Abstract
Social interactions are a crucial aspect of human behaviour. Numerous neurophysiological studies have focused on socio-cognitive processes associated with the so-called theory of mind—the ability to attribute mental states to oneself and others. Theory of mind is closely related to social intelligence defined as a set of abilities that facilitate effective social interactions. Social intelligence encompasses multiple theory of mind components and can be measured by the Four Factor Test of Social Intelligence (the Guilford-Sullivan test). However, it is unclear whether the differences in social intelligence are reflected in structural brain differences. During the experiment, 48 healthy right-handed individuals completed the Guilford-Sullivan test. T1-weighted structural MRI images were obtained for all participants. Voxel-based morphometry analysis was performed to reveal grey matter volume differences between the two groups (24 subjects in each)—with high social intelligence scores and with low social intelligence scores, respectively. Participants with high social intelligence scores had larger grey matter volumes of the bilateral caudate. The obtained results suggest the caudate nucleus involvement in the neural system of socio-cognitive processes, reflected by its structural characteristics.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10548-021-00837-1.
Keywords: Theory of mind, Social cognition, Social intelligence, Caudate, Voxel-based morphometry
Introduction
Social interactions are a crucial part of everyday life. There is increasing support for so-called “social intelligence”, which is dissociable from general intelligence. Social intelligence is defined as a set of human abilities that facilitates effective interaction with other people, including the ability to infer the mental and emotional states of others, to understand their intentions, and to predict their behaviour (Thorndike 1920; Moss and Hunt 1927; Vernon 1933). Evidence points to an association of this skill with socio-cognitive processes of the so-called theory of mind (TOM), the ability of a person to attribute mental states (for example, thoughts, beliefs, and intentions) to oneself and to others (Premack and Woodruff 1978).
TOM processes are the focus of many neurophysiological studies, and various experimental tasks have been used to define their neural correlates. A classic example of such a task is making predictions or assumptions based on a story in which a protagonist has a false belief (Saxe et al. 2006; Dodell-Feder et al. 2011). Other studies used different stimulus modalities, in particular, cartoons (Völlm et al. 2006), photographs (de Lange et al. 2008; Canessa et al. 2012), videos (Wolf et al. 2010; Boccadoro et al. 2019), and animations (Gobbini et al. 2007), representing protagonists’ actions or social interactions with the task, either to passively view the stimuli or to make assumptions based on the information presented. Another test measuring TOM ability is Reading the Mind in the Eyes Test (RMET), where a participant is presented with photographs of the eye region and is asked to decide which word better describes what a person in the picture is thinking or feeling (Baron‐Cohen et al. 2001). Some studies also use interactive game designs, such as the prisoner’s dilemma game for engaging TOM (Kircher et al. 2009). According to a meta-analytic study, neurophysiological correlates of TOM processes reported in different papers can vary as a result of task-related activation because studies focus on different aspects of TOM (e.g., implicit versus explicit, cognitive versus affective, visual versus verbal TOM) (Molenberghs et al. 2016).
The issues mentioned above can be potentially improved by using more sophisticated psychological tasks that engage multiple components of TOM. An appropriate example is the standardised J. Guilford and M. O’Sullivan Four Factor Test of Social Intelligence (Guilford-Sullivan test) that aims to give a composite evaluation of a person’s social intelligence isolated from general intelligence. This test is based on Guilford’s structure-of-intellect model. According to this model, social intelligence includes 30 different abilities, four of which are quantitatively measured in four subtests of the Guilford-Sullivan test (O’Sullivan 1965). The subtests estimate (1) the ability to group other people’s mental states based on similarity—Expression Grouping subtest, (2) the ability to interpret sequences of social behaviour—Missing Cartoons subtest, (3) the ability to respond flexibly in interpreting changes in social behaviour—Cartoon Prediction subtest, and (4) the ability to predict what will happen in an interpersonal situation—Social Translations subtest. These subtests include different stimuli modalities (verbal and visual) and various tasks (grouping, predicting, and interpreting), measuring several components of socio-cognitive processes. They are closely related to tasks frequently utilized to measure TOM. For example, the Expression Grouping subtest is closely associated with RMET, while Missing Cartoons and Cartoon Prediction subtests are widely and independently used to estimate TOM (Gallagher et al. 2000; Brunet et al. 2000; Völlm et al. 2006). To summarize, the Guilford-Sullivan test encompasses four measurements obtained by different TOM-related tasks. Thus, it can be a good psychological scale for studying the neural correlates of socio-cognitive processes.
Several clinical neuroimaging studies used the Guilford-Sullivan test to assess its correlation with neuroanatomical characteristics based on voxel-based morphometry (VBM). VBM allows to estimate grey matter volume (GMV) differences on a voxel-wise basis using the statistical approach of parametric mapping (Ashburner and Friston 2000). It was demonstrated that impairments in this test's performance in first-episode psychosis were significantly correlated with reduced grey matter density in the left middle frontal gyrus, the right supplementary motor cortex, the left superior temporal gyrus, and the left inferior parietal lobule (Bertrand et al. 2008). Along with that, Cartoon Prediction subtest performance in patients with social and executive disorders, in the case of frontotemporal dementia, was positively correlated with GMV in the orbital frontal, superior temporal, visual association, and posterior cingulate regions of the right hemisphere (Eslinger et al. 2007). Although the Guilford-Sullivan test was only used to study pathologies, mentioned brain structures can also be associated with social intelligence in healthy people.
At the same time, social intelligence is a measure of socio-cognitive abilities. And a broader list of brain regions is reported in association with social cognition processes. This evidence is gained partly from analyses of the human brain’s structural characteristics using VBM analysis. In particular, performance in RMET was positively correlated with the GMV of the dorsomedial prefrontal cortex (PFC), inferior parietal lobule (or temporoparietal junction, TPJ), and precuneus in the left hemisphere (Sato et al. 2016) as well as with the volume of the caudate nucleus and putamen (Warrier et al. 2018). In another study, performance in a similar facial expression recognition test was positively correlated with the volume of the right inferior frontal gyrus (IFG) (Cabinio et al. 2015). In a task providing conditions for spontaneous social interactions, a significant positive correlation was shown between the participant’s performance and cortical thickness in the medial PFC, right IFG, and right TPJ (Rice and Redcay 2013).
Along with structural findings, functional studies expanded the set of brain regions attributed to socio-cognitive processes. For example, the striatum was implicated in different aspects of social behaviour, such as social reward and learning about others’ preferences, according to the results of human and animal studies (Báez-Mendoza and Schultz 2013). In addition, the insula, as a part of the limbic cortex, was reported to be involved in socio-emotional processes, decision making in a social context, and social pain processing (Lamm and Singer 2010; Menon and Uddin 2010; Eisenberger 2012; Uddin et al. 2017; Riečanský and Lamm 2019). In a meta-analysis of 350 fMRI studies on social cognition, Van Overwalle et al. found activation of the cerebellum during abstract mentalizing (Van Overwalle et al. 2014). Named structural and functional studies did not target social intelligence directly, but their results can potentially include its neural correlates, because it falls within the definition of socio-cognitive processes.
It was mentioned that the Guilford-Sullivan test includes tasks used to measure TOM abilities. Therefore, social intelligence can be associated with neural correlates similar to those of TOM. There is evidence of involvement of the several brain regions in TOM-specific socio-cognitive processes. In the meta-analysis, Molenberghs et al. (2016) defined core areas of the TOM neural system repeatedly engaged in all types of tasks found in the TOM-related research literature: the medial PFC and bilateral TPJ. Along with the precuneus and the right superior temporal sulcus, these areas were also reported in a study that utilized a task with stories about false beliefs on a substantial sample (N = 462) of individuals (Dufour et al. 2013). Named areas are believed to constitute the TOM neural system and, as a result, can be related to social intelligence.
Thus, studies applying the Guilford-Sullivan test for identifying neural correlates of socio-cognitive processes are scarce, and their results are controversial. To our knowledge, there is no study on the association between GMV and the level of social intelligence in a healthy population. Besides, the results of studies using classical TOM tasks, together with other structural and functional analyses, cumulatively demonstrate that, depending on the type of experimental task, different brain areas can be attributed to the TOM neural system. Thus, this study aims to confirm and to expand the list of neuroanatomical correlates of socio-cognitive processes using the Guilford-Sullivan test and VBM analysis. Based on prior studies, it can be hypothesized that this test’s performance for healthy people will correlate with the GMV in previously defined nodes of the TOM neural system according to fMRI studies in the medial PFC, the TPJ in both hemispheres, the precuneus and the right superior temporal sulcus (Dufour et al. 2013; Molenberghs et al. 2016). In addition, it can be assumed that the level of social intelligence will correlate with the GMV in regions reported in clinical studies that utilized the Guilford-Sullivan test, such as the cingulate cortex and orbitofrontal cortex.
Materials and Methods
Subjects
A total of 48 healthy right-handed volunteers (32 women and 16 men) participated in the study. All participants were 24.9 ± 5.5 years old, with no history of neurological or psychological disorders and no contraindications for magnetic resonance imaging. All subjects provided written informed consent prior to the study. All procedures were conducted in accordance with the Declaration of Helsinki and were approved by the Ethics Committee of the N.P. Bechtereva Institute of the Human Brain, Russian Academy of Sciences.
Social Intelligence Testing
The Russian adaptation of the four-factor test of social intelligence, developed by J. Guilford and M. Sullivan (Guilford-Sullivan test), was used to measure the level of social intelligence (Mikhaylova 2001). This test consists of four subtests: (1) Cartoon Predictions, (2) Expression Grouping, (3) Social Translations, and (4) Missing Cartoons (see Fig. 1). In the first subtest (Cartoon Predictions), participants need to choose one out of three cartoons, which appropriately continues the suggested situation. The task in the expression grouping subtest is to find the facial expression that best fits a group of three other expressions. In the social translations subtest, a statement between a pair of people in certain social situations is presented; participants need to choose one out of three situations in which a suggested statement has a different meaning. Within the Missing Cartoons subtest, participants’ task is to choose one out of four cartoons, which completes the suggested scenario in the right way. The first subtest consisted of 14 tasks, while the second, third, and fourth subtests consisted of 15, 12, and 14 tasks, respectively. Considering that these subtests vary in the type of activity and can be expressed to varying degrees in different subjects, a cumulative measure was utilised to obtain the balanced individual characteristic of social intelligence rather than to investigate its particular components.
Fig. 1.
Examples of measurements in the Guilford-Sullivan test. Task A represents the Cartoon Predictions subtest, where participants must select one of three cartoons that most appropriately describes the outcome of the suggested situation. Task B represents the Missing Cartoons subtest, where participants are required to choose one of four cartoons, which correctly fills the suggested sequence of cartoons
Additionally, the sum of raw scores for all subtests can be transformed into standard scores according to the manual for the Russian adaptation of Four Factor test of social intelligence. The main idea of this transformation, according to manual instructions, is to highlight five levels of social intelligence, from the lowest (1) to the highest (5). where the score of 3 corresponds to the average level of social intelligence (see Table 1). Standard scores for every subject were used as a criterion of group formation for the VBM analysis. Volunteers were divided into two groups (24 subjects in each): values equal to or below the score of 3 were considered low social intelligence, while values above the score of 3 were considered high social intelligence (see Table 2). Groups did not differ significantly in age and gender.
Table 1.
Conversion of raw Guilford-Sullivan test scores into standard scores
| Raw score | Standard score |
|---|---|
| 0–12 | 1 |
| 13–26 | 2 |
| 27–37 | 3 |
| 38–46 | 4 |
| 47–55 | 5 |
Table 2.
The demographics and the Guilford-Sullivan test scores
| High social tellinigence | Low social intelligence | Total | |
|---|---|---|---|
| Female (people) | 18 | 14 | 32 |
| Male (people) | 6 | 10 | 16 |
| Age (years) | 23.8 ± 5 | 26.1 ± 5.6 | 24.9 ± 5.5 |
Data Acquisition and Quality Control
Magnetic resonance imaging was performed using a 3 T Philips Achieva (Philips Medical Systems, Best, The Netherlands). Structural images were acquired using a T1-weighted pulse sequence (T1W-3D-FFE; repetition time [TR] = 2.5 ms; TE = 3.1 ms; 30◦ flip angle), recording 130 axial slices (field of view [FOV] = 240 × 240 mm; 256 × 256 scan matrix) of 0.94 mm thickness. All MRI scans were inspected for image artefacts and incidental brain abnormalities. All subjects were included in the study.
Voxel-Based Morphometry Analysis (VBM-analysis)
The VBM analysis of structural data was performed with Statistical Parametric Mapping software (SPM12, Wellcome Department of Imaging Neuroscience, London, UK (www.fil.ion.ucl.ac.uk/spm) and the Computational Anatomy Toolbox 12 (CAT12; http://dbm.neuro.uni-jena.de/cat.html) running in MATLAB (MathWorks, Natick, MA). All structural data were manually reoriented to place their native-space origin at the anterior commissure. Then, the default parameters of the CAT12 toolbox were used. Images were corrected for magnetic field inhomogeneities and segmented into grey matter, white matter, and cerebrospinal fluid. Normalisation to MNI space using the DARTEL (Diffeomorphic Anatomical Registration Through Exponentiated Lie algebra) algorithm to a 1.5 mm isotropic adult template provided by the CAT12 toolbox was performed for segmented grey matter data. Finally, the grey matter segments were smoothed with a Gaussian smoothing kernel of 8 mm. The CAT12 toolbox provides an automated quality check protocol. Therefore, quality check control for all structural data was performed to obtain so-called image quality rating (IQR) scores, which were later used as an additional covariate in the statistical analysis. In addition, total intracranial volumes (TIVs) were calculated to be used as a covariate.
Statistical Analysis
The statistical analysis was performed for two groups of subjects: (1) those with a high social intelligence score (> 3) and (2) those with a low social intelligence score (≤ 3). For the VBM analysis, we included the following confounders (as covariates), which can affect VBM results: sex (male/female), age, TIV, and IQR scores. The two-sample t-test was performed to test the hypothesized differences in the GMV. In addition, we conducted multiple regression analysis for total score and separately for each subtest using z-scores as a covariate of interest. Statistical parametric maps were created with the uncorrected p-value (< 0.001) threshold and a subsequent cluster-level family-wise error (FWE) correction with p < 0.05. The SPM results were visualized using the MRIcron toolbox (https://www.nitrc.org/projects/mricron).
Results
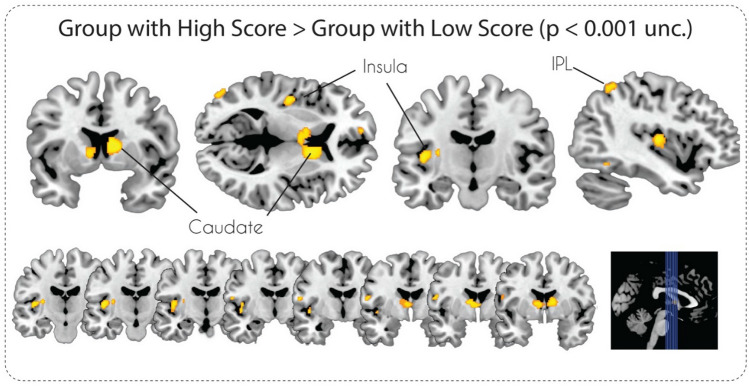
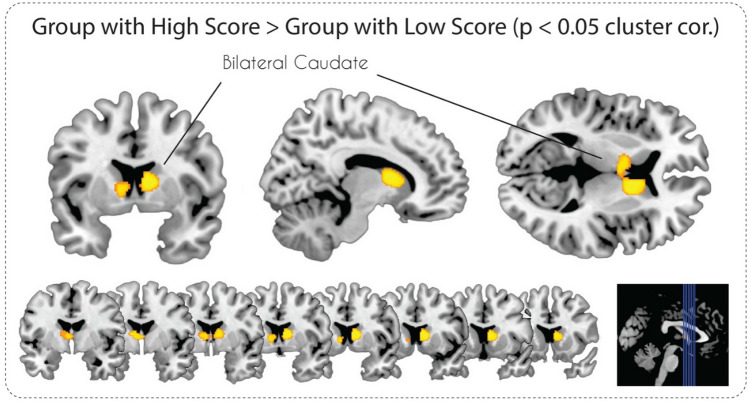
The VBM analysis with the voxel-wise uncorrected p-value (< 0.001) threshold for the “High Social Intelligence > Low Social Intelligence” contrast revealed significant GMV differences in the bilateral caudate nucleus, left insula, left inferior temporal gyrus, and left angular gyrus (see Table 3 and Fig. 2). In the case of the “Low Social Intelligence > High Social Intelligence” contrast, two clusters in the left inferior temporal gyrus and left middle frontal gyrus were revealed (Table 3). After applying a stricter cluster-level FWE-correction (p < 0.05) only GMV differences in the bilateral caudate nucleus survived for the “High Social Intelligence > Low Social Intelligence” contrast (Fig. 3) and nothing survived for the “Low Social Intelligence > High Social Intelligence” contrast.
Table 3.
Clusters of the grey matter volume differences associated with both [High Social Intelligence > Low Social Intelligence] and [Low Social Intelligence > High Social Intelligence] contrasts, minimal cluster size (k = 30)
| Region (L—left, R—right) | cluster size (k) | T score | MNI coordinates | ||
|---|---|---|---|---|---|
| x | y | z | |||
| High social intelligence > Low social intelligence | |||||
| R Caudate, L caudate | 824 | 4.85 | 11 | 6 | 9 |
| L Posterior insula | 183 | 4.61 | − 42 | − 14 | 8 |
| L Inferior parietal lobule | 113 | 4.08 | − 41 | − 54 | 57 |
| L Inferior temporal gyrus | 115 | 3.89 | − 48 | − 57 | − 8 |
| L Middle occipital gyrus | 68 | 3.87 | − 48 | − 83 | 9 |
| L Precentral gyrus | 66 | 3.84 | − 57 | − 6 | 15 |
| Low social intelligence > High social intelligence | |||||
| L Middle frontal gyrus | 67 | 4.59 | − 36 | 38 | 42 |
| L Inferior temporal gyrus | 185 | 4.12 | − 38 | − 5 | − 41 |
Fig. 2.
Statistical parametric maps of grey matter volume differences in subjects with High and Low Social Intelligence at p < 0.001, uncorrected
Fig. 3.
Statistical parametric maps of grey matter volume differences in subjects with High and Low Social Intelligence at p < 0.05, FWE cluster-level corrected
The multiple regression analysis revealed a number of clusters of positive correlations between the GMV and z-transformed scores for total Guilford-Sullivan test score and for the fourth “Missing Cartoons” subtest score, but no clusters were survived after FWE cluster-level correction (see Supplementary Figs. 1, 2 and Supplementary Tables 1, 2).
Discussion
The main goal of this study was to identify structural changes in the human brain associated with social intelligence, measured by the Guilford-Sullivan test. We observed that participants with high scores in the test demonstrated more GMV in the bilateral caudate, insula, IPL, ITG, and MOG. However, after applying conservative cluster-level correction, only findings in the bilateral caudate survived. Additional correlational analysis revealed the possible impact of the fourth Guilford-Sullivan subtest in GMV changes in caudate nuclei and left insula, but only at the uncorrected level of significance.
The cluster revealed in the present study occupies the head of the caudate nucleus. According to diffusion tensor imaging and retrograde tracing studies, this area is connected to the medial PFC (Lehéricy et al. 2004; Besson et al. 2017), which is considered to be responsible for emotional processing, decision-making, memory, self-perception, and social cognition in general (Bicks et al. 2015). Notably, the medial PFC is part of the TOM network (Carrington and Bailey 2009; Dufour et al. 2013; Molenberghs et al. 2016). Structural connections with regions of the TOM system suggest the involvement of the caudate nucleus in brain mechanisms of social cognition.
This notion is supported by structural, functional, and clinical studies on brain mechanisms of social cognition. A genome-wide meta-analysis identified a positive correlation between RMET scores and volumes of the caudate nucleus and putamen (Warrier et al. 2018). The activity of the left caudate nucleus, among other areas, was revealed during observing animations of mental interactions in the Triangles Task (Martin et al. 2016), during joint attention (induced by video stimuli) (Williams et al. 2005), and during guessing the mental state of a subject featuring first-person perspective sentences (Otsuka et al. 2011). In addition, several studies associated the activity in the caudate with understanding and classifying verbal expressions, which can be regarded as the verbal aspect of the TOM ability (corresponding with the social translations subtest in the Guilford-Sullivan test) (Shibata et al. 2010). A clinical study also demonstrated that focal left caudate nucleus lesions lead to impairment in TOM-related social cognition: poorer performance in the Faux-Pas test, the RMET, the Emotion Recognition Test (particularly for facial expressions of fear and sadness), accompany signs of alexithymia, social anhedonia, and reduced empathy abilities (Kemp et al. 2013). In another study, interpersonal schizotypy, characterised by impairments in social processes, was significantly negatively correlated with activation in the bilateral precuneus and right caudate nucleus during mentalising in the prisoner's dilemma game (Acosta et al. 2019). Thus, the results of structural and functional studies on healthy persons and patients as well as our own results indicate that the caudate nucleus participates in brain mechanisms of TOM-related socio-cognitive processes.
It is important to note the role of the caudate nucleus in brain mechanisms of socially oriented behaviour (e.g., trust and cooperation). Its activity was increased during signalling and integrating reputations gained through experience into trust decisions (Wardle et al. 2013). Being faced with choices, whether to be cooperative or not, was associated with activity in the medial PFC, anterior cingulate gyrus, caudate, and insula (Lemmers-Jansen et al. 2018). Specifically, cooperative choices were associated with activity in the right parietal cortex and caudate. A similar study on first-episode psychosis patients (characterized by reduced TOM abilities) showed reduced activation of the caudate and medial PFC during cooperative choices (Lemmers-Jansen et al. 2019). Another study on patients with early psychosis confirmed reduced caudate activation du ivity and differential activity in patients and healthy subjects.ring cooperative actions (Fett et al. 2019). Therefore, the caudate nucleus demonstrates involvement in trust and cooperation through increased local neuronal act
Moreover, changes in GMV, activation, and functional connectivity in the caudate were associated with impairments in social functioning. Some studies have found enlargement of the caudate in cases of autistic spectrum disorder (ASD), bipolar affective disorder, and early-onset schizophrenia, which are characterised by social behaviour impairments (Ong et al. 2012; Juuhl-Langseth et al. 2012; Qiu et al. 2016). In addition, a meta-analysis of atypical emotional face processing found ASD-related hyperactivation in the bilateral thalamus and bilateral caudate (Aoki et al. 2015). Furthermore, there was a less pronounced or absent functional connectivity between caudate nuclei and the cerebral cortex in ASD patients (Turner et al. 2006). It was shown that the volume of the caudate was correlated with the level of socio-cognitive processes. In the case of major depressive disorder (MDD), higher SAS-scores (social anhedonia scale) were associated with reduced GMV in the bilateral caudate nucleus in both, the MDD group and healthy controls (Enneking et al. 2019). Moreover, the level of social potency was correlated positively with GMV in the left parahippocampal gyrus, left cingulate gyrus, left caudate and left precentral gyrus (Grodin and White 2015). An ALE meta-analysis showed significant GMV reduction of the left caudate nucleus and insula in the group of schizophrenia patients with persistent negative symptoms relative to controls (Li et al. 2018). Another meta-analysis detected reduced right globus pallidus and putamen volumes in VBM studies as well as decreased caudate volumes in manual tracing studies in children with ADHD (Frodl and Skokauskas 2012). In conclusion, there is strong evidence for the association between social cognition deficits and structural/morphological characteristics of the caudate nucleus.
It is important to note that the relationship between grey matter volume and strength of functional activity or functional connectivity is not so straightforward as it could be expected. On the one hand, there is a substantial number of studies in which positive relationships between GMV and local activity (Braskie et al. 2009; Trivedi et al. 2011; Maillet and Rajah 2011) or functional connectivity (Wang et al. 2017) was reported. In some cases, increases in GMV can be caused by training or improving skills. For instance, Ilg and colleagues (2008) showed an increase of in grey matter volume in the right dorsolateral occipital cortex, which correlated with increased activation strength at this region (Ilg et al. 2008) that was caused by mirror reading training. In another study influence of cortical thickness on age-related BOLD-signal changes was shown (Pur et al. 2019). However, there were also many findings that could not reveal any correlation between function and grey matter volume (Takeuchi et al. 2017; Avinun et al. 2020).
Another line of research suggested the inverse relationship between grey matter volume and strength of functional connectivity in cases of different pathological conditions. For example, trigeminal neuropathic pain and trigeminal neuralgia were associated with decreased grey matter volume and increased resting-state functional organization in the thalamus (Henssen et al. 2019). Patients with chronic spontaneous urticaria had an increased GMV in the cerebellum and left fusiform cortex and decreased functional connectivity with the right supplementary motor area and medial prefrontal cortex, premotor cortex, the primary motor cortex, respectively (Wang et al. 2021). To sum up, the relationship between GMV and functional activity is still an unresolved question, but there are pieces of evidence for a positive correlation between GMV and functional activity in the healthy population.
In summary, the results of previous neuroanatomical and neurophysiological studies on healthy persons and patients provide evidence for the implication of the caudate nuclei in brain mechanisms of social cognition. However, this region was not revealed in meta-analytic studies assessing TOM-related experimental tasks. Thus, our findings of a relationship between the GMV in the caudate nuclei and the Guilford-Sullivan test of social intelligence scores can potentially be explained by the difference in structural characteristics of this area depending on the level of social intelligence.
While findings in the left insula did not survive conservative cluster-level thresholding, it was the second largest cluster. We suggest that it may play a role in neural mechanisms of social intelligence. For example, the anterior insular cortex is known to be the core element of empathy, which is closely related to affective TOM (Fan et al. 2011). In addition, activations in the insula were observed during pain judgement in TOM-measuring tasks (Corradi-Dell’Acqua et al. 2014). In addition, it was one of the regions implicated in decision making on whether to be cooperative or not (Lemmers-Jansen et al. 2018). Interestingly, the activation in both regions, revealed in the current study (the bilateral caudate and left insula), was associated with romantic love, which is an important social behaviour. Right caudate activation was correlated with the intensity of romantic passion, and left insula-putamen-globus pallidus activation was correlated with trait affect intensity (Aron et al. 2005). Importantly, there is evidence of involvement of the insula in pathologies associated with social cognition deficits. In schizophrenia patients, its activation was positively correlated with social loneliness (Lindner et al. 2014), while a meta-analysis of neuroimaging studies of social functions showed, compared to healthy controls, hypoactivation of the right anterior insula in ASD patients (Di Martino et al. 2009). In another study, reduced connectivity between the insula and brain regions involved in emotional and sensory processing was observed in patients with ASD (Ebisch et al. 2011). A VBM study has also revealed the decreased volume of insular subregions in cases of social anxiety disorder (Kawaguchi et al. 2016).
Thus, the implication of the insula in neural mechanisms of socio-cognitive processes is reflected by its structural and functional characteristics as well as by differential involvement in patients. Therefore, although findings in the insula did not survive conservative cluster-level thresholding in the current study, further research is needed to clarify its implication in social intelligence.
Our VBM analysis did not reveal regions, such as the cingulate cortex and orbitofrontal cortex, reported in clinical studies that utilized the Guilford-Sullivan test. This can be explained by the fact that findings revealed by studying pathologies do not always extend to healthy individuals and furthermore, the evidence is limited (Eslinger et al. 2007, Bertrand et al. 2008). Another reason might be modality-related differences, such that one study measured the density and another applied the short version of the Guilford-Sullivan test. Our experiment also did not find GMV changes in brain structures, attributed to the TOM neural system, in particular, the medial PFC, the TPJ in both hemispheres, the precuneus, and the right superior temporal sulcus. There are several reasons potentially explaining this fact. First, to our knowledge, there are no studies on the correlation between performances in TOM-related tests and the Guilford-Sullivan test. The latter is a specific measure of social intelligence. This ability and TOM, although closely related to each other, are not identical and, therefore, can be associated with different brain structures.
Second, a recent meta-analytic study demonstrated that although there are common regions shared across all TOM tasks, different types of TOM tasks reliably elicit activity in unique brain areas and are associated with distinct neural systems (Molenberghs et al. 2016). The authors found dissociation based on tasks with different instructional focuses (implicit versus explicit TOM tasks), types of mentalising inferences (cognitive versus affective TOM tasks), and modalities of presentation (visual versus verbal TOM tasks). For example, explicit tasks elicited more activation in the posterior medial frontal cortex and left TPJ, while implicit tasks elicited significantly greater activation in the dorsal medial PFC and right lateral orbitofrontal cortex. Accordingly, the Guilford-Sullivan test can be viewed as a specific type of TOM task or a more sophisticated task, measuring multiple components of TOM. Therefore, it can be associated with unique brain structures.
Studies that most accurately defined the nodes of the TOM system (using meta-analysis or large data samples) were based on functional data (Dufour et al. 2013; Molenberghs et al. 2016), while this study utilized VBM analysis to estimate the brain’s structural characteristics. A possible assumption is that brain areas demonstrating their involvement in the execution of action through changing their functional activity are not the same as those involved by changing their GMV. On a similar note, recent studies have demonstrated the existence of the so-called hidden nodes of neural systems that demonstrate their involvement by changing functional interactions with other brain areas without changing their local activity (Medvedev et al. 2019).
In summary, the reason our hypothesis was not supported may lie in the type of task (the Guilford-Sullivan test) or in the applied methodology (the VBM analysis).
Limitations
Despite its advantages, this study has some limitations. One of them is associated with the nature of the Guilford-Sullivan test. Although its authors claim the absence of dependency between the social intelligence and general cognitive abilities, some studies have revealed the correlation between them for some subtests of the Guilford-Sullivan test (Shanley et al. 1971; Riggio et al. 1991). This problem can potentially be resolved by using the general intelligence level as an additional covariate in the VBM analysis in future studies. In addition, it could be advantageous to consider other measures, reflecting levels of empathy, altruism, or the tendency for prosocial behaviour (e.g., trust and cooperation), as analysis covariates in future research.
Conclusion
This VBM study provides new data suggesting the role of the bilateral caudate in social intelligence by demonstrating its higher GMV in people with high, compared to low, scores on the Guilford-Sullivan test. According to this result and previous data, there is strong evidence for the involvement of the caudate nucleus in the process of social cognition. Considering its structural and functional characteristics, the caudate nucleus can serve as a node in the neural system, underlying socio-cognitive processes and, in particular, TOM-related brain processes. However, future investigation is needed to confirm this assumption.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgment
We thank Maria Starchenko for her help with obtaining and preparation of psychological data.
Funding
Open Access funding enabled and organized by Projekt DEAL. The study was supported by the Russian Science Foundation Grant No 19-18-00436.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflicts of Interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Ethics Approval
Approval was obtained from the ethics committee of the N.P. Bechtereva Institute of the Human Brain, Russian Academy of Sciences. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Acosta H, Straube B, Kircher T. Schizotypy and mentalizing: an fMRI study. Neuropsychologia. 2019;124:299–310. doi: 10.1016/j.neuropsychologia.2018.11.012. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Cortese S, Tansella M. Neural bases of atypical emotional face processing in autism: a meta-analysis of fMRI studies. World J Biol Psychiatry. 2015;16:291–300. doi: 10.3109/15622975.2014.957719. [DOI] [PubMed] [Google Scholar]
- Aron A, Fisher H, Mashek DJ, et al. Reward, motivation, and emotion systems associated with early-stage intense romantic love. J Neurophysiol. 2005;94:327–337. doi: 10.1152/jn.00838.2004. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry: the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Avinun R, Israel S, Knodt AR, Hariri AR. Little evidence for associations between the big five personality traits and variability in brain gray or white matter. Neuroimage. 2020;220:117092. doi: 10.1016/j.neuroimage.2020.117092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Báez-Mendoza R, Schultz W. The role of the striatum in social behavior. Front Neurosci. 2013;7:233. doi: 10.3389/fnins.2013.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Hill J, et al. The “Reading the Mind in the Eyes” test Revised Version: a Study with Normal Adults, and Adults with Asperger Syndrome or High-functioning Autism. J Child Psychol Psychiatry. 2001;42:241–251. doi: 10.1111/1469-7610.00715. [DOI] [PubMed] [Google Scholar]
- Bertrand MC, Achim A, Harvey PO, et al. Structural neural correlates of impairments in social cognition in first episode psychosis. Soc Neurosci. 2008;3:79–88. doi: 10.1080/17470910701563491. [DOI] [PubMed] [Google Scholar]
- Besson P, Carrière N, Bandt SK, et al. Whole-brain high-resolution structural connectome: inter-subject validation and application to the anatomical segmentation of the striatum. Brain Topogr. 2017;30:291–302. doi: 10.1007/s10548-017-0548-0. [DOI] [PubMed] [Google Scholar]
- Bicks LK, Koike H, Akbarian S, Morishita H. Prefrontal cortex and social cognition in mouse and man. Front Psychol. 2015;6:1805. doi: 10.3389/fpsyg.2015.01805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccadoro S, Cracco E, Hudson AR, et al. Defining the neural correlates of spontaneous theory of mind (ToM): An fMRI multi-study investigation. Neuroimage. 2019;203:116193. doi: 10.1016/j.neuroimage.2019.116193. [DOI] [PubMed] [Google Scholar]
- Braskie MN, Small GW, Bookheimer SY. Entorhinal cortex structure and functional MRI response during an associative verbal memory task. Hum Brain Mapp. 2009;30:3981–3992. doi: 10.1002/hbm.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet E, Sarfati Y, Hardy-Baylé MC, Decety J. A PET investigation of the attribution of intentions with a nonverbal task. Neuroimage. 2000;11:157–166. doi: 10.1006/nimg.1999.0525. [DOI] [PubMed] [Google Scholar]
- Cabinio M, Rossetto F, Blasi V, et al. Mind-reading ability and structural connectivity changes in aging. Front Psychol. 2015;6:1808. doi: 10.3389/fpsyg.2015.01808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canessa N, Alemanno F, Riva F, et al. The neural bases of social intention understanding: the role of interaction goals. PLoS ONE. 2012;7:e42347. doi: 10.1371/journal.pone.0042347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington SJ, Bailey AJ. Are there theory of mind regions in the brain? A review of the neuroimaging literature. Hum Brain Mapp. 2009;30:2313–2335. doi: 10.1002/hbm.20671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradi-Dell’Acqua C, Hofstetter C, Vuilleumier P. Cognitive and affective theory of mind share the same local patterns of activity in posterior temporal but not medial prefrontal cortex. Soc Cogn Affect Neurosci. 2014;9:1175–1184. doi: 10.1093/scan/nst097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange FP, Spronk M, Willems RM, et al. Complementary systems for understanding action intentions. Curr Biol. 2008;18:454–457. doi: 10.1016/j.cub.2008.02.057. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Ross K, Uddin LQ, et al. Functional brain correlates of social and nonsocial processes in autism spectrum disorders: an activation likelihood estimation meta-analysis. Biol Psychiatry. 2009;65:63–74. doi: 10.1016/j.biopsych.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodell-Feder D, Koster-Hale J, Bedny M, Saxe R. FMRI item analysis in a theory of mind task. Neuroimage. 2011;55:705–712. doi: 10.1016/j.neuroimage.2010.12.040. [DOI] [PubMed] [Google Scholar]
- Dufour N, Redcay E, Young L, et al. Similar brain activation during false belief tasks in a large sample of adults with and without autism. PLoS ONE. 2013;8:e75468. doi: 10.1371/journal.pone.0075468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisch SJH, Gallese V, Willems RM, et al. Altered intrinsic functional connectivity of anterior and posterior insula regions in high-functioning participants with autism spectrum disorder. Hum Brain Mapp. 2011;32:1013–1028. doi: 10.1002/hbm.21085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI. The neural bases of social pain: evidence for shared representations with physical pain. Psychosom Med. 2012;74:126–135. doi: 10.1097/PSY.0b013e3182464dd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enneking V, Krüssel P, Zaremba D, et al. Social anhedonia in major depressive disorder: a symptom-specific neuroimaging approach. Neuropsychopharmacology. 2019;44:883–889. doi: 10.1038/s41386-018-0283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslinger PJ, Moore P, Troiani V, et al. Oops! resolving social dilemmas in frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2007;78:457–460. doi: 10.1136/jnnp.2006.098228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Duncan NW, de Greck M, Northoff G. Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neurosci Biobehav Rev. 2011;35:903–911. doi: 10.1016/j.neubiorev.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Frodl T, Skokauskas N. Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta Psychiatr Scand. 2012;125:114–126. doi: 10.1111/j.1600-0447.2011.01786.x. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Happé F, Brunswick N, et al. Reading the mind in cartoons and stories: an fMRI study of “theory of mind” in verbal and nonverbal tasks. Neuropsychologia. 2000;38:11–21. doi: 10.1016/S0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Gobbini MI, Koralek AC, Bryan RE, et al. Two takes on the social brain: a comparison of theory of mind tasks. J Cogn Neurosci. 2007;19:1803–1814. doi: 10.1162/jocn.2007.19.11.1803. [DOI] [PubMed] [Google Scholar]
- Grodin EN, White TL. The neuroanatomical delineation of agentic and affiliative extraversion. Cogn Affect Behav Neurosci. 2015;15:321–334. doi: 10.3758/s13415-014-0331-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henssen D, Dijk J, Knepflé R, et al. Alterations in grey matter density and functional connectivity in trigeminal neuropathic pain and trigeminal neuralgia: a systematic review and meta-analysis. NeuroImage Clin. 2019;24:102039. doi: 10.1016/j.nicl.2019.102039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilg R, Wohlschläger AM, Gaser C, et al. Gray matter increase induced by practice correlates with task-specific activation: a combined functional and morphometric magnetic resonance imaging study. J Neurosci. 2008;28:4210–4215. doi: 10.1523/JNEUROSCI.5722-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juuhl-Langseth M, Rimol LM, Rasmussen IA, et al. Comprehensive segmentation of subcortical brain volumes in early onset schizophrenia reveals limited structural abnormalities. Psychiatry Res Neuroimaging. 2012;203:14–23. doi: 10.1016/j.pscychresns.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Kawaguchi A, Nemoto K, Nakaaki S, et al. Insular volume reduction in patients with social anxiety disorder. Front Psychiatry. 2016;7:3. doi: 10.3389/fpsyt.2016.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher T, Blümel I, Marjoram D, et al. Online mentalising investigated with functional MRI. Neurosci Lett. 2009;454:176–181. doi: 10.1016/j.neulet.2009.03.026. [DOI] [PubMed] [Google Scholar]
- Lamm C, Singer T. The role of anterior insular cortex in social emotions. Brain Struct Funct. 2010;214:579–591. doi: 10.1007/s00429-010-0251-3. [DOI] [PubMed] [Google Scholar]
- Lehéricy S, Ducros M, Van De Moortele PF, et al. Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Ann Neurol. 2004;55:522–529. doi: 10.1002/ana.20030. [DOI] [PubMed] [Google Scholar]
- Li Y, Li W-X, Xie D-J, et al. Grey matter reduction in the caudate nucleus in patients with persistent negative symptoms: An ALE meta-analysis. Schizophr Res. 2018;192:9–15. doi: 10.1016/j.schres.2017.04.005. [DOI] [PubMed] [Google Scholar]
- Lindner C, Dannlowski U, Walhöfer K, et al. Social alienation in schizophrenia patients: association with insula responsiveness to facial expressions of disgust. PLoS ONE. 2014 doi: 10.1371/journal.pone.0085014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillet D, Rajah MN. Age-related changes in the three-way correlation between anterior hippocampus volume, whole-brain patterns of encoding activity and subsequent context retrieval. Brain Res. 2011;1420:68–79. doi: 10.1016/j.brainres.2011.08.071. [DOI] [PubMed] [Google Scholar]
- Martin AK, Dzafic I, Robinson GA, et al. Mentalizing in schizophrenia: a multivariate functional MRI study. Neuropsychologia. 2016;93:158–166. doi: 10.1016/j.neuropsychologia.2016.10.013. [DOI] [PubMed] [Google Scholar]
- Medvedev SV, Korotkov AD, Kireev MV. Hidden nodes of the brain systems. Hum Physiol. 2019;45:552–556. doi: 10.1134/S0362119719050104. [DOI] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhaylova ES (2001) Test Dzh.Gilforda i M.Sallivena:diagnostika sotsial'nogo intellekta [The J. Guilford and M. O'Sullivan test: diagnosing social intelligence]. Imaton, St.Petersburg
- Molenberghs P, Johnson H, Henry JD, Mattingley JB. Understanding the minds of others: a neuroimaging meta-analysis. Neurosci Biobehav Rev. 2016;65:276–291. doi: 10.1016/j.neubiorev.2016.03.020. [DOI] [PubMed] [Google Scholar]
- Moss FA, Hunt T. Are you socially intelligent? Sci Am. 1927;137:108–110. doi: 10.1038/scientificamerican0827-108. [DOI] [Google Scholar]
- O’Sullivan M. Four factor tests of social intelligence (behavior cognition) Beverly Hills Calif: Sheridan Supply Co.; 1965. [Google Scholar]
- Ong D, Walterfang M, Malhi GS, et al. Size and shape of the caudate nucleus in individuals with bipolar affective disorder. Aust N Z J Psychiatry. 2012;46:340–351. doi: 10.1177/0004867412440191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka Y, Osaka N, Yaoi K, Osaka M. First-person perspective effects on theory of mind without self-reference. PLoS ONE. 2011;6:e19320. doi: 10.1371/journal.pone.0019320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premack D, Woodruff G. Does the chimpanzee have a theory of mind? Behav Brain Sci. 1978;1:515–526. doi: 10.1017/S0140525X00076512. [DOI] [Google Scholar]
- Pur DR, Eagleson RA, de Ribaupierre A, et al. Moderating effect of cortical thickness on BOLD signal variability age-related changes. Front Aging Neurosci. 2019;11:46. doi: 10.3389/fnagi.2019.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu T, Chang C, Li Y, et al. Two years changes in the development of caudate nucleus are involved in restricted repetitive behaviors in 2–5-year-old children with autism spectrum disorder. Dev Cogn Neurosci. 2016;19:137–143. doi: 10.1016/j.dcn.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice K, Redcay E. Spontaneous mentalizing captures variability in the cortical thickness of social brain regions. Soc Cogn Affect Neurosci. 2013;10:327–334. doi: 10.1093/scan/nsu081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riečanský I, Lamm C. The role of sensorimotor processes in pain empathy. Brain Topogr. 2019;32:965–976. doi: 10.1007/s10548-019-00738-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggio RE, Messamer J, Throckmorton B. Social and academic intelligence: conceptually distinct but overlapping constructs. Pers Individ Dif. 1991;12:695–702. doi: 10.1016/0191-8869(91)90225-Z. [DOI] [Google Scholar]
- Sato W, Kochiyama T, Uono S, et al. structural neural substrates of reading the mind in the eyes. Front Hum Neurosci. 2016;10:1–7. doi: 10.3389/fnhum.2016.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R, Moran JM, Scholz J, Gabrieli J. Overlapping and non-overlapping brain regions for theory of mind and self reflection in individual subjects. Soc Cogn Affect Neurosci. 2006;1:229–234. doi: 10.1093/scan/nsl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanley LA, Walker RE, Foley JM. Social Intelligence: a concept in search of data. Psychol Rep. 1971;29:1123–1132. doi: 10.2466/pr0.1971.29.3f.1123. [DOI] [Google Scholar]
- Shibata M, Toyomura A, Itoh H, Abe J-I. Neural substrates of irony comprehension: a functional MRI study. Brain Res. 2010;1308:114–123. doi: 10.1016/j.brainres.2009.10.030. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Nouchi R, et al. Global associations between regional gray matter volume and diverse complex cognitive functions: evidence from a large sample study. Sci Rep. 2017;7:1–16. doi: 10.1038/s41598-017-10104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorndike EL. Intelligence and its uses Harper’s Mag. 1920;140:227–235. [Google Scholar]
- Trivedi MA, Stoub TR, Murphy CM, et al. Entorhinal cortex volume is associated with episodic memory related brain activation in normal aging and amnesic mild cognitive impairment. Brain Imaging Behav. 2011;5:126–136. doi: 10.1007/s11682-011-9117-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner KC, Frost L, Linsenbardt D, et al. Atypically diffuse functional connectivity between caudate nuclei and cerebral cortex in autism. Behav Brain Funct 2. 2006 doi: 10.1186/1744-9081-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Nomi JS, Hébert-Seropian B, et al. Structure and Function of the Human Insula. J Clin Neurophysiol. 2017;34:300–306. doi: 10.1097/WNP.0000000000000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F, Baetens K, Mariën P, Vandekerckhove M. Social cognition and the cerebellum: a meta-analysis of over 350 fMRI studies. Neuroimage. 2014;86:554–572. doi: 10.1016/j.neuroimage.2013.09.033. [DOI] [PubMed] [Google Scholar]
- Vernon PE. Some characteristics of the good judge of personality. J Soc Psychol. 1933;4:42–57. doi: 10.1080/00224545.1933.9921556. [DOI] [Google Scholar]
- Völlm BA, Taylor ANW, Richardson P, et al. Neuronal correlates of theory of mind and empathy: A functional magnetic resonance imaging study in a nonverbal task. Neuroimage. 2006;29:90–98. doi: 10.1016/j.neuroimage.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Wang Y, Gao D, Cui B, et al. Increased grey matter volume and associated resting-state functional connectivity in chronic spontaneous urticaria: a structural and functional MRI study. J Neuroradiol. 2021 doi: 10.1016/j.neurad.2021.01.011. [DOI] [PubMed] [Google Scholar]
- Wang Y, Guo N, Zhao L, et al. The structural and functional correlates of the efficiency in fearful face detection. Neuropsychologia. 2017;100:1–9. doi: 10.1016/j.neuropsychologia.2017.04.004. [DOI] [PubMed] [Google Scholar]
- Wardle MC, Fitzgerald DA, Angstadt M, et al. The caudate signals bad reputation during trust decisions. PLoS ONE. 2013;8:e68884. doi: 10.1371/journal.pone.0068884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrier V, Grasby KL, Uzefovsky F, et al. Genome-wide meta-analysis of cognitive empathy: heritability, and correlates with sex, neuropsychiatric conditions and cognition. Mol Psychiatry. 2018;23:1402–1409. doi: 10.1038/mp.2017.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JHG, Waiter GD, Perra O, et al. An fMRI study of joint attention experience. Neuroimage. 2005;25:133–140. doi: 10.1016/j.neuroimage.2004.10.047. [DOI] [PubMed] [Google Scholar]
- Wolf I, Dziobek I, Heekeren HR. Neural correlates of social cognition in naturalistic settings: a model-free analysis approach. Neuroimage. 2010;49:894–904. doi: 10.1016/j.neuroimage.2009.08.060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.