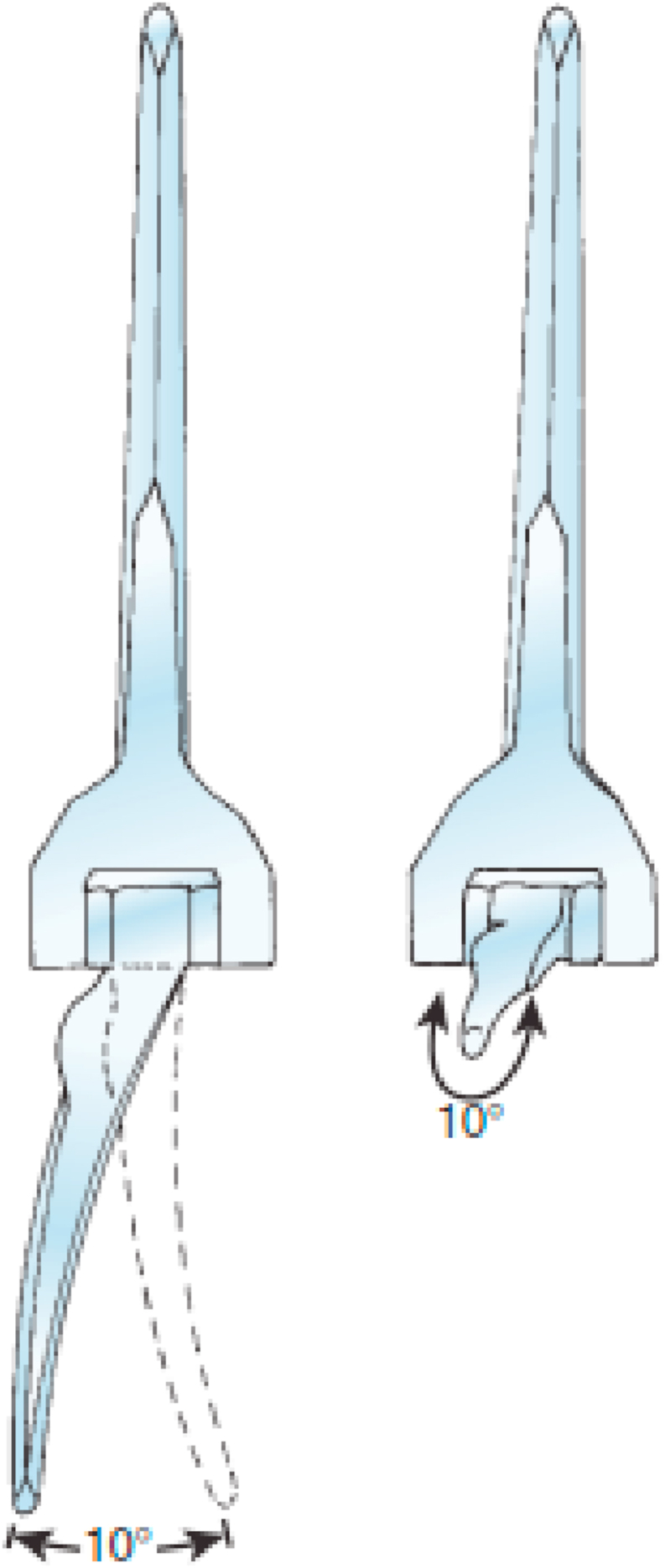
A “sloppy” hinge semi-constrained prosthesis allows for 5–10° of varus-valgus and internal-external rotation laxity. (Reproduced with permission from Morrey BF, Sanchez-Sotelo J, ed. The Elbow and Its Disorders, Fourth ed., Page 766, Philadelphia: Saunders Elsevier, 2009).
The linked (semi-constrained) prostheses use pins and bushings which allow for some out-of-plane motion of approximately 6–8° of varus-valgus and axial rotation. This allows for less stresses at the implant-cement-bone interface compared to the fully constrained prostheses. However, there are theoretically more stresses at the implant-cement-bone interface compared to unlinked prostheses. The advantages of linked implants are immediate stability from the linkage mechanism and the ability to be used in the setting of ligamentous insufficiency or extensive bone loss (Fig. 2). Moreover, these implants allow for a more aggressive soft tissue release in the setting of stiffness or deformity without the risk of instability. Commonly used linked TEA systems include: the Coonrad-Morrey (Zimmer Biomet), the Nexel (Zimmer Biomet), the Discovery (DJO) and the GSB III Elbow (Zimmer Biomet).
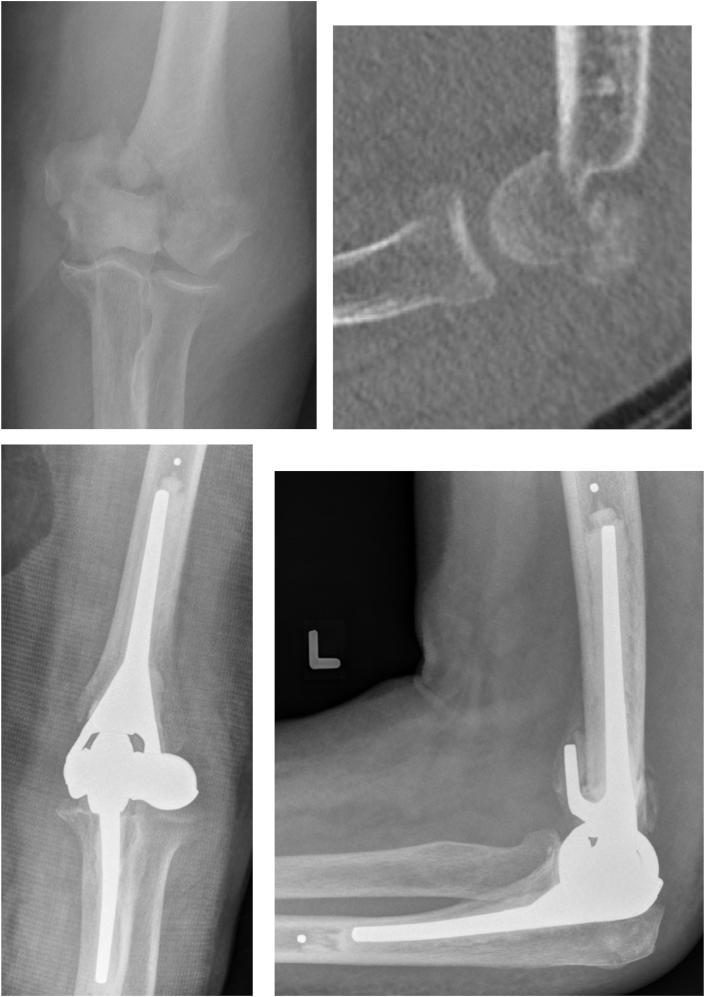
Fig. 2.
Pre and postoperative images of an 88-year-old male with a comminuted intra-articular distal humerus fracture treated with total elbow arthroplasty. A linked arthroplasty system allows for stability without the need for fixation of condylar fragments.
Unlinked prostheses were designed with the potential to further decrease the stresses at the bone-cement interface. These implants have no mechanical link between the ulnar and humeral components, and they rely solely on implant shape, capsuloligamentous integrity, and precise surgical technique for stability. The early unlinked prostheses were designed as resurfacing implants as their humeral components did not have stems. These earlier designs had high failure rates from mechanical loosening which led to the development of stemmed designs with improved results.7 The unlinked TEA has the theoretical but clinically unproven advantage of reduced wear, osteolysis, and aseptic loosening due to lesser stresses at the bone-cement-implant interface. Commonly used unlinked TEA systems include: the Souter-Strathclyde (Stryker) and the Kudo/iBP (Biomet) elbow.
Convertible TEA prostheses allow the surgeon to choose between an unlinked and linked prosthesis depending on the status of the osseous and capsuloligamentous structures and the intraoperative assessment of implant stability (Fig. 3). Moreover, these implants allow for easy revision of an unstable unlinked elbow arthroplasty to a linked implant without removing well-fixed components. The Latitude system (Wright Medical) is a convertible TEA system that also has the option of performing a hemiarthroplasty (Fig. 4). The articular shape of the distal humerus hemiarthroplasty implant is designed to match the native spool of the trochlea and the articular surface of the capitellum more anatomically. Distal humerus hemiarthroplasty is an option for the management of comminuted distal humerus fractures, nonunions, and avascular necrosis in specific situations.
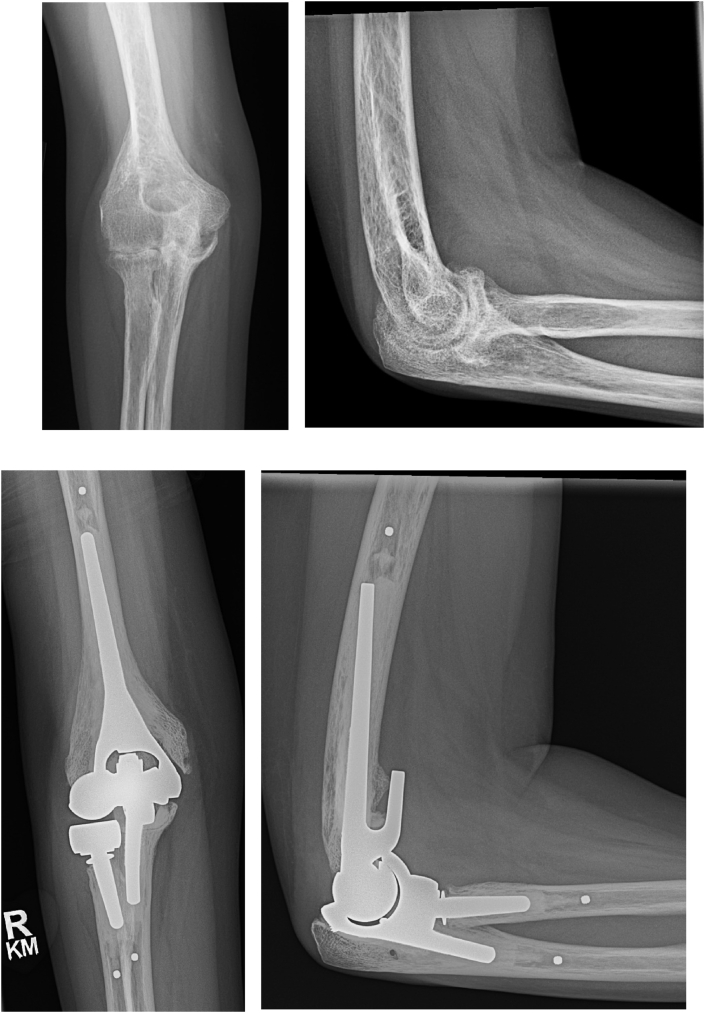
Fig. 3.
Pre and postoperative images of a 41-year-old female with end-stage rheumatoid arthritis and significant preoperative stiffness (arc of motion 40–75°) affecting even activities of daily living. An unlinked total elbow arthroplasty was used due to the patient’s young age and potential for reduced wear. At one-year postoperative follow-up, the patient is pain-free with an arc of motion of 20–135°.
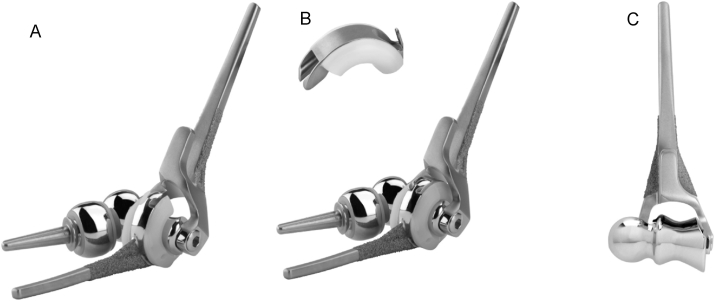
Fig. 4.
Latitude convertible elbow prosthesis has the option to be used as A) unliked, B) linked, or C) distal humerus hemiarthroplasty. (Reproduced with permission from Wright Medical).
More recently designed implants employ many features that have been shown to improve outcomes such as:
-
1.
The anterior flange of the humeral component has been shown to resist posterosuperior and rotatory forces8
-
2.
Surface treatment of cemented stems has been shown to improve stem fixation and long-term stability.9 Moreover, it’s been shown that plasma-spray treatment outperforms beaded stems.9,10
-
3.
The use of thicker ultra-high molecular weight polyethylene (UHMWPE) reduces contact pressure and wear.11
-
4.
More attention is being paid to the design and instrumentation of implants to reproduce the anatomic axis of rotation of the native elbow.12
2. Indications and patient selection
Classically, TEA was used for the management of patients with end-stage inflammatory arthropathies, such as rheumatoid arthritis, of the elbow.12 Historically, these individuals were generally low demand allowing for successful outcomes with low rates of wear and loosening.13 However, the indications for TEA have continued to expand with improved surgical techniques and implant design. Additional indications for TEA include acute comminuted distal humerus fractures in the elderly, post-traumatic arthritis or salvage of distal humerus nonunion, dysfunctional instability, primary osteoarthritis, and elbow reconstruction in the setting of primary or metastatic tumors.
Global trends from national registry data demonstrate that inflammatory arthritis continues to be the most common indication for TEA.14 However, other indications particularly acute trauma, post-traumatic sequelae, and primary osteoarthritis have been gaining increasing popularity with regional variations.14 A recent systematic review evaluating global trends from 6 national registry data (UK, Australia, New Zealand, Norway, Sweden, and the Netherlands) between 2000-2009 and 2010–2017 showed that the proportion of TEAs performed for inflammatory arthritis decreased from 61% to 46%.14 With a decreasing proportion of TEAs being performed for inflammatory arthritis due to advances in medical management, more TEAs are being performed for acute distal humerus fractures (23% vs. 38%), post-traumatic sequelae, and primary osteoarthritis (5% vs. 8%).14 However, there are some notable regional variations. In the United States, Norway and Australia, an acute fracture is the most common indication for TEA ahead of both inflammatory arthritis and post-traumatic sequelae.15, 16, 17 Moreover, primary osteoarthritis is a more common indication in Australia (33.9%), UK (33.2%), and Dutch (27%) registries compared to Scandinavian countries (3%).14,16,18,19
A detailed discussion regarding patient’s functional demands and expectations is of paramount importance when considering TEA. Although there are no published consensus recommendations for postoperative activity after TEA, many surgeons recommend lifelong postoperative activity restrictions to decrease wear and implant failure. These restrictions generally include not lifting anything weighing more than 10 lb or repetitive lifting of more than 5 lb. However, a study evaluating patients’ compliance with activity restrictions after TEA found that 94% of patients engaged in moderate-demand activities (most commonly carrying groceries and gardening) and 40% of patients engaged in high-demand activities (most commonly snow shoveling, dirt shoveling, and placing luggage in an overhead compartment).20 Male gender and patients who underwent TEA for fracture/nonunion were risk factors for engaging in high-demand activities. Other factors that have been associated with increased risk of mechanical failure include younger age, obesity, preoperative deformity, and ankylosis.21, 22, 23, 24, 25
If an unlinked TEA is being considered, a careful evaluation of elbow stability and collateral ligament integrity, bone stock, and periarticular muscle function is required.
3. Surgical technique overview
3.1. Surgical approaches
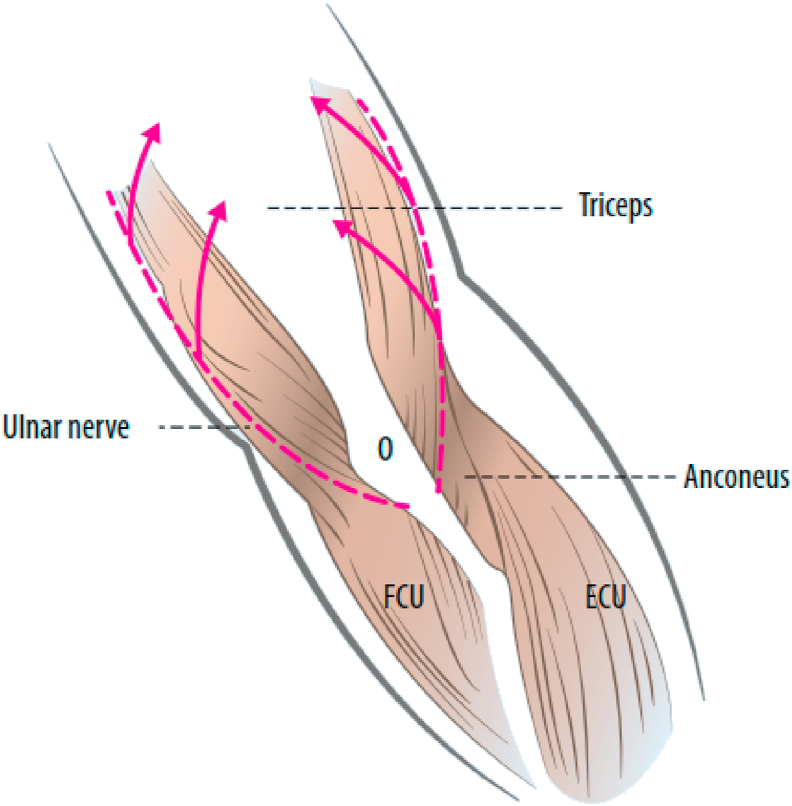
Multiple approaches have been described for total elbow arthroplasty which can be broadly categorized into triceps off, in which the triceps is detached from the olecranon, and triceps on, in which most of the triceps insertion on the olecranon is preserved.
Bryan-Morrey triceps reflecting approach is a commonly used triceps off approach for performing TEA (Fig. 5).26 It was described in 1982 to address triceps weakness associated with earlier approaches for arthroplasty which involved triceps split or triceps turn down. In this approach, the entire extensor mechanism along with the anconeus is elevated from medial to lateral. Although detaching the triceps insertion maximizes joint exposure and facilitate ulnar preparation, triceps off approaches have been associated with a higher risk of triceps insufficiency and rupture with clinically relevant weakness reported in up to 29% of patients.27
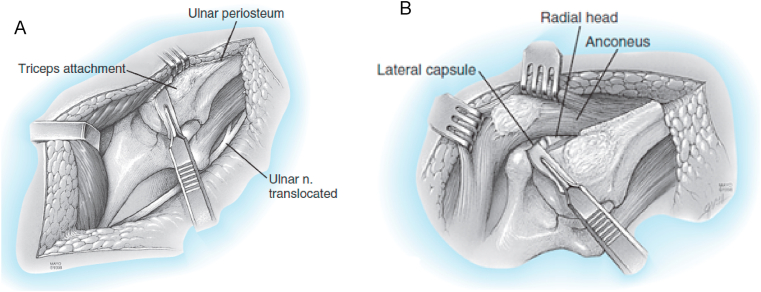
Fig. 5.
Bryan-Morrey triceps reflection approach. A) Once the ulnar nerve is identified and protected, the extensor mechanism and the anconeus are reflected from medial to lateral by releasing the Sharpeys fibers from the tip of the olecranon. B) Once the extensor mechanism is subluxated to the lateral aspect of the lateral epicondyle, the collateral ligaments are released to allows for appropriate exposure to perform the arthroplasty. (Copyright © 2018 Mayo Foundation for Medical Education and Research. Published by Elsevier Inc. All rights reserved.)
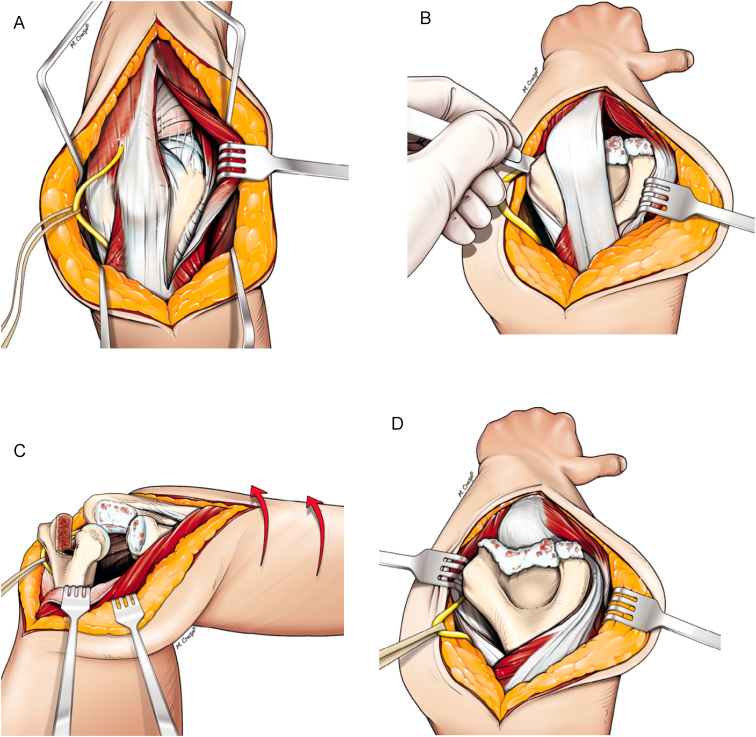
In recent years, triceps on approaches for TEA have been gaining popularity. Alonso-Llames paratricipital approach and the lateral para-olecranon approach are commonly used triceps on approaches.28,29 In the paratricipital approach, the triceps is elevated from the intermuscular septa and the posterior aspect of the humerus medially and laterally. On the medial side, the approach is extended distally through the floor of the ulnar nerve between the two heads of the flexor carpi ulnaris. Laterally, the approach can be extended either between the olecranon and anconeus (Boyd interval) or between the anconeus and extensor carpi ulnaris (i.e. Kocher interval) (Fig. 6). The lateral para-olecranon approach, described by the senior author, involves a Boyd approach distally by elevating anconeus muscle from the lateral margin of the proximal ulna leaving a cuff of forearm fascia on the ulna for repair at the end of the procedure. This is extended proximally as a triceps split separating the portion of the triceps tendon that inserts directly on the olecranon tip from the portion that blends with the anconeus fascia (Fig. 7). The medial window is the same as the medial window of the paratricipital approach as described above. Once, the medial and lateral collateral ligaments are released and the anterior and posterior capsules are detached, the elbow can be dislocated allowing for the preparation and placement of the humeral, ulnar, and if desired radial components. Better elbow extension strength has been reported in triceps on approaches compared to triceps off approaches.29,30
Fig. 6.
Paratricipital approach. Medial and lateral windows are made by elevating the triceps from the intermuscular septa and posterior humerus. Medially, the ulnar nerve is identified, and the dissection is continued distally through the floor of the ulnar nerve. Laterally, the dissection is continued distally between the anconeus and olecranon. (Reproduced with permission from Dey Hazra RO, Lill H, Jensen G, Imrecke J, Ellwein A. Fracture-pattern-related therapy concepts in distal humeral fractures. Obere Extrem. 2018; 13(1):23–32.).
Fig. 7.
Lateral paraolecranon approach. A) The lateral window involves the Boyd approach distally along the lateral margin of the ulna which is extended proximally as a triceps split. B) The medial and lateral collateral ligaments and the common flexor and extensor origins are sectioned from their insertions to allow for dislocation of the elbow. C) Supination of the forearm allows for visualization of the trochlear notch and radius for preparation. D) The distal humerus can be dislocated through the triceps split or medially for preparation. (Reproduced with permission from Studer A, Athwal GS, MacDermid JC, Faber KJ, King GJ. The lateral para-olecranon approach for total elbow arthroplasty. J Hand Surg Am. 2013 Nov; 38(11):2219–2226).
3.2. Procedural steps and tips
The detailed steps in preparation and implantation of the components vary depending on the system used; however, there are some key steps and principles which are covered here.
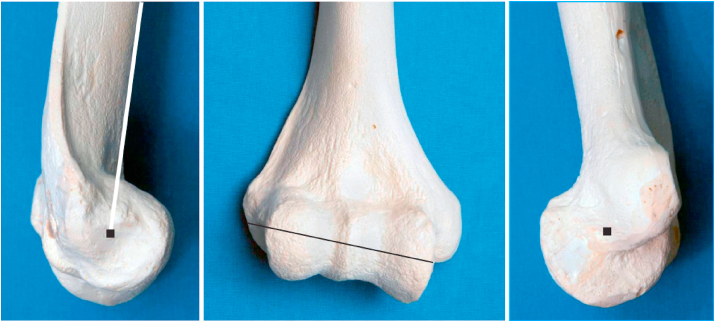
All currently available systems have stemmed components that require canal preparation. The authors prefer to prepare the ulnar canal first to avoid potential fracture of thinned or weakened humeral condyles during ulnar preparation if the humeral canal is prepared first. Restoration of flexion-extension axis and appropriate size, alignment, and rotation of the components are key to the success and theoretically the longevity of the arthroplasty. The ulnar canal is opened at the base of the coronoid midway along the trochlear notch in line with the ulnar shaft. The ulnar canal is prepared with the help of flexible reamers and then sequential broaching. Proper sizing of the component depends on the system used. Some systems (e.g. Latitude) use the geometry of the articular surfaces of the distal humerus or proximal ulna and radius while others (e.g. Coonrad-Morrey, Nexel, Discovery) rely on serial broaching to determine implant size. The posterior flat surface of the olecranon is used as a reference point to guide the rotation of ulnar canal preparation during broaching.31 To prepare the humeral component, the central portion of the trochlea is resected to aid in opening the humeral canal. The humeral canal is again prepared using series of flexible reamers and sequential broaching. The flexion-extension axis of the elbow, which is defined by the geometric centres of the trochlea and capitellum, is used to guide the rotation and depth of insertion of the humeral component.32 Intraoperatively, the flexion-extension axis is determined by extending a line from the tubercle on the lateral aspect of the capitellum at the site of origin of the lateral collateral ligament to the anterior-inferior aspect of the medial epicondyle at the site of origin of the ulnar collateral ligament (Fig. 8).33 If these anatomic landmarks are not present, such as in cases of comminuted distal humerus fractures or revision arthroplasty, the posterior cortex of the distal humerus just proximal to the olecranon fossa is relatively flat and can be used to judge the rotation of the humeral component.34 The humeral component should be internally rotated by an average of 14° with respect to this posterior humeral flat spot.34 Determining the depth of insertion of the implant is also a challenge; however, in most current devices the proximal portion of the yoke of the humeral component sits at the level of the proximal portion of the olecranon fossa. Soft tissue tensioning during examination with trial components can also be used to estimate the depth of implant positioning.
Fig. 8.
The flexion-extension axis of the elbow extending from the site of origin of the lateral collateral ligament to that of the ulnar collateral ligament. (Copyright © 2018 Mayo Foundation for Medical Education and Research. Published by Elsevier Inc. All rights reserved.)
After the trial components have been placed, the range of motion, tracking, and stability should be assessed. If pistoning of the components is noted during the range of motion assessment, impingement from the coronoid tip, olecranon tip should be ruled out. If no sites of impingement are noted, pistoning may be due to the components being placed too deep allowing the anterior soft tissue to act as a fulcrum. If an unlinked prosthesis is trialed, it is particularly important to assess the tracking of the ulnohumeral and radiocapitellar joints. If there is any maltracking of the articulations which cannot be corrected with repositioning of the components, a linked prosthesis should be used.
Once correct implant positioning and stability have been determined, the trial components are removed, and the final components are implanted. Proper cementation technique, which includes the use of cement restrictors, washing and drying the canal, retrograde filling of the canal with a cement gun through a narrow flexible nozzle is crucial in optimizing the fixation of the implant.35 A bone graft is then placed under the anterior flange of the humeral stem. If a linked arthroplasty is planned, the linking mechanism is inserted. If an unlinked arthroplasty is planned or in some cases of linked arthroplasty depending on the surgeon’s preference, the collateral ligaments with the corresponding common muscular origins are repaired. Finally, the extensor mechanism must be repaired if detached or split.
3.3. Postoperative rehabilitation
The rehabilitation plan depends on the surgical approach used and the implant constraint selected.36 Rehabilitation is structured to minimize early complications while restoring elbow function. In the immediate postoperative period, the elbow is immobilized in a semi-extended position with an anterior splint to avoid tension and pressure on the posterior wound. For a linked arthroplasty performed through a triceps on approach, most surgeons start an active range of motion protocol once the wound is healed. If a triceps off approach was used, resisted exercises are avoided for 3 months. Limiting flexion initially to 90° with a gradual increase in flexion and using gravity-assisted extension may help prevent triceps avulsion. If an unlinked arthroplasty was used, the postoperative rehabilitation plan is focused on protecting the collateral ligament repair. An overhead rehabilitation program with the use of a lightweight thermoplastic resting splint in between exercises for the first 6 weeks may be helpful.36 If there is a limitation of elbow motion, a static progressive extension splint at night or flexion cuff may be used in both linked and unlinked arthroplasty patients after 6 weeks.
Most surgeons recommend lifetime weight restrictions after elbow arthroplasty; however, the exact limitation varies and there is no empirical evidence to guide these restrictions. The authors typically recommend that the patient not lift more than 10 lb as a single event or more than 5 lb repeatedly. The patients should also avoid engaging in impact sports involving the upper extremity (e.g. golf) after an elbow arthroplasty.
4. Outcomes
Outcomes after TEA are not as successful as hip or knee arthroplasty and vary substantially depending on the indication. TEA performed for rheumatoid arthritis has been shown to have superior functional outcomes, lower complication, and revision rates compared to other indications.37 Survivorship data has been reported in a small number of studies with 10-year survivorship following primary TEA of 85–92% for rheumatoid arthritis, 89% for acute fracture, 89% for primary osteoarthritis, 69–80% for juvenile inflammatory arthropathy, and 42% for hemophilia.37 Complications of TEA remain problematic; however, most are minor and do not have a major impact on outcomes. For rheumatoid arthritis reported complications range from 5 to 30%, while rates of up to 50% have been reported for acute fracture, primary osteoarthritis, and trauma sequelae.37 Revision rates are higher for trauma sequelae (up to 30%) compared to rheumatoid arthritis (11–13%), acute fractures (10–11%), and primary osteoarthritis (11%).37 The most common indications for revision arthroplasty have been aseptic loosening, deep infection, and periprosthetic fractures.38
Patient’s age, sex, and body habitus have been shown to have an impact on the outcomes of TEA. Younger more active patients and males are at higher risks of mechanical failure requiring revision.39,40 Obesity has been correlated with lower implant survivorship, higher infection, dislocation, and periprosthetic fracture rates.22,23 Multiple previous surgeries, preoperative deformity, or ankylosis have higher complication and revision rates.24,25,39,40 Finally, the surgeon’s experience and volume, and duration of the procedure have an impact on TEA outcomes.39,41,42 The data from the Finnish arthroplasty registry showed a lower risk of revision if TEA was performed in specialized hospitals versus nonspecialized hospitals.41 Moreover, the Scottish arthroplasty registry data suggest a better implant survival rate if the surgeon’s volume was more than 10 cases per year.42
5. Conclusion
Advances in implant design, surgical techniques, and appropriate patient selection have resulted in the expansion of the use of TEA and improved outcomes. TEA was initially used mainly for the management of patients with the end-stage rheumatoid arthritis. Over the last couple of decades, the indications have expanded to include acute comminuted distal humerus fractures in the elderly, primary osteoarthritis, post-traumatic arthritis, nonunion, primary or metastatic tumors, among others. Future innovations in the design, instrumentation, and surgical technique will ensure further improvement in its survivorship and lower complication rates.
References
- 1.Dee R., Sweetnam D.R. Total replacement arthroplasty of the elbow joint for rheumatoid arthritis: two cases. Proc Roy Soc Med. 1970;63(7):653–655. [PMC free article] [PubMed] [Google Scholar]
- 2.Dee R. Total replacement arthroplasty of the elbow for rheumatoid arthritis. J Bone Joint Surg Br. 1972;54(1):88–95. [PubMed] [Google Scholar]
- 3.Dee R. Total replacement of the elbow joint. Orthop Clin N Am. 1973;4(2):415–433. [PubMed] [Google Scholar]
- 4.Souter W.A. Arthroplasty of the elbow with particular reference to metallic hinge arthroplasty in rheumatoid patients. Orthop Clin N Am. 1973;4(2):395–413. [PubMed] [Google Scholar]
- 5.Garrett J.C., Ewald F.C., Thomas W.H., Sledge C.B. Loosening associated with G.S.B. hinge total elbow replacement in patients with rheumatoid arthritis. Clin Orthop Relat Res. 1977;127:170–174. [PubMed] [Google Scholar]
- 6.Schlein A.P. Semiconstrained total elbow arthroplasty. Clin Orthop Relat Res. 1976;121:222–229. [PubMed] [Google Scholar]
- 7.Kudo H., Iwano K. Total elbow arthroplasty with a non-constrained surface-replacement prosthesis in patients who have rheumatoid arthritis. A long-term follow-up study. J Bone Joint Surg Am. 1990;72(3):355–362. [PubMed] [Google Scholar]
- 8.Kedgley A.E., Takaki S.E., Lang P., Dunning C.E. The effect of cross-sectional stem shape on the torsional stability of cemented implant components. J Biomech Eng. 2006;129(3):310–314. doi: 10.1115/1.2720907. [DOI] [PubMed] [Google Scholar]
- 9.Jeon I.H., Morrey B.F., Sanchez-Sotelo J. Ulnar component surface finish influenced the outcome of primary Coonrad-Morrey total elbow arthroplasty. J Shoulder Elbow Surg. 2012;21(9):1229–1235. doi: 10.1016/j.jse.2011.08.062. [DOI] [PubMed] [Google Scholar]
- 10.Hosein Y.K., King G.J., Dunning C.E. The effect of stem surface treatment and material on pistoning of ulnar components in linked cemented elbow prostheses. J Shoulder Elbow Surg. 2013;22(9):1248–1255. doi: 10.1016/j.jse.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Willing R., King G.J., Johnson J.A. The effect of implant design of linked total elbow arthroplasty on stability and stress: a finite element analysis. Comput Methods Biomech Biomed Eng. 2014;17(11):1165–1172. doi: 10.1080/10255842.2012.739161. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez-Sotelo J. Total elbow arthroplasty. Open Orthop J. 2011;5:115–123. doi: 10.2174/1874325001105010115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanchez-Sotelo J., Baghdadi Y.M., Morrey B.F. Primary linked semiconstrained total elbow arthroplasty for rheumatoid arthritis: a single-institution experience with 461 elbows over three decades. J Bone Joint Surg Am. 2016;98(20):1741–1748. doi: 10.2106/JBJS.15.00649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macken A.A., Prkic A., Kodde I.F., Lans J., Chen N.C., Eygendaal D. Global trends in indications for total elbow arthroplasty: a systematic review of national registries. EFORT Open Rev. 2020;5(4):215–220. doi: 10.1302/2058-5241.5.190036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poff C.B., Kothandaraman V., Kunkle B.F., Friedman R.J., Eichinger J.K. JSES; 2021. Trends in Total Elbow Arthroplasty Utilization in the United States from 2002-2017. Seminars in Arthroplasty. [Google Scholar]
- 16.Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR) Hip, Knee & Shoulder Arthroplasty: 2020 Annual Report. AOA; Adelaide: 2020. Demographics and outcome of elbow and wrist arthroplasty: supplementary report; pp. 1–31.https://aoanjrr.sahmri.com/annual-reports-2020/supplementary [Google Scholar]
- 17.Norwegian national advisory unit on arthroplasty and hip fractures. Norwegian arthroplasty registry: 2020 report. http://nrlweb.ihelse.net/eng/ June 2020:137-142.
- 18.UK National Joint Registry . 2020. National Joint Registry: 17th Annual Report; pp. 206–225.https://reports.njrcentre.org.uk/ [PubMed] [Google Scholar]
- 19.Dutch Arthroplasty Register (LROI) 2019. Online LROI Annual Report 2019; pp. 125–134.https://www.lroi-report.nl/previous-reports/ [Google Scholar]
- 20.Barlow J.D., Morrey B.F., O’Driscoll S.W., Steinmann S.P., Sanchez-Sotelo J. Activities after total elbow arthroplasty. J Shoulder Elbow Surg. 2013;22(6):787–791. doi: 10.1016/j.jse.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 21.Throckmorton T., Zarkadas P., Sanchez-Sotelo J., Morrey B. Failure patterns after linked semiconstrained total elbow arthroplasty for posttraumatic arthritis. J Bone Joint Surg Am. 2010;92(6):1432–1441. doi: 10.2106/JBJS.I.00145. [DOI] [PubMed] [Google Scholar]
- 22.Baghdadi Y.M., Veillette C.J., Malone A.A., Morrey B.F., Sanchez-Sotelo J. Total elbow arthroplasty in obese patients. J Bone Joint Surg Am. 2014;96(9):e70. doi: 10.2106/JBJS.M.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffin J.W., Werner B.C., Gwathmey F.W., Chhabra A.B. Obesity is associated with increased postoperative complications after total elbow arthroplasty. J Shoulder Elbow Surg. 2015;24(10):1594–1601. doi: 10.1016/j.jse.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 24.Schneeberger A.G., Adams R., Morrey B.F. Semiconstrained total elbow replacement for the treatment of post-traumatic osteoarthrosis. J Bone Joint Surg Am. 1997;79(8):1211–1222. doi: 10.2106/00004623-199708000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Mansat P., Morrey B.F. Semiconstrained total elbow arthroplasty for ankylosed and stiff elbows. J Bone Joint Surg Am. 2000;82(9):1260–1268. doi: 10.2106/00004623-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Bryan R.S., Morrey B.F. Extensive posterior exposure of the elbow. A triceps-sparing approach. Clin Orthop Relat Res. 1982;166:188–192. [PubMed] [Google Scholar]
- 27.Pierce T.D., Herndon J.H. The triceps preserving approach to total elbow arthroplasty. Clin Orthop Relat Res. 1998;(354):144–152. doi: 10.1097/00003086-199809000-00017. [DOI] [PubMed] [Google Scholar]
- 28.Alonso-Llames M. Bilaterotricipital approach to the elbow. Its application in the osteosynthesis of supracondylar fractures of the humerus in children. Acta Orthop Scand. 1972;43(6):479–490. doi: 10.3109/17453677208991270. [DOI] [PubMed] [Google Scholar]
- 29.Studer A., Athwal G.S., MacDermid J.C., Faber K.J., King G.J. The lateral para-olecranon approach for total elbow arthroplasty. J Hand Surg Am. 2013;38(11):2219–2226. doi: 10.1016/j.jhsa.2013.07.029. e2213. [DOI] [PubMed] [Google Scholar]
- 30.Booker S.J., Smith C.D. Triceps on approach for total elbow arthroplasty: worth preserving? A review of approaches for total elbow arthroplasty. Shoulder Elbow. 2017;9(2):105–111. doi: 10.1177/1758573216682479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duggal N., Dunning C.E., Johnson J.A., King G.J. The flat spot of the proximal ulna: a useful anatomic landmark in total elbow arthroplasty. J Shoulder Elbow Surg. 2004;13(2):206–207. doi: 10.1016/j.jse.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Deland J.T., Garg A., Walker P.S. Biomechanical basis for elbow hinge-distractor design. Clin Orthop Relat Res. 1987;(215):303–312. doi: 10.1097/00003086-199702000-00044. [DOI] [PubMed] [Google Scholar]
- 33.Morrey B., Larson A.N., Morrey M. Hinged external fixators of the elbow. In: Morrey B., editor. fourth ed. Saunders; Philadelphia: 2008. p. 505. (The Elbow and its Disorders). [Google Scholar]
- 34.Sabo M.T., Athwal G.S., King G.J. Landmarks for rotational alignment of the humeral component during elbow arthroplasty. J Bone Joint Surg Am. 2012;94(19):1794–1800. doi: 10.2106/JBJS.J.01740. [DOI] [PubMed] [Google Scholar]
- 35.Faber K.J., Cordy M.E., Milne A.D., Chess D.G., King G.J., Johnson J.A. Advanced cement technique improves fixation in elbow arthroplasty. Clin Orthop Relat Res. 1997;334:150–156. [PubMed] [Google Scholar]
- 36.Pipicelli J.G., King G.J.W. Rehabilitation of elbow instability. Hand Clin. 2020;36(4):511–522. doi: 10.1016/j.hcl.2020.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Samdanis V., Manoharan G., Jordan R.W. Indications and outcome in total elbow arthroplasty: a systematic review. Shoulder Elbow. 2020;12(5):353–361. doi: 10.1177/1758573219873001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prkic A., Welsink C., The B., van den Bekerom M.P.J., Eygendaal D. Why does total elbow arthroplasty fail today? A systematic review of recent literature. Arch Orthop Trauma Surg. 2017;137(6):761–769. doi: 10.1007/s00402-017-2687-x. [DOI] [PubMed] [Google Scholar]
- 39.Mansat P., Bonnevialle N., Rongières M., Mansat M., Bonnevialle P. Experience with the Coonrad-Morrey total elbow arthroplasty: 78 consecutive total elbow arthroplasties reviewed with an average 5 years of follow-up. J Shoulder Elbow Surg. 2013;22(11):1461–1468. doi: 10.1016/j.jse.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 40.Cil A., Veillette C.J., Sanchez-Sotelo J., Morrey B.F. Linked elbow replacement: a salvage procedure for distal humeral nonunion. J Bone Joint Surg Am. 2008;90(9):1939–1950. doi: 10.2106/JBJS.G.00690. [DOI] [PubMed] [Google Scholar]
- 41.Skyttä E.T., Eskelinen A., Paavolainen P., Ikävalko M., Remes V. Total elbow arthroplasty in rheumatoid arthritis: a population-based study from the Finnish Arthroplasty Register. Acta Orthop. 2009;80(4):472–477. doi: 10.3109/17453670903110642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jenkins P.J., Watts A.C., Norwood T., Duckworth A.D., Rymaszewski L.A., McEachan J.E. Total elbow replacement: outcome of 1,146 arthroplasties from the Scottish Arthroplasty Project. Acta Orthop. 2013;84(2):119–123. doi: 10.3109/17453674.2013.784658. [DOI] [PMC free article] [PubMed] [Google Scholar]