Summary
Protein transport toward the nucleus is important for translating molecular signals into gene expression changes. Interestingly, the unconventional motor protein myosin VI regulates RNA polymerase II-dependent gene transcription. Whether actin-filament-dependent myosins are actively transported to nuclear compartments remains unknown.
Here, we report that neurons also contain myosin VI inside their nucleus. Notably, nuclear appearance of this actin-dependent motor depends on functional cytoplasmic dynein, a minus end-directed microtubule motor. We find that the trafficking factor muskelin assists in the formation of dynein-myosin VI interactions and further localizes to nuclear foci, enriched in the myosin. Impairment of dynein, but not myosin VI function, reduces nuclear muskelin levels. In turn, muskelin represents a critical determinant in regulating myosin VI nuclear targeting.
Our data reveal that minus end-directed microtubule transport determines myosin VI subcellular localization. They suggest a pathway of cytoplasm-to-nucleus trafficking that requires muskelin and is based on dynein-myosin cross talk.
Subject Areas: Biological Sciences, Neuroscience, Molecular Neuroscience, Cellular Neuroscience, Cell Biology
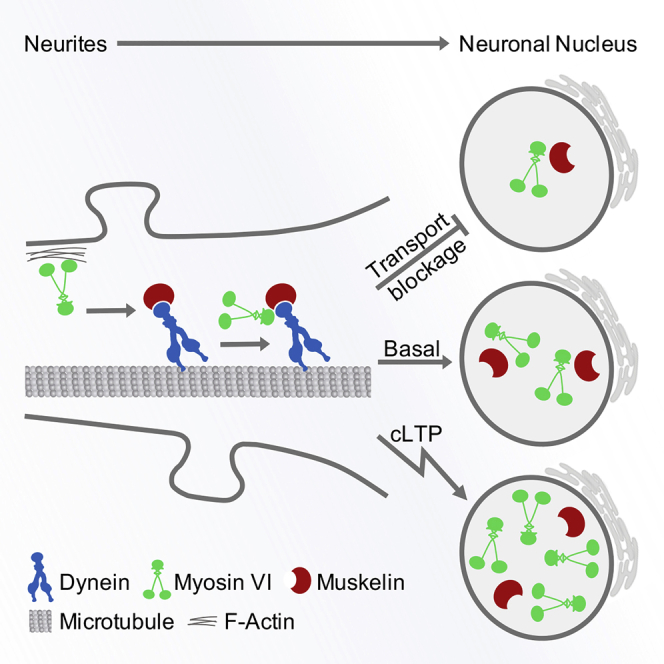
Graphical abstract
Highlights
-
•
Myosin VI and muskelin are recruited to the neuronal nucleus
-
•
Active cytoplasmic dynein is required for myosin VI and muskelin nuclear targeting
-
•
Muskelin regulates myosin VI to dynein binding and myosin VI nuclear translocation
-
•
Dynein mediates nuclear translocation of myosin VI during cLTP
Biological Sciences ; Neuroscience ; Molecular Neuroscience ; Cellular Neuroscience ; Cell Biology
Introduction
Molecular motors execute a variety of critical processes within various cell types. The families of kinesins and dyneins represent microtubule (MT)-associated ATPases, whereas the myosin family motor proteins perform cellular processes associated with the actin cytoskeleton (Hirokawa et al., 2010; Schliwa and Woehlke, 2003; Vale, 2003). Although kinesins and dynein mainly act within the cytoplasm, recent evidence points to important roles of specific myosins in mammalian nuclei (Cook et al., 2020).
Cytoplasmic dynein is the key MT minus end-directed motor essential for long-distance transport and positioning of organelles, vesicular cargoes, and cytoplasmic molecules (Reck-Peterson et al., 2018). The dynein transport machinery consists of the dynein complex, the dynactin complex required for movement along MTs, and coiled-coil-containing activating proteins. In neurons, due to their highly polarized architecture, dynein often transports cargoes from the cell periphery to the cell body, as observed for the endo-lysosomal trafficking of neurotransmitter receptors (Heisler et al., 2011; Maas et al., 2006) and other synaptic proteins (Boecker and Holzbaur, 2019; Heisler et al., 2018; Schapitz et al., 2010). The specificity of cargo selection and the regulation of motor activities are often achieved through adaptor and accessory transport factors (Heisler et al., 2014; Kneussel et al., 2014; Olenick and Holzbaur, 2019; Reck-Peterson et al., 2018). Although dynein is suggested to functionally associate with kinesin-1 motor complexes for bidirectional cargo trafficking and self-positioning (Olenick and Holzbaur, 2019), the cross talk of molecular motors between the actin and MT cytoskeleton remains largely unexplored (Coles and Bradke, 2015).
Cytoplasmic dynein mainly promotes long-distance transport of cargoes along MT tracks, whereas actin-based myosin VI functions include the regulation of Golgi complex integrity, F-actin dynamics, and cell migration (Kneussel and Wagner, 2013; Tumbarello et al., 2013). Besides myosin VI's ability to act as a load-dependent anchor (Altman et al., 2004), it represents a unique myosin family member that moves to F-actin minus ends (Wells et al., 1999). At the cell cortex and in synaptic spines, myosin VI regulates vesicular trafficking and the endocytosis of neurotransmitter receptors (Buss et al., 2001; Heisler et al., 2011; Morris et al., 2002; Osterweil et al., 2005; Wagner et al., 2019). The cytoplasmic role of myosin VI and its structural properties have been thoroughly investigated (Tumbarello et al., 2013), whereas actin and myosin VI notably exist in the mammalian nucleus with their nuclear functions only being about to be unraveled (Cook et al., 2020).
Within nuclei, myosin VI was identified to associate with RNA polymerase II (RNAPII) (Vreugde et al., 2006) and to functionally regulate hormone and cytokine receptor-dependent gene transcription (Fili et al., 2017, 2020; Loikkanen et al., 2009; Zorca et al., 2015). Mechanistically, myosin VI was suggested to function as an auxiliary motor for RNAPII to drive gene expression while undergoing regulated and direct DNA binding (Fili et al., 2017, 2020; Vreugde et al., 2006). Only recently, the active movement of myosin VI along actin filaments in the nucleus was also detected (Grosse-Berkenbusch et al., 2020). The functions of myosin VI inside the nucleus include transcription-dependent chromatin rearrangements (Grosse-Berkenbusch et al., 2020), gene pairing (Zorca et al., 2015), and the spatial organization of transcription initiation (Hari-Gupta et al., 2020). A role of the myosin in gene transcription is further supported by studies of the p53-dependent pro-survival pathway (Cho and Chen, 2010; Jung et al., 2006) where it was found to redistribute from cytoplasmic to nuclear compartments upon DNA damage. Interestingly, myosin VI nuclear accumulation was also increased after stimulation of neuroendocrine PC12 cells (Majewski et al., 2018) and after serum stimulation of HeLa cells (Hari-Gupta et al., 2020). These observations suggest that active molecular processes might underlie myosin VI nuclear appearance. Whether myosin VI localizes to neuronal nuclei in mammalian brain as well as the molecular factors that might transport the myosin for its potential active nuclear targeting in general remain unknown.
Muskelin is an evolutionary conserved protein expressed in various tissues. It was originally identified to mediate cell adhesive and actin cytoskeletal responses (Adams et al., 1998) and to act as an integrator of cell morphology and nucleocytoplasmic communication in skeletal myoblasts (Valiyaveettil et al., 2008). Of note, in muscle cells myosin VI localization to nuclei was also observed (Karolczak et al., 2013). Recent studies described muskelin as a functional component of the CTLH complex, a macromolecular assembly suggested to reside either in the cytoplasm or in the nucleus and to integrate fundamental processes including energy metabolism, proliferation, survival, cell adhesion, and migration in response to extracellular stimuli (Huffman et al., 2019; Lampert et al., 2018; Liu and Pfirrmann, 2019; Maitland et al., 2019; Qiao et al., 2020).
In neurons, muskelin critically regulates the bidirectional vesicular transport of GABAA receptors and of the cellular prion protein PrPC (Heisler et al., 2011, 2018). It acts as a cargo adaptor, directly linking synaptic proteins to cytoplasmic dynein and has been suggested to regulate the activity of vesicular motor protein assemblies (Heisler et al., 2011, 2018). Interestingly, muskelin represents one of the few transport factors that associates with both MT-based motor proteins and the actin-dependent motor myosin VI to regulate GABAA receptor endocytosis (Heisler et al., 2011, 2018). Whether muskelin or the MT motor dynein affect transport and positioning of actin-dependent myosin motor proteins is presently unknown.
In this study we show that the actin-dependent motor protein myosin VI localizes to neuronal nuclei, depending on active transport through the MT-dependent dynein motor complex. Myosin VI nuclear targeting critically requires muskelin, a trafficking regulator known to associate with both motor systems. Our data suggest a cellular pathway that is based on myosin-dynein cross talk.
Results
Myosin VI associates with dynein and is transported to the neuronal nucleus
Myosin VI was found in the nucleus of various cell types and is suggested to functionally regulate nuclear processes (Karolczak et al., 2013; Majewski et al., 2018; Vreugde et al., 2006; Zorca et al., 2015). In neurons, the myosin motor mediates critical functions at the cellular cortex in regulating synaptic transport and plasticity (Heisler et al., 2011; Wagner et al., 2019), but whether and how myosin VI undergoes nuclear translocation in neuronal cell types has remained unknown.
We therefore isolated nuclear proteins from mouse whole brain tissue. Detection of the NMDA receptor subunit GluN2B and the neuron-specific nuclear protein NeuN (Mullen et al., 1992) served as quality controls, as they are restricted to the cytoplasmic (C) or nuclear extract (N), respectively (Figure 1A). Besides the presence of myosin VI in the cytoplasm, we detected prominent proportions in the nuclei of brain cells (Figure 1A). Likewise, actin was present in both, cytoplasmic and nuclear fractions. To verify the specificity of the myosin VI antibody used in this experiment, we prepared nuclear extracts derived from brains of homozygous (sv/sv) Snell's waltzer mice, representing spontaneous myosin VI null mutants (Avraham et al., 1995). Myosin VI was detectable in the nuclear brain extract from heterozygous animals (sv/se), whereas the myosin VI signal was lost in myosin VI-depleted fractions (sv/sv), when compared with actin, used as control (Figure 1B). We therefore conclude that brain myosin VI generally undergoes translocation to the nuclear compartment.
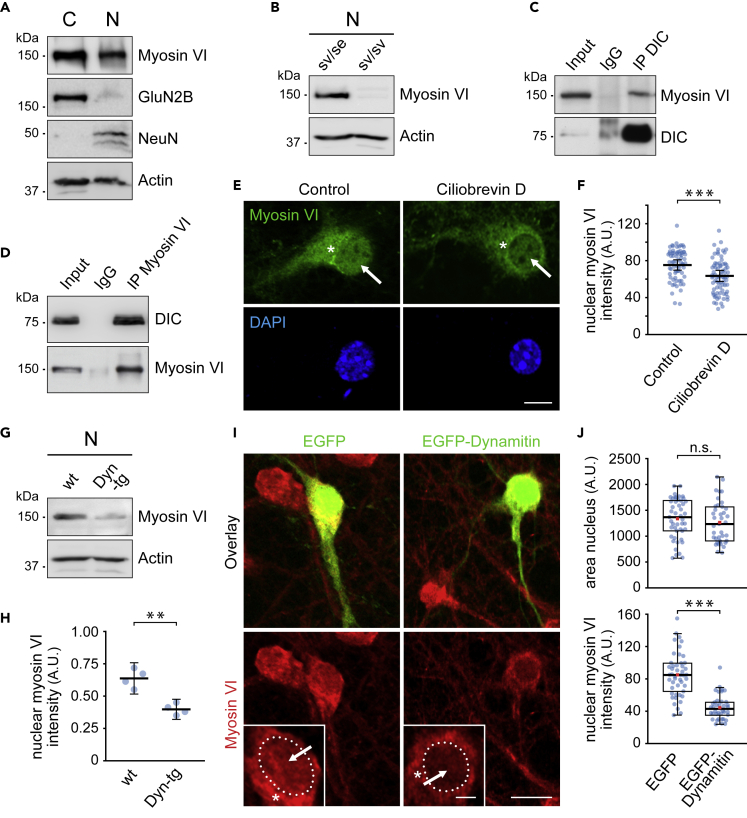
Figure 1.
Myosin VI nuclear targeting depends on dynein
(A) Myosin VI is detected in cytoplasmic (C) and nuclear fractions (N) from adult mouse brain (n = 3 experiments).
(B) Anti-myosin VI antibody specificity control in nuclear fractions (N) from homozygous (sv/sv) and heterozygous (sv/se) Snell's waltzer mice. Control: Actin.
(C) Immunoprecipitation using DIC-specific antibodies coprecipitates myosin VI from whole brain lysate in the presence of 1% Triton X-100 (n = 3 experiments).
(D) Coimmunoprecipitation of DIC with myosin VI from whole brain lysate in the presence of 1% Triton X-100 (n = 3 experiments).
(E) Confocal images showing myosin VI nuclear intensity (arrows) after immunostaining of days in vitro (DIV) 12–14 hippocampal neurons treated for 80 min with 0.4% DMSO (control) or 20 μM ciliobrevin D. Scale bar, 10 μm.
(F) Reduced nuclear myosin VI intensity in ciliobrevin D (62.25 ± 4.15) when compared with control (75.74 ± 3.98) treated neurons. Control: n = 69 cells; ciliobrevin D: n = 77 cells; 3 experiments. Data represent mean ± the 95% confidence intervals for the mean. Independent samples t test, p < 0.001, ∗∗∗ indicates statistical significance.
(G) Nuclear fractions (N) from brain of dynamitin-overexpressing (Dyn-tg) and wild-type (wt) mice.
(H) Reduced nuclear myosin VI levels in Dyn-tg (0.40 ± 0.07) when compared with wt mice (0.64 ± 0.12) (n = 4 experiments). Myosin VI intensities normalized to Actin. Data represent mean ± the 95% confidence intervals for the mean. Independent samples t test, p < 0.01, ∗∗ indicates statistical significance.
(I) Confocal images showing myosin VI nuclear intensity (arrows) after immunostaining of DIV 12–14 hippocampal neurons overexpressing EGFP or EGFP-dynamitin. Scale bar: 20 μm and 5 μm in insets.
(J) No significant differences in nuclear area between EGFP-dynamitin- (median = 1,225.00) and EGFP- (median = 1,358.00) overexpressing neurons, Mann-Whitney U test, U = 784.000, Z = −1.149, p = 0.250. Reduced nuclear myosin VI intensity in EGFP-dynamitin- (median = 43.15) when compared with EGFP- (median = 84.82) overexpressing neurons. Distributions differed between groups, two-sample Kolmogorov-Smirnov, p < 0.001, and nuclear myosin VI intensity significantly differed between groups, Mann-Whitney U test, U = 162.00, Z = −6.545, p < 0.001, ∗∗∗ indicates statistical significance. EGFP: n = 47 cells; EGFP-dynamitin: n = 39 cells; 4 experiments. Box borders represent the 25th and 75th percentiles, horizontal lines inside boxes indicate median, and whiskers represent values less than 1.5 times the interquartile range lower or higher than the 25th and 75th percentiles, respectively. Red squares indicate the mean.
Whether mammalian myosin VI motors reach the nucleus via passive diffusion or active transport is presently unknown. To gain mechanistic insights into the process of its nuclear accumulation, we asked whether the myosin might bind to other motor proteins, known to mediate active transport toward neuronal somata. Notably, precipitation of endogenous dynein intermediate chain (DIC) from brain lysate resulted in coprecipitation of endogenous myosin VI (Figure 1C), indicating that the myosin motor is found in a protein complex containing the dynein motor. Accordingly, precipitation of myosin VI, vice versa, led to coprecipitation of DIC (Figure 1D). As the experiments were performed in the presence of detergent, we exclude that both motors just share the same transport vesicle. To test whether dynein affects myosin VI subcellular localization, we first treated cultured hippocampal neurons with ciliobrevin D (Cilio D), a specific inhibitor of the AAA+ ATPase activity of the dynein motor domain (Firestone et al., 2012). Under control conditions, immunostaining revealed high myosin VI signals in the somatic regions close to the neuronal nucleus (Figures 1E and 1I, asterisks), which likely represent the role of myosin VI in Golgi organization (Jung et al., 2006; Sahlender et al., 2005; Tumbarello et al., 2013). In addition, neuronal myosin VI was detectable as punctate signals inside the nuclei (Figures 1E and 1I, arrows). However, the acute treatment of hippocampal neurons with Cilio D caused a moderate but significant reduction in myosin VI nuclear signal intensity (Figures 1E and 1F). These data suggest that the nuclear delivery of myosin VI requires active dynein-based cytoskeleton transport.
To validate this finding, we next applied an independent loss-of-function approach using transgenic mice that overexpress dynamitin in the brain (LaMonte et al., 2002; Perlson et al., 2009). Dynamitin is a subunit of the dynactin complex associated with dynein, and its overexpression leads to dynein-dynactin dissociation (Burkhardt et al., 1997; King and Schroer, 2000; Palazzo et al., 2001; Valetti et al., 1999). Overexpressed dynamitin has been shown to effectively interfere with dynactin- and dynein-mediated processes such as Golgi dynamics and cargo transport, including neuronal trafficking. Notably, the levels of myosin VI but not of actin (control) were significantly decreased in nuclear fractions derived from dynamitin-overexpressing mice (Dyn-tg), when compared with wild-type fractions (Figures 1G and 1H). We next aimed to test whether these results would similarly apply to hippocampal neurons. To interfere with dynein-mediated transport, we therefore overexpressed EGFP-dynamitin (Burkhardt et al., 1997). Although this condition did not alter the average area of neuronal nuclei, it strongly reduced myosin VI signal intensity within the nuclear compartment (Figures 1I and 1J).
Together, our data suggest that the MT-dependent motor dynein piggybacks the myosin VI motor to promote its nuclear translocation.
The microtubule- and actin-associated transport factor muskelin enters neuronal nuclei
The transport factor muskelin is one of the few cargo adaptors that associates with both actin-dependent myosin VI and MT-based dynein motor complexes, to interconnect subsequent steps of cytoplasmic cargo trafficking (Heisler et al., 2011, 2018). Interestingly, fluorescent fusion proteins of muskelin mutants were reported to differentially enter the nucleus in skeletal myoblasts and neurons (Delto et al., 2015; Valiyaveettil et al., 2008).
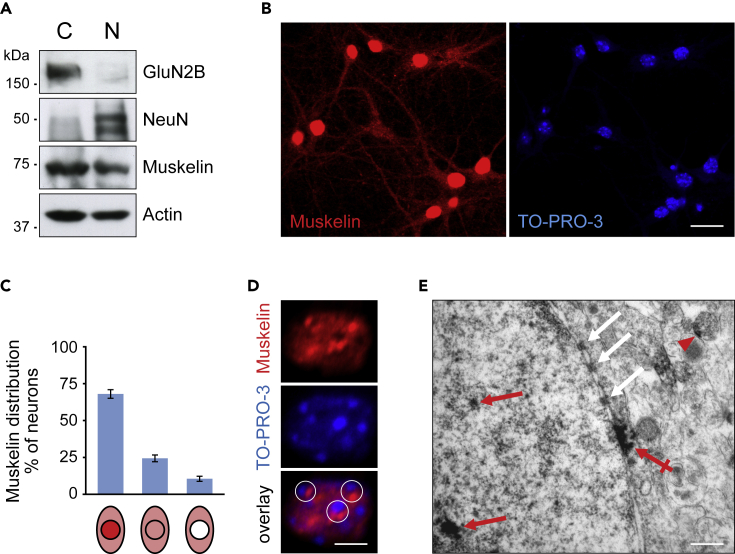
To assess whether muskelin could be involved in dynein-mediated nuclear targeting of myosin VI, we initially performed cellular fractionation experiments to further characterize the subcellular localization of endogenous muskelin in murine brain. Following isolation of proteins from whole brain tissue, we identified muskelin in both cytoplasmic (C) and nuclear (N) extracts (Figure 2A). In contrast, the NMDA receptor subunit GluN2B and the neuron-specific nuclear protein NeuN, used as fractionation controls, were restricted to the cytoplasmic or nuclear extract, respectively (Figure 2A). Upon immunostaining of endogenous protein in cultured hippocampal neurons, we further confirmed the nuclear localization of muskelin (Figure 2B). Quantification revealed that about 67% of all cells showed an enrichment of the transport factor within their nuclei, whereas only 7% of the cells turned out negative for nuclear muskelin under neuron culture conditions (Figure 2C). Within the neuronal nucleus, muskelin displayed a punctate distribution. Areas of highest muskelin intensities were frequently detected adjacent to foci of TO-PRO-3-labeled DNA (Figure 2D), representing potential regions of high chromatin density (Falk et al., 2008). We further performed electron microscopy (EM) to analyze cytoplasmic and nuclear muskelin at higher resolution. Consistent with its role as a transport regulator (Heisler et al., 2011, 2018), immunodetection at the ultrastructural level confirmed muskelin at vesicular structures in the cytoplasm (Figure 2E, red arrowhead). In addition, EM revealed distinct muskelin-positive aggregates within the neuronal nucleus (Figure 2E, red arrows) and at the nuclear envelope (Figure 2E, red crossed arrow) of mouse hippocampal slices. Together, these results suggest a potential contribution of muskelin to nuclear functions in neurons.
Figure 2.
Localization of muskelin in neuronal nuclei
(A) Cytoplasmic (C) and nuclear extracts (N) after cell fractionation from adult mouse brain contain muskelin (n = 3 experiments).
(B) Immunostainings of muskelin in DIV 12–14 hippocampal neurons with TO-PRO-3 colabeling reveals strong nuclear muskelin signals. Scale bar, 20 μm.
(C) Quantification of (B) indicating that 67.17% ± 0.03% of neurons show muskelin nuclear enrichment, 25.35% ± 0.02% cells show equal muskelin intensities across soma and nuclei, and 7.48% ± 0.01% show less muskelin in nuclear when compared to somatic regions (331 neurons, n = 3 experiments). Data represent mean ± SEM.
(D) Immunostaining of muskelin with TO-PRO-3 labeling in DIV 12–14 hippocampal neurons show punctate muskelin signals adjacent to TO-PRO-3 foci (circles) within the nucleus (n = 3 experiments). Scale bar, 5 μm.
(E) Electron microscopy showing muskelin-specific immunoperoxidase signals in hippocampal tissue slices from adult mice. Muskelin signals are detected at vesicular structures outside the nucleus (red arrowhead), at the nuclear envelope (crossed red arrow) and within the nucleus (red arrows). White arrows indicate nuclear pores. Scale bar, 50 nm.
Cytoplasmic dynein regulates nuclear muskelin levels
The neuron-specific dynein intermediate chain isoform 1a (DIC1a) directly links muskelin to the dynein motor complex (Heisler et al., 2011). In addition to muskelin's role as a coordinator of dynein-mediated transport (Heisler et al., 2011, 2018), we asked whether muskelin itself might require active dynein for its own nuclear delivery.
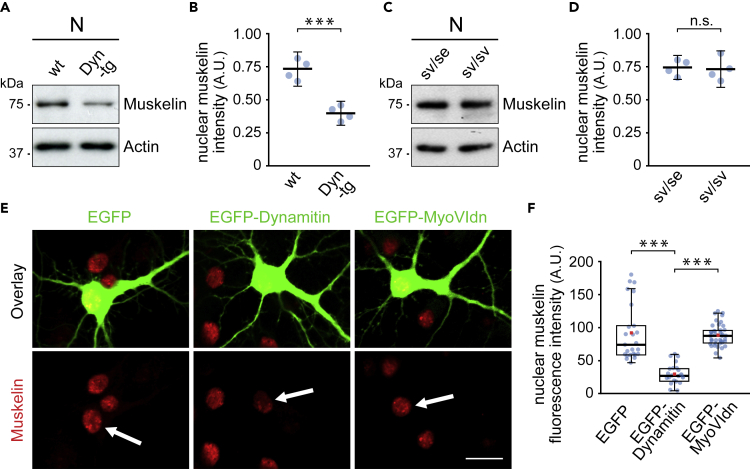
Using dynamitin-overexpressing transgenic mice, impaired in neuronal dynein-mediated transport (LaMonte et al., 2002; Perlson et al., 2009), we found that muskelin levels were significantly reduced in nuclear fractions of transgenes (Dyn-tg), when compared with wild-type and loading controls (Figures 3A and 3B). These data indicate that muskelin is actively translocated to the neuronal nucleus and that this process depends on functional dynein.
Figure 3.
Dynein drives muskelin nuclear targeting
(A) Nuclear fractions (N) from brain of dynamitin-overexpressing (Dyn-tg) and wild-type (wt) mice.
(B) Reduced nuclear muskelin levels in Dyn-tg (0.41 ± 0.09) when compared with wt mice (0.73 ± 0.13) (n = 4 experiments). Muskelin intensities normalized to Actin. Data represent mean ± the 95% confidence intervals for the mean. Independent samples t test, p < 0.001, ∗∗∗ indicates statistical significance.
(C) Nuclear fractions (N) from brain of homozygous (sv/sv) and heterozygous (sv/se) Snell's waltzer mice.
(D) No significant differences in muskelin nuclear levels in sv/sv (0.73 ± 0.14) when compared with sv/se mice (0.74 ± 0.09) (n = 4 experiments). Muskelin intensities normalized to Actin. Data represent mean ± 95% confidence intervals for the mean. Independent samples t test, p = 0.833, n.s.: not statistically significant.
(E) Confocal images of nuclear muskelin intensity (arrows) upon immunostaining of DIV 12–14 hippocampal neurons overexpressing EGFP, EGFP-dynamitin, or EGFP-MyoVIdn as indicated. Scale bar, 20 μm.
(F) Nuclear muskelin fluorescence intensity was significantly different between at least one pair of groups, Kruskal-Wallis test, χ2 (2) = 45.869, p < 0.001. Dunn's pairwise multiple comparisons test (p values adjusted using Bonferroni correction) revealed significant differences between EGFP-dynamitin (median = 24.36) and EGFP (median = 72.21), z = 5.113, p < 0.001, and between EGFP-dynamitin and EGFP-MyoVIdn (median = 85.78), z = −6.616, p < 0.001. ∗∗∗ indicates statistical significance. There was no evidence of difference between EGFP and EGFP-MyoVIdn, p = 0.872. EGFP: n = 25 cells; EGFP-dynamitin: n = 21 cells; EGFP-MyoVIdn: n = 40 cells; 3 experiments. Box borders indicate the 25th and 75th percentiles, horizontal lines inside boxes indicate median, and whiskers represent values less than 1.5 times the interquartile range lower or higher than the 25th and 75th percentiles, respectively. Red squares indicate the mean.
Based on the fact that muskelin also associates with the actin-dependent motor myosin VI (Heisler et al., 2011), we further aimed to investigate whether the myosin may potentially contribute to regulate nuclear muskelin levels. However, in contrast to the functional inhibition of the MT-dependent motor dynein, depletion of the actin-dependent motor myosin VI in homozygous Snell's waltzer mice (sv/sv) (Avraham et al., 1995) did not affect muskelin levels in nuclear brain fractions (Figures 3C and 3D). In an independent assay, we overexpressed EGFP-dynamitin (Burkhardt et al., 1997) or a dominant-negative myosin VI mutant EGFP-MyoVIdn (Heisler et al., 2011; Osterweil et al., 2005), to test whether these observations would also apply to hippocampal neurons. The fluorescence intensity of nuclear muskelin remained unaffected through EGFP-MyoVIdn, whereas it appeared strongly reduced in neurons overexpressing EGFP-dynamitin (Figures 3E and 3F). Our findings therefore indicate that dynein, but not myosin VI, is the critical driver to power muskelin translocation toward the neuronal nucleus.
Muskelin regulates myosin VI nuclear targeting
A former study on neurotransmitter receptor endocytosis had revealed subsequent steps of muskelin association with either myosin VI or dynein motor complexes (Heisler et al., 2011). In addition, we find that myosin VI motor can in general interact with cytoplasmic dynein (compare with Figures 1C and 1D). We therefore aimed to understand whether muskelin may be part of a triple myosin VI-dynein complex and whether it would act on myosin VI nuclear delivery.
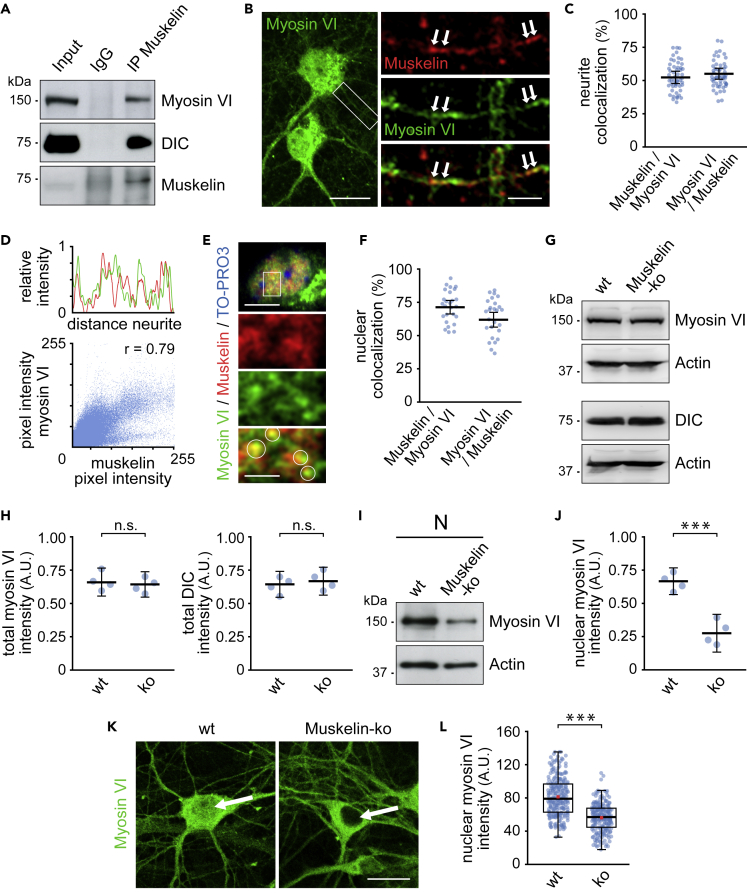
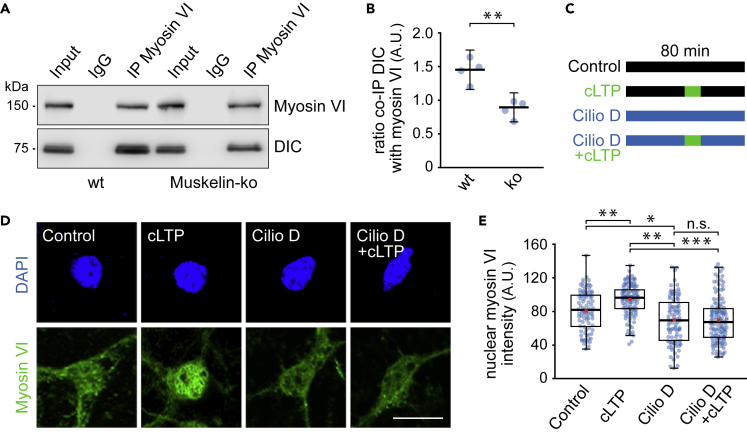
Indeed, coimmunoprecipitation experiments detected dynein and myosin VI to coprecipitate with muskelin (Figure 4A), suggesting that muskelin associates with both molecular motors at a given time. As these experiments were performed in the presence of detergent, we exclude that the proteins just share identical vesicles. Furthermore, we observed a strong correlation and frequent colocalization of muskelin and myosin VI signals in proximal dendrites of hippocampal neurons following coimmunostaining (Figures 4B–4D). Based on muskelin's role in neuronal trafficking, the colocalized signals potentially represent molecules in transit. In addition to colocalization in dendrites, we detected a frequent overlap of muskelin and myosin VI puncta in the neuronal nucleus (Figures 4E and 4F). To test whether muskelin controls myosin VI nuclear targeting, we then analyzed brain fractions from muskelin knockout mice (Muskelin-ko) (Heisler et al., 2011). The overall levels of myosin VI and dynein in total brain fractions equaled between genotypes, indicating that muskelin does not affect the expression or degradation of the motor proteins (Figures 4G and 4H). However, nuclear protein levels of myosin VI were strongly reduced in muskelin-deficient mice, when compared with wild-type and loading controls (Figures 4I and 4J). These results were also confirmed in a second and neuron-specific approach. Following immunostaining of cultured hippocampal neurons, we found that muskelin deficiency (Muskelin-ko) caused a significant decrease in myosin VI signals in the neuronal nucleus (Figures 4K and 4L).
Figure 4.
Muskelin regulates myosin VI nuclear targeting
(A) Myosin VI and dynein coprecipitate with muskelin after immunoprecipitation from whole brain lysate in the presence of 1% Triton X-100 (n = 3 experiments).
(B) Coimmunostaining of myosin VI and muskelin in DIV 12–14 hippocampal neurons. Arrows in magnified region (boxed region) indicate myosin VI and muskelin colocalization in dendrites. Scale bars: 20 μm in overview, 5 μm in boxes.
(C) Quantification: % of muskelin signals overlapping myosin VI (52.22 ± 3.06), % of myosin VI signals overlapping muskelin (54.94 ± 2.75), n = 52 cells; 4 experiments. Data represent mean ± 95% confidence intervals for the mean.
(D) Upper plot: Line scan of (B) showing muskelin (red) and myosin VI (green) intensities along the dendrite segment. Lower plot: Scatterplot showing correlation (Pearson correlation coefficient r = 0.79) between myosin VI and muskelin pixel intensities.
(E) Coimmunostaining detects muskelin and myosin VI colocalized puncta (circles) in nuclei (TO-PRO-3 labeling) of DIV 12–14 hippocampal neurons. Boxed nuclear region is shown at higher magnification. Scale bars: 10 μm in overview, 3 μm in boxes.
(F) Quantification: % of muskelin signals overlapping myosin VI (70.91 ± 4.73), % of myosin VI signals overlapping muskelin (62.49 ± 5.61), n = 26 cells; 4 experiments. Data represent mean ± 95% confidence intervals for the mean.
(G) Whole brain lysate of muskelin knockout (Muskelin-ko) and wild-type (wt) mice.
(H) No statistically significant differences in total myosin VI or DIC levels between wt (Myo VI: 0.67 ± 0.11; DIC: 0.64 ± 0.10) and ko brain lysate (Myo VI: 0.64 ± 0.08; DIC: 0.68 ± 0.11) (n = 4 experiments). Myosin VI intensities normalized to Actin. Data represent mean ± the 95% confidence intervals for the mean. Independent samples t test, p = 0.441 (Myo VI); p = 0.433 (DIC), n.s.: not statistically significant.
(I) Nuclear extracts (N) from adult brains of muskelin knockout and wild-type mice.
(J) Reduced nuclear myosin VI levels in Muskelin-ko (0.28 ± 0.15) compared with wt mice (0.66 ± 0.10) (n = 4 experiments). Myosin VI intensities normalized to Actin. Data represent mean ± 95% confidence intervals for the mean. Independent samples t test, p < 0.001, ∗∗∗ indicates statistical significance.
(K) Confocal images of nuclear myosin VI intensity (arrows) upon immunostaining of DIV 12–14 hippocampal neurons from muskelin knockout or wild-type mice. Scale bar, 20 μm.
(L) Reduced nuclear myosin VI intensity in Muskelin-ko (median = 56.41) when compared with wt (median = 78.21) neurons. Distributions differed between both groups, two-sample Kolmogorov-Smirnov, p < 0.001, and nuclear myosin VI intensity significantly differed between both groups, Mann-Whitney U test, U = 10,324.00, Z = −11.115, p < 0.001, ∗∗∗ indicates statistical significance. Muskelin-ko: n = 219 cells; wt: n = 237 cells; 4 experiments. Box borders represent the 25th and 75th percentiles, horizontal lines inside boxes indicate median, and whiskers represent values less than 1.5 times the interquartile range lower or higher than the 25th and 75th percentiles, respectively. Red squares indicate the mean.
With respect to a functional triple complex harboring muskelin and the two motor proteins (compare with Figure 4A), we hypothesized that reduced nuclear myosin VI levels in muskelin-depleted neurons might be due to impaired myosin VI to dynein binding in the absence of muskelin. Notably, coprecipitation of DIC with myosin VI was significantly reduced in muskelin-depleted brain lysate (Muskelin-ko), compared with lysate obtained from wild-type mice (wt) (Figures 5A and 5B). This suggests that muskelin assists in the formation of a dynein-myosin VI interaction. As myosin VI has been shown to undergo stimuli-dependent nuclear translocation in several cell lines (Cho and Chen, 2010; Hari-Gupta et al., 2020; Jung et al., 2006; Majewski et al., 2018), we finally asked whether the localization of neuronal myosin VI would respond to neuron-specific stimulation paradigms. Indeed, immunostainings of hippocampal neurons revealed a significant increase of nuclear myosin VI intensity, following 30 min of recovery after induction of chemical long-term potentiation (cLTP) (Figures 5C–5E) (Franchini et al., 2019; Otmakhov et al., 2004). Furthermore, whereas nuclear myosin VI levels were reduced in neurons treated with Cilio D, induction of cLTP in the presence of Cilio D (Cilio D + cLTP) did not cause an increase in nuclear myosin VI levels that was observed for neurons treated with cLTP alone (Figures 5C–5E). These data indicate that dynein-dependent processes contribute to the nuclear translocation of myosin VI following cLTP stimulation.
Figure 5.
cLTP-induced nuclear translocation of myosin VI
(A) Coprecipitation of DIC with myosin VI after immunoprecipitation in the presence of 1% Triton X-100 using brain lysate from wild-type or muskelin knockout mice.
(B) Reduced coprecipitation of DIC with myosin VI using Muskelin-ko (0.87 ± 0.21) when compared with wt lysate (1.44 ± 0.29). DIC coprecipitation was normalized to myosin VI immunoprecipitation (n = 4 experiments). Data represent mean ± 95% confidence intervals for the mean. Independent samples t test, p < 0.01, ∗∗ indicates statistical significance.
(C and D) (C) cLTP and ciliobrevin treatment scheme (D) Confocal images showing nuclear myosin VI intensity upon immunostaining of DIV 12–14 hippocampal neurons treated with 0.4% DMSO (control) or 20 μM ciliobrevin D (cilio D) for 80 min or with additional cLTP induction after 40 min during control (cLTP) or ciliobrevin D treatment (cilio D + cLTP). Scale bar, 20 μm.
(E) Nuclear myosin VI intensity was significantly different between at least one pair of groups, Kruskal-Wallis test, χ2 (2) = 72.561, p < 0.001. Dunn's pairwise multiple comparisons test (p values adjusted using Bonferroni correction) revealed significant differences between control (median = 80.95) and cLTP (median = 95.85), z = −3.572, p < 0.01, ∗∗ indicates statistical significance; between cilio D (median = 68.77) and control, z = 2.821, p < 0.05, ∗ indicates statistical significance; between cilio D and cLTP, z = −6.678, p < 0.01, ∗∗ indicates statistical significance; and between cilio D + cLTP (median = 66.46) and cLTP, z = 7.775, p < 0.001, ∗∗∗ indicates statistical significance. There was no evidence of difference between cilio D + cLTP and cilio D. Control: n = 86 cells; cilio D: n = 93 cells; cLTP: n = 115 cells; cilio D + cLTP: n = 130 cells; 3 experiments. Box borders indicate the 25th and 75th percentiles, horizontal lines inside boxes indicate median, and whiskers represent values less than 1.5 times the interquartile range lower or higher than the 25th and 75th percentiles, respectively. Red squares indicate the mean.
In summary, our findings identify myosin VI in neuronal nuclei and suggest a mechanism, by which dynein piggybacks myosin VI to regulate its nuclear trafficking. Using transgenic, mutant, and KO mice, we characterized muskelin's and myosin VI's nuclear entry and suggest muskelin to regulate the active transport of myosin VI toward the nuclear compartment.
Discussion
In this study, we report the association of actin-dependent myosin VI with the MT-based motor protein dynein. Myosin VI shows a punctate localization in the nucleus of hippocampal neurons and transgenic mice impaired in dynein motor function, or acute inhibition of the dynein ATPase reveals a reduced nuclear targeting of myosin VI. The trafficking factor muskelin assists in the formation of a dynein-myosin VI interaction and also displays a punctate nuclear distribution in the majority of neurons. In contrast, the genetic knockout of muskelin interferes with myosin VI nuclear targeting. Together our findings indicate that myosin VI undergoes active transport toward the nuclear compartment mediated by cytoplasmic dynein in a muskelin-dependent manner.
The presented data connect to previous findings of elevated nuclear myosin VI levels after stimulation of mammalian cell lines (Hari-Gupta et al., 2020; Jung et al., 2006; Majewski et al., 2018). They extend these observations by showing that at least a prominent portion of myosin VI depends on an active transfer toward the mammalian nucleus. Furthermore, the association of the myosin with cytoplasmic dynein and its cargo adaptor muskelin might enable a coordinated nuclear targeting of myosin VI under certain cellular conditions. In this respect, our data identify that neuron-specific stimuli, such as the induction of cLTP, can trigger myosin VI recruitment to the neuronal nucleus. They also provide a first hint that the dynein-muskelin-assisted transport pathway contributes to stimuli-induced myosin VI nuclear translocation.
Although the latter hypothesis requires further investigation, it is supported by the role of dynein in cytoplasm-to-nucleus communication in neuronal tissue. Neurotrophins and their receptors, such as TrkB-BDNF signaling endosomes, undergo nerve terminal-to-soma-directed transport through dynein underlying their downstream nuclear signaling (Gauthier et al., 2004; Heerssen et al., 2004; Olenick et al., 2019). Dynein also associates with cytoplasmic importins and kinases after nerve injury and mediates subsequent retrograde transport to enable nuclear injury responses (Hanz et al., 2003; Perlson et al., 2005). With respect to synaptic signal-to-nucleus communication, dynein binding to the soluble messenger proteins Jacob or CRTC1 was shown to constitute a prerequisite in transmission of stimulus-dependent information from synaptic-to-nuclear compartments (Herbst and Martin, 2017; Karpova et al., 2013; Lever et al., 2015). Together, it is well possible that in addition to cLTP induction, other stimuli might trigger myosin VI nuclear translocation in neurons. Based on myosin VI observations from cell lines, these stimuli might include axonal injury, DNA damage, and pathways of hormone or nutrient signaling in neurons.
In general, it is reasonable to speculate that myosin VI nuclear targeting will underlie a two-step process, by which active dynein first mediates transport toward the nuclear compartment, followed by the shuttling of the myosin across the nuclear envelope. Our data, together with our previous findings (Heisler et al., 2011), suggest that muskelin, as a direct dynein adaptor and myosin VI binding partner at the cell cortex, already coordinates the long-distance delivery of myosin VI toward neuronal somata. In this respect, muskelin might facilitate the association of myosin VI with dynein intermediate chain through direct interaction with DIC involving its LisH/CTLH domain (Heisler et al., 2011, 2018).
However, adding a second level of complexity, proteins harboring these kinds of motifs were in addition reported to substantially provide nuclear localization activity (Emes and Ponting, 2001; Lampert et al., 2018; Qiao et al., 2020; Valiyaveettil et al., 2008). Consistent with this view, the disruption of muskelin dimerization through mutations of its LisH/CTLH domain, thereby exposing this element, triggered the translocation of mRFP-muskelin fusion proteins into the nucleus (Delto et al., 2015). Furthermore, Valiyaveettil et al. also observed nuclear localization activity of muskelin's LisH/CTLH motif by studying GFP fusion proteins and showed that other proteins lacking nuclear import activity were shuttled into mammalian nuclei, when fused to muskelin's LisH/CTLH motif. In summary, (1) the dynein-dependent delivery of myosin VI toward the nucleus and (2) the nuclear import of myosin VI likely represent two distinct highly regulated processes. In parts, these sequential steps might overlap and share some of its multifunctional molecules such as, for instance, muskelin.
In this context it is noteworthy that myosin VI itself also harbors several nuclear localization sequence motifs that seem to be functional (Majewski et al., 2018). A fraction of myosin VI, which already localizes close to the nucleus, might therefore also undergo nuclear entry independent of muskelin. In general, it is plausible that not only one mechanism of nuclear import exists for the myosin (Cook et al., 2020), but also that it undergoes nuclear entry via different pathways. Beside the possibility of an nuclear localization sequence and eventually importin-assisted shuttling across the nuclear envelope, myosin VI nuclear import is likely to be controlled through interactions with other proteins (Cook et al., 2020; Fili et al., 2017; Majewski et al., 2018). Based on our data, we suggest that the dynein-muskelin-assisted pathway is dedicated to coordinate the long-distance delivery of myosin VI toward the nucleus, rather than its nuclear import. However, the prominent and punctate localization of muskelin itself, as detected in the majority of neuronal nuclei, suggests that it potentially stays associated with myosin VI and perhaps supports the process of nuclear import. Furthermore, we found muskelin and myosin VI puncta to frequently colocalize in neuronal nuclei, and myosin VI was reported to accumulate in discrete puncta in the nucleus associated with RNAPII, PML nuclear bodies and nuclear speckles. Given the role of myosin VI in cellular processes such as proliferation, survival, cell adhesion, and migration (Cho and Chen, 2010; Fili et al., 2017; Hari-Gupta et al., 2020; Jung et al., 2006; Loikkanen et al., 2009; Vreugde et al., 2006; Zorca et al., 2015), it is noteworthy that muskelin and other CTLH complex-associated proteins that contain the LisH/CTLH motifs were reported to essentially affect the same fundamental processes as discussed for myosin VI (Huffman et al., 2019; Lampert et al., 2018; Liu and Pfirrmann, 2019; Maitland et al., 2019; Qiao et al., 2020). Consistent with these cellular functions both, myosin VI and muskelin are reported to show significant association with breast and prostate cancers (Chen et al., 2019; Dunn et al., 2006; Gueron et al., 2014; Jung et al., 2019; Wang et al., 2015; Yoshida et al., 2004). Intriguingly, an affinity-based approach to screen for binding partners of the Drosophila homolog of myosin VI (Jaguar) identified muskelin and the other six CTLH complex members to associate with myosin VI (Finan et al., 2011). Therefore, the possible nuclear functions of muskelin and other CTLH complex members, as well as the question to what extent these functions may overlap with myosin VI-regulated nuclear processes, warrants further investigation.
In summary, our data identify that myosin VI nuclear localization is regulated through the dynein motor complex and muskelin. They suggest a mechanism by which MT-dependent dynein piggybacks the myosin motor to reach the neuronal nucleus. To our knowledge, this is one of the first reports providing evidence that myosin motors can actively be positioned to reach specific subcellular compartments by means of MT-based cytoskeleton transport.
Limitations of the study
In this study, we analyzed cultured primary hippocampal neurons and whole brain fractions of genetically modified mice for myosin VI nuclear targeting. In the future, it will be interesting to see whether dynein and muskelin regulate myosin VI nuclear delivery also in other cell types of the brain. The dynein-muskelin-assisted trafficking pathway might further be important in tissues where myosin VI nuclear functions were initially described, including cervical-, breast-, and adrenal gland cell lines. Although myosin VI was suggested to regulate hormone- and cytokine receptor-dependent nuclear functions in these tissues, future studies are warranted to investigate whether these triggers also affect myosin VI nuclear translocation and function in neurons.
In addition, we like to mention the technical limitations of the study. As the dynein transport machinery is important for a variety of cellular processes, the tools to only interfere with one isolated dynein-mediated process are limited. With respect to our study, overexpression of dynamitin in transgenic mice or transiently in cultured cells has previously been shown not only to inhibit MT-based dynein-mediated transport but also to affect Golgi morphology (Burkhardt et al., 1997; LaMonte et al., 2002; Valetti et al., 1999). A subpopulation of myosin VI reported to associate with the Golgi (Tumbarello et al., 2013) might therefore also undergo redistribution following dynamitin overexpression. Although we additionally applied the first dynein-specific chemical antagonist ciliobrevin in acute treatments (80 min, 20 μM), a dose-dependent effect of ciliobrevins (4-h treatment) on Golgi morphology has previously been observed (See et al., 2016). Importantly, depletion of the direct dynein adaptor muskelin, which does not localize to the Golgi, but regulates long-distance transport of synaptic cargo (Heisler et al., 2011, 2018), revealed comparable and similarly pronounced effects on myosin VI nuclear targeting when compared with dynein inhibition.
Resource availability
Lead contact
Further information and request for resources should be directed to and will be fulfilled by the lead contact, Frank F. Heisler (frank.heisler@zmnh.uni-hamburg.de).
Materials availability
All unique/stable reagents generated in this study are available from the lead contact with a completed Materials Transfer Agreement.
Data and code availability
All data are included in the manuscript.
Methods
All methods can be found in the accompanying transparent methods supplemental file.
Acknowledgments
We are grateful to Erika L. F. Holzbaur for tissue lysates of transgenic mice overexpressing dynamitin. We also thank Michaela Schweizer for expertise in electron microscopy and Yvonne Pechmann for technical support. This work was supported by the Deutsche Forschungsgemeinschaft grant KN556/16-1 to M.K. and by the Deutsche Forschungsgemeinschaft FOR 2419 grants, project KN556/11-2 to M.K. and project HE8413/1-1 to F.F.H., Landesforschungsförderung Hamburg LFF-FV74 and LFF-FV76 to M.K., and the Ritz-Stiftung to F.F.H.
Author contribution
F.F.H. and M.K. designed and coordinated the project. F.F.H., N.S.-R., and M.M. performed the experiments. F.F.H. and M.K. analyzed the data. F.F.H. and M.K. wrote the manuscript and prepared the figures.
Declaration of interest
The authors declare no competing interests.
Published: May 21, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102416.
Contributor Information
Matthias Kneussel, Email: matthias.kneussel@zmnh.uni-hamburg.de.
Frank F. Heisler, Email: frank.heisler@zmnh.uni-hamburg.de.
Supplemental information
References
- Adams J.C., Seed B., Lawler J. Muskelin, a novel intracellular mediator of cell adhesive and cytoskeletal responses to thrombospondin-1. EMBO J. 1998;17:4964–4974. doi: 10.1093/emboj/17.17.4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman D., Sweeney H.L., Spudich J.A. The mechanism of myosin VI translocation and its load-induced anchoring. Cell. 2004;116:737–749. doi: 10.1016/s0092-8674(04)00211-9. [DOI] [PubMed] [Google Scholar]
- Avraham K.B., Hasson T., Steel K.P., Kingsley D.M., Russell L.B., Mooseker M.S., Copeland N.G., Jenkins N.A. The mouse Snell's waltzer deafness gene encodes an unconventional myosin required for structural integrity of inner ear hair cells. Nat. Genet. 1995;11:369–375. doi: 10.1038/ng1295-369. [DOI] [PubMed] [Google Scholar]
- Boecker C.A., Holzbaur E.L. Vesicular degradation pathways in neurons: at the crossroads of autophagy and endo-lysosomal degradation. Curr. Opin. Neurobiol. 2019;57:94–101. doi: 10.1016/j.conb.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt J.K., Echeverri C.J., Nilsson T., Vallee R.B. Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J. Cell Biol. 1997;139:469–484. doi: 10.1083/jcb.139.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss F., Arden S.D., Lindsay M., Luzio J.P., Kendrick-Jones J. Myosin VI isoform localized to clathrin-coated vesicles with a role in clathrin-mediated endocytosis. EMBO J. 2001;20:3676–3684. doi: 10.1093/emboj/20.14.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B., Zhang C., Wang Z., Chen Y., Xie H., Li S., Liu X., Liu Z., Chen P. Mechanistic insights into Nav1.7-dependent regulation of rat prostate cancer cell invasiveness revealed by toxin probes and proteomic analysis. FEBS J. 2019;286:2549–2561. doi: 10.1111/febs.14823. [DOI] [PubMed] [Google Scholar]
- Cho S.J., Chen X. Myosin VI is differentially regulated by DNA damage in p53- and cell type-dependent manners. J. Biol. Chem. 2010;285:27159–27166. doi: 10.1074/jbc.M110.142117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles C.H., Bradke F. Coordinating neuronal actin-microtubule dynamics. Curr. Biol. 2015;25:R677–R691. doi: 10.1016/j.cub.2015.06.020. [DOI] [PubMed] [Google Scholar]
- Cook A.W., Gough R.E., Toseland C.P. Nuclear myosins - roles for molecular transporters and anchors. J. Cell Sci. 2020;133:jcs242420. doi: 10.1242/jcs.242420. [DOI] [PubMed] [Google Scholar]
- Delto C.F., Heisler F.F., Kuper J., Sander B., Kneussel M., Schindelin H. The LisH motif of muskelin is crucial for oligomerization and governs intracellular localization. Structure. 2015;23:364–373. doi: 10.1016/j.str.2014.11.016. [DOI] [PubMed] [Google Scholar]
- Dunn T.A., Chen S., Faith D.A., Hicks J.L., Platz E.A., Chen Y., Ewing C.M., Sauvageot J., Isaacs W.B., De Marzo A.M., Luo J. A novel role of myosin VI in human prostate cancer. Am. J. Pathol. 2006;169:1843–1854. doi: 10.2353/ajpath.2006.060316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emes R.D., Ponting C.P. A new sequence motif linking lissencephaly, Treacher Collins and oral-facial-digital type 1 syndromes, microtubule dynamics and cell migration. Hum. Mol. Genet. 2001;10:2813–2820. doi: 10.1093/hmg/10.24.2813. [DOI] [PubMed] [Google Scholar]
- Falk M., Lukasova E., Kozubek S. Chromatin structure influences the sensitivity of DNA to gamma-radiation. Biochim. Biophys. Acta. 2008;1783:2398–2414. doi: 10.1016/j.bbamcr.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Fili N., Hari-Gupta Y., Aston B., Dos Santos A., Gough R.E., Alamad B., Wang L., Martin-Fernandez M.L., Toseland C.P. Competition between two high- and low-affinity protein-binding sites in myosin VI controls its cellular function. J. Biol. Chem. 2020;295:337–347. doi: 10.1074/jbc.RA119.010142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fili N., Hari-Gupta Y., Dos Santos A., Cook A., Poland S., Ameer-Beg S.M., Parsons M., Toseland C.P. NDP52 activates nuclear myosin VI to enhance RNA polymerase II transcription. Nat. Commun. 2017;8:1871. doi: 10.1038/s41467-017-02050-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan D., Hartman M.A., Spudich J.A. Proteomics approach to study the functions of Drosophila myosin VI through identification of multiple cargo-binding proteins. Proc. Natl. Acad. Sci. U S A. 2011;108:5566–5571. doi: 10.1073/pnas.1101415108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestone A.J., Weinger J.S., Maldonado M., Barlan K., Langston L.D., O'Donnell M., Gelfand V.I., Kapoor T.M., Chen J.K. Small-molecule inhibitors of the AAA+ ATPase motor cytoplasmic dynein. Nature. 2012;484:125–129. doi: 10.1038/nature10936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchini L., Stanic J., Ponzoni L., Mellone M., Carrano N., Musardo S., Zianni E., Olivero G., Marcello E., Pittaluga A. Linking NMDA receptor synaptic retention to synaptic plasticity and cognition. iScience. 2019;19:927–939. doi: 10.1016/j.isci.2019.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier L.R., Charrin B.C., Borrell-Pages M., Dompierre J.P., Rangone H., Cordelieres F.P., De Mey J., MacDonald M.E., Lessmann V., Humbert S., Saudou F. Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell. 2004;118:127–138. doi: 10.1016/j.cell.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Grosse-Berkenbusch A., Hettich J., Kuhn T., Fili N., Cook A., Hari-Gupta Y., Palmer A., Streit L., Ellis P.J.I., Toseland C.P., Gebhardt J.C.M. Myosin VI moves on nuclear actin filaments and supports long-range chromatin rearrangements. bioRxiv. 2020 doi: 10.1101/2020.04.03.023614. [DOI] [Google Scholar]
- Gueron G., Giudice J., Valacco P., Paez A., Elguero B., Toscani M., Jaworski F., Leskow F.C., Cotignola J., Marti M. Heme-oxygenase-1 implications in cell morphology and the adhesive behavior of prostate cancer cells. Oncotarget. 2014;5:4087–4102. doi: 10.18632/oncotarget.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanz S., Perlson E., Willis D., Zheng J.Q., Massarwa R., Huerta J.J., Koltzenburg M., Kohler M., van-Minnen J., Twiss J.L., Fainzilber M. Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron. 2003;40:1095–1104. doi: 10.1016/s0896-6273(03)00770-0. [DOI] [PubMed] [Google Scholar]
- Hari-Gupta Y., Fili N., Dos Santos A., Cook A., Gough R.E., Reed H.C.W., Wang L., Aaron J., Venit T., Wait E. Nuclear myosin VI regulates the spatial organization of mammalian transcription initiation. bioRxiv. 2020 doi: 10.1101/2020.04.21.053124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerssen H.M., Pazyra M.F., Segal R.A. Dynein motors transport activated Trks to promote survival of target-dependent neurons. Nat. Neurosci. 2004;7:596–604. doi: 10.1038/nn1242. [DOI] [PubMed] [Google Scholar]
- Heisler F.F., Lee H.K., Gromova K.V., Pechmann Y., Schurek B., Ruschkies L., Schroeder M., Schweizer M., Kneussel M. GRIP1 interlinks N-cadherin and AMPA receptors at vesicles to promote combined cargo transport into dendrites. Proc. Natl. Acad. Sci. U S A. 2014;111:5030–5035. doi: 10.1073/pnas.1304301111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler F.F., Loebrich S., Pechmann Y., Maier N., Zivkovic A.R., Tokito M., Hausrat T.J., Schweizer M., Bahring R., Holzbaur E.L. Muskelin regulates actin filament- and microtubule-based GABA(A) receptor transport in neurons. Neuron. 2011;70:66–81. doi: 10.1016/j.neuron.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler F.F., Pechmann Y., Wieser I., Altmeppen H.C., Veenendaal L., Muhia M., Schweizer M., Glatzel M., Krasemann S., Kneussel M. Muskelin coordinates PrP(C) lysosome versus exosome targeting and impacts prion disease progression. Neuron. 2018;99:1155–1169 e1159. doi: 10.1016/j.neuron.2018.08.010. [DOI] [PubMed] [Google Scholar]
- Herbst W.A., Martin K.C. Regulated transport of signaling proteins from synapse to nucleus. Curr. Opin. Neurobiol. 2017;45:78–84. doi: 10.1016/j.conb.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N., Niwa S., Tanaka Y. Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron. 2010;68:610–638. doi: 10.1016/j.neuron.2010.09.039. [DOI] [PubMed] [Google Scholar]
- Huffman N., Palmieri D., Coppola V. The CTLH complex in cancer cell plasticity. J. Oncol. 2019;2019:4216750. doi: 10.1155/2019/4216750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung E.J., Liu G., Zhou W., Chen X. Myosin VI is a mediator of the p53-dependent cell survival pathway. Mol. Cell. Biol. 2006;26:2175–2186. doi: 10.1128/MCB.26.6.2175-2186.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S.Y., Papp J.C., Sobel E.M., Yu H., Zhang Z.F. Breast cancer risk and insulin resistance: post genome-wide gene-environment interaction study using a random survival forest. Cancer Res. 2019;79:2784–2794. doi: 10.1158/0008-5472.CAN-18-3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karolczak J., Sobczak M., Majewski L., Yeghiazaryan M., Jakubiec-Puka A., Ehler E., Slawinska U., Wilczynski G.M., Redowicz M.J. Myosin VI in skeletal muscle: its localization in the sarcoplasmic reticulum, neuromuscular junction and muscle nuclei. Histochem. Cell Biol. 2013;139:873–885. doi: 10.1007/s00418-012-1070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpova A., Mikhaylova M., Bera S., Bar J., Reddy P.P., Behnisch T., Rankovic V., Spilker C., Bethge P., Sahin J. Encoding and transducing the synaptic or extrasynaptic origin of NMDA receptor signals to the nucleus. Cell. 2013;152:1119–1133. doi: 10.1016/j.cell.2013.02.002. [DOI] [PubMed] [Google Scholar]
- King S.J., Schroer T.A. Dynactin increases the processivity of the cytoplasmic dynein motor. Nat. Cell Biol. 2000;2:20–24. doi: 10.1038/71338. [DOI] [PubMed] [Google Scholar]
- Kneussel M., Triller A., Choquet D. SnapShot: receptor dynamics at plastic synapses. Cell. 2014;157:1738–1738 e1731. doi: 10.1016/j.cell.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Kneussel M., Wagner W. Myosin motors at neuronal synapses: drivers of membrane transport and actin dynamics. Nat. Rev. Neurosci. 2013;14:233–247. doi: 10.1038/nrn3445. [DOI] [PubMed] [Google Scholar]
- LaMonte B.H., Wallace K.E., Holloway B.A., Shelly S.S., Ascano J., Tokito M., Van Winkle T., Howland D.S., Holzbaur E.L. Disruption of dynein/dynactin inhibits axonal transport in motor neurons causing late-onset progressive degeneration. Neuron. 2002;34:715–727. doi: 10.1016/s0896-6273(02)00696-7. [DOI] [PubMed] [Google Scholar]
- Lampert F., Stafa D., Goga A., Soste M.V., Gilberto S., Olieric N., Picotti P., Stoffel M., Peter M. The multi-subunit GID/CTLH E3 ubiquitin ligase promotes cell proliferation and targets the transcription factor Hbp1 for degradation. Elife. 2018;7:e35528. doi: 10.7554/eLife.35528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever M.B., Karpova A., Kreutz M.R. An Importin Code in neuronal transport from synapse-to-nucleus? Front. Mol. Neurosci. 2015;8:33. doi: 10.3389/fnmol.2015.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Pfirrmann T. The Gid-complex: an emerging player in the ubiquitin ligase league. Biol. Chem. 2019;400:1429–1441. doi: 10.1515/hsz-2019-0139. [DOI] [PubMed] [Google Scholar]
- Loikkanen I., Toljamo K., Hirvikoski P., Vaisanen T., Paavonen T.K., Vaarala M.H. Myosin VI is a modulator of androgen-dependent gene expression. Oncol. Rep. 2009;22:991–995. doi: 10.3892/or_00000526. [DOI] [PubMed] [Google Scholar]
- Maas C., Tagnaouti N., Loebrich S., Behrend B., Lappe-Siefke C., Kneussel M. Neuronal cotransport of glycine receptor and the scaffold protein gephyrin. J. Cell Biol. 2006;172:441–451. doi: 10.1083/jcb.200506066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitland M.E.R., Onea G., Chiasson C.A., Wang X., Ma J., Moor S.E., Barber K.R., Lajoie G.A., Shaw G.S., Schild-Poulter C. The mammalian CTLH complex is an E3 ubiquitin ligase that targets its subunit muskelin for degradation. Sci. Rep. 2019;9:9864. doi: 10.1038/s41598-019-46279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewski L., Nowak J., Sobczak M., Karatsai O., Havrylov S., Lenartowski R., Suszek M., Lenartowska M., Redowicz M.J. Myosin VI in the nucleus of neurosecretory PC12 cells: stimulation-dependent nuclear translocation and interaction with nuclear proteins. Nucleus. 2018;9:125–141. doi: 10.1080/19491034.2017.1421881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris S.M., Arden S.D., Roberts R.C., Kendrick-Jones J., Cooper J.A., Luzio J.P., Buss F. Myosin VI binds to and localises with Dab2, potentially linking receptor-mediated endocytosis and the actin cytoskeleton. Traffic. 2002;3:331–341. doi: 10.1034/j.1600-0854.2002.30503.x. [DOI] [PubMed] [Google Scholar]
- Mullen R.J., Buck C.R., Smith A.M. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Olenick M.A., Dominguez R., Holzbaur E.L.F. Dynein activator Hook1 is required for trafficking of BDNF-signaling endosomes in neurons. J. Cell Biol. 2019;218:220–233. doi: 10.1083/jcb.201805016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olenick M.A., Holzbaur E.L.F. Dynein activators and adaptors at a glance. J. Cell Sci. 2019;132:jcs227132. doi: 10.1242/jcs.227132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterweil E., Wells D.G., Mooseker M.S. A role for myosin VI in postsynaptic structure and glutamate receptor endocytosis. J. Cell Biol. 2005;168:329–338. doi: 10.1083/jcb.200410091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otmakhov N., Khibnik L., Otmakhova N., Carpenter S., Riahi S., Asrican B., Lisman J. Forskolin-induced LTP in the CA1 hippocampal region is NMDA receptor dependent. J. Neurophysiol. 2004;91:1955–1962. doi: 10.1152/jn.00941.2003. [DOI] [PubMed] [Google Scholar]
- Palazzo A.F., Joseph H.L., Chen Y.J., Dujardin D.L., Alberts A.S., Pfister K.K., Vallee R.B., Gundersen G.G. Cdc42, dynein, and dynactin regulate MTOC reorientation independent of Rho-regulated microtubule stabilization. Curr. Biol. 2001;11:1536–1541. doi: 10.1016/s0960-9822(01)00475-4. [DOI] [PubMed] [Google Scholar]
- Perlson E., Hanz S., Ben-Yaakov K., Segal-Ruder Y., Seger R., Fainzilber M. Vimentin-dependent spatial translocation of an activated MAP kinase in injured nerve. Neuron. 2005;45:715–726. doi: 10.1016/j.neuron.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Perlson E., Jeong G.B., Ross J.L., Dixit R., Wallace K.E., Kalb R.G., Holzbaur E.L. A switch in retrograde signaling from survival to stress in rapid-onset neurodegeneration. J. Neurosci. 2009;29:9903–9917. doi: 10.1523/JNEUROSCI.0813-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao S., Langlois C.R., Chrustowicz J., Sherpa D., Karayel O., Hansen F.M., Beier V., von Gronau S., Bollschweiler D., Schafer T. Interconversion between anticipatory and active GID E3 ubiquitin ligase conformations via metabolically driven substrate receptor assembly. Mol. Cell. 2020;77:150–163 e159. doi: 10.1016/j.molcel.2019.10.009. [DOI] [PubMed] [Google Scholar]
- Reck-Peterson S.L., Redwine W.B., Vale R.D., Carter A.P. The cytoplasmic dynein transport machinery and its many cargoes. Nat. Rev. Mol. Cell Biol. 2018;19:382–398. doi: 10.1038/s41580-018-0004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlender D.A., Roberts R.C., Arden S.D., Spudich G., Taylor M.J., Luzio J.P., Kendrick-Jones J., Buss F. Optineurin links myosin VI to the Golgi complex and is involved in Golgi organization and exocytosis. J. Cell Biol. 2005;169:285–295. doi: 10.1083/jcb.200501162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapitz I.U., Behrend B., Pechmann Y., Lappe-Siefke C., Kneussel S.J., Wallace K.E., Stempel A.V., Buck F., Grant S.G., Schweizer M. Neuroligin 1 is dynamically exchanged at postsynaptic sites. J. Neurosci. 2010;30:12733–12744. doi: 10.1523/JNEUROSCI.0896-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliwa M., Woehlke G. Molecular motors. Nature. 2003;422:759–765. doi: 10.1038/nature01601. [DOI] [PubMed] [Google Scholar]
- See S.K., Hoogendoorn S., Chung A.H., Ye F., Steinman J.B., Sakata-Kato T., Miller R.M., Cupido T., Zalyte R., Carter A.P. Cytoplasmic dynein antagonists with improved potency and isoform selectivity. ACS Chem. Biol. 2016;11:53–60. doi: 10.1021/acschembio.5b00895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumbarello D.A., Kendrick-Jones J., Buss F. Myosin VI and its cargo adaptors - linking endocytosis and autophagy. J. Cell Sci. 2013;126:2561–2570. doi: 10.1242/jcs.095554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R.D. The molecular motor toolbox for intracellular transport. Cell. 2003;112:467–480. doi: 10.1016/s0092-8674(03)00111-9. [DOI] [PubMed] [Google Scholar]
- Valetti C., Wetzel D.M., Schrader M., Hasbani M.J., Gill S.R., Kreis T.E., Schroer T.A. Role of dynactin in endocytic traffic: effects of dynamitin overexpression and colocalization with CLIP-170. Mol. Biol. Cell. 1999;10:4107–4120. doi: 10.1091/mbc.10.12.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiyaveettil M., Bentley A.A., Gursahaney P., Hussien R., Chakravarti R., Kureishy N., Prag S., Adams J.C. Novel role of the muskelin-RanBP9 complex as a nucleocytoplasmic mediator of cell morphology regulation. J. Cell Biol. 2008;182:727–739. doi: 10.1083/jcb.200801133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreugde S., Ferrai C., Miluzio A., Hauben E., Marchisio P.C., Crippa M.P., Bussi M., Biffo S. Nuclear myosin VI enhances RNA polymerase II-dependent transcription. Mol. Cell. 2006;23:749–755. doi: 10.1016/j.molcel.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Wagner W., Lippmann K., Heisler F.F., Gromova K.V., Lombino F.L., Roesler M.K., Pechmann Y., Hornig S., Schweizer M., Polo S. Myosin VI drives clathrin-mediated AMPA receptor endocytosis to facilitate cerebellar long-term depression. Cell Rep. 2019;28:11–20 e19. doi: 10.1016/j.celrep.2019.06.005. [DOI] [PubMed] [Google Scholar]
- Wang H., Wang B., Zhu W., Yang Z. Lentivirus-mediated knockdown of myosin VI inhibits cell proliferation of breast cancer cell. Cancer Biother. Radiopharm. 2015;30:330–335. doi: 10.1089/cbr.2014.1759. [DOI] [PubMed] [Google Scholar]
- Wells A.L., Lin A.W., Chen L.Q., Safer D., Cain S.M., Hasson T., Carragher B.O., Milligan R.A., Sweeney H.L. Myosin VI is an actin-based motor that moves backwards. Nature. 1999;401:505–508. doi: 10.1038/46835. [DOI] [PubMed] [Google Scholar]
- Yoshida H., Cheng W., Hung J., Montell D., Geisbrecht E., Rosen D., Liu J., Naora H. Lessons from border cell migration in the Drosophila ovary: a role for myosin VI in dissemination of human ovarian cancer. Proc. Natl. Acad. Sci. U S A. 2004;101:8144–8149. doi: 10.1073/pnas.0400400101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorca C.E., Kim L.K., Kim Y.J., Krause M.R., Zenklusen D., Spilianakis C.G., Flavell R.A. Myosin VI regulates gene pairing and transcriptional pause release in T cells. Proc. Natl. Acad. Sci. U S A. 2015;112:E1587–E1593. doi: 10.1073/pnas.1502461112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are included in the manuscript.