Abstract
Background
Optimal pain management is key to successful recovery in revision total hip arthroplasty. Lumbar plexus blocks (LPBs) have traditionally been used for postoperative pain management. Recently, the lumbar erector spinae plane block (LESPB) has emerged as a promising regional anesthesia technique and is relatively simple to perform. Our study aimed to evaluate whether continuous LESPB provided better analgesia and clinical outcomes than continuous LPB in revision hip arthroplasty.
Material and methods
We compared 25 LPBs with 25 LESPBs performed from October 2017 to November 2018 for revision hip arthroplasty. The primary outcome of this study was difference in opioid consumption between the groups at 24 hours postoperatively. Secondary outcomes include pain scores, hospital lengths of stay, pain adjunct consumption, and incidence of postoperative nausea and vomiting.
Results
There was no significant difference in average opioid consumption between the LPB and LESPB groups during the first 24 hours postoperatively (73.8 ± 68.1 mg vs 85.1 ± 69.7 mg, respectively, P = .57). Similarly, there was no significant difference in average pain scores (3.3 ± 2.1 vs 3.7 ± 1.8, respectively, P = .42).
Conclusions
There was no significant difference in opioid consumption and pain scores in patients with continuous LESPB compared with those with continuous LPB. While our study did not show a difference in these outcomes, the LESPB is a straightforward regional block that avoids many of the risks of LPBs and may be as effective for pain control.
Keywords: Hip arthroplasty, Erector spinae plane block, Lumbar plexus block, Regional anesthesia, Analgesia, Nerve block
Introduction
Revision total hip arthroplasty (THA) procedures have increased steadily in the United States over the past decade as more patients undergo joint replacement [1]. Optimal anesthetic management and pain control are critical to enhanced recovery, successful rehabilitation, and prevention of chronic pain [[2], [3], [4], [5]]. Pain control after THA can be challenging because of complex innervation of the hip joint from both the lumbar and sacral nerve plexus [6]. Lumbar plexus blocks (LPBs) have been shown to reduce opioid requirements, opioid-related side effects, and enhance satisfaction in patients undergoing THA compared with patients without a block [[7], [8], [9]]. However, there are several disadvantages of LPBs. They are considered to be anatomically deeper blocks that can be challenging to perform and similar to neuraxial procedures, must adhere to American Society of Regional Anesthesia anticoagulation guidelines [10,11]. The lumbar paravertebral region is also highly vascular, and inadvertent intravascular injection and formation of hematomas have been described [12,13]. Although ultrasound can be used during these blocks, visualization of the lumbar plexus with ultrasound is not always successful because of deep anatomy and can be technically challenging to perform [11]. Furthermore, adverse effects such as bilateral or epidural spread of local anesthetic are not uncommon [14].
The erector spinae plane block was first described by Forero for neuropathic thoracic pain in 2016, and since then, this interfascial plane block has been used successfully in a variety of surgeries [[15], [16], [17]]. Tulgar and Senturk modified this block to be performed at the lumbar level in 2018 [18]. Several case reports and an observational study have demonstrated effective pain control in THA when a lumbar erector spinae plane block (LESPB) with or without catheter has been used [[19], [20], [21], [22]]. Imaging studies suggest that local anesthetic in LESPBs surrounds the psoas muscle, leading to blockade of the lumbar plexus and, thus, essentially functions as a LPB by proxy [20,21,23,24]. LESPBs are also routinely performed under ultrasound, which has improved the overall performance, accuracy, and safety of peripheral nerve blocks in arthroplasty [25].
Based on these reported benefits, some anesthesiologists at our institution have recently chosen to perform an LESPB along with nerve catheter placement for revision THA in lieu of a LPB with catheter. However, because the LESPB is still a relatively new regional technique, there is a paucity of data evaluating the analgesic efficacy of this block compared with LPBs. Given the proposed mechanism of action of the LESPB described previously, we hypothesize that the continuous LESPB would provide better analgesia than continuous LPB. To evaluate this hypothesis, we performed a retrospective cohort study of patients undergoing revision THA and reviewed postoperative opioid requirements and pain scores.
Material and methods
We obtained approval from the institutional review board before this study. We evaluated 50 revision THAs performed between October 2017 and November 2018. Twenty-five of these patients received an LPB and peripheral nerve catheter, and the other 25 patients received an LESPB with catheter. Patients undergoing revision THA at our institution historically received an LPB with catheter in the preoperative area before surgery. However, starting in May 2018, we slowly transitioned to performing preoperative LESPBs with catheters for these patients. For this study, we did a preliminary analysis of the first 25 revision THAs performed with continuous LESPB and compared these patients with the last 25 patients who received continuous LPB for revision THA.
Inclusion criteria were patient age 18 years or older undergoing revision hip surgery who received a preoperative LPB or LESPB with peripheral nerve catheter placement. Two conversion hip revision surgeries were included in our study. These conversions were similar in technical complexity, surgical length, blood loss, and need for revision-type approaches and implants compared with our other revision hip surgeries and, thus, were included in the study. We did not include patients with opioid tolerance, ie, those patients taking more than 60 morphine milligram equivalents (MMEs) per day, as defined by the Food and Drug Administration [26]. In order to limit data collection error, multiple study personnel collected data on each patient.
The primary outcome of this study was difference in opioid consumption, as measured in MMEs, between the LESPB and LPB groups at 24 hours postoperatively, starting from the time the patient first entered the postanesthesia care unit (PACU). Secondary outcomes included MME consumption at 24-48 hours postoperatively, Numeric Rating Scale (NRS) pain scores at 0-24 and 24-48 hours postoperatively, PACU and hospital lengths of stay, procedure time for regional block, and incidence of postoperative nausea and vomiting (PONV). We also compared the 2 groups in terms of demographic characteristics (age, gender, height, weight, body mass index, American Society of Anesthesiologists [ASA] class, comorbidities, baseline opioid use, type of anesthesia, duration of local anesthetic infusion, type of revision surgery, and length of surgical procedure).
We used our institutional Epic (Epic Systems Corporation, Verona, WI) electronic medical records (EMRs) to record opioids consumed by the patients. Total opioid consumption was converted into MMEs through MDCalc application [27]. NRS pain scores (on a scale of 0 – 10, with 0 being no pain and 10 being the worst pain imaginable) were documented into the EMR by nursing staff every 15 minutes to 1 hour in the PACU and every 4 hours on the hospital floor as per standard at our institution. We collected these pain scores from PACU stay, defined as the 0-hour time point, up to 48 hours postoperatively. Individual patient pain scores were averaged over a 24-hour period, and then the average pain score for the entire group was calculated. If a pain score at a particular time point was not documented, it was not included in the calculation of an individual’s average pain score.
All other pertinent data were also collected from the EMR. Time length of block placement was collected from the standardized anesthesiologist procedure note. The anesthesia intraoperative record was used to document surgery length. We used the administration of any antiemetic drug during the patient’s hospitalization as a surrogate parameter to estimate the rates of PONV. Duration of postoperative local anesthetic infusion through the nerve catheter was obtained from the nursing medication administration record.
As per our usual institutional practice, all patients were written for a multimodal analgesic regimen before anesthesia consisting of celecoxib 200 mg, gabapentin 600 mg, and oral acetaminophen 1000 mg, provided there were no contraindications. The nerve stimulator–guided LPBs were placed either in the lateral decubitus or the sitting position, using one of the 2 established approaches—the classic approach by Chayen et al. [28] or a modification of this approach by Hadzic [29]. The block needle tip was directed into the posterior aspect of the psoas muscle where the plexus runs to elicit a twitch of the quadratus femoris muscle. Once this was achieved, 20-30 milliliters of ropivacaine 0.2% was injected, and an indwelling peripheral nerve catheter was placed.
For the LESPBs, the patients were placed in the prone position, and a curvilinear ultrasound probe was used to identify the L4 transverse process on the ipsilateral side of surgery [18,20]. A block needle was inserted from caudad to cephalad, and once the needle tip contacted the L4 transverse process, 20-40 milliliters of ropivacaine 0.2% was injected into the plane underneath the erector spinae muscle, and an indwelling nerve catheter was inserted.
Intraoperatively, the patients underwent either a general or a spinal anesthetic. Anesthesia technique and administration of intravenous fentanyl or hydromorphone were at the discretion of the anesthesia provider. Upon arrival in the PACU, a continuous infusion of ropivacaine 0.1% or 0.2% at 8 milliliters per hour was initiated through the indwelling peripheral nerve catheter. Patients were also written for additional intravenous fentanyl or hydromorphone as well as oral opioids as needed, per our usual institutional PACU orders. Postoperative pain adjunct administration in the PACU or on the floor was recorded for 48 hours after surgery. All patients were followed up by the acute pain service postoperatively, and if not contraindicated, received acetaminophen, gabapentin, and either celecoxib or intravenous ketorolac. Peripheral nerve catheters were typically removed on postoperative day 1.
For the statistical analysis of the results of the 2 groups, we used student’s two-sided t-tests for continuous variables with P < .05 as threshold for statistical significance. To compare categorical variables, we used Pearson’s chi-squared test or Fisher’s exact test, depending on the number of events per group. The two-sided t-test analyses were performed using Microsoft Excel, Version 16.20 (Microsoft Corporation, Redmond, WA). We used Social Science Statistics (Jeremy Stangroom; https://www.socscistatistics.com/) for Pearson’s chi-squared tests and Fischer’s exact test [30]. For our study time frame, placement of an LESPB with catheter was solely dependent on the availability of a few anesthesiologists who were trained and competent in performing this block. If none of these anesthesiologists were available on a particular day, the patient would receive a standard LPB with catheter. We hypothesized that this would lead to a quasi-random group assignment and, as such, used univariate analysis to compare outcomes between the LPB and LESPB groups.
Results
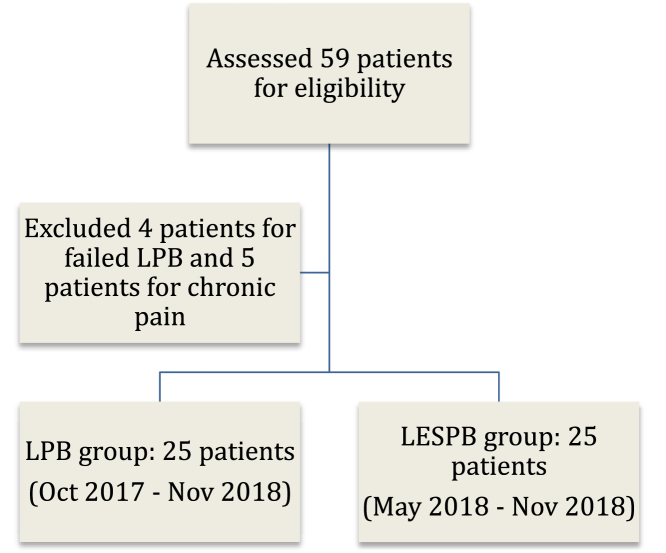
We analyzed 50 patients who underwent revision THAs. We evaluated a total of 59 patients and excluded 9 (Fig. 1). The LPB and LESPB groups were comparable in terms of demographic data and patient characteristics, as shown in Table 1.
Figure 1.
Flow diagram depicting creation of study groups.
Table 1.
Patient characteristics.
| Patient variables | LPB (n = 25) | LESPB (n = 25) | P value |
|---|---|---|---|
| Age | 66.1 (9.9) | 66.5 (13.9) | .91 |
| Female gender | 13 (52) | 16 (64) | .39 |
| Height (cm) | 168.6 (11.6) | 169.2 (10.1) | .85 |
| Weight (kg) | 80.6 (18.8) | 81 (20.4) | .94 |
| BMI (kg/m2) | 28.3 (6.1) | 28.4 (7.6) | .96 |
| Baseline pain score (NRS) | 3.1 (3.3) | 3.5 (3.2) | .67 |
| Baseline MME usage (mg) | 11.8 (17.2) | 10.7 (14.3) | .81 |
| ASA classification | .96 | ||
| I | 2 (8) | 2 (8) | |
| II | 12 (48) | 11 (44) | |
| III | 11 (44) | 12 (48) | |
| Hypertension | 12 (48) | 15 (60) | .39 |
| Diabetes mellitus | 5 (20) | 8 (32) | .33 |
| Obesity (BMI ≥ 30) | 6 (24) | 7 (28) | .75 |
| Depression/anxiety | 4 (16) | 3 (12) | .68 |
| Atrial fibrillation | 2 (8) | 4 (16) | .38 |
| Chronic kidney disease | 3 (12) | 4 (16) | .68 |
Continuous variables are presented as mean (standard deviation); categorical variables are presented as count (percentage). P < .05 is statistically significant.
ASA, American Society of Anesthesiologists; BMI, body mass index.
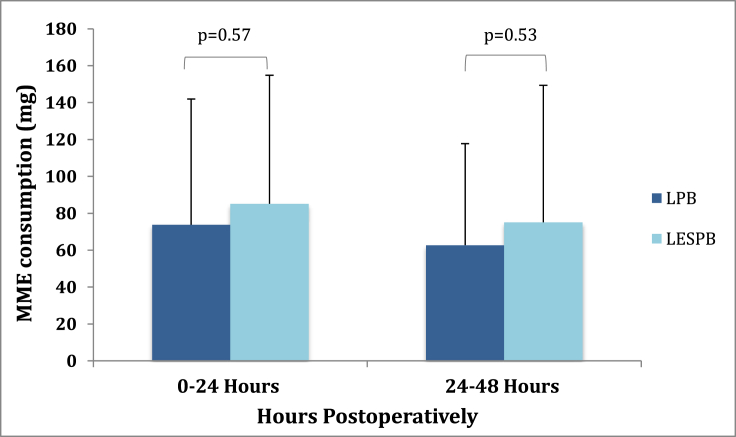
At 0-24 hours postoperatively, there was no significant difference in opioid consumption between the LPB and LESPB groups (73.8 ± 68.1 mg vs 85.1 ± 69.7 mg, respectively, P = .57; shown in Figure 2). Mean difference between MME consumption in this time period was 11.3 mg (95% confidence interval [CI] -27.89 to 50.49). During the 24- to 48-hour postoperative time period, the LPB groups averaged 62.7 ± 55.0 mg in MME consumption, while the LESPB group averaged 75.1 ± 74.3 mg (P = .53). Mean difference was 12.4 mg (95% CI -24.77 to 49.57).
Figure 2.
MME consumption in milligrams (mg) at 0-24 and 24-48 hours after surgery. Error bars indicate standard deviations.
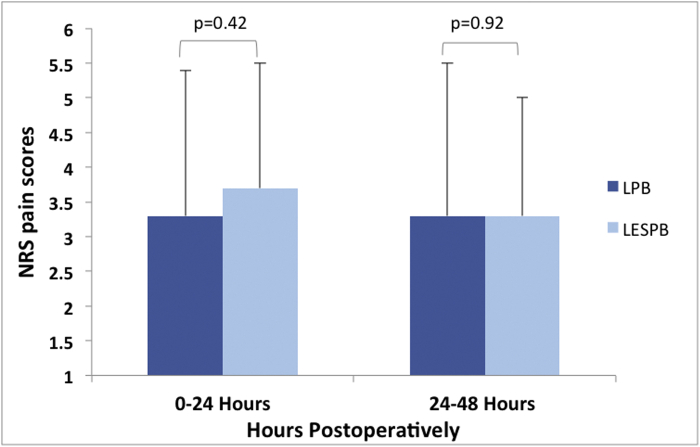
The average NRS pain scores (Fig. 3) during the 0- to 24-hour postoperative period in the LPB group was 3.3 ± 2.1 vs 3.7 ± 1.8 in the LESPB patients (P = .42). Average pain score during the 24- to 48-hour postoperative period was 3.3 ± 2.2 in the LPB group vs 3.3 ± 1.7 in the LESPB group (P = .92).
Figure 3.
Average NRS pain scores (0-10) at 0-24 and 24-48 hours after surgery. Error bars indicate standard deviations.
In the 4 patients in whom technical difficulties precluded a successful LPB, they received either an alternative block or no blocks at all. Of note, there were no technical difficulties that resulted in an aborted LESPB. No regional block complications were reported for either group. Duration of postoperative local anesthetic infusion averaged 26.1 ± 8.8 hours in the LPB group and 20 ± 9.5 hours in the LESPB group (mean difference 6.1 hours, 95% CI 0.89 to 11.31 hours, P = .03; also shown in Table 2).
Table 2.
Surgery and perioperative data.
| Patient variables | LPB (n = 25) | LESPB (n = 25) | P value |
|---|---|---|---|
| General anesthesia (GA) | 19 (76) | 21 (84) | .48 |
| Spinal anesthesia | 5 (20) | 4 (16) | .71 |
| Conversion from spinal to GA | 1 (4) | 0 (0) | 1.00 |
| Hospital length of stay (h) | 74.8 (31.9) | 80.9 (75.3) | .71 |
| Block procedure time (min) | 22.6 (6.2) | 27.4 (18.1) | .22 |
| Surgery time (min) | 185.1 (86.8) | 164.2 (49.9) | .30 |
| Performed by primary surgeon | 11 (44) | 13 (52) | .57 |
| Duration of local anesthetic infusion (h) | 26.1 (8.8) | 20 (9.5) | .03 |
| PONV | 7 (28) | 8 (32) | .76 |
Continuous variables are presented as mean (standard deviation); categorical variables are presented as count (percentage). P < .05 is statistically significant.
The percentage of patients consuming perioperative adjunct pain medications, such as acetaminophen, gabapentin, and nonsteroidal anti-inflammatory drug (NSAID), is recorded in Table 3. There was a significant difference in the number of patients receiving postoperative NSAID. All 25 patients in the LPB group received postoperative NSAID, while 18 patients in the LESPB group did (P = .01). There were no significant differences in other adjunct pain medication consumption. Antiemetics were administered to 7 LPB patients vs 8 LESPB patients, as shown in Table 2.
Table 3.
Perioperative pain adjunct consumption.
| Patient variables | LPB (n = 25) | LESPB (n = 25) | P-value |
|---|---|---|---|
| Preoperative adjunct | |||
| Acetaminophen | 22 (88) | 23 (92) | 1.00 |
| Gabapentin | 19 (76) | 17 (68) | .53 |
| Celecoxib | 18 (72) | 12 (48) | .08 |
| Postoperative adjunct | |||
| Acetaminophen | 25 (100) | 25 (100) | 1.00 |
| Gabapentin | 25 (100) | 24 (96) | 1.00 |
| NSAID PO and/or IVa | 25 (100) | 18 (72) | .01 |
Adjuncts presented as count (percentage). P < .05 is statistically significant.
PO, per os; IV, intravenous.
Patient received PO celecoxib and/or IV ketorolac.
Hospital length of stay averaged 74.8 ± 31.9 hours among the LPB patients and 80.9 ± 75.3 hours among the LESPB patients (mean difference 6.1 hours, 95% CI −26.79 to 38.99). The average surgery time in the LPB group was 185 ± 86.8 minutes and 164 ± 49.9 minutes in the LESPB group (mean difference -21 minutes, 95% CI −19.26 to 61.26). In regard to anesthesia type, 19 patients (76%) in the LPB group and 21 patients (84%) in the LESPB group received general anesthesia. There was one conversion from spinal anesthesia to general anesthesia in the LPB group due to failed spinal. The catheter was left in place for postoperative pain control.
We collected data on the surgical aspects of each revision hip arthroplasty as well. In terms of the complexity of each procedure, both groups had similar numbers of each type of revision such as femur and/or acetabular exchanges, isolated head or liner exchanges, soft tissue reconstruction, and need for extended trochanteric osteotomy. These characteristics are documented in Table 4. As described earlier, the 2 conversion surgeries were similar to our other revision hip surgeries in regard to technical complexity and need for revision-type approaches and implants. One conversion hip revision consisted of an extended trochanteric osteotomy with revision femoral components and open reduction internal fixation of the osteotomy. Another conversion required extended surgical dissection and exposure as well as revision-type femoral component. Finally, while these cases were performed by multiple surgeons, one surgeon (E.N.H.) performed the highest number of surgeries in both groups; 12 out of 25 of the LPB surgeries and 13 out of 25 of the LESPB group (Table 2).
Table 4.
Revision hip arthroplasty characteristics.
| Patient variables | LPB | LESPB |
|---|---|---|
| Isolated head/liner exchange | 3 | 4 |
| Single component-femur | 4 | 3 |
| Single component-acetabulum | 9 | 7 |
| Both components | 8 | 9 |
| Conversion | 0 | 2 |
| Soft tissue reconstruction | 2 | 3 |
| ETO | 3 | 4 |
This table shows the number of patients receiving each type of revision.
ETO, extended trochanteric osteotomy; LPB, lumbar plexus block; LESPB, lumbar erector spinae plane block.
Discussion
Our retrospective study comparing LPBs and LESPBs in revision hip arthroplasty showed no significant difference in opioid consumption in the first 24 hours postoperatively. Furthermore, we found no significant difference in our secondary outcomes of postoperative NRS pain scores, opioid consumption at 24-48 hours postoperatively, PACU and hospital lengths of stay, procedure block time, and rates of PONV. The LPB patients had a significantly longer duration of ropivacaine infusion postoperatively than the LESPB patients. In addition, there were significantly fewer patients who received postoperative NSAID in the LESPB group, likely due to a contraindication or allergy. Despite having a shorter duration of ropivacaine infusion, patients in the LESPB group did not have significantly higher opioid consumption or pain scores than the LPB group. If this had any influence on opioid consumption and pain scores, the results would favor LESPB for postoperative pain management in revision THA.
As it is a relatively new regional anesthesia technique, there are currently few published studies investigating the effectiveness of LESPB for pain control in hip surgery. This is in contrast to LPBs, which have been used for decades and shown to reduce opioid requirements and enhance satisfaction in patients undergoing THA [[7], [8], [9]]. Tulgar first described the LESPB at the level of the L4 transverse process for a patient undergoing THA, and a subsequent case series demonstrated adequate postoperative pain control [18,20]. An observational study analyzed 15 patients who underwent hemiarthroplasty or intramedullary nailing with an LESPB and sedation. None of the patients had to be converted to general anesthesia or required local anesthetic infiltration at the surgical site, suggesting that LESPB can be used as the main anesthetic in elderly hip fracture patients with multiple medical comorbidities [21]. Finally, in a randomized, prospective feasibility study, investigators found that patients who received an LESPB for hip surgery had significantly lower pain scores, tramadol consumption, and rescue opioid requirement than patients with no block, demonstrating that LESPB can be an effective mode of postoperative pain control [31]. All these studies suggest potential benefits of LESPB in hip surgery.
Kinjo and Schultz reported 2 cases in which patients received continuous LESPBs for revision hip arthroplasty with both patients demonstrating satisfactory pain control [32]. As there are few studies on LESPB in hip surgery, we decided to compare the effectiveness of this relatively new block with the well-described LPB. To our knowledge, there has not been a study comparing continuous LESPBs to continuous LPBs for postoperative pain control in revision hip arthroplasty.
The LESPB has a low risk of complications based on existing studies and is relatively straightforward to perform [33]. The more readily identifiable anatomy of the LESPB facilitates the use of ultrasound and may therefore have a lower risk of injury to blood vessels and other deep structures such as the kidney [34]. Blocks performed under ultrasound guidance can greatly reduce risk of vascular puncture and hematoma formation [35]. Paravertebral spread is also thought be to less likely with injection of local anesthetic in lumbar erector spinae region, and the risk of neuraxial spread is predicted to be much lower than LPB [24]. Furthermore, in regard to patient comfort, several studies demonstrated significantly lower procedure-related pain scores in patients receiving an ultrasound-guided nerve block than those in patients undergoing the same block with nerve stimulator, which is how LPBs are often placed [36,37]. The LPB must also adhere to American Society of Regional Anesthesia anticoagulation guidelines and is an anatomically deeper block that can be challenging to place [10,11]. In addition to the higher risk of hematoma formation, inadvertent intravascular injection has also been described. One study reported one event of cardiac arrest, 2 respiratory failures, one seizure, and one death after LPB in a sample size of only 394 patients [12]. Even with the use of ultrasound in LPB and reduction in injectate volume, epidural spread of local anesthetic can still occur [38].
Our study has several limitations. The retrospective nature of our study did not allow for randomization, and confounders cannot be excluded. We were not able to conclude equivalence between the 2 blocks. We attempted to assess potential differences between LPBs and LESPBs by evaluating patients undergoing a type of surgery at our institution, where patients typically receive standardized perioperative pain adjuncts. However, some medications, particularly NSAIDs, were not administered because of patient allergy or other contraindication such as chronic kidney disease, which is a limitation and could have potentially affected postoperative MME consumption or pain scores. In addition, a few patients in both LPB and LESPB groups received a slightly higher local anesthetic concentration for their catheter infusion. No standardized study protocol was followed because of the retrospective nature of this study, and some natural variability in clinical care occurred.
Conclusions
We did not find a significant difference in postoperative opioid consumption and pain scores in patients undergoing revision THA with continuous LESPB compared with those with continuous LPB. While our study did not show a difference in these outcomes, the LESPB is a relatively straightforward regional block that might avoid many of the risks of LPBs, and we currently have no evidence that it is less effective for pain control. Further randomized controlled trials are warranted to support the role of LESPBs in hip surgery.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Appendix A. Supplementary data
References
- 1.Kremers H.M., Larson D.R., Crowson C.S. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2014;97:1386. doi: 10.2106/JBJS.N.01141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hebl J.R., Dilger J.A., Byer D.E. A pre-emptive multimodal pathway featuring peripheral nerve block improves perioperative outcomes after major orthopedic surgery. Reg Anesth Pain Med. 2008;33:510. [PubMed] [Google Scholar]
- 3.Horlocker T.T., Kopp S.L., Pagnano M.W., Hebl J.R. Analgesia for total hip and knee arthroplasty: a multimodal pathway featuring peripheral nerve block. J Am Acad Orthop Surg. 2006;14:126. doi: 10.5435/00124635-200603000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Gaffney C.J., Pelt C.E., Gililland J.M., Peters C.L. Perioperative pain management in hip and knee arthroplasty. Orthop Clin North Am. 2017;48:407. doi: 10.1016/j.ocl.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Stein B.E., Srikumaran U., Tan E.W., Freehill M.T., Wilckens J.H. Lower-extremity peripheral nerve blocks in the perioperative pain management of orthopaedic patients. J Bone Joint Surg Am. 2012;94:e167. doi: 10.2106/JBJS.K.01706. [DOI] [PubMed] [Google Scholar]
- 6.Birnbaum K., Prescher A., Heßler S., Heller K.D. The sensory innervation of the hip joint - an anatomical study. Surg Radiol Anat. 1997;19:371. doi: 10.1007/BF01628504. [DOI] [PubMed] [Google Scholar]
- 7.Siddiqui Z.I., Cepeda M.S., Denman W., Schumann R., Carr D.B. Continuous lumbar plexus block provides improved analgesia with fewer side effects compared with systemic opioids after hip arthroplasty: a randomized controlled trial. Reg Anesth Pain Med. 2007;32:393. doi: 10.1016/j.rapm.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Stevens R.D., Van Gessel E., Flory N., Fournier R., Gamulin Z. Lumbar plexus block reduces pain and blood loss associated with total hip arthroplasty. Anesthesiology. 2000;93:115. doi: 10.1097/00000542-200007000-00021. [DOI] [PubMed] [Google Scholar]
- 9.Marino J., Russo J., Kenny M., Herenstein R., Livote E., Chelly J.E. Continuous lumbar plexus block for postoperative pain control after total hip arthroplasty. J Bone Joint Surg Am. 2009;91:29. doi: 10.2106/JBJS.H.00079. [DOI] [PubMed] [Google Scholar]
- 10.Horlocker T.T., Vandermeuelen E., Kopp S.L., Gogarten W., Leffert L.R., Benzon H.T. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Cociety of Regional Anesthesia and Pain Medicine evidence-based guidelines (Fourth Edition) Reg Anesth Pain Med. 2018;43:263. doi: 10.1097/AAP.0000000000000763. [DOI] [PubMed] [Google Scholar]
- 11.Hadzic A. McGraw-Hill Education LLC.; New York, NY: 2017. Hadzic’s textbook of regional anesthesia and acute pain management. [Google Scholar]
- 12.Auroy Y., Benhamou D., Bargues L. Major complications of regional anesthesia in France: the SOS regional anesthesia hotline service. Anesthesiology. 2002;97:1274. doi: 10.1097/00000542-200211000-00034. [DOI] [PubMed] [Google Scholar]
- 13.Pham-Dang C., Beaumont S., Floch H., Bodin J., Winer A., Pinaud M. Acute toxic accident following lumbar plexus block with bupivacaine. Ann Fr Anesth Reanim. 2000;19:356. [PubMed] [Google Scholar]
- 14.Gadsden J.C., Lindenmuth D.M., Hadzic A., Xu D., Somasundarum L., Flisinski K.A. Lumbar plexus block using high-pressure injection leads to contralateral and epidural spread. Anesthesiology. 2008;109:683. doi: 10.1097/ALN.0b013e31818631a7. [DOI] [PubMed] [Google Scholar]
- 15.Forero M., Adhikary S.D., Lopez H., Tsui C., Chin K.J. The erector spinae plane block: a novel analgesic technique in thoracic neuropathic pain. Reg Anesth Pain Med. 2016;41:621. doi: 10.1097/AAP.0000000000000451. [DOI] [PubMed] [Google Scholar]
- 16.Tsui B.C.H., Fonseca A., Munshey F., McFadyen G., Caruso T.J. The erector spinae plane (ESP) block: a pooled review of 242 cases. J Clin Anesth. 2019;53:29. doi: 10.1016/j.jclinane.2018.09.036. [DOI] [PubMed] [Google Scholar]
- 17.Kot P., Rodriguez P., Granell M. The erector spinae plane block: a narrative review. Korean J Anesthesiol. 2019;72:209. doi: 10.4097/kja.d.19.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tulgar S., Senturk O. Ultrasound guided Erector Spinae Plane block at L-4 transverse process level provides effective postoperative analgesia for total hip arthroplasty. J Clin Anesth. 2018;44:68. doi: 10.1016/j.jclinane.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Tulgar S., Senturk O. Ultrasound guided Erector Spinae Plane block at L-4 transverse process level provides effective postoperative analgesia for total hip arthroplasty. J Clin Anesth. 2018;44:68. doi: 10.1016/j.jclinane.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Tulgar S., Selvi O., Senturk O., Ermis M.N., Cubuk R., Ozer Z. Clinical experiences of ultrasound-guided lumbar erector spinae plane block for hip joint and proximal femur surgeries. J Clin Anesth. 2018;47:5. doi: 10.1016/j.jclinane.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 21.Ahiskalioglu A., Tulgar S., Celik M., Ozer Z., Alici H.A., Aydin M.E. Lumbar erector spinae plane block as a main anesthetic method for hip surgery in high risk elderly patients: initial experience with a magnetic resonance imaging. Eur J Med. 2020;52:16. doi: 10.5152/eurasianjmed.2020.19224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bugada D., Zarcone A.G., Manini M., Lorini L.F. Continuous Erector Spinae Block at lumbar level (L4) for prolonged postoperative analgesia after hip surgery. J Clin Anesth. 2019;52:24. doi: 10.1016/j.jclinane.2018.08.023. [DOI] [PubMed] [Google Scholar]
- 23.Kose H.C., Kose S.G., Thomas D.T. Lumbar versus thoracic erector spinae plane block: similar nomenclature, different mechanism of action. J Clin Anesth. 2018;48:1. doi: 10.1016/j.jclinane.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 24.Tulgar S., Balaban O. Spread of local anesthetic in erector spine plane block at thoracic and lumbar levels. Reg Anesth Pain Med. 2019;44:134. doi: 10.1136/rapm-2018-000027. [DOI] [PubMed] [Google Scholar]
- 25.Elmofty D.H., Buvanendran A. Regional anesthesia in total joint arthroplasty: what is the evidence? J Arthroplasty. 2017;32:S74. doi: 10.1016/j.arth.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 26.FDA briefing document joint meeting of the arthritis advisory committee and the drug safety and risk management advisory committee. 2018. https://www.fda.gov/advisory-committees/advisory-committee-calendar/june-11-12-2019-joint-meeting-drug-safety-and-risk-management-advisory-committee-and-anesthetic-and [accessed 01.08.20]
- 27.Motov S.M. Morphine milligram equivalents (MME) calculator - MDCalc. MD+Calc. 2020. https://www.mdcalc.com/morphine-milligram-equivalents-mme-calculator#use-cases [accessed 06.05.20]
- 28.Chayen D., Nathan H., Chayen Psoas compartment block. Anesthesiology. 1976;41:95. doi: 10.1097/00000542-197607000-00019. [DOI] [PubMed] [Google Scholar]
- 29.Hadzic A. McGraw-Hill Professional; New York; Toronto: 2012. Anesthesia. NYS of R. Hadzic’s peripheral nerve blocks and anatomy for ultrasound-guided regional anesthesia. [Google Scholar]
- 30.Stangroom J. Social Science Statistics. 2018. https://www.socscistatistics.com/ [accessed 06.05.20]
- 31.Tulgar S., Kose H.C., Selvi O. Comparison of ultrasound-guided lumbar erector spinae plane block and transmuscular quadratus lumborum block for postoperative analgesia in hip and proximal femur surgery: a prospective randomized feasibility study. Anesth Essays Res. 2018;12:825. doi: 10.4103/aer.AER_142_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kinjo S., Schultz A. Continuous lumbar erector spinae plane block for postoperative pain management in revision hip surgery: a case report. Braz J Anesthesiol. 2019;69:420. doi: 10.1016/j.bjane.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kot P., Rodriguez P., Granell M. The erector spinae plane block: a narrative review. Korean J Anesthesiol. 2019;72:209. doi: 10.4097/kja.d.19.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chin K.J., Adhikary S., Forero M. Is the erector spinae plane (ESP) block a sheath block? A reply. Anaesthesia. 2017;72:916. doi: 10.1111/anae.13926. [DOI] [PubMed] [Google Scholar]
- 35.Warman P., Nicholls B. Ultrasound-guided nerve blocks: efficacy and safety. Best Pract Res Clin Anaesthesiol. 2009;23:313. doi: 10.1016/j.bpa.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 36.Tran D.Q.H., Clemente A., Tran D.Q., Finlayson R.J. A comparison between ultrasound-guided infraclavicular block using the “double bubble” sign and neurostimulation-guided axillary block. Anesth Analg. 2008;107:1075. doi: 10.1213/ane.0b013e31817ef259. [DOI] [PubMed] [Google Scholar]
- 37.Casati A., Danelli G., Baciarello M. A prospective, randomized comparison between ultrasound and nerve stimulation guidance for multiple injection axillary brachial plexus block. Anesthesiology. 2007;106:992. doi: 10.1097/01.anes.0000265159.55179.e1. [DOI] [PubMed] [Google Scholar]
- 38.Sauter A.R., Ullensvang K., Niemi G. The Shamrock lumbar plexus block. Eur J Anaesthesiol. 2015;32:764. doi: 10.1097/EJA.0000000000000265. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.